Abstract
OBJECTIVE
Low levels of physical activity appear to be associated with insulin resistance. However, the detailed associations of these complex relationships remain elusive. We examined the prospective associations between self-reported TV viewing time, objectively measured time spent sedentary, at light-intensity activity, and at moderate- and vigorous-intensity physical activity (MVPA) with insulin resistance.
RESEARCH DESIGN AND METHODS
In 192 individuals (81 men and 111 women) with a family history of type 2 diabetes, we measured physical activity and anthropometric and metabolic variables at baseline and after 1 year of follow-up in the ProActive UK trial. Physical activity was measured objectively by accelerometry. Insulin resistance was expressed as fasting insulin and the homeostasis model assessment score (HOMA-IR).
RESULTS
Baseline MVPA was a significant predictor of fasting insulin at follow-up (β = −0.004 [95% CI −0.007 to −0.0001], P = 0.022), and the association approached significance for HOMA-IR (β = −0.003 [−0.007 to 0.000002], P = 0.052), independent of time spent sedentary, at light-intensity activity, sex, age, smoking status, waist circumference, and self-reported TV viewing. Time spent sedentary and at light-intensity activity were not significantly associated with insulin resistance. The change in MVPA between baseline and follow-up was inversely related to fasting insulin (β = −0.003 [−0.007 to −0.0003], P = 0.032) and the HOMA-IR score (β = −0.004 [−0.008 to −0.001], P = 0.015) at follow-up, after adjustment for baseline phenotype in addition to the same confounders as above.
CONCLUSIONS
These results highlight the importance of promoting moderate-intensity activity such as brisk walking for improving insulin sensitivity and possibly other metabolic risk factors to prevent type 2 diabetes.
Insulin resistance, considered by some to be the underlying cause of the metabolic syndrome, is an important factor in the etiology of type 2 diabetes and is also a strong independent predictor of the disease, even in individuals with normal glucose levels (1).
Potentially modifiable risk factors for insulin resistance include age, overall and abdominal obesity, dietary factors, sedentary behavior, and low levels of physical activity (1). Sedentary behavior, defined as self-reported TV viewing or total sitting time, is associated with abnormal glucose metabolism (2,3) and the metabolic syndrome (4) and predicts obesity and type 2 diabetes in women (5). More recent studies using motion sensors based on accelerometry for assessing time spent sedentary have suggested that total time and uninterrupted sedentary time are associated with metabolic risk and 2-h postchallenge glucose values (6,7).
Other studies using individually calibrated heart rate monitoring for assessing physical activity energy expenditure (PAEE) have shown that there is a cross-sectional association between PAEE with a clustered metabolic risk score (i.e., the standardized sum of the individual components) (8), that PAEE predicts progression toward the metabolic syndrome (9), and that a change in PAEE predicts clustered metabolic risk independent of changes in adiposity and cardiorespiratory fitness (10). In agreement with these observations, there is evidence to suggest that total physical activity measured by accelerometry is associated with insulin resistance and clustered metabolic risk (11,12) and that small increases in total physical activity are associated with a reduction in metabolic risk (13).
However, none of these studies examined the independent prospective associations between different subcomponents of objectively measured physical activity, i.e., time spent sedentary, at light-intensity activity and at moderate- and vigorous-intensity physical activity (MVPA), and self-reported TV viewing with insulin resistance. This association is important because existing guidelines on physical activity for public health emphasize the importance of MVPA but do not consider the potential harmful effects of sedentary living.
Therefore, the aim of the present study was to examine the independent prospective associations between time spent sedentary, at light-intensity activity and at MVPA, and self-reported TV viewing with insulin resistance in a cohort analysis of the ProActive UK trial (14). We hypothesized that objectively measured time spent at MVPA predicts insulin resistance independent of time spent at lower-intensity levels and self-reported TV viewing.
RESEARCH DESIGN AND METHODS
The present study is a cohort analysis of the ProActive UK trial, which was extensively described previously (14). In brief, the aim of the ProActive UK trial was to evaluate the efficacy of a theoretical, evidence-based, andfamily-based intervention program to increase physical activity among individuals defined as high-risk through having a parental history of type 2 diabetes. Of the 465 individuals who were eligible, 399 were recruited for baseline measurements and randomly assigned to one of three interventions as described previously (14). Complete data on anthropometry and biochemistry were available for 365 participants at baseline and 321 participants at the 1-year follow-up, in addition to sociodemographic information. The main trial results indicated no significant difference between the three trial arms in the 1-year change in objectively measured daytime physical activity or in the outcomes included in the present report (14). Consequently, the three trial arms were pooled and a cohort analysis was conducted. Physical activity was measured by accelerometry in a subsample of participants (n = 192) at baseline and follow-up and constitutes the sample for the present study. The measure of socioeconomic status was based on age at finishing full-time education (older or younger than 16 years). Time (hours per week) spent viewing TV and video and smoking status (current, former, or never) were assessed with a questionnaire at baseline and follow-up. All participants provided written informed consent, and ethics permission for the study was granted by the Eastern England Multi-center Research Ethics Committee.
Anthropometric and metabolic tests
After an overnight fast, a sample of venous blood was taken from each individual. Fasting plasma glucose and serum insulin levels were measured using the hexokinase method at baseline, and follow-up as described previously (14). We used both fasting insulin and homeostasis model assessment (HOMA) as indicators of insulin resistance. HOMA of insulin resistance (HOMA-IR) was calculated as fasting plasma glucose (millimoles per liter) times fasting serum insulin (milliunits per liter) divided by 22.5.
Weight was measured on standard calibrated scales, and height was measured using a rigid stadiometer. BMI was calculated as weight in kilograms divided by the square of height in meters. Overweight and obesity were defined as BMI >25 and >30 kg/m2, respectively. Waist circumference (centimeters) was measured over light indoor clothing as the midpoint between the lower costal margin and the level of the anterior superior iliac crests. Resistance (ohms) was assessed using a standard bioimpedance technique (Bodystat; Isle of Man, U.K.). Total body water and fat-free mass (FFM) were calculated using the impedance index (height2/resistance) and body weight and resistance. Fat mass was calculated as body weight minus FFM. Systolic and diastolic blood pressures were measured using an automated Accutorr sphygmomanometer (Accutorr, Cambridge, U.K.). Exactly the same measurements and analytical procedures were applied at baseline and at follow-up.
Physical activity by accelerometry
Physical activity was measured with accelerometry (MTI Actigraph, model WAM 7164; Manufacturing Technology, Fort Walton Beach, FL) over 4 consecutive days at baseline and follow-up as described previously (11). Nonwear time was identified as continuous zero movement lasting >60 min. By using this criterion, seven individuals were excluded because they did not manage to record at least 500 min/day of activity for at least 3 days during either baseline or follow-up measurements. Outcome variables from the activity monitor included time (minutes per day) spent at different activity intensity categories averaged per day over the measurement period. Intensity thresholds for moderate- (1,952–5,724 counts/min) and vigorous-intensity activity (>5,725 counts/min) were defined (15). Sixty percent of participants did not accumulate any time in vigorous-intensity physical activity at baseline. We therefore constructed a single variable by combining accumulated time in MVPA. Sedentary behavior was defined as <100 counts/min and light-intensity activity as 101–1,951 counts/min. The cutoff for sedentary behavior is an arbitrary threshold, which we and others have used previously (6,7,11). Data reduction and cleaning and analyses of accelerometer data were performedusing a specially written program (MAHUffe; http://www.mrc-epid.cam.ac.uk). Individuals were also categorized above or below accumulation of at least 30 min/day of time spent at MVPA according to current recommendations for public health (16).
Statistical analyses
Descriptive characteristics are summarized as means ± SD at baseline and follow-up. Fasting insulin and HOMA were logarithmically transformed owing to their skewed distributions (geometric means [95% CI] are presented in the results). Associations between variables were examined using Pearson correlation coefficients and partial correlation coefficients. Differences between sex and between baseline and follow-up were analyzed by ANOVA.
To examine which, if any, of the objectively measured subcomponents of physical activity and self-reported TV and video viewing were independently associated with insulin resistance, we fitted multiple linear regression models with either fasting insulin or HOMA as the outcome and objectively measured time spent sedentary, at light-intensity activity, at MVPA, and self-reported TV viewing as exposure variables. In the first cross-sectional model we adjusted for age, sex, smoking status (current, former, or never), and waist circumference. To examine whether physical activity subcomponents and video and TV viewing independently predicted insulin resistance at follow-up, we modeled insulin resistance (fasting insulin or HOMA) at follow-up as outcome variables and objectively measured time spent sedentary, at light-intensity activity, at MVPA, and self-reported TV viewing measured at baseline as exposure variables. In addition to the confounders described above, we also adjusted our prospective models for follow-up time and baseline insulin resistance (i.e., fasting insulin or HOMA). Finally, we examined whether changes in physical activity subcomponents and TV viewing (follow-up minus baseline values) were associated with insulin resistance at follow-up after adjustment for all confounding factors described above. In all multiple linear regression models, multicollinearity was controlled for by means of the variance inflation factor. Including the intervention arm (three categories) in our analyses did not change the direction or magnitude of associations observed, and it was therefore removed from our final models. All data were analyzed in a continuous form but stratified above and below accumulation of at least 30 min/day of time spent at MVPA and obesity status (normal weight, overweight, or obese) for illustrative purposes. All analyses were performed using SPSS for Windows (version 13; SPSS, Chicago, IL).
RESULTS
Table 1 displays the descriptive characteristics of participants at baseline and follow-up. The mean follow-up time was 405 ± 103 days. Men were heavier and taller and had a higher FFM than women (P < 0.001). Fasting glucose (P < 0.01), insulin (P < 0.001), and the HOMA-IR score (P < 0.001) were also significantly higher in men than in women, whereas fat mass was higher in women (P < 0.001). Forty-nine percent of men and 36% of women were overweight, and, in addition, 27% of both men and women were obese.
Table 1.
Descriptive characteristics of participants at baseline and follow-up
| Men |
Women |
|||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| n | 81 | 111 | ||
| Weight (kg) | 89.6 ± 15.4 | 90.3 ± 16.1 | 73.3 ± 13.9 | 73.6 ± 14.3*** |
| Height (cm) | 177.9 ± 6.3 | 177.7 ± 6.4 | 163.5 ± 6.2 | 163.3 ± 5.8*** |
| BMI (kg/m2) | 28.3 ± 4.5 | 28.5 ± 4.7 | 27.5 ± 5.0 | 27.7 ± 5.2 |
| Fat mass (kg) | 23.8 ± 8.3 | 24.5 ± 8.9 | 25.9 ± 9.7 | 26.2 ± 10.0*** |
| FFM (kg) | 65.9 ± 8.8 | 65.8 ± 8.6 | 47.4 ± 5.8 | 47.4 ± 5.9*** |
| Waist circumference (cm) | 101.3 ± 11.6 | 101.6 ± 12.2 | 88.8 ± 11.2 | 89.4 ± 11.9*** |
| Glucose | 5.0 ± 0.8 | 5.1 ± 1.1 | 4.8 ± 0.5 | 4.9 ± 0.5** |
| Insulin (pmol/l) | 56.3 (49.8–62.3) | 63.1 (56.9–70.0) | 48.1 (41.2–55.2) | 48.6 (41.5–55.5)*** |
| HOMA | 1.79 (1.56–2.02) | 2.04 (1.84–2.24) | 1.46 (1.24–1.68) | 1.50 (1.29–1.82)*** |
| Sedentary (min/day) | 452 ± 84 | 435 ± 89 | 419 ± 85 | 418 ± 87** |
| Light (min/day) | 297 ± 77 | 302 ± 81 | 321 ± 70 | 310 ± 74* |
| MVPA (min/day) | 29 ± 16 | 30 ± 18 | 25 ± 17 | 29 ± 21 |
| TV and video (h/week) | 16.8 ± 9.0 | 14.8 ± 8.1† | 20.7 ± 9.8 | 18.7 ± 9.4†*** |
| Current smokers (%) | 21.0 | 16.0** | 16.2 | 13.6**†† |
Data are means ± SD, geometric means (95% CI), or χ2 for time and sex differences.n = 192. ANOVA for between-sex differences:
*P < 0.05;
**P < 0.01;
***P < 0.001; ANOVA for between-time differences:
†P < 0.05;
††P < 0.01.
Men spent more time sedentary (P < 0.01), whereas women spent more time at light-intensity activity (P < 0.05). In contrast, men reported significantly lower levels of TV viewing (P < 0.001). Self-reported TV viewing decreased significantly between baseline and follow-up (P < 0.05), whereas all other variables remained unchanged. We did not observe any significant sex by time interactions. Therefore, all subsequent analyses were performed with men and women combined, adjusted for sex.
Time spent sedentary was significantly and inversely associated with time spent at light-intensity activity at baseline and follow-up (r = −0.52, P < 0.0001; r = −0.48, P < 0.0001) and time spent at light-intensity activity was significantly and positively correlated with time spent at MVPA at follow-up (r = 0.24, P = 0.009) but not at baseline. Self-reported TV viewing was significantly and inversely associated with time spent at light-intensity activity at baseline and follow-up (r = −0.16, P = 0.027; r = −0.24, P = 0.001) but was not associated with any of the other objectively measured time estimates. Self-reported TV viewing at baseline was significantly correlated with TV viewing at follow-up (r = 0.78, P < 0.001). Similarly, all objectively measured time estimates at baseline were significantly correlated with their corresponding time estimates at follow-up (r = 0.61 to 0.63, P < 0.0001), indicating a high degree of stability of patterns of physical activity.
Table 2 shows the cross-sectional and prospective associations between time estimates of physical activity and self-reported TV viewing with insulin resistance. In cross-sectional analyses, time (minutes per day) spent at MVPA was significantly and inversely associated with HOMA (β = −0.004 [95% CI −0.008 to −0.00001], P = 0.048) and fasting insulin (β = −0.005 [−0.008 to −0.001], P = 0.017), independent of time spent sedentary, time spent at light-intensity activity, sex, age, smokingstatus, waist circumference, and self-reported TV viewing. TV viewing was significantly and positively associated with HOMA (β = 0.01 [95% CI 0.004–0.019], P = 0.002) and fasting insulin (β = 0.01 [0.004–0.017], P = 0.002), independent of objectively measured time estimates and the same confounders as above.
Table 2.
Associations between objectively measured time estimates of physical activity and self-reported TV and video viewing at baseline with the HOMA score and fasting insulin in adults with a family history of type 2 diabetes
| Baseline HOMA score | P value | Baseline fasting insulin | P value | Follow-up HOMA score | P value | Follow-up fasting insulin | P value | |
|---|---|---|---|---|---|---|---|---|
| Sedentary (min/day) | 0.0004 (−0.0006 to 0.001) | 0.42 | 0.0004 (−0.0006 to 0.001) | 0.39 | 0.001 (−0.002 to 0.00004) | 0.21 | 0.001 (−0.0013 to 0.00005) | 0.07 |
| Light (min/day) | 0.0001 (−0.001 to 0.001) | 0.83 | 0.0002 (−0.001 to 0.001) | 0.73 | −0.001 (−0.001 to 0.0002) | 0.16 | −0.001 (−0.0013 to 0.0002) | 0.18 |
| MVPA (min/day) | −0.004 (−0.008 to −0.00001) | 0.048 | −0.005 (−0.008 to −0.001) | 0.017 | −0.003 (−0.007 to 0.000002) | 0.052 | −0.004 (−0.007 to −0.001) | 0.022 |
| TV/video (h/week) | 0.01 (0.004 to 0.019) | 0.002 | 0.01 (0.004 to 0.017) | 0.002 | −0.001 (−0.007 to 0.006) | 0.88 | 0.0002 (−0.006 to 0.006) | 0.99 |
Data are β coefficients (95% CI).n = 192. Data are adjusted for sex, age, smoking status (current, former, or never), and waist circumference. Prospective data are additionally adjusted for baseline phenotypes and follow-up time.
We thereafter examined whether time spent sedentary, at light-intensity activity and at MVPA, and TV viewing predicted insulin resistance at follow-up. Time spent at MVPA was a significant predictor of fasting insulin (β = −0.004 [95% CI −0.007 to −0.0001], P = 0.022), and the association approached significance for HOMA (β = −0.003 [−0.007 to 0.000002], P = 0.052), independent of baseline phenotype, follow-up time, and other confounding factors. Similar to results for the cross-sectional analyses, time spent sedentary and at light-intensity activity were not significantly associated with insulin resistance at follow-up. In contrast to the cross-sectional analyses, TV viewing did not predict insulin resistance at follow-up (β = −0.0006 [−0.007 to 0.006], P = 0.84, and β = 0.00007 [−0.006 to −0.006], P = 0.94, for HOMA and fasting insulin, respectively).
Then we examined whether the change in MVPA and TV viewing between baseline and follow-up was associated with insulin resistance. The change in MVPA was significantly and inversely related to the change in fasting insulin (β = −0.003 [95% CI −0.007 to −0.0003], P = 0.032) and the HOMA score (β = −0.004 [−0.008 to −0.001], P = 0.015) after adjustment for sex, baseline phenotype, age, waist, smoking status, TV viewing, and follow-up time. In contrast, the change in TV viewing was not associated with either fasting insulin (β = 0.003 [−0.006 to 0.011], P = 0.55) or the HOMA score (β = 0.004 [−0.005 to 0.013], P = 0.42).
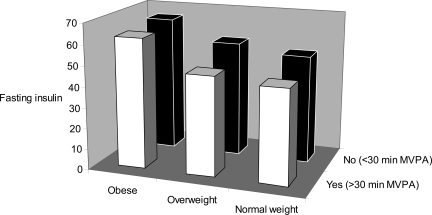
Finally, we analyzed whether meeting the recommendations of accumulating 30 min/day of MVPA at baseline was associated with fasting insulin at follow-up. Meeting this activity guideline was associated with a significantly lower mean value for fasting insulin (geometric mean difference between groups 1.12 pmol/l [95% CI 1.02–1.24], P = 0.002), independent of baseline insulin levels and the same confounders as above.Figure 1 shows fasting insulin levels at follow-up, stratified according to baseline BMI group (normal, overweight, or obese) and the dichotomous variable of meeting/not meeting the physical activity guidelines at baseline. BMI group (Ptrend = 0.004) and meeting activity guidelines (Ptrend = 0.050) predicted fasting insulin at follow-up, independent of each other and of baseline fasting insulin, age, sex, smoking status, TV viewing, and follow-up time.
Figure 1.
Fasting insulin (geometric mean) at follow-up stratified by BMI group (normal weight, overweight, and obese) and according to achieving at least 30 min of MVPA per day (yes vs. no). Data are adjusted for sex, baseline age, baseline fasting insulin, baseline smoking status, baseline TV viewing, and follow-up time (n = 192).
CONCLUSIONS
Our results suggest that time spent at MVPA is associated with indicators of insulin resistance independent of time spent sedentary, at light-intensity activity, and TV viewing. These results were consistent in both cross-sectional and prospective analyses androbust to confounding by adiposity,baseline insulin resistance, and other confounding factors. Consistently, an increase in MVPA over 1 year was associated with improved insulin sensitivity.
This is the first study to examine the prospective associations between TV viewing, objectively measured time spent sedentary, and time spent at MVPA with insulin resistance. Previous studies have suggested that objectively measured overall physical activity or PAEE is inversely associated with insulin resistance and other features of the metabolic syndrome (6–13), and in some studies these associations were independent of adiposity and cardiorespiratory fitness (9–11). Evidence is also emerging that TV viewing and sedentary behavior are associated with abnormal glucose metabolism, clustered metabolic risk, the metabolic syndrome, and type 2 diabetes (2–5). However, none of these previous studies controlled for objectively measured time spent at MVPA.
In cross-sectional analysis, we observed a significant association between self-reported amount of time watching TV with insulin resistance. However, this association was attenuated in the prospective model, suggesting that TV viewing does not predict insulin resistance independent of objectively measured time spent at MVPA. Indeed, self-reported TV time may be a weak indicator of overall sedentary behavior. In this dataset, TV viewing was significantly but weakly correlated with light-intensity activity but not with objectively measured time spent sedentary or time spent at MVPA. This suggests that TV viewing do not displace MVPA and that some individuals may combine relatively high levels of physical activity with high levels of TV viewing. However, TV viewing may be associated with other unhealthy behaviors that affect obesity and metabolic variables (17).
We recently reported an inverse cross-sectional association between time spent at MVPA and insulin resistance that was independent of PAEE in middle-aged U.K. Caucasians in whom physical activity was measured with individually calibrated minute-by-minute heart rate monitoring (18). We and others have suggested previously that total daily physical activity (counts per minute) measured by accelerometry is a significant determinant of insulin sensitivity, whereas time spent sedentary and at light-intensity activity are not (11,12). These previous cross-sectional observations corroborate our present prospective observation because time spent at MVPA explains most of the variance in total physical activity (counts per minute) (R2= 0.67, P < 0.0001 in the present study), suggesting that activities of moderate intensity, such as brisk walking, are the main contributors to overall levels of physical activity when measured by accelerometry. Because of issues of multicollinearity, we were not able to adjust our analyses for total daily physical activity volume (i.e., total counts).
The results from our study should be interpreted with the following limitations in mind. First, our results may only be generalizable to relatively sedentary, overweight, middle-aged, U.K. whites with a family history of type 2 diabetes. However, given the epidemic increase in overweight and obesity in adult U.K. men and women (19) and the large proportion of U.K. adults not being sufficiently active (20), it is likely that our results are generalizable to a larger part of the adult U.K. population. Second, our assessment of visceral adiposity by waist circumference lacks precision compared with more sophisticated measurement techniques such as magnetic resonance imaging and computed tomography. It is therefore possible that some residual confounding by central adiposity may persist. However, measurement error such as this is likely to be small and may be less than that in studies in which BMI has been used as a measure of adiposity. Third, we used fasting insulin and the HOMA score as markers of insulin resistance. Although these methods are less accurate than the hyperinsulinemic-euglycemic clamp, they serve as valuable surrogates for insulin resistance in normoglycemic individuals (21). The apparent discrepancy between the reduction in time spent viewing TV between baseline and follow-up without a corresponding increase in objectively measured time spent at MVPA is probably explained by the use of different methods when one assesses TV viewing (self-report) and time spent at MVPA (accelerometry). It is also plausible that the difference in self-reported time spent TV viewing between baseline and follow-up is explained by misreporting. Finally, although we controlled for many potential confounding factors, we cannot rule out the possibility that unmeasured factors such as genotype, birth weight, and growth in early life explain the observed associations.
Our study also has some unique strength. The longitudinal design allows statistical control for confounders, measured or unmeasured, which do not change over time. Furthermore, we reduced the potential for recall bias and differential measurement error, which is an unavoidable component of self-reported sedentary behavior and physical activity, by measuring time spent sedentary and at different intensity levels of activity with accelerometry. Our observations of an independent cross-sectional association between accumulated time spent at MVPA with insulin sensitivity was confirmed in our prospective analysis, indicating a causal association. Taken together, the observed associations between time spent at MVPA and insulin resistance are not likely to be due to measurement error, bias, or chance.
The independent association between time spent at MVPA with insulin sensitivity is biologically plausible. The mechanisms by which physical activity may affect insulin sensitivity independent of fat mass include increased glucose transport into skeletal muscle through increases in GLUT4 protein content and insulin-stimulated trafficking but also through a noninsulin hypoxia-dependent pathway (22). Further, physical activity may increase skeletal muscle capacity to oxidize fat, thereby decreasing the available amount of nonesterified fatty acids to the liver, which may improve hepatic insulin sensitivity (23). Conversely, prolonged time spent sedentary may have distinct physiological effects compared with physical activity (24). The relative importance of time spent at different subcomponents of physical activity, including time spent sedentary and at MVPA, in association with various metabolic health outcomes needs further study. Such studies are not likely to be successful without precise measurements of exposure variables including subdimensions of physical activity and different types of sedentary behavior.
Recent guidelines for health-enhancing physical activity state that all adults (18–65 years) should engage in at least moderate-intensity aerobic physical activity for a minimum of 30 min on 5 days each week or vigorous-intensity activity for a minimum of 20 min on 3 days each week (16). Our results support the recommendation of moderate-intensity physical activity proposed in these guidelines. However, because of the limited amount of time devoted to vigorous-intensity activity in our sample, we are not able to comprehensively evaluate the relative importance of vigorous-intensity activity on insulin resistance.
In summary, time spent at MVPA, objectively measured by accelerometry, predicts insulin resistance, independent of time spent sedentary, at light-intensity activity, and self-reported TV viewing. These results highlight the importance of promoting moderate-intensity activity such as brisk walking to improve insulin sensitivity and other metabolic risk factors and to prevent type 2 diabetes, at least in individuals with a high risk of developing this disease.
Acknowledgments
The U.K. Medical Research Council (Ref. No. ISRCTN 61323766), U.K. National Health Service Research and Development, the U.K. Royal College of General Practitioners Scientific Foundation, and Diabetes UK (Ref. No. RG35259) funded the development and execution of the ProActive UK trial.
No potential conflicts of interest relevant to this article were reported.
We thank the study participants and practice teams for their collaboration and work in helping with recruitment. Thanks are also due to S. Sharp, J. Luan, and T. Fanshawe for statistical advice, to Dr. M. Hennings for helping develop the MAHUffe software, and to A.L. Kinmonth for her helpful comments.
APPENDIX
The ProActive UK research group includes, besides the authors, K. Williams, J. Grant, A.T. Prevost, W. Hollingworth, D. Spiegelhalter (principal investigator), W. Hardeman (principal investigator), S. Sutton (principal investigator), and A.L. Kinmonth (principal investigator).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Kumar S, O'Rahilly S: (Eds.). Insulin Resistance: Insulin Action and Its Disturbances in Disease Chichester, U.K., Wiley, 2005 [Google Scholar]
- 2.Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, Owen N: AusDiab Steering Committee Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care 2007; 30: 516– 522 [DOI] [PubMed] [Google Scholar]
- 3.Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, Cameron AJ, Dwyer T, Jolley D, Shaw JE: AusDiab Steering Committee Physical activity and television viewing in relation to risk of undiagnosed abnormal glucose metabolism in adults. Diabetes Care 2005; 27: 2603– 2609 [DOI] [PubMed] [Google Scholar]
- 4.Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, Cameron AJ, Dwyer T, Jolley D, Shaw JE: AusDiab Steering Committee Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia 2005; 48: 2254– 2261 [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE: Television watching and other sedentary behaviours in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003; 289: 1785– 1791 [DOI] [PubMed] [Google Scholar]
- 6.Healy GN, Dunstan DW, Shaw JE, Zimmet PZ, Owen N: Beneficial associations of physical activity with 2-h but not fasting blood glucose in Australian adults: the AusDiab study. Diabetes Care 2006; 29: 2598– 2604 [DOI] [PubMed] [Google Scholar]
- 7.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N: Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008; 31: 369– 371 [DOI] [PubMed] [Google Scholar]
- 8.Franks PW, Ekelund U, Brage S, Wong MJ, Wareham NJ: Does the association of habitual physical activity with the metabolic syndrome differ by level of fitness? Diabetes Care 2004; 27: 1187– 1193 [DOI] [PubMed] [Google Scholar]
- 9.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ: Physical activity energy expenditure predicts progression towards the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the MRC Ely study. Diabetes Care 2005; 28: 1195– 2000 [DOI] [PubMed] [Google Scholar]
- 10.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ: Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness: the MRC Ely study. Diabetes Care 2007; 30: 2101– 2106 [DOI] [PubMed] [Google Scholar]
- 11.Ekelund U, Griffin SG, Wareham NJ: Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care 2007; 30: 337– 342 [DOI] [PubMed] [Google Scholar]
- 12.Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E: EGIR-RISC Study Group Physical activity and insulin sensitivity: the RISC study. Diabetes 2008; 57: 2613– 2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons R, Griffin S, Steele R, Wareham NJ, Ekelund U: Increasing overall physical activity and aerobic fitness is associated with improvements in metabolic risk: cohort analysis of the ProActive trial. Diabetologia 2008; 51: 787– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinmonth AL, Wareham NJ, Hardemann W, Sutton S, Prevost T, Fanshawe T, Williams KM, Ekelund U, Spiegelhalter D, Griffin S: The ProActive (UK) trial; no evidence of efficacy of a theory-based behavioural intervention to increase physical activity in an at-risk group in primary care. Lancet 2008; 371: 41– 48 [DOI] [PubMed] [Google Scholar]
- 15.Freedson PS, Melanson E, Sirard J: Calibration of the Computer Science and Applications, Inc., accelerometer. Med Sci Sports Exerc 1998; 30: 777– 781 [DOI] [PubMed] [Google Scholar]
- 16.Haskell WL, Lee IM, Part RR, Powell KE, Blain SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A: Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007; 39: 1423– 1434 [DOI] [PubMed] [Google Scholar]
- 17.Gore SA, Foster JA, DiLillo VG, Kirk K, Smith West D: Television viewing and snacking. Eat Behav 2003; 4: 399– 405 [DOI] [PubMed] [Google Scholar]
- 18.Assah F, Ekelund U, Brage S, Wareham NJ: The association between intensity and overall level of physical activity energy expenditure with a marker of insulin resistance. Diabetologia 2008; 51: 1399– 1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennie K, Jebb S: Prevalence of obesity in Great Britain. Obes Rev 2005; 6: 11– 12 [DOI] [PubMed] [Google Scholar]
- 20.Stamatakis A, Ekelund U, Wareham NJ: Temporal trends in physical activity in England: the Health Survey for England 1991 to 2004. Prev Med 2007; 45: 416– 423 [DOI] [PubMed] [Google Scholar]
- 21.Laakso M: How good a marker is insulin level for insulin resistance? Am J Epidemiol 1993; 137: 959– 965 [DOI] [PubMed] [Google Scholar]
- 22.Hollozy JO: Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 2005; 99: 338– 345 [DOI] [PubMed] [Google Scholar]
- 23.Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, Stolinski M, Whyte M, Lovell D, Bowes SB, Gibney J, Jones RH, Umpleby AM: Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 2007; 50; 404– 413 [DOI] [PubMed] [Google Scholar]
- 24.Hamilton MT, Hamilton DG, Zderic TW: Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes and cardiovascular disease. Diabetes 2007; 56: 2655– 2667 [DOI] [PubMed] [Google Scholar]