Abstract
OBJECTIVE
Glucose fluctuations trigger activation of oxidative stress, a main mechanism leading to secondary diabetes complications. We evaluated the relationship between glycemic variability and β-cell dysfunction.
RESEARCH DESIGN AND METHODS
We conducted a cross-sectional study in 59 patients with type 2 diabetes (aged 64.2 ± 8.6 years, A1C 6.5 ± 1.0%, and BMI 29.8 ± 3.8 kg/m2[mean ± SD]) using either oral hypoglycemic agents (OHAs) (n = 34) or diet alone (nonusers). As a measure of glycemic variability, the mean amplitude of glycemic excursions (MAGE) was computed from continuous glucose monitoring data recorded over 3 consecutive days. The relationships between MAGE, β-cell function, and clinical parameters were assessed by including postprandial β-cell function (PBCF) and basal β-cell function (BBCF) obtained by a model-based method from plasma C-peptide and plasma glucose during a mixed-meal test as well as homeostasis model assessment of insulin sensitivity, clinical factors, carbohydrate intake, and type of OHA.
RESULTS
MAGE was nonlinearly correlated with PBCF (r = 0.54, P < 0.001) and with BBCF (r = 0.31, P = 0.025) in OHA users but failed to correlate with these parameters in nonusers (PBCF P = 0.21 and BBCF P = 0.07). The stepwise multiple regression analysis demonstrated that PBCF and OHA combination treatment were independent contributors to MAGE (R2 = 0.50, P < 0.010), whereas insulin sensitivity, carbohydrate intake, and nonglycemic parameters failed to contribute.
CONCLUSIONS
PBCF appears to be an important target to reduce glucose fluctuations in OHA-treated type 2 diabetes.
Of genetic and environmental origin, defective insulin secretion and insulin sensitivity are the main factors causing the development and progression of type 2 diabetes. The UK Prospective Diabetes Study (UKPDS) showed that after an initial improvement, glycemic control continues to deteriorate despite the use of oral agents to enhance insulin secretion and to reduce insulin resistance (1). This deterioration can be attributed to the progressive decline of β-cell function. Even in subjects with well-controlled type 2 diabetes, 70% of the variability of A1C can be explained by abnormalities in postprandial glucose (2). Chronic sustained hyperglycemia has been shown to exert deleterious effects on the β-cells and the vascular endothelium (3). Monnier et al. (4) and Brownlee and Hirsch (5) have recently emphasized that another component of dysglycemia, i.e., glycemic variability, is even more important than chronic sustained hyperglycemia in generating oxidative stress and contributing to the development of secondary diabetes complications. In vivo studies have convincingly demonstrated that hyperglycemic spikes induce increased production of free radicals and various mediators of inflammation, leading to dysfunction of both the vascular endothelium (3) and the pancreatic β-cell (6). Furthermore, by evaluating hard end points in prospective analyses, Shiraiwa et al. (7) and Cavalot et al. (8) have reported deleterious effects of glucose excursions on diabetic vascular complications.
Prolonged postprandial glucose excursions have been linked to several factors such as inadequate insulin secretion, insulin deficiency, or an abnormal release of counterregulatory hormones (9). However, glycemic variability in type 2 diabetes appears to result from the complex interplay between pathophysiological factors and behavioral and treatment factors (10).
In clinically established type 2 diabetes, the degree of association of glycemic variability with pancreatic β-cell dysfunction remains unclear. To address this issue, we used continuous glucose monitoring to assess glycemic variability and used an insulin secretion model during a mixed-meal test (MMT) to measure basal β-cell function (BBCF) and postprandial β-cell function (PBCF) in subjects with type 2 diabetes after withdrawal of oral hypoglycemic agents or treated with diet alone.
RESEARCH DESIGN AND METHODS
This cross-sectional study enrolled 59 consecutive outpatients with type 2 diabetes. All patients were Caucasians and were recruited from seven practices of primary care physicians/internists in the district of Greifswald, Germany, from 2004 to 2006. Their antihyperglycemic therapy consisted of oral hypoglycemic agents (OHAs), either sulfonylurea (n = 12) and metformin (n = 10) alone or a combination of both (n = 12), or of diet alone (n = 25). None were taking other medications known to alter glucose metabolism, and all were otherwise in good health.
Criteria for inclusion were a diagnosis of type 2 diabetes for at least 1 year but <20 years, age 35–79 years, BMI of 24–38 kg/m2, A1C of 5.0–9.0%, and treatment with OHAs or diet. Exclusion criteria were need for insulin use; circulating islet cell antibodies; concomitant chronic disease, including kidney, liver, and cardiovascular disease; recent acute illness; or changes in diet, treatment, or lifestyle within 3 months before the inclusion examination.
Before commencement of the study procedures, OHA medication was withdrawn and substituted with placebo for 8 days to allow for the pharmacological effects of sulfonylureas and metformin to dissipate (11,12), taking into account the possibility that a more prolonged withdrawal might cause deleterious effects on glucose control. During the OHA withdrawal and the 4-day study period, subjects were under medical supervision and were advised to continue their regular lifestyle and to maintain their usual exercise and dietary patterns.
The study protocol was approved by the ethics review board at the University of Greifswald, Greifswald, Germany, and was conducted in accordance with the rules in the European Community and the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Study procedures
At study entry, a continuous glucose monitoring (CGM) system (CGMS) sensor (Medtronic MiniMed, Northridge, CA) was inserted and calibrated according to the standard operating guidelines, and CGM was performed for 60 ± 10 h (mean ± SD). At day 2 of the study, following a 12-h overnight fast, a 500-ml standardized liquid MMT, containing 75 g carbohydrate, 58 g fat, and 30 g protein to total 1,000 kcal (caloric contribution: 37% carbohydrate, 51% fat, and 12% protein) (13), was given at the physician's practice as described previously (14). Patients were required to consume the test meal within 10–15 min. Standardization of diet was not performed during CGMS measurements, but subjects entered type and amount of food consumed into their logbooks. Blood samples were taken via an indwelling intravenous cannula, which was inserted into an arm vein, at −15, 0, 30, 60, 90, 120, and 150 min relative to the meal ingestion for the measurement of glucose and C-peptide.
Blood drawn for measurement of standard laboratory values was shipped to a central laboratory, where A1C was analyzed by the Bio-Rad Diamat analyzer system using ion-exchange high-performance liquid chromatography (normal range 4.6–6.0%). Plasma glucose was measured by the glucose oxidase enzymatic method on a DiaSys Super G Analyzer (Hitado Diagnostic Systems, Moehnesee-Delecke, Germany). The Medical Diagnostic Laboratory at the University of Greifswald performed serum insulin (INS enzyme immunoassay; IBL, Hamburg, Germany) and C-peptide analyses (HCP enzyme immunoassay, DPC Biermann, Bad Nauheim, Germany). Cross-reactivity of the insulin antibody with human proinsulin was 3% and with the C-peptide antibody was 17%.
CGM data and glycemic variability
Subjects used home blood glucose monitors that were calibrated with Accu-Chek glucose standards (Roche Diagnostics, Mannheim, Germany) and entered at least four glucometer readings per day into the CGMS monitor for calibration. The glucose profiles obtained from CGMS measurements were analyzed usingMiniMedSolution Software (MedtronicMiniMed).Profiles with less than four glucometer entries were disregarded. Mean sensor glucose, sensor glucose range, median sensor glucose, and the times patients were hyperglycemic (>10 mmol/l) and hypoglycemic (<3.3 mmol/l) were calculated from the CGMS datasets. The value 10.0 mmol/l as the upper limit of glucose at postprandial times was chosen according to recommendations of the American Diabetes Association (15). The area under the curve for sensor glucose (AUCCGM) was calculated with the trapezoidal method for a 24-h time period. MAGE, considered as the “gold standard” of glycemic variability, was calculated as described by Service et al. (16) from the glucose excursions of the CGMS profiles. Carbohydrate intake (bread exchange units) per day was calculated from the subjects' logbooks, according to standard tables containing the nutrient composition with bread exchange units for diabetic subjects.
Glucose and insulin levels during MTT
Fasting glucose and fasting plasma insulin were obtained as mean values of pretest MMT measurements. The difference between fasting and peak plasma concentrations of glucose and insulin during the MMT are denoted as incremental glucose and insulin peak, respectively. The incremental areas under the curve (IAUCs) for glucose and insulin were calculated with the trapezoidal method for the 0- to 150-min postmeal time interval.
Model of C-peptide kinetics during MTT
BBCF and PBCF were estimated from glucose and C-peptide time-concentration profiles during the MMT using an insulin secretion model validated in healthy subjects and subjects with newly diagnosed type 2 diabetes (17). The model parameters M0 and MI were estimated using weighted nonlinear regression analysis (18).M0 is an index of the BBCF and represents the ability of fasting glucose to stimulate β-cells. It is calculated as fasting C-peptide secretion (per unit volume of the central compartment) divided by fasting plasma glucose concentration.
MI is an index of PBCF and represents the ability of postprandial glucose to step up β-cell secretion. It equals the increment in secretion (again per unit volume of the central compartment) in response to a unit increment in glucose concentration.
Insulin sensitivity
Insulin sensitivity was calculated from duplicate fasting insulin and fasting glucose samples using the computer program for the homeostasis model assessment (HOMA2) of insulin resistance (19).
Statistical analysis
The variables are summarized either as means ± SD or as medians (25th–75th percentile) as appropriate. Differences in baseline clinical and biochemical characteristics were tested using an unpaired t test. Spearman correlation analysis and nonlinear regression were performed to relate glycemic variability to β-cell function. The stepwise multiple regression analysis was used to explore the influence of β-cell function on MAGE including various clinical factors. The variables were tested for normality and, where appropriate, were logarithmically transformed as indicated.P < 0.05 was considered statistically significant.
RESULTS
Clinical and biochemical characteristics
Of the 69 subjects with type 2 diabetes who volunteered for the study, 10 were excluded: 2 tested positive for GAD antibodies, 3 aborted CGMS measurements, and 5 refused the MMT. The 59 participating subjects were grouped into OHA users and nonusers. As shown in Table 1, diabetes duration was significantly longer and A1C was higher in OHA users than in nonusers. All other characteristics, including carbohydrate intake during the study, were not significantly different between the treatment groups.
Table 1.
Clinical characteristics of the study groups
| OHA users | Nonusers | P value | |
|---|---|---|---|
| n | 34 | 25 | |
| Sex (male/female) | 15/19 | 16/9 | 0.13 |
| Age (years) | 65.0 (57.0–71.0) 8.5 | 64.0 (62.0–69.0) | 0.65 |
| Diabetes duration (years) | 8.5 (3.0–11.0) | 2.0 (1.0–6.0) | 0.003 |
| First-degree relatives with diabetes | 13 (37) | 13 (52) | |
| BMI (kg/m2) | 29.5 ± 3.9 | 30.2 ± 3.6 | 0.42 |
| Waist circumference (cm) | 101.2 ± 12.6 | 103.8 ± 12.5 | 0.45 |
| Blood pressure (mmHg) | |||
| Systolic | 140.0 (125.0–145.0) | 130.0 (130.0–142.5) | 0.98 |
| Diastolic | 80.0 (80.0–90.0) | 80.0 (80.0–86.3) | 0.39 |
| A1C (%) | 6.8 ± 1.2 | 6.1 ± 0.6 | 0.013 |
| Fasting C-peptide (nmol/l) | 0.92 (0.70–1.26) | 0.91 (0.71–1.25) | 0.90 |
| Triglyceride (mmol/l) | 1.8 (1.3–2.5) | 1.8 (1.5–2.2) | 0.57 |
| HDL cholesterol (mmol/l) | 1.3 (1.1–1.4) | 1.4 (1.0–1.6) | 0.45 |
| Carbohydrate intake (BU/day) | 10.0 (9.3–12.5) | 12.0 (10.0–14.0) | 0.14 |
Data are means ± SD, medians (25th–75th percentile), or n (%). Significance level OHA users vs. nonusers, P <0.05. BU, bread exchange units.
CGMS measurements and glycemic variability
Table 2 shows the results of CGM measurements. All sensor glucose values were significantly higher in OHA users than in nonusers. Likewise, between-group differences were observed in the glucose area values (mean AUCCGM). Compared with the OHA users, nonusers spent a significantly lower amount of time in the hyperglycemic range (10.3 vs. 0.9 h/day, P < 0.001), whereas the duration of hypoglycemia was almost negligible. The MAGE was significantly higher in the group of OHA users than in nonusers and exceeded the proposed 4 mmol/l target level.
Table 2.
CGM data, glycemic variability, MMT-derived parameters, and insulin sensitivity
| OHA user | Nonusers | P value | |
|---|---|---|---|
| CGM data | |||
| Mean sensor glucose (mmol/l) | 9.8 (8.6–13.1) | 7.0 (6.2–7.6) | <0.001 |
| Mean sensor glucose range (mmol/l) | 8.7 ± 2.5 | 5.8 ± 2.3 | <0.001 |
| Median sensor glucose (mmol/l) | 9.7 (8.2–12.8) | 6.8 (6.2–7.9) | <0.001 |
| Mean AUCCGM(mmol · l−1· 24 h−1) | 236.0 (207.6–313.7) | 166.8 (147.6–184.8) | <0.001 |
| Duration of hyperglycemia (h/day) | 10.3 (5.7–20.9) | 0.9 (0.0–2.4) | <0.001 |
| Duration of hypoglycemia (h/day) | 0.0 (0.0–1.2) | 0.0 (0.0–0.0) | 0.033 |
| Glycemic variability | |||
| MAGE (mmol/l) | 5.7 ± 1.8 | 3.6 ± 1.9 | <0.001 |
| MMT-derived parameters | |||
| Fasting glucose (nmol/l) | 8.1 (7.4–11.5) | 7.0 (6.0–7.8) | <0.001 |
| Incremental glucose peak (mmol/l) | 4.2 (3.3–4.8) | 2.4 (1.6–3.2) | <0.001 |
| IAUCGlucose(mmol · l−1· 150 min−1) | 254.5 (187.0–305.0) | 117.5 (54.5–156.5) | <0.001 |
| Fasting plasma insulin (nmol/l) | 0.11 (0.07–0.14) | 0.10 (0.09–0.13) | 0.77 |
| Incremental insulin peak (mmol/l) | 0.55 (0.27–0.81) | 0.67 (0.45–0.80) | 0.62 |
| IAUCInsulin(mmol · l−1· 150 min−1) | 38.0 (25.0–42.9) | 43.8 (21.5–44.9) | 0.06 |
| Fasting β-cell function (10−9/min) | 7.1 (4.9–8.8) | 9.0 (8.1–11.1) | 0.005 |
| Postprandial β-cell function (10−9/min) | 27.4 (15.7–46.3) | 71.4 (50.4–108.8) | <0.001 |
| Insulin sensitivity | |||
| HOMA-%S | 44.6 ± 17.1 | 53.0 ± 13.1 | 0.045 |
Data are means ± SD or median (25th–75th percentiles). Significance level OHA users vs. nonusersP <0.05. HOMA-%S, homeostatic model assessment insulin sensitivity index.
Plasma glucose, plasma insulin, and estimates of pancreatic β-cell function during the MMT
The statistical comparisons of measurements characterizing the glycemic status, β-cell function, and insulin sensitivity are summarized in Table 2. Fasting glucose, the incremental glucose peak, and IAUC for glucose were significantly higher in OHA users than in nonusers during the MMT. Although fasting insulin levels, incremental insulin peak, and IAUC for insulin were not significantly different between the two subject groups, IAUCInsulin/IAUCGlucose was significantly decreased in OHA users (−171.3 pmol · l−1· 150 min−1, P = 0.010).
Relationships between glycemic variability and β-cell function
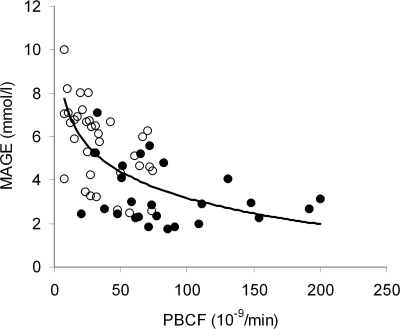
When the relationship between MAGE and β-cell function was analyzed by nonlinear regression analysis, including both OHA users and nonusers, a significant relationship was found with the model indexes of β-cell function. As shown in Fig. 1, the relationships of MAGE with PBCF as a group (r = 0.66, P < 0.001) and BBCF (r = 0.44, P < 0.001) were nonlinear and a decrease in PBCF <25 × 10−9/min was associated with a steep increase in MAGE. A separate analysis of the two subject groups showed that the relationship between MAGE and PBCF remained significant for OHA users (r = 0.54, P < 0.001) but failed to achieve significance for nonusers (r = 0.26, P = 0.21). A statistically significant nonlinear association was also observed with BBCF in OHA users (r = 0.39, P = 0.025) but not in nonusers (r = 0.37, P = 0.07). MAGE did not correlate with fasting insulin (r = 0.08, P = 0.56) or postprandial insulin (r = −0.01, P = 0.93).
Figure 1.
The relationship between MAGE and PBCF in OHA users (r = 0.54, P < 0.001) and nonusers (r = 0.26, P = 0.21) with type 2 diabetes. The overall regression line was obtained by nonlinear regression analysis as y = −1.7435 ln(x) + 11.208 (r = 0.66, P < 0.001). ○, OHA users; ●, nonusers.
Multivariate regression analysis with MAGE as the dependent variable
Multivariate regression analyses were performed to assess the independent effects of β-cell function as well as glycemic and other factors on MAGE. These included A1C, the degree of insulin sensitivity, age, sex, duration of diabetes, carbohydrate consumption (bread exchange units), and OHA therapy. Because PBCF and BBCF are highly correlated, two independent models were tested: model 1 including PBCF and model 2 including BBCF. The results of the stepwise regression analyses are provided in Table 3. The log-transformed PBCF was the strongest independent contributor to MAGE, whereas the sulfonylurea plus metformin combination treatment accounted for a smaller portion of the variability. The other independent variables failed to enter. The BBCF substitution for PBCF in model 2 showed that only the type of OHA treatment, of which the sulfonylurea plus metformin combination therapy was the most influential, remained independently associated with MAGE. The basal β-cell function, BBCF, and other factors did not enter this model.
Table 3.
Results of stepwise forward regression analysis with MAGE as the dependent variable
| Explanatory variable | Regression coefficient (β) | SEM | P value | Coefficient of determination (R2) |
|---|---|---|---|---|
| Model 1 | ||||
| Postprandial β-cell function | −2.928 | 0.616 | <0.001 | 0.443 |
| SU + MET treatment | 1.610 | 0.607 | 0.010 | 0.504 |
| Model 2 | ||||
| SU + MET treatment | 4.020 | 0.572 | <0.001 | 0.307 |
| SU treatment | 2.106 | 0.517 | <0.001 | 0.398 |
| MET treatment | 1.575 | 0.491 | 0.002 | 0.491 |
Variables of β-cell function were log transformed to assure normality. MET, metformin; SU, sulfonylurea.
CONCLUSIONS
Data in the literature suggest a relationship between glucose fluctuations during postprandial periods and the development of diabetes-related macrovascular complications (20). Although of unclear etiology, the failing β-cell function unequivocally contributes to the glycemic instability (21). However, the degree of association between glycemic variability and β-cell function in type 2 diabetes remains unclear. The present study was performed to investigate these relationships using the CGMS to determine glycemic variability and using the insulin secretion model (17) to determine β-cell function. Our results show that in a carefully investigated cohort of primary care subjects with fairly well-controlled type 2 diabetes, glycemia in the segment of OHA users is characterized by MAGE values above the proposed target of 4.0 mmol/l (22) and higher-than-recommended glucose levels after a standard meal (15). The values for PBCF and BBCF in our study are consistent with data from previous studies (17). Although the immediate pharmacological effects of OHAs could be washed out, post hoc exploratory analysis (unpublished results) revealed that 4 weeks after resumption of OHA therapy, median PBCF values were still 26% lower (P = 0.047) than before the 8-day OHA withdrawal period and were significantly lower compared with those for OHA nonusers (P < 0.001). This result suggests that the increased glucose levels during the period of OHA withdrawal exerted a longer lasting glucotoxic effect on the β-cell. Because we investigated the association between β-cell function and glycemic variability after OHA withdrawal, even if the subjects may have not been at baseline, this observation does not directly affect the relationship between these parameters.
We demonstrate for the first time that glycemic variability strongly correlates with PBCF in a segment of type 2 diabetic patients using OHAs and that the relationship is nonlinear. Our results extend knowledge about postprandial insulin secretion and glucose control. In newly diagnosed type 2 diabetes, Albarrak et al. (23) showed that glucose meal responses correlate with postprandial β-cell responsiveness but not with insulin resistance. The nonlinear relationship between the MAGE and PBCF suggests that, in OHA users, postprandial β-cell response at or <∼25 × 10−9/min is associated with a drastic increase in glycemic variability, whereas above this threshold, glycemic variability is improved and affected by other factors apart from β-cell dysfunction. Using multivariate regression models, we found that PBCF explained 44% of the interindividual glycemic variability. As expected, the type of OHAs was also independently associated with MAGE. Unexpectedly, other factors such as diabetes duration, insulin sensitivity, and carbohydrate intake failed to enter the regression models.
The interindividual variability of glucose excursions could not be fully elucidated by the explanatory variables included in the present study. It appears that other genetic and environmental factors such as treatment duration and medication compliance may be responsible for the residual amount of unexplained variability. However, the influence of these factors is difficult to assess in a cross-sectional study for which the primary aim is to investigate the relationship between MAGE and β-cell dysfunction. These environmental factors are associated with glycemic variability and most likely with postprandial hyperglycemia as has been recently demonstrated in insulin-treated type 2 diabetes (24). Although the subjects in the present study consumed comparable amounts of carbohydrate and underwent a standardized MMT, it was beyond the scope of our investigation to analyze medication compliance or other behavioral factors in detail.
A weakness of the current study is its cross-sectional nature. The strengths of the study include CGM measurements, use of a standardized MMT, and central laboratory analyses.
In summary, we demonstrate a nonlinear relationship between glycemic variability and β-cell dysfunction in a segment of type diabetic patients using OHAs. The PBCF correlates most strongly with glycemic variability. Our data suggest that PBCF is an important therapeutic target in controlling glucose excursions during postprandial periods to prevent secondary complications of type 2 diabetes. In this regard, treatment regimens including incretin mimetics (22,25) might have the potential of longer-term improvement in PCBF.
Acknowledgments
This study was funded by the Bundesministerium für Bildung und Forschung (Inno Regio Project 03i 2709).
No potential conflicts of interest relevant to this article were reported.
We are grateful to our colleagues in the outpatient centers who contributed their patients to the study and the patient volunteers for their participation.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Turner RC, Holman RR, Cull CA, Stratton IM, Mattthews RR, Frighi V, Manley E, Neil A, McElroy H, Wright D, Kohner E, Fox C, Hadden D: Intensive blood-glucose control with sulphonylureasor insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 2.Monnier L, Lapinski H, Colette C: Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients—variations with increasing levels of HbA1c. Diabetes Care 2003; 26: 881– 885 [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A: Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1– 7 [DOI] [PubMed] [Google Scholar]
- 4.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C: Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681– 1687 [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M, Hirsch IB: Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006; 295: 1707– 1708 [DOI] [PubMed] [Google Scholar]
- 6.Robertson RP: Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 2004; 279: 42351– 42354 [DOI] [PubMed] [Google Scholar]
- 7.Shiraiwa T, Kaneto H, Miyatsuka T, Kato K, Yamamoto K, Kawashima A, Kanda T, Suzuki M, Imano E, Matsuhisa M, Hori M, Yamasaki Y: Postprandial hyperglycemia is a better predictor of the progression of diabetic retinopathy than HbA1c in Japanese type 2 diabetic patients. Diabetes Care 2005; 28: 2806– 2807 [DOI] [PubMed] [Google Scholar]
- 8.Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M: Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Study. J Clin Endocrinol Metab 2006; 91: 813– 819 [DOI] [PubMed] [Google Scholar]
- 9.Del PS, Marchetti P, Bonadonna RC: Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 2002; 51 ( Suppl. 1): S109– S116 [DOI] [PubMed] [Google Scholar]
- 10.Service FJ, Nelson RL: Characteristics of glycemic stability. Diabetes Care 1980; 3: 58– 62 [DOI] [PubMed] [Google Scholar]
- 11.Haupt E, Etti H, Bamberg J, Hilgenfeld J, Schöffling K: Blutzuckersenkende Sulfonamide: Eine Placebo-Auslassstudie zur Objektivierung der Wirsamkeit oraler Antidiabetika. Akt Endokrinol Stoffw 1984; 5: 23– 31[article in German] [Google Scholar]
- 12.Diabetes Prevention Program Research Group Effects of withdrawal from metformin on development of diabetes in the Diabetes Prevention Program. Diabetes Care 2003; 26: 977– 980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrezenmeir J, Fenselau S, Keppler I, Abel J, Orth B, Laue C, Sturmer W, Fauth O, Halmagyi M: Postprandial triglyceride high response and the metabolic syndrome. Ann N Y Acad Sci 1997; 827: 353– 368 [DOI] [PubMed] [Google Scholar]
- 14.Kohnert KD, Augstein P, Heinke P, Zander E, Peterson K, Freyse EJ, Salzsieder E: Chronic hyperglycemia but not glucose variability determines HbA1c levels in well-controlled patients with type 2 diabetes. Diabetes Res Clin Pract 2000; 77: 420– 426 [DOI] [PubMed] [Google Scholar]
- 15.Postprandial blood glucose. American Diabetes Association Diabetes Care 2001; 24: 775– 778 [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF: Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970; 19: 644– 655 [DOI] [PubMed] [Google Scholar]
- 17.Hovorka R, Chassin L, Luzio SD, Playle R, Owens DR: Pancreatic β-cell responsiveness during meal tolerance test: model assessment in normal subjects and subjects with newly diagnosed noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998; 83: 744– 750 [DOI] [PubMed] [Google Scholar]
- 18.Van Cauter E, Mestrez F, Sturis J, Polonsky KS: Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992; 41: 368– 377 [DOI] [PubMed] [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487– 1495 [DOI] [PubMed] [Google Scholar]
- 20.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M: Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1clevel. Diabetes Care 2000; 23: 1830– 1834 [DOI] [PubMed] [Google Scholar]
- 21.Cremer GM, Molnar GD, Taylor WF, Moxness KE, Service FJ, Gatewood LC, Ackerman E, Rosevear JW: Studies of diabetic instability. II. Tests of insulinogenic reserve with infusions of arginine, glucagon, epinephrine, and saline. Metabolism 1971; 20: 1083– 1098 [DOI] [PubMed] [Google Scholar]
- 22.Monnier L, Colette C: Glycemic variability—should we and can we prevent it? Diabetes Care 2008; 31: S150– S154 [DOI] [PubMed] [Google Scholar]
- 23.Albarrak AI, Luzio SD, Chassin LJ, Playle RA, Owens DR, Hovorka R: Associations of glucose control with insulin sensitivity and pancreatic β-cell responsiveness in newly presenting type 2 diabetes. J Clin Endocrinol Metab 2002; 87: 198– 203 [DOI] [PubMed] [Google Scholar]
- 24.Murata GH, Duckworth WC, Shah JH, Wendel CS, Hoffman RM: Sources of glucose variability in insulin-treated type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES). Clin Endocrinol (Oxf) 2004; 60: 451– 456 [DOI] [PubMed] [Google Scholar]
- 25.Bloomgarden ZT: Exploring treatment strategies for type 2 diabetes. Diabetes Care 2007; 30: 2737– 2745 [DOI] [PubMed] [Google Scholar]