Abstract
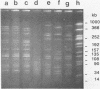
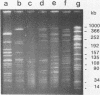
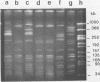
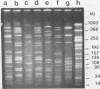
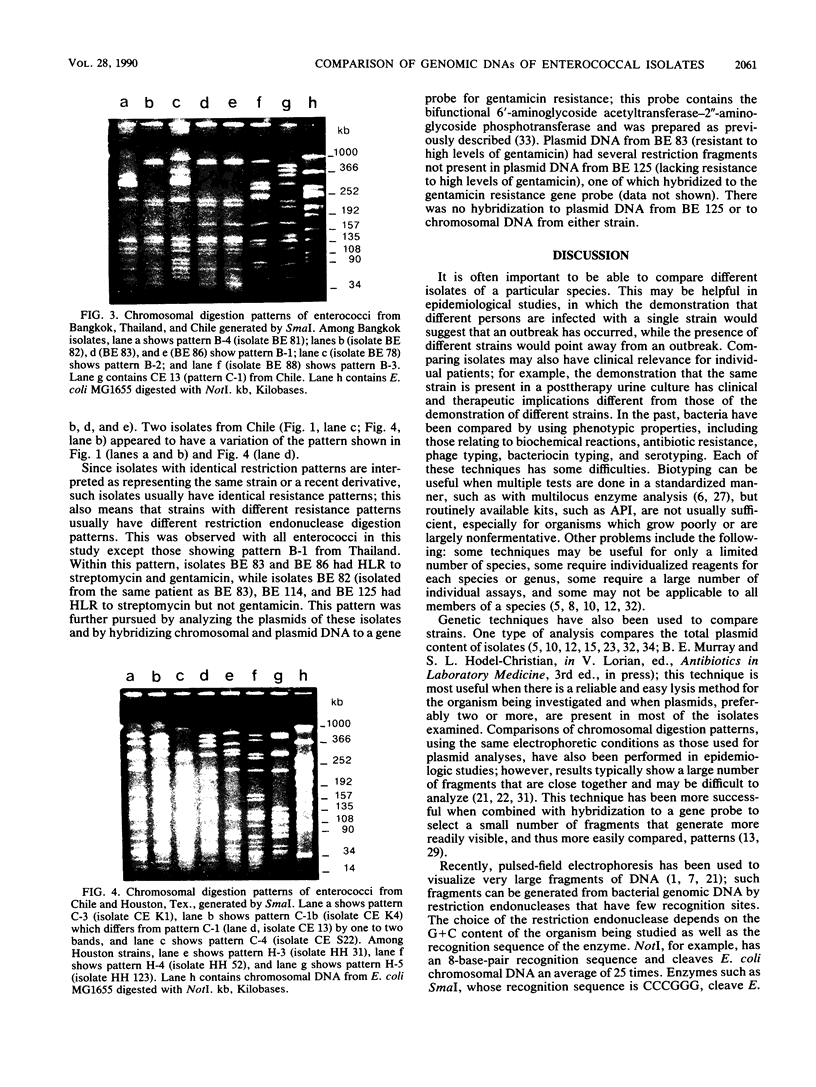
Epidemiologic evaluation of enterococci has been limited by the lack of a simple and effective method for comparing strains. In this study, we have compared chromosomal restriction endonuclease digestion patterns of 27 isolates of Enterococcus faecalis from three different locations by using pulsed-field electrophoresis of large chromosomal fragments (14 to 1,000 kilobases). All but two isolates generated a clear, evaluable pattern with a single lysis and digestion, and the remaining two were visualized when a larger quantity of bacteria was used. All isolates from different locations generated different restriction patterns, as did most isolates within a single location; there was also evidence of spread of strains between individuals in each location. The ease with which this analysis can be performed, together with the clarity and polymorphism seen in the patterns, suggests that this technique will be very useful for epidemiological evaluations of nosocomial enterococcal infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. L., Wylie B. A., Sooka A., Koornhof H. J. Bacteremia caused by a lactose-fermenting, multiply resistant Salmonella typhi strain in a patient recovering from typhoid fever. J Clin Microbiol. 1987 Aug;25(8):1516–1518. doi: 10.1128/jcm.25.8.1516-1518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Baker C. J., Troup N. J., Tompkins L. S. Restriction endonuclease analysis of human and bovine group B streptococci for epidemiologic study. J Clin Microbiol. 1989 Jun;27(6):1352–1356. doi: 10.1128/jcm.27.6.1352-1356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne J., Brun Y., el Solh N., Delorme V., Mouren C., Bes M., Fleurette J. Characterization of clinically significant isolates of Staphylococcus epidermidis from patients with endocarditis. J Clin Microbiol. 1988 Apr;26(4):613–617. doi: 10.1128/jcm.26.4.613-617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo-Viola D. Enzyme polymorphism, prodigiosin production, and plasmid fingerprints in clinical and naturally occurring isolates of Serratia marcescens. J Clin Microbiol. 1989 May;27(5):860–868. doi: 10.1128/jcm.27.5.860-868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstein A. I., Morthland V. H., Eng S., Archer G. L., Schoenknecht F. D., Rashad A. L. Restriction enzyme analysis of plasmid DNA and bacteriophage typing of paired Staphylococcus aureus blood culture isolates. J Clin Microbiol. 1989 Aug;27(8):1874–1879. doi: 10.1128/jcm.27.8.1874-1879.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Mobilization of the gentamicin resistance gene in Enterococcus faecalis. Antimicrob Agents Chemother. 1990 Jun;34(6):1278–1280. doi: 10.1128/aac.34.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg S. D., Wachsmuth I. K., Hickman-Brenner F. W., Cohen M. L. Comparison of plasmid profile analysis, phage typing, and antimicrobial susceptibility testing in characterizing Salmonella typhimurium isolates from outbreaks. J Clin Microbiol. 1984 Feb;19(2):100–104. doi: 10.1128/jcm.19.2.100-104.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnen E., Richter F., Richter K., Andries L. Establishment of a typing system for group D streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):322–330. doi: 10.1016/s0176-6724(88)80048-8. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Licitra C. M., Brooks R. G., Terry P. M., Shaw K. J., Hare R. S. Use of plasmid analysis and determination of aminoglycoside-modifying enzymes to characterize isolates from an outbreak of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1989 Nov;27(11):2535–2538. doi: 10.1128/jcm.27.11.2535-2538.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuhl L. M., Rotbart H. A., Facklam R. R., Roe M. H., Elliot J. A. Neonatal enterococcal sepsis: case-control study and description of an outbreak. Pediatr Infect Dis J. 1987 Nov;6(11):1022–1026. [PubMed] [Google Scholar]
- Mayer L. W. Use of plasmid profiles in epidemiologic surveillance of disease outbreaks and in tracing the transmission of antibiotic resistance. Clin Microbiol Rev. 1988 Apr;1(2):228–243. doi: 10.1128/cmr.1.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr In-vivo acquisition of two different types of aminoglycoside resistance by a single strain of Klebsiella pneumoniae causing severe infection. Ann Intern Med. 1982 Feb;96(2):176–180. doi: 10.7326/0003-4819-96-2-176. [DOI] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J. Chromosomal DNA fingerprinting--a new method of species and strain identification applicable to microbial pathogens. J Med Microbiol. 1989 Oct;30(2):89–99. doi: 10.1099/00222615-30-2-89. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Patterson T. F., Farrel P., Hierholzer W. J., Jr, Zervos M. J. Evaluation of restriction endonuclease analysis as an epidemiologic typing system for Branhamella catarrhalis. J Clin Microbiol. 1989 May;27(5):944–946. doi: 10.1128/jcm.27.5.944-946.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado D., Murray B. E., Cleary T. G., Pickering L. K. Limitations of using the plasmid pattern as an epidemiological tool for clinical isolates of Shigella sonnei. J Infect Dis. 1987 Feb;155(2):314–316. doi: 10.1093/infdis/155.2.314. [DOI] [PubMed] [Google Scholar]
- Richards H., Datta N. Plasmids and transposons acquired by Salmonella typhi in man. Plasmid. 1982 Jul;8(1):9–14. doi: 10.1016/0147-619x(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Farrar W. E., Jr, McGee Z. A., Schaffner W. Evolution of a plasmid mediating resistance to multiple antimicrobial agents during a prolonged epidemic of nosocomial infections. J Infect Dis. 1981 Feb;143(2):170–181. doi: 10.1093/infdis/143.2.170. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Wachsmuth I. K., Shangkuan Y. H., Schmidt E. V., Barrett T. J., Schrader J. S., Scherach C. S., McGee H. B., Feldman R. A., Brenner D. J. Salmonellosis associated with marijuana: a multistate outbreak traced by plasmid fingerprinting. N Engl J Med. 1982 May 27;306(21):1249–1253. doi: 10.1056/NEJM198205273062101. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N. J., Woods T., Bibb W., McKinney R. M. Molecular epidemiology of Legionella species by restriction endonuclease and alloenzyme analysis. J Clin Microbiol. 1987 Oct;25(10):1875–1880. doi: 10.1128/jcm.25.10.1875-1880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S., Madhavan T., Reuman P., Tewari R., Duckworth D. Plasmid profiles and klebocin types in epidemiologic studies of infections by Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):279–284. doi: 10.1007/BF01963102. [DOI] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E. Comparison of enterococcal and staphylococcal beta-lactamase plasmids. J Infect Dis. 1990 Jan;161(1):54–58. doi: 10.1093/infdis/161.1.54. [DOI] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E., Echeverria P., Mathewson J. J., DuPont H. L. Enteroinvasive Escherichia coli in travelers with diarrhea. J Infect Dis. 1988 Sep;158(3):640–642. doi: 10.1093/infdis/158.3.640. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]