Abstract
OBJECTIVE
We developed a field procedure using personal digital assistant (PDA) technology to test the hypothesis that naturally occurring episodes of hypo- and hyperglycemia are associated with deterioration in cognitive function in children with type 1 diabetes.
RESEARCH DESIGN AND METHODS
A total of 61 children aged 6–11 years with type 1 diabetes received a PDA programmed with two brief cognitive tests (mental math and choice reaction time), which they completed just before home glucose readings. The computer recorded time to complete each test and number of correct responses. Children completed several trials per day over 4–6 weeks for a total of 70 trials. Performance variables were compared across glucose ranges. Individual impairment scores (IISs) were also computed for each child by calculating the SD between performance during euglycemia and that during glucose extremes.
RESULTS
Time to complete both mental math and reaction time was significantly longer during hypoglycemia. During hyperglycemia, time to complete math was significantly longer and reaction time was marginally significant (P = 0.053). There were no differences on task accuracy. Decline in mental math performance was equivalent at glucose levels <3.0 and >22.2 mmol/l. IISs varied greatly across children, with no age or sex differences.
CONCLUSIONS
A decrease in mental efficiency occurs with naturally occurring hypo- and hyperglycemic glucose fluctuations in children with type 1 diabetes, and this effect can be detected with a field procedure using PDA technology. With blood glucose levels >22.2 mmol/l, cognitive deterioration equals that associated with significant hypoglycemia.
In adults with type 1 diabetes, the negative impact of acute glucose extremes on cognitive and motor function is well documented. This is especially true for the disruptive effects of hypoglycemia, which have been demonstrated in numerous laboratory studies using insulin clamp techniques (1–5) and in field studies (6). More recently, there is growing evidence that acute hyperglycemia can also disrupt cognitive performance in adults with both type 1 and type 2 diabetes (6–8), although there are some discrepant findings (5). Evidence for cognitive deterioration has obvious clinical implications for people living withdiabetes, many of whom experiencehypo- and hyperglycemia on a relatively frequent basis. Such disruptions would also have clinical significance for children with type 1 diabetes; however, surprisingly few studies have examined these effects in pediatric populations. Only one published laboratory study (9) tested the impact of acute hypoglycemia in adolescents and found that even with relatively mild hypoglycemia (3.1–3.6 mmol/l), mental efficiency significantly decreased. Two laboratory studies have examined the impact of acute hyperglycemia in pediatric patients with mixed results, with one finding no effect in adolescents tested at 20 mmol/l (10) and the other finding a significant decline at glucose levels above 20 mmol/l (11).
One major barrier to investigating the effects of acute hypo- and hyperglycemia on cognitive function in pediatric populations is reluctance to induce extreme glucose levels, and possible neurological insult, in younger patients with developing brains (12). However, glucose excursions more extreme than those induced in studies occur routinely outside of the laboratory in children with type 1 diabetes. Therefore, one way to bypass the ethical problems that arise in neurocognitive research involving pediatric patients is to develop experimental procedures that take advantage of these naturally occurring hypo- and hyperglycemic episodes. We have previously used personal digital assistant (PDA) technology to investigate the impact of daily hyperglycemia, including postprandial increases, on cognitive performance in adults with type 1 and type 2 diabetes (6,7). Patients performed brief cognitive tests on a PDA before home glucose measurements, repeating 50–70 trials over 1 month. A comparison of performance during hyperglycemia (>15 mmol/l) and euglycemia showed a significant decline at higher glucose levels. There was, however, large individual variability in the effects of hyperglycemia, with ∼50% of adults showing clinically significant disruption.
The purpose of this study was to develop and test a similar computerized field procedure to assess cognitive performance at different glucose levels in school-aged youth with type 1 diabetes. This field procedure was then used to test the hypothesis that children experience significant disruptions in cognitive performance in their daily lives due to naturally occurring episodes of both acute hypo- and hyperglycemia. Children performed two brief cognitive tests on a PDA just before home glucose measurements three to five times per day over a period of ∼1 month, completing a total of 70 trials. After completing each test, children made subjective ratings of the difficulty of performing the task to determine the degree to which they were subjectively aware of changes in cognitive function. Individual impairment scores (IISs) were computed for each child to examine relationships between cognitive disruption and several demographic and clinical variables,including age, sex, diabetes duration, history of severe hypoglycemia, and metabolic control.
RESEARCH DESIGN AND METHODS
Children participating in this study were taking part in a larger project investigating family management of diabetes, which required them to complete surveys programmed on PDA computers several times each day. Although later phases of the larger project involved interventions, including blood glucose awareness training, data for this study was collected during an earlier phase of the project that did not involve any intervention. Families were recruited for the study through pediatric endocrine clinics at the University of Virginia and Joslin Diabetes Center in Boston, as well as through advertisements and regional parent support groups. Inclusion criteria for children were age 6–11 years, a diagnosis of type 1 diabetes for at least 1 year, willingness to perform glucose measurements 3–5 times daily, and ability to read, complete questionnaires, and use the PDA. Inclusion criteria for parents included ability to complete the study protocol, role as main diabetes caregiver for the child, and the absence of any self-reported significant psychiatric disorder, including depression and substance abuse. Eligible families were invited to orientation meetings during which the study was explained and institutional review board–approved informed consent/assent was obtained.
A total of 77 families entered the study, and 66 parent-child pairs completed the protocol. Reasons for withdrawal for nine families included relocation, family stressors, and illness in the child. Two other families withdrew because the child did not want to continue completing the PDA surveys. For two families, PDA data were lost in the mail, and they chose not to repeat the procedure. An additional three families had missing data that excluded them from data analysis. Thus, the final sample of children with complete data was 61 (University of Virginia n = 31, Joslin n = 30). There were no significant demographic or clinical differences between families who completed and did not complete the study. Children received a $35 toy or bookstore gift card for completing data collection.
The final sample included 31 girls and 30 boys, whose mean ± SD age was 8.83 ± 1.6 years and diabetes duration was 4.7 ± 2.6 years, with 25 children aged 6–8 and 36 aged 9–11 years. A1C measures ranged from 6.7 to 10.4% (7.9 ± 0.68%). No children had repeated a grade in school. Almost all families were Caucasian (95%), and most parents had at least some college education (mean years of school: 15.8 ± 2.6).
Questionnaires
Children completed the State-Trait Anxiety Scale and the Children's Depression Inventory, measures that are widely used in research, with well-documented reliability and validity (13,14). Parents also completed a questionnaire about the child's diabetes history, including the past frequency of mild, moderate, and severe hypoglycemic episodes. Severe hypoglycemia was defined for parents as episodes during which their child was incapable of self-treatment or asking for treatment due to mental confusion, stupor, unconsciousness, or seizure. Moderate episodes were defined as those in which hypoglycemia significantly disrupted routine or ongoing behavior (e.g., the child could not continue with current activities). Mild episodes were defined as those associated with warning symptoms that quickly resolved after treatment and did not significantly disrupt function. Parents reported the frequency of severe and moderate episodes over the past year and reported the frequency of mild episodes over the past month.
Cognitive performance assessment
Families were provided with a Visor PDA programmed with cognitive tests and linked to a Freestyle Tracker BG meter to collect and store glucose readings (Abbot Diabetes Care, Abbott Labs, Alameda, CA). Families were asked to complete 3–5 PDA trials each day, for a total of 70 trials over the next 4–6 weeks. Children were unable to complete trials unless the parent was present and entered a password to start the program. After children completed the cognitive tests, the computer instructed parents and children to measure glucose. All data were automatically stored and time stamped, providing a validity check to insure that children completed cognitive tests before glucose measurement.
The PDA presented two cognitive tests, a mental math task and choice reaction time. These tests were chosen based on previous studies (3,6,7) showing that these tasks were sensitive to cognitive-motor disruptions in performance caused by blood glucose extremes in adults. Both tests were adapted for use by children. The mental math task consisted of 10 math problems, 5 additions and 5 subtractions, presented in random order. Children entered their solution by tapping numbers displayed on a number pad on the screen. For children ages 6–8 years, math problems and solutions contained only single-digit numbers. Children aged 9–11 years were given math problems containing one double-digit number <20 and one single-digit number. In the choice reaction-time task, the symbols for the four card suits (hearts, diamonds, spades, and clubs) were shown in color in the four corners of the screen. One card suit would appear in the center of the screen, and children “tapped” the matching card suit in one of the corners, with a total of 10 to match presented in random order. The computer tracked two performance measures, time to complete the task (in seconds) and number of problems correct. After completing each task, children rated their difficulty completing the task (e.g., how hard it was) on a visual analogue scale, where 0 = not at all and 6 = very hard.
After orientation and informed consent/assent, parents and children were given Visor computers and Freestyle meters and instructed on their use and were also given, questionnaires to complete and a stamped envelope for returning data. After return of PDA data, a blood sample kit was mailed to parents, who obtained blood from their children and returned the sample to the University of Virginia Clinical Laboratories for A1C measurement.
Data analysis
Two dependent measures were computed for each cognitive test: 1) time (in seconds) to complete the task (math time and reaction time) and 2) number of correct responses (math correct and reaction time correct). For math time and reaction time, higher numbers indicate more seconds to complete the task and therefore poorer performance. For math correct and reaction time correct, higher numbers indicate more accuracy and better performance. The data were analyzed 1) across subjects at different blood glucose ranges to examine the impact of glycemic status on cognitive function and 2) within subject, using Z scores, to determine whether individual subjects showed differences in the degree to which cognitive function declined at glucose extremes. For analyses across glucose levels, means for performance measures were computed for six clinically relevant ranges: <3.0 mmol/l (<54 mg/dl), 3.0–3.8 mmol/l (54–69 mg/dl), 3.9–9.9 mmol/l (70–179 mg/dl), 10–16.6 mmol/l (180–299 mg/dl), 16.7–22.1 mmol/l (300–399 mg/dl), and >22.2 mmol/l (>400 mg/dl). The ranges 16.7–22.1 mmol/l and higher than 22.2 mmol/l were specifically chosen for investigation because, in the state of Virginia, parents are called when children's glucose readings at school are higher than 16.7 mmol/l and sent home from school when readings are higher than 22.2 mmol/l (15).
IISs were also computed for each child, using the child's mean performance during euglycemia (4.3–9.9 mmol/l) as their individual baseline or “normal” performance. The difference, in Z scores, between mean baseline performance and mean performance during hypo- and hyperglycemia was then computed. IISs were computed for blood glucose levels <3.0 and >22.2 mmol/l. Thus, these impairment scores represented the mean number of SDs between performance during euglycemia and the most extreme blood glucose levels. The calculation of similar measures for individual impairment in adults with diabetes has been previously described in detail (6,7).
RESULTS
Performance across blood glucose levels
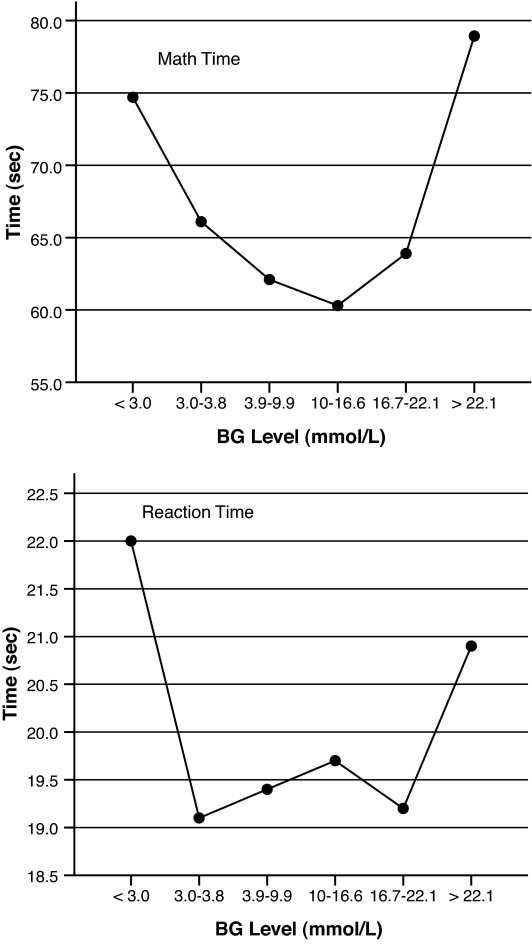
Fig. 1 shows the means and SDs for each of the four performance measures across blood glucose ranges. There were significant main effects for math time (F = 5.0, P < 0.001) and a strong trend for reaction time (F = 2.2, P = 0.053). Contrasts showed that compared with performance at euglycemia, math time was significantly longer when blood glucose was <3.0 mmol/l (P = 0.017) and >22.2 mmol/l (P = 0.0001). Reaction time was significantly longer at glucose levels <3.0 mmol/l (P = 0.01), with a trend toward significance when blood glucose was >22.2 mmol/l (P = 0.08). For both math time and reaction time, seconds to task completion did not differ for glucose levels <3.0 and >22.2 mmol/l, indicating that performance was equally poor in the lowest and highest blood glucose ranges for both tasks. Compared with performance during euglycemia, math time was an average of 12.6 and 16.8 s longer when blood glucose levels were <3.0 and >22.2 mmol/l, respectively. Reaction time was an average of 2.6 and 1.5 s slower when glucose levels were <3.0 and >22.2 mmol/l, respectively. In contrast to the results for time to perform tasks, there were no significant differences in the number of correct responses across ranges.
Figure 1.
Mental math time and reaction time across blood glucose (BG) ranges.
Exploratory analyses were also conducted to identify practice effects over time on the two tasks. Math time and reaction time over the first 35 trials were compared with the second 35 trials at each of the above three blood glucose ranges: euglycemia (3.9–9.9 mmol/l), hypoglycemia (<3.0 mmol/l), and hyperglycemia (>22.2 mmol/l). As expected, during euglycemia math time was significantly shorter for the second of 35 trials (56.9 vs. 66.8 s; F = 19.6, P = 0.001), but there was no improvement for reaction time (P = 0.13). When blood glucose was <3.0 or >22.2 mmol/l, there were no significant differences in math time or reaction time between the first and second half of the study, indicating no practice effects over time.
Perceived difficulty of task performance
Mean difficulty ratings tended to be low across all blood glucose ranges; however, significant differences were still found. For the math task, average ratings were 0.44, 0.77, and 0.58 for euglycemia, hypoglycemia, and hyperglycemia, respectively, with a significant main effect across blood glucose levels (F = 4.3, P < 0.0001). Mean difficulty ratings for the reaction time task were 0.15, 0.40, and 0.13, respectively, which also differed across blood glucose levels (F = 3.9, P < 0.001). However, contrasts showed that for both tasks, difficulty ratings were significantly higher only when glucose was <3.0 mmol/l (P < 0.01). This indicates that children perceived greater difficulty performing tasks during hypoglycemia but not when glucose levels were >22.2 mmol/l, even though time to complete tasks increased significantly and equivalently at both blood glucose extremes.
Individual differences
To examine individual differences in the impact of hypo- and hyperglycemia on performance, IISs were computed as described above for math time at blood glucose levels <3.0 and >22.2 mmol/l. These scores were only computed for those children who had blood glucose readings <3.0 mmol/l (n = 34) or >22.2 mmol/l (n = 41). Positive Z scores indicated poorer performance compared with euglycemia, and negative scores indicated better performance. Mean IIS for math time when blood glucose was <3.0 and >22.2 mmol/l were 0.57 ± 1.6 and 0.33 ± 1.1, respectively. There were no sex or age differences in IIS at either hypo- or hyperglycemia. A total of 21% of children had IISs higher than 1.0, indicating that performance deteriorated on average >1 SD when blood glucose was <3.0 mmol/l. When blood glucose was >22.2 mmol/l, 27% of children showed this degree of decline.
Exploratory correlations were computed between IIS and several clinical variables. Separate correlations were computed for glucose ranges <3.0 and >22.2 mmol/l. Impairment scores were not related to diabetes duration, blood glucse variability (as determined by several measures including the interquartile range, low and high blood glucose risk indexes), or depression and anxiety measures. The child's A1C correlated withimpairment scores for reaction time when blood glucose was >22.2 mmol/l (r = 0.40, P = 0.02), indicating more impairment with poorer diabetes control. Number of severe hypoglycemic episodes over the past year correlated with impairment scores for both math time (r = 0.39, P = 0.04) and reaction time (r = 0.40, P = 0.02) when blood glucose was >22.2 mmol/l, indicating that more frequent episodes were associated with more impairment. Neither A1C nor frequency of severe hypoglycemia correlated with performance impairment exhibited when blood glucose was <3.0 mmol/l.
CONCLUSIONS
Based on these findings, naturally occurring episodes of acute hypo- and hyperglycemia during daily routine can be associated with cognitive-motor disruptions in school-aged children with diabetes. To our knowledge, this is the first study comparing the negative impact of hypo- and hyperglycemia on cognitive function in this pediatric population. Somewhat surprisingly, the decline in performance at both glucose extremes was equivalent. However, a significant decline in performance was not seen until hyperglycemia became quite profound. In addition, blood glucose extremes affected only the time to complete tasks and not the number of correct responses. This finding replicates results from adult studies (2,3) and adds to the data suggesting that the initial effect of blood glucose extremes is a decrease in mental efficiency and speed and not a decrease in accuracy. Thus, people with diabetes of all ages may compensate behaviorally for blood glucose–related cognitive disruptions by first slowing down their performance and consequently sacrificing efficiency to preserve accuracy. A similar type of behavioral compensation plays a key role in models of aging and cognitive functioning (16).
The extent to which people with diabetes are subjectively aware of these effects remains unclear and is an important area for ongoing research. In this study, children were aware that they were having more difficulty completing tasks during hypoglycemia but not during hyperglycemia, even though performance was equally affected. However, children's difficulty ratings were also extremely low (average <1.0 on a six-point scale) across all blood glucose ranges, which may indicate that they were reluctant to acknowledge problems in their performance. In a previous article (17), we have reported that young children show very poor ability to recognize mild to moderate hypoglycemia, failing to detect on average over 40% of blood glucose readings <3.0 mmol/l. Given this, it is somewhat surprising that children in this study showed some awareness of increased difficulty performing the tasks when blood glucose levels were low.
Although this study found statistically significant differences in performance at blood glucose extremes, it is also important to consider whether the observed level of deterioration is clinically significant. One approach to this question is to examine the degree of disruption by whatever objective standards are available. During euglycemia, it took children an average of just over 1 min to complete 10 relatively simple mental math problems. During hypo- and hyperglycemia, respectively, this task took an average of ≥12 and ≥16 seconds longer to complete, representing an ∼20% decrease in speed. It is not difficult to imagine that a 20% decrease in mental efficiency could be clinically meaningful, especially with more complex, demanding, or time-consuming tasks. Another approach to this question is to evaluate the number of individual children who showed effects that might be considered clinically significant. In this study, IISs indicated that performance declined >1 SD during hypo- and hyperglycemia for >20% of children.
The finding that children varied greatly in the extent to which they were affected by glucose extremes replicates findings from studies of adults with diabetes (3,6). The mechanisms underlying these individual differences in vulnerability remain difficult to identify. In this study, demographic variables such as age and sex were not associated with individual differences. Exploratory correlations to examine the role of clinical variables indicated that higher A1C levels and frequency of severe hypoglycemia may be related to more impairment when blood glucose levels are very high. This finding is in contrast to the predictions some clinicians would make based on anecdotal evidence that type 1 diabetic individuals who are in better glycemic control experience more symptomatology and disruption with hyperglycemia. Neither A1C or history of severe hypoglycemia were related to the degree of impairment during hypoglycemia. Obviously, more research with much larger numbers of children is needed to gather more conclusive information regarding risk factors for acute blood glucose–related cognitive disruption.
More research is also needed to identify the neurobiological mechanismsunderlying the impact of acute hyperglycemia on cognitive function. While the effect of neuroglycopenia secondary to hypoglycemia is well defined, there is controversy about the neurological effect of hyperglycemia. However, there are several possibilities including microvascular dysfunction in the blood-brain barrier and alterations in the synthesis, availability, or reuptake of neurotransmitters, such as serotonin (18). Recent studies have identified a significant reduction in the plasma free fraction of l-tryptophan in children with type 1 diabetes, as well as differences in auditory cortical responses between children with and without diabetes, which may indicate brain differences in serotonergic neurotransmission (19,20). Other recent studies show that changes in extracellular brain glucose have a direct effect on orexin neurons in the lateral hypothalamus, which play a critical role in the regulation of wakefulness and arousal (21).
Another purpose of this study was to determine whether PDA procedures could provide an alternative field method for studying cognitive function in children at different blood glucose levels. Two questions need to be addressed for this purpose: feasibility and efficacy. In terms of feasibility, it appears that in general children can successfully perform repeated trials of brief PDA-administered cognitive tests over a period of several weeks. Of 77 families who entered the study, 78% completed the protocol. In terms of efficacy, the mental math task appears to be sensitive enough to detect differences in children's cognitive performance associated with glycemic extremes. This replicates findings in previous studies of adults with diabetes (5,6) and indicates that even relatively simple tasks requiring working memory and problem solving can be disrupted by hyperglycemia. The reaction time task showed less sensitivity to the disruptive effects of blood glucose extremes and also no practice effects during euglycemia, which may indicate that it was not complex or difficult enough. Failure to find an effect on performance accuracy may also have occurred because of a ceiling effect for these relatively easy tasks, which were designed to avoid producing psychological burden and frustration for these young children. Future studies need to incorporate more complex and demanding cognitive tasks, while balancing the need to not overburden pediatric populations.
The current study has several important methodological limitations that should be considered when interpreting these findings. First, we tested a relatively small number of children over a relatively short period of time, which yielded a limited number of extreme blood glucose readings to analyze. Only 56 and 67% of the children had blood glucose values in the lowest and highest ranges, respectively, for data analysis. Future studies are needed to test a larger number of children over a longer time period, or for repeated short periods over longer time, to capture more measures of performance during extreme blood glucose fluctuations. In addition, future studies would benefit greatly by using continuous glucose-monitoring devices in order to obtain a more comprehensive picture of glucose dynamics preceding cognitive testing. This approach would allow, for example, the opportunity to assess the impact of blood glucose variabililty and of antecedent episodes of hypo- and/or hyperglycemia on cognitive function. Finally, this study is limited by testing a fairly homogenous sample of almost all Caucasian children with well-educated parents.
Even with these limitations, the finding that routinely occurring episodes of acute hypo- and hyperglycemia can disrupt cognitive motor function in children, and that the impact of significant hyperglycemia equals that of significant hypoglycemia, has important implications. Nonetheless, these findings should be considered preliminary and interpreted with great caution. For example, these findings should not be interpreted as evidence that children's cognitive performance cannot be affected by blood glucose levels <22.2 mmol/l. Nor are these findings evidence that all children will experience significant impairments at 22.2 mmol/l. This study found large individual differences in degree of impairment at different blood glucose levels, and there are likely numerous, unidentified variables that influence the impact of an episode of acute hyperglycemia on cognitive function. What these findings do strongly indicate is that more research into the effects of acute hyperglycemia on cognitive function in children is warranted.
Acknowledgments
This research was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases R01 060039).
This research was also supported by Abbott Diabetes Care, Abbott Labs, Alameda, California, who provided blood glucose meters and testing supplies. L.G.-F., B.K., and W.C. have received research funding from Abbott Diabetes Care, Abbott Labs, Alameda, CA. No other potential conflicts of interest relevant to this article were reported.
The authors also thank Tiana Bolden and Steven Mortimer for editorial assistance; Nancy Kechner, Likun Hou, Victoria Parsons, Rupa Dasgupta, and Mi-Young Ryee for their help in data collection, management, and analysis; and Bob Chase from Sweetbriar University in Amherst, VA, for programming the PDA survey.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Holmes CS, Hayford JT, Gonzalez JL, Weydert JA: A survey of cognitive functioning at difference glucose levels in diabetic persons. Diabetes Care 1983; 6: 180– 185 [DOI] [PubMed] [Google Scholar]
- 2.Cox DJ, Gonder-Frederick LA, Schroeder DB, Cryer PE, Clarke WL: Disruptive effects of acute hypoglycemia on speed of cognitive and motor performance. Diabetes Care 1993; 16: 1391– 1393 [DOI] [PubMed] [Google Scholar]
- 3.Gonder-Frederick LA, Cox DJ, Driesen N, Ryan CM, Clarke WL: Individual differences in neurobehavioral disruption during mild and moderate hypoglycemia in adults with IDDM. Diabetes 1994; 43: 1407– 1412 [DOI] [PubMed] [Google Scholar]
- 4.Deary IJ: Symptoms of hypoglycaemia and effects on mental performance and emotions. In Hypoglycaemia in Clinical Diabetes Frier BM, Fisher BM: Eds. John Wiley & Sons, 1999, pp. 29– 54 [Google Scholar]
- 5.Draelos MT, Jacobsen Am, Weinger K, Widom B, Ryan C, Finkelstein D, Simonsen D: Cognitive function in patients with insulin dependent diabetes mellitus during hyperglycemia and hypoglycemia Am J Med 1995; 98: 135– 144 [DOI] [PubMed] [Google Scholar]
- 6.Cox DJ, Gonder-Frederick LA, McCall A, Kovatchev BP, Clarke WL: The effects of glucose fluctuation on cognitive function and QOL: the functional costs of hypoglycaemia and hyperglycaemia among adults with type 1 or type 2 diabetes. Int J Clin Pract Suppl 2002; 129: 20– 26 [PubMed] [Google Scholar]
- 7.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL: Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 2005; 28: 71– 77 [DOI] [PubMed] [Google Scholar]
- 8.Sommerfield AJ, Deary IJ, Frier BM: Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care 2004; 27: 2335– 2340 [DOI] [PubMed] [Google Scholar]
- 9.Ryan CM, Atchison J, Puczynski S, Puczynaki M, Arslanian S, Becker D: Mild hypoglycemia associated with deterioration of mental efficiency in children with insulin-dependent diabetes mellitus. J Pediatr 1990; 117: 32– 38 [DOI] [PubMed] [Google Scholar]
- 10.Gschwend S, Ryan C, Atchison J, Arslanian S, Becker D: Effects of acute hyperglycaemia on mental efficiency and counterregulatory hormones in adolescents with insulin-dependent diabetes mellitus. J Pediatr 1995; 126: 178– 184 [DOI] [PubMed] [Google Scholar]
- 11.Davis EA, Soong SA, Byrne GC, Jones TW: Acute hyperglycaemia impairs cognitive function in children with IDDM. J Pediatr Endocrinol Metab 1996; 9: 455– 461 [DOI] [PubMed] [Google Scholar]
- 12.Ryan CM: Effects of diabetes mellitus on neuropsychological functioning: a lifespan perspective. Semin Clin Neuropsychiatry 1997; 2: 4– 14 [DOI] [PubMed] [Google Scholar]
- 13.Spielberger CD: State-Trait Anxiety Inventory for Children: Preliminary Manual Palo Alto, CA, Consulting Psychologists Press, 1973 [Google Scholar]
- 14.Kovacs M: Rating scales to assess depression in school-aged children. Acta Paedopsychiatr 1981; 46: 305– 315 [PubMed] [Google Scholar]
- 15.Virginia School Health Guidelines Virginia Department of Health, Virginia Department of Education 1992, Virginia [Google Scholar]
- 16.Baltes PB, Staudinger UM, Lindenberger U: Lifespan psychology: theory and application to intellectual functioning. Ann Rev Psychol 1999; 50: 471– 507 [DOI] [PubMed] [Google Scholar]
- 17.Gonder-Frederick L, Zrebiec J, Bauchowitz A, Lee J, Cox D, Kovatchev B, Ritterband L, Clarke W: Detection of hypoglycemia by children with type 1 diabetes 6 to 11 years of age and their parents: a field study. Pediatrics 2008; 121: e489– e495 [DOI] [PubMed] [Google Scholar]
- 18.McCall AL, Figlewicz DP: How does diabetes mellitus produce brain dysfunction? Diabetes Spectrum 1997; 10: 25– 32 [Google Scholar]
- 19.Herrera R, Manjarrez G, Nishimura E, Hernandez J: Serotonin-related tryptophan in children with insulin-dependent diabetes. Pediatr Neurol 2003; 28: 20– 23 [DOI] [PubMed] [Google Scholar]
- 20.Manjarrez G, Cisneros I, Herrera R, Vazquez F, Robles A, Hernandez J: Prenatal impairment of brain serotonergic transmission in infants. J Pediatr 2005; 147: 592– 596 [DOI] [PubMed] [Google Scholar]
- 21.Burdakov D, Jensen L, Alexopoulos H, Williams R, Fearon I, O'Kelly I, Feraslmenko O, Fugger L, Verkhratsky A: Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 2006; 50: 711– 722 [DOI] [PubMed] [Google Scholar]