Abstract
OBJECTIVE
High levels of dietary fiber, especially soluble fiber, are recommended to lower serum cholesterol levels and improve glycemic control in patients with type 2 diabetes. It is not clear, however, how high levels of fiber affect mineral balance.
RESEARCH DESIGN AND METHODS
In a randomized crossover study, 13 patients with type 2 diabetes were fed a high-fiber (50 g total and 25 g soluble fiber) and a moderate-fiber (24 g total and 8 g soluble fiber) diet of the same energy, macronutrient, calcium, magnesium, and phosphorus content for 6 weeks each. Intestinal calcium absorption was determined by fecal recovery of 47Ca. Stool weight and mineral content were assessed during 3 days, and 24-h urinary mineral content and serum chemistry were assessed over 5 days at the end of each phase. The results were compared by repeated-measures ANOVA.
RESULTS
Compared with the moderate-fiber diet, the high-fiber diet increased stool weight (165 ± 53 vs. 216 ± 63 g/day, P = 0.02) and reduced 24-h urinary calcium (3.3 ± 1.7 vs. 2.4 ± 1.2 mmol/day, P = 0.003) and phosphorus (29.2 ± 5.5 vs. 26.0 ± 3.2 mmol/day, P = 0.003) excretion and serum calcium concentration (2.33 ± 0.06 vs. 2.29 ± 0.07 mmol/l, P = 0.04). Calcium absorption, stool calcium, magnesium, and phosphorus content and serum phosphorus concentration were not significantly different with the two diets.
CONCLUSIONS
A high-fiber diet rich in soluble fiber has a small impact on calcium and phosphorus balance in subjects with type 2 diabetes. It may be prudent to ensure adequate intake of calcium and other minerals in individuals consuming a high-fiber diet.
Diets high in fiber, especially soluble fiber, are associated with an improvement in serum lipid, glucose, and insulin concentrations (1,2). Because of the beneficial effects of soluble fiber on the LDL cholesterol concentration, the National Cholesterol Education Program Adult Treatment Panel III recommends consumption of 10–25 g soluble fiber per day, through dietary changes, in individuals with high LDL cholesterol concentrations (3). Fiber consumption is also emphasized by the American Diabetes Association for individuals with diabetes or glucose intolerance to help manage their glucose and lipid concentrations (4).
There is some concern that a high intake of fiber may impair the absorption of minerals, especially calcium (5,6). It is postulated that dietary fiber binds with polyvalent mineral ions forming unabsorbable fiber-mineral complexes (5,6). The relationship between dietary fiber and calcium, magnesium, and phosphorus absorption or balance is controversial, however, with some studies reporting impaired mineral absorption or balance and other studies reporting no change or a positive balance with a high-fiber diet compared with a moderate-fiber diet (7–11). The conflicting results may be due to one or more methodological issues such as not closely matching the mineral content of the diets, not providing the diets, lack of randomization, and short study duration. In addition, most of the above-mentioned studies looked at the effect of fiber supplements rather than fiber-rich natural foods on mineral absorption, and thus there is limited understanding of the effect of increasing the intake of fiber-rich natural foods on mineral absorption.
The objective of our study was to compare the long-term effect of a high-fiber diet particularly rich in soluble fiber with that of a moderate-fiber diet on mineral absorption and metabolism in subjects with type 2 diabetes in a randomized crossover study. The diets consisted of natural foods, were matched for calcium, magnesium, and phosphorus content, and were prepared in the metabolic kitchen. The effects of these diets on lipid and glycemic control have been published previously (2).
RESEARCH DESIGN AND METHODS
We recruited 13 patients (12 men and 1 woman) with type 2 diabetes for this study. The study was conducted at the General Clinical Research Center of the University of Texas Southwestern Medical Center at Dallas. The protocol for this study was approved by the institutional review board of the medical center, and all patients gave written informed consent. Nine of the 13 subjects were non-Hispanic whites, and the remaining subjects were African American. Their mean ± SD age was 60 ± 8 years (range 45–70 years) and mean BMI (weight in kilograms divided by the square of the height in meters) was 34.9 ± 8.7. None of the patients were receiving insulin therapy. Ten patients were taking glyburide daily; one or more patients were using nonsteroidal anti-inflammatory drugs, anticlotting, antihypertensive, antipsychotic, antidepressant, sleep, and gout medications, or pyridoxine daily, and the doses of these medications did not change throughout the study. None of the patients had thyroid, renal, or hepatic disease or were taking any vitamin D or mineral supplements.
All of the patients received the two isoenergic study diets, a high-fiber and a moderate-fiber diet, for a period of 6 weeks each, in a randomized crossover design. There was a median interval of 7 days between the two diet periods during which the subjects were instructed to follow an isoenergic diet. The daily energy intake needed for weight maintenance during the two diets was estimated by multiplying the calculated basal energy expenditure by an activity factor (12).
All meals were prepared in the metabolic kitchen. On weekdays, all of the patients ate at least one meal per day at the General Clinical Research Center. The remaining food was supplied in packages to be consumed at home. To monitor compliance, the patients were instructed to bring back any unconsumed food, were interviewed by a dietitian, and were weighed during their visits.
The patients were hospitalized during the last week of each dietary period (days 36–42) for evaluation. The patients were instructed to maintain their usual level of physical activity during the entire study period.
Diets
A set of three standard menus for each study diet were prepared for a 2,000-kcal intake. For a different energy requirement, all food items were adjusted accordingly. The nutrient intakes during the two diets are presented in Table 1. The moderate-fiber diet provided 8 g/day of soluble fiber and 16 g/day of insoluble fiber, and the high-fiber diet provided 25 g/day each of soluble and insoluble fiber. The fiber goal for the high-fiber diet was achieved by including natural sources of fiber such as fruit, beans, oat bran, oatmeal, sweet potato, winter squash, and vegetables such as okra and zucchini. Percent energy from macronutrients was the same for the two diets. Both of the diets were designed to provide 800 mg calcium, 400 mg magnesium, 1,600 mg inorganic phosphorus, 120 mmol sodium, and 100 mmol potassium per day. Homogenates of the diets were prepared, and the mineral content of the diets was determined by ash analysis. Thereafter, both diets were matched for calcium, magnesium, phosphorus, sodium, and potassium by adding calcium gluconate, magnesium gluconate, sodium chloride, dibasic sodium phosphate, potassium chloride, or dibasic potassium phosphate to the diets, if needed. The intake of fluids was limited to 3 l/day of distilled water. Plain tea or coffee was allowed in restricted amounts. Sample menus for both of the study diets have been published previously (2). The energy intake was adjusted if needed to maintain a constant body weight during the study.
Table 1.
Composition of the study diets
| Moderate-fiber diet | High-fiber diet | |
|---|---|---|
| Total fiber (g/day) | 24 | 50 |
| Soluble fiber (g/day) | 8 | 25 |
| Insoluble fiber (g/day) | 16 | 25 |
| Carbohydrate (% of total energy) | 55 | 55 |
| Protein (% of total energy) | 15 | 15 |
| Fat (% of total energy) | 30 | 30 |
| Calcium (mg/day) | 800 | 800 |
| Magnesium (mg/day) | 400 | 400 |
| Inorganic phosphate (mg/day) | 1,600 | 1,600 |
| Sodium (mmol/day) | 120 | 120 |
| Potassium (mmol/day) | 100 | 100 |
Study protocol
Fractional intestinal calcium absorption was measured by recovery of 47Ca in the feces after oral administration of isotope (13) on day 36. 47Ca was given orally with 250 ml distilled water and 4.3 ml Neo-Calglucon (Sandoz Pharmaceuticals, East Hanover, NJ). Food was withheld for 4 h thereafter. Stool was collected for 3 days until the disappearance of carmine, which was given 24 h after isotope administration. Polyethylene glycol was given with the isotope as nonabsorbable marker.
The patients collected 24-h urine specimens for determination of urine volume and chemistry on days 38 through 42 of each dietary phase. Stools were collected for determination of stool weight and mineral content for 3 days at the end of each dietary phase. Blood for serum chemistry analyses was drawn, after an overnight fast, daily on days 38 through 42 during each dietary period.
Biochemical analyses
Urinary, stool, and plasma calcium content and urinary and stool magnesium content were determined by atomic absorption spectrophotometry. Urinary, stool, and plasma phosphorus content was measured using the method of Fiske and SubbaRow (14). Urinary sodium and potassium were measured by specific ion electrode using a Beckman Astra analyzer (Beckman Instruments, Fullerton, CA). Plasma sodium, potassium, creatinine, and uric acid were measured as a part of the systematic multichannel analysis (SMA-20). Urinary creatinine was estimated by the rate-Jaffe method using Beckman Astra, oxalate by the method of Hodgkinson and Williams (15), uric acid by the enzymatic method of Liddle et al. (16), and citrate by an enzymatic method using kits (Boehringer-Mannheim Biochemicals, Indianapolis, IN). Urinary sulfate was determined by the turbidimetric procedure of Ma and Chan (17). The 47Ca in stool was determined by a well-type NaI-crystal gamma-scintillation counter equipped with a single-channel pulse-height analyzer at the 47Ca peak of 1.29 MeV. Parathyroid hormone (PTH) was assessed by a Nichols Allegro Intact PTH Assay (Nichols Diagnostics, San Juan Capistrano, CA).
Statistical analyses
The repeated measures analysis of variance model was used to assess the effect of the study diets and the order in which the diets were given on stool weight and mineral content, percent 47Ca absorption from the gastrointestinal tract, 24-h urinary volume and chemistry, and fasting serum chemistry. The results were not affected by the order in which the diets were given, and so only the effect of the diets will be presented in the article. All statistical analyses were performed using SAS (version 9.1.3).
RESULTS
The stool weight and mineral content and percent absorption of 47Ca in the gastrointestinal tract are presented in Table 2. Stool weight was about one-third higher (P = 0.02) during the high-fiber diet than during the moderate-fiber diet. There was no statistically significant difference in stool calcium, magnesium, and phosphorus content between the two diets, although there was a tendency for magnesium and phosphorus stool content to be slightly higher with the high-fiber diet than with the moderate-fiber diet. 47Ca absorption in the gastrointestinal tract was slightly lower with the high-fiber diet than with the moderate-fiber diet, but the difference was not statistically significant.
Table 2.
Stool mineral content and intestinal 47Ca absorption, 24-h urinary chemistry, and fasting serum chemistry during the study
| Moderate-fiber diet | High-fiber diet | High-fiber − moderate-fiber | P value* | |
|---|---|---|---|---|
| Stool mineral content and % absorption of 47Ca | ||||
| Weight (g/day)† | 165 ± 53 | 216 ± 63 | 59 (17 to 101) | 0.02 |
| Ca (mmol/day)† | 7.9 ± 2.2 | 7.7 ± 2.5 | −0.5 (−2.5 to 1.6) | 0.57 |
| Mg (mmol/day)† | 5.8 ± 2.4 | 6.7 ± 1.7 | 0.7 (−1.4 to 2.7) | 0.40 |
| P (mmol/day)† | 9.9 ± 3.0 | 11.3 ± 2.7 | 1.0 (−2.0 to 4.1) | 0.31 |
| % absorption of 47Ca | 54.9 ± 21.5 | 51.6 ± 18.1 | −3.4 (−18 to 11.2) | 0.63 |
| Urinary chemistry‡ | ||||
| Volume (ml/day) | 2,430 ± 632 | 2,597 ± 484 | 167 (−81 to 415) | 0.17 |
| Ca (mmol/day) | 3.3 ± 1.7 | 2.4 ± 1.2 | −0.9 (−1.4 to −0.4) | 0.003 |
| Mg (mmol/day) | 4.6 ± 1.2 | 4.6 ± 0.9 | −0.004 (−0.47 to 0.46) | 0.83 |
| P (mmol/day) | 29.2 ± 5.5 | 26.0 ± 3.2 | −3.2 (−5.4 to −1.0) | 0.003 |
| Na (mmol/day) | 116 ± 22 | 102 ± 13 | −14 (−24 to −3) | 0.02 |
| K (mmol/day) | 88 ± 23 | 94 ± 10 | 6 (−4 to 17) | 0.23 |
| Cr (mmol/day) | 14.2 ± 2.4 | 13.8 ± 1.7 | −0.5 (−1.2 to 0.1) | 0.12 |
| Urate (mmol/day) | 3.8 ± 0.9 | 3.9 ± 0.7 | 0.1 (−0.3 to 0.5) | 0.47 |
| Sulfate (mmol/day) | 19.2 ± 2.6 | 15.2 ± 2.5 | −4.1 (−5.4 to −2.7) | <0.0001 |
| Citrate (μmol/day) | 39.4 ± 16.8 | 37.7 ± 14.1 | −1.7 (−6.1 to 2.6) | 0.39 |
| Oxalate (μmol/day) | 341 ± 56 | 464 ± 115 | 123 (68 to 177) | 0.0006 |
| CrCl (ml/s) | 1.9 ± 0.5 | 1.8 ± 0.3 | −0.1 (−0.2 to 0.02) | 0.12 |
| pH | 5.8 ± 0.5 | 6.1 ± 0.4 | 0.3 (0.1 to 0.4) | 0.001 |
| Serum chemistry§ | ||||
| Ca (mmol/l) | 2.33 ± 0.06 | 2.29 ± 0.07 | −0.04 (−0.07 to −0.002) | 0.04 |
| P (mmol/l) | 1.20 ± 0.15 | 1.18 ± 0.15 | −0.01 (−0.06 to 0.03) | 0.43 |
| Na (mmol/l) | 139 ± 1 | 139 ± 2 | −0.2 (−1.3 to 0.9) | 0.53 |
| K (mmol/l) | 4.45 ± 0.26 | 4.38 ± 0.18 | −0.07 (−0.17 to 0.03) | 0.16 |
| PTH (ng/l) | 32.7 ± 11.6 | 35.4 ± 13.6 | 2.7 (−3.1 to 8.5) | 0.33 |
| Cr (μmol/l) | 92 ± 18 | 93 ± 17 | 0.8 (−3.0 to 4.5) | 0.71 |
All values are shown as the mean ± SD or mean difference (95% CI).
*Repeated-measures ANOVA model was used for comparison.
†Stool weight was assessed in 11 subjects during the moderate-fiber phase and 12 subjects during the high-fiber phase, and stool Ca, Mg, and P contents were assessed in 11 subjects during the moderate-fiber phase and 10 subjects during the high-fiber phase.
‡Urine values represent mean values for five daily collections of urine for each patient.
§Serum values represent mean values for five daily collections of blood for each patient except for PTH levels, which were determined on a single sample. Ca, calcium; Cr, creatinine; CrCl, creatinine clearance; K, potassium; Mg, magnesium; Na, sodium, P, phosphorus.
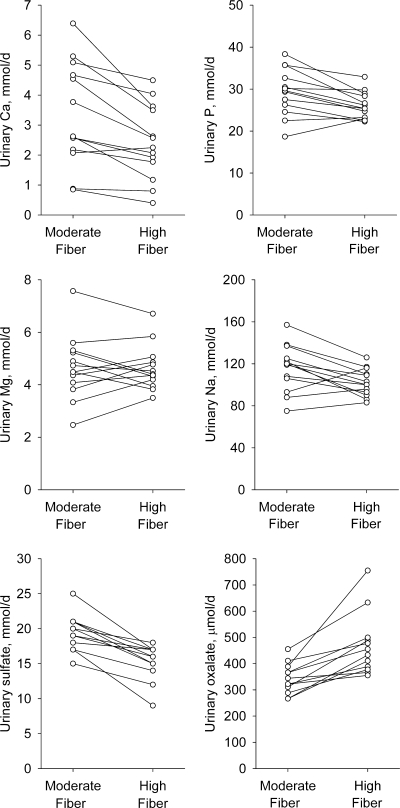
The 24-h urinary volume and chemistry data are presented in Table 2. The 24-h urinary calcium, phosphorus, magnesium, sodium, sulfate, and oxalate results for each individual are shown in Fig. 1. Urinary volume was similar for the two diets. Compared with the moderate-fiber diet, the high-fiber diet was associated with lower urinary calcium (27%; P = 0.003), phosphorus (11%; P = 0.003), and sodium (12%; P = 0.02) excretion. No difference was observed for urinary magnesium and potassium excretion. Urinary excretion of oxalate was higher (36%; P = 0.0006) and that of sulfate was lower (21%; P <0.0001) with the high-fiber diet compared with the moderate-fiber diet. No difference was found in urinary excretion of urate, citrate, and creatinine or in creatinine clearance with the two diets. Urinary pH was higher with the high-fiber diet than with the moderate-fiber diet (5%; P = 0.001).
Figure 1.
The 24-h urinary calcium (Ca), phosphorus (P), magnesium (Mg), sodium (Na), sulfate, and oxalate concentrations for each individual during consumption of the moderate-fiber and high-fiber diets. Each value represents mean of five daily collections of urine during the inpatient stay at the General Clinical Research Center.
The mean fasting serum calcium level was slightly but significantly lower (1.5%; P = 0.04) with the high-fiber diet than with the moderate-fiber diet (Table 2). There was a tendency for the serum phosphorus level and potassium level to be slightly lower and the PTH level to be higher with the high-fiber diet than with the moderate-fiber diet, but the differences were not statistically significant. Serum sodium and creatinine levels were similar with the two diets.
CONCLUSIONS
Our data suggest that a high intake of fiber results in reduced urinary calcium excretion and a slight but significant reduction in serum calcium levels. These effects are most likely due to effects of dietary fiber on reducing calcium absorption. However, we did not find a significant reduction in intestinal calcium absorption using the 47Ca isotope method. Analysis of minerals in stool also did not reveal increased fecal calcium excretion despite a 31% increase in the wet weight of stool with the high-fiber diet. Similarly, Spencer et al. (9) also observed a significant decrease in urinary calcium excretion and no change in the intestinal absorption of 47Ca in ambulatory men after consumption of an additional 21 g fiber/day in the form of oat bran muffins for 32 days. Balasubramanian et al. (7) reported a decrease in apparent calcium absorption (calcium intake − fecal calcium excretion and expressed as a percentage of intake) in elderly subjects during the last 10 days after consumption of 30 g of wheat bran supplements for 21days. Wisker et al. (8), on the other hand, found no change in urinary or fecal calcium content in healthy subjects after consumption of diets enriched with 15 g barley fiber for 22 days. This study, however, did not closely match the calcium content of the two diets. Similarly, Behall et al. (10) reported no change in apparent calcium balance (intake − fecal and urinary excretion) in subjects with type 2 diabetes after consumption of a diet enriched with 32 g/day of guar for 6 months, whereas Coudray et al. (11) reported a positive calcium balance in healthy men consuming diets enriched with 58 g fiber in the form of inulin or sugar beet for 28 days. These studies also did not match the calcium content of the different diets. Furthermore, Behall et al. (10) did not provide the study diets for the subjects and therefore probably had less control of the overall diet.
The lower urinary calcium excretion with the high-fiber diet may also be partly explained by increased alkalization of urine as evidenced by the increased pH with the high-fiber diet. This could be due to the reduced animal protein and higher plant food content of the high-fiber diet. The high-fiber diet contained about 15 g more plant protein than the moderate-fiber diet for an energy intake of 2,000 kcal/day. Animal protein is rich in sulfur-containing amino acids that are oxidized to sulfate (18). Plant foods, on the other hand, contain alkali that buffers the moderate acidosis caused by sulfate. Alkali enhances renal tubular reabsorption of calcium and reduces bone resorption, thereby lowering the urinary calcium excretion (19). The reduced urinary sulfate excretion with the high-fiber diet may have also contributed to the reduced urinary calcium excretion because sulfate binds with calcium ions and prevents renal tubular reabsorption of calcium (20). A reduced sulfate level would lead to greater renal tubular reabsorption and reduced excretion of calcium.
The lower urinary calcium excretion with the high-fiber diet may also be partly due to the lower urinary sodium excretion. The role of urinary sodium is likely to be minor, however, because there was only a small difference in urinary sodium excretion with the two diets and because sodium intake, which is highly correlated with urinary sodium excretion, was held constant across the two diets. Caffeine intake, which slightly reduces calcium absorption but has no effect on 24- h urinary calcium excretion (21), was kept constant throughout the study and could not have affected urinary calcium excretion.
Stool and urinary magnesium content were not significantly higher with the high-fiber diet compared with the moderate-fiber diet. These results are similar to a number of studies that reported no change in apparent magnesium balance with diets enriched with oat bran (9), guar (10), inulin, or sugar beet (11) fiber. Only Wisker et al. (8) reported a slight negative magnesium balance following consumption of diets rich in barley fiber.
The decreased urinary phosphorus excretion with the high-fiber diet was probably due to the effect of dietary fiber on phosphorus absorption. Our study, however, did not show a significant increase in the phosphorus content of stools with the high-fiber diet. Our results contradict the results from another study (9), in which consumption of a diet rich in oat bran led to a significant increase in urinary phosphorus level; however, the phosphorus content was about twice as high with the high-fiber diet than with the moderate-fiber diet (9).
It is not possible to distinguish the role of soluble from insoluble fiber on mineral absorption in our study because the high-fiber diet, although particularly rich in soluble fiber, also contained insoluble fiber in greater quantity than in the moderate-fiber diet. Also not possible to differentiate from our study is the role of dietary fiber from the effect of phytate and oxalate on mineral absorption. The high-fiber diet in our study had nearly 3 times more phytate and ∼4 times more oxalate than the moderate-fiber diet. Both phytate and oxalate form insoluble complexes with minerals such as calcium, thereby reducing their absorbability (22,23). Designing diets with the same amount of insoluble fiber, phytate, and oxalate would be challenging because foods rich in soluble fiber tend to be rich in the above components. In addition, the purpose of our study was not to match the insoluble fiber, phytate, and oxalate content of the two diets but to examine the effect of a diet high in fiber derived from natural foods on lipids, glycemic response, and mineral balance. We did not assess the impact of the high-fiber diet on absorption of trace minerals. Whether fiber derived from natural foods affects absorption of these minerals needs further study. The small sample size in our study is probably not an issue because we had a crossover design. According to Garcia et al. (24), who evaluated the efficiency of crossover designs, a crossover design needs about 4–10 times fewer subjects than a similarly powered parallel design. In their article, Garcia et al. also noted that our study (with the primary data) (2) incorporated an efficient crossover design. Furthermore, our current analysis did have sufficient power to statistically detect the clinically important differences in the mineral balance data presented in this article.
Our study had several strengths. The mineral contents, especially the calcium, phosphorus, and magnesium contents, of the two diets were carefully matched because high-fiber foods, such as legumes and green leafy vegetables, are naturally rich in calcium and other minerals, and excessive calcium intake decreases magnesium absorption and vice versa (25) and increased intakes of calcium and phosphate decrease magnesium absorption due to formation of an insoluble calcium-magnesium-phosphate complex in the intestinal lumen (26). Other strengths included providing the diets and thus controlling the overall food intake, hospitalizing the patients during the last week of each phase for data collection, assessing the long-term effect of fiber intake on mineral absorption and metabolism by feeding each diet for 6 weeks, and determining the calcium absorption through fecal recovery of calcium isotope, the gold-standard method for assessing calcium absorption.
In summary, a diet high in fiber, especially soluble fiber, lowers urinary calcium and phosphorus levels and slightly reduces serum calcium concentration. The high intake of fiber did not have an effect on magnesium levels. These results do not justify modifying the dietary recommendations made by the National Cholesterol Education Program Adult Treatment Panel III and the American Diabetes Association. Nevertheless, it may be prudent to ensure adequate amounts of calcium and other minerals with long-term consumption of a high-fiber diet.
Acknowledgments
This study was funded by National Institutes of Health Grant M01-RR00633 and by the Southwestern Medical Foundation.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the annual meeting of the American College of Nutrition, Arlington, Virginia, 2–5 October 2008.
We thank Alan Stewart for performing all the chemical analyses.
Footnotes
Clinical trial reg. no. NCT00825383, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Anderson JW: Physiological and metabolic effects of dietary fiber. Fed Proc 1985; 44: 2902– 2906 [PubMed] [Google Scholar]
- 2.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ: Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000; 342: 1392– 1398 [DOI] [PubMed] [Google Scholar]
- 3.Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Panel III). JAMA 2001; 285: 2486– 2497 [DOI] [PubMed] [Google Scholar]
- 4.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31 ( Suppl 1): S61– S78 [DOI] [PubMed] [Google Scholar]
- 5.James WP, Branch WJ, Southgate DA: Calcium binding by dietary fibre. Lancet 1978; 1: 638– 639 [DOI] [PubMed] [Google Scholar]
- 6.Oku T, Konishi F, Hosoya N: Mechanism of inhibitory effect of unavailable carbohydrate on intestinal calcium absorption. J Nutr 1982; 112: 410– 415 [DOI] [PubMed] [Google Scholar]
- 7.Balasubramanian R, Johnson EJ, Marlett JA: Effect of wheat bran on bowel function and fecal calcium in older adults. J Am Coll Nutr 1987; 6: 199– 208 [DOI] [PubMed] [Google Scholar]
- 8.Wisker E, Nagel R, Tanudjaja TK, Feldheim W: Calcium, magnesium, zinc, and iron balances in young women: effects of a low-phytate barley-fiber concentrate. Am J Clin Nutr 1991; 54: 553– 559 [DOI] [PubMed] [Google Scholar]
- 9.Spencer H, Norris C, Derler J, Osis D: Effect of oat bran muffins on calcium absorption and calcium, phosphorus, magnesium and zinc balance in men. J Nutr 1991; 121: 1976– 1983 [DOI] [PubMed] [Google Scholar]
- 10.Behall KM, Scholfield DJ, McIvor ME, Van Duyn MS, Leo TA, Michnowski JE, Cummings CC, Mendeloff AI: Effect of guar gum on mineral balances in NIDDM adults. Diabetes Care 1989; 12: 357– 364 [DOI] [PubMed] [Google Scholar]
- 11.Coudray C, Bellanger J, Castiglia-Delavaud C, Remesy C, Vermorel M, Rayssignuier Y: Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur J Clin Nutr 1997; 51: 375– 380 [DOI] [PubMed] [Google Scholar]
- 12.Harris JA, Benedict FG: A biometric study of human basal metabolism. Proc Natl Acad Sci USA 1918; 4: 370– 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslau NA, McGuire JL, Zerwekh JE, Frenkel EP, Pak CY: Hypercalcemia associated with increased serum calcitriol levels in three patients with lymphoma. Ann Intern Med 1984; 100: 1– 6 [DOI] [PubMed] [Google Scholar]
- 14.Fiske CH, SubbaRow Y: The colorimetric determination of phosphorus. J Biol Chem 1925; 66: 375– 400 [Google Scholar]
- 15.Hodgkinson A, Williams A: An improved colorimetric procedure for urine oxalate. Clin Chim Acta 1972; 36: 127– 132 [DOI] [PubMed] [Google Scholar]
- 16.Liddle L, Seegmiller JE, Laster L: The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med 1959; 54: 903– 913 [PubMed] [Google Scholar]
- 17.Ma RS, Chan CJ: Endogenous sulphuric acid production: a method of measurement by extrapolation. Clin Biochem 1973; 6: 82– 87 [DOI] [PubMed] [Google Scholar]
- 18.Tschope W, Ritz E: Sulfur-containing amino acids are a major determinant of urinary calcium. Miner Electrolyte Metab 1985; 11: 137– 139 [PubMed] [Google Scholar]
- 19.Sakhaee K, Maalouf NM, Abrams SA, Pak CY: Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab 2005; 90: 3528– 3533 [DOI] [PubMed] [Google Scholar]
- 20.Schuette SA, Zemel MB, Linkswiler HM: Studies on the mechanism of protein-induced hypercalciuria in older men and women. J Nutr 1980; 110: 305– 315 [DOI] [PubMed] [Google Scholar]
- 21.Heaney RP: Effects of caffeine on bone and the calcium economy. Food Chem Toxicol 2002; 40: 1263– 1270 [DOI] [PubMed] [Google Scholar]
- 22.Graf E, Eaton JW: Effects of phytate on mineral bioavailability in mice. J Nutr 1984; 114: 1192– 1198 [DOI] [PubMed] [Google Scholar]
- 23.Kelsay JL, Prather ES: Mineral balances of human subjects consuming spinach in a low-fiber diet and in a diet containing fruits and vegetables. Am J Clin Nutr 1983; 38: 12– 19 [DOI] [PubMed] [Google Scholar]
- 24.Garcia R, Benet M, Arnau C, Cobo E: Efficiency of the crossover design: an empirical estimation. Stat Med 2004; 23: 3773– 3780 [DOI] [PubMed] [Google Scholar]
- 25.Spencer H: Minerals and mineral interactions in human beings. J Am Diet Assoc 1986; 86: 864– 867 [PubMed] [Google Scholar]
- 26.Brink EJ, Beynen AC, Dekker PR, van Beresteijn EC, van der Meer R: Interaction of calcium and phosphate decreases ileal magnesium solubility and apparent magnesium absorption in rats. J Nutr 1992; 122: 580– 586 [DOI] [PubMed] [Google Scholar]