Abstract
Aim
To investigate the role of heat shock proteins 70 (HSP70) in radiosensitivity and invasiveness of endometrial cancer in vitro.
Methods
HSP70 expression was silenced in relatively radioresistant, well-differentiated human endometrial cancer cell line ISK, using small interference RNA method, or by HSP70 overexpression after transfecting a HSP70-expressing vector. The effect of HSP70 on ISK cell line response to irradiation was evaluated. The surviving fraction was measured using colony-formation assay. Apoptosis was detected by flow cytometry and HSP70 expression was determined by quantitative real-time polymerase chain reaction, western-blot, and/or immuocytochemistry. Cell invasiveness was measured using transwell invasion assay.
Results
HSP70 silencing caused a significant increase in irradiation-induced cell killing in comparison with control cells, with an enhancement factor of 1.27, and in the percentage of apoptotic cells (14.22% vs 6.74%, P = 0.021). After 4 Gy irradiation, mean ± standard deviation survival fraction in ISK cells was reduced to 0.32 ± 0.04 in comparison with control values but in ISK/siRNA-HSP70 cells the survival fraction was higher and amounted to 0.51 ± 0.08 (P = 0.026). Silencing HSP70 significantly inhibited cell invasion before and after irradiation (106 ± 19 vs 219 ± 18 and 119 ± 16 vs 256 ± 31, P = 0.007). On the contrary, ectopic overexpression of HSP70 attenuated irradiation-induced apoptosis (7.15% vs 4.08%, P = 0.043) and induced more ISK/HSP70 cells invaded through the filters than mock-infected cells before and after irradiation (274 ± 21 vs 194 ± 16 before irradiation, and 298 ± 24 vs 227 ± 19 after irradiation, respectively, P = 0.032).
Conclusion
Disruption of HSP70-induced cytoprotection during irradiation enhances therapeutic effect of irradiation, which makes HSP70 a promising target in the research of endometrial cancer.
Endometrial cancer is one of the most common gynecologic malignancies worldwide, with radiation therapy as a very important treatment option (1). However, the efficacy of radiation therapy is often limited by radioresistance, ie, diminished susceptibility of the irradiated cells to undergo apoptosis. In our previous studies, we found that human heat shock protein 70 (HSP70) was significantly overexpressed in radioresistant endometrial cancer cells line ISK compared with relatively radiosensitive cells after irradiation (2). This suggested that irradiation-induced HSP70 content may play an important role in the development of radioresistance.
The HSP70 family contains at least 8 homologous chaperone proteins (3). Endoplasmatic reticulum and mitochondria have their specific HSP70 proteins, whereas the remaining 6 family members reside mainly in the cytosol and nucleus. HSP70B' is the major human isoform in the HSP70 family that is strictly stress-inducible and therefore available to function only in stressed cells (4). Phylogenetic analysis indicated that the HSP70B’ protein sequence was most closely related to another major inducible human HSP70 – HSP72. HSP70B' and HSP72 together play a role in cell survival after proteotoxic stress. Inducible HSP70 are up-regulated in the majority of human tumors and are believed to have an important role in cell proliferation, tumor growth, and cancer invasiveness (5-8). Overexpression of HSP70 in human tumors is associated with poor prognosis and poor response to radiation therapy (9,10). Some in vitro studies suggested that the viability of cells pretreated with exogenous HSP70 before radiation was indeed protected and that transfection of cells with a siRNA designed to interfere with HSP70 synthesis increased the irradiation-induced apoptosis (11,12). Despite this, the role of HSP70 in endometrial cancer is largely unknown. Understanding of HSP70s function in endometrial cancer may provide clues to the development of radioresistance and offer alternatives for clinical treatment. Meanwhile, it is well known that metastasis is highly dependent on tumor cell invasion. Previous studies reported that overexpression of HSP70 induced the expression of matrix metallopeptidase 9 (MMP-9) and thereby improved the cell invasiveness (13). In the present study, we investigated the role of HSP70 in radiosensitivity and invasiveness of a well defined endometrial cancer cell in vitro.
Materials and methods
Cell culture
Three human endometrial cancer cell lines were obtained from the Type Culture Collection of Chinese Academy of Sciences (Beijing, China): ISK (well differentiated, relatively radioresistant), RL95-2 (moderately differentiated, radiosensitive), and KLE (poorly differentiated, radiosensitive). RL95-2 and KLE cells were cultured in DMEM (Gibco BRL, Gaithersburg, MD, USA), supplemented with 100 U/mL penicillin/streptomycin. ISK cells, stably transfected with pcDNA3.1, pcDNA3-HSP70, and pcDNA3-siHSP70, were maintained in DMEM in the presence of G-418 (400 µg/mL). All media were supplemented with 10% fetal bovine serum (FBS) and 2 mmol/L glutamine. The cultures were incubated at 37°C in a humidified 5% CO2 incubator.
Irradiation
Cells were cultured in 60-mm dishes until 70% to 80% confluent and then exposed to x-rays from a VARIAN clinical 2100C/D linear accelerator (VARIAN, Palo Alto, CA, USA) at a dose rate of 2.0 Gy/min.
Clonogenic cell survival assay
The surviving fraction was measured using a standard colony-formation assay. Cells were transfected as above with vehicle, siHSP70, and pcDNA3-HSP70. Seventy-two hours after transfection, cells were trypsinized, counted, and approximately 200 cells were plated in six-well plates and allowed to attach for 6 hours. After 6 hours, cells were irradiated and incubated for 12 days. Colonies were stained with crystal violet and those with ≥50 cells were counted. Five replicate dishes were counted for each treatment.
Creation of ISK/siRNA-HSP70 cells.
A 21-nucleotide DNA sequence 5′-CACAAGAAGGACATCAGCCTT-3′ targeting HSP70 was designed by Kangchen (Shanghai, China). The 21-nucleotide oligonucleotide 5′-CTTAGGCTGTTACATCTCTCC-3′, which had no significant homology to any known human mRNA, was used as a negative control. The sequence of each insert was confirmed by automated sequencing. Cells were transfected with HSP70 siRNA (ISK/siRNA) or control siRNA (ISK/control) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Stable integrants were selected by G-418 (400 µg/mL) starting 72 hours after transfection. After 2 weeks, one pool was isolated and subjected to further cloning procedures by the limiting dilution method. After 8 weeks, 2 clones with a >95% reduction in HSP70 protein levels compared with control were isolated. Cells were frequently tested for the expression of HSP70 by Western blot analysis. One stable clone expressing reduced levels of HSP70 protein was repeatedly selected and maintained in 400 µg/mL G418 selection medium. The G-418 selective pressure was removed 24 hours before experimental procedures.
Creation of ISK/HSP70 cells.
The HSP70 expression vector pcDNA3-HSP70 containing the cDNA for full-length human HSP70 was designed by Kangchen (Kangchen, Shanghai, China). Cells grown on 60-mm dishes were transfected with pcDNA3-HSP70 (ISK/HSP70) or pcDNA3.1 (ISK/Neo) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Clones resistant to G418 (400 µg/mL) were selected and then subcloned by limiting dilution. Stable clones were formed and maintained in 400 μg/mL G418 selection medium.
Quantitative real-time polymerase chain reaction (RT-PCR) data analysis
Total RNA was harvested from cells before and 1 hour after 4 Gy radiation exposure using TRIzol (Invitrogen), concentrated using isopropyl alcohol precipitation, and further purified using RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocols. The quantity and quality of the purified RNA were assessed by 260/280 optical density (OD) measurements on a spectrophotometer and the integrity was determined by formaldehyde denatured gel electrophoresis.
To determine the level of HSP70 mRNA, quantitative RT-PCR was performed on an ABI Prism 7000 Sequence Detection System using SYBR Green RT-PCR Kit (Applied Biosystems, Foster City, CA, USA). PCR reaction was performed using 20 µL of total reaction mixture volume, containing 1 µL of cDNA reaction products, 10 µL SYBR Green PCR Master Mix, and 500 nM of forward and reverse primers. The gene-specific primers were as follows: HSP70, 5′-TGTTCCGTTTCCAGCCCCCAA-3′ (sense) and 5′-GGGCTTGTCTCCGTCGTTGAT-3′ (antisense); and GAPDH, 5′-GGGAGCCAAAAGGGTCATCATCTC-3′(sense) and 5′-CCATGCCAGTGAGCTTCCCGTTC-3′ (antisense). The protocol of RT-PCR was as follows: initiation with a 10 minutes of denaturation at 95°C, followed by 40 cycles of amplification at 95°C (10 seconds) for denaturation, 10 seconds of hybridization at 58°C for HSP70 or 57°C for GAPDH, and 20 seconds of extension at 72°C. The RT-PCR amplification product was analyzed by melting curve analysis and 1.2% agarose gel electrophoresis, and the fold-change was determined based on average cycle threshold (Ct) values for triplicates. Gene expression levels were calculated and presented with 2-△△Ct values (14).
Protein extraction and Western blotting
Seventy-five percent confluent cells were irradiated with 4 Gy x-ray. After 1 hour, cells were harvested and lysed with cold RIPA buffer. Total cell lysates were clarified by centrifugation (14 000 rpm, 20 minutes) at 4°C. Total protein concentration was determined by BCA Protein Assay kit (Pierce, Rockford, IL, USA). Twenty micrograms of each soluble protein sample was separated by 15% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. After blocking with 5% TBST-milk (20 mmol/L Tris-HCl, 137 mM NaCl, 1.5% nonfat dry milk, and 0.1% Tween20, pH 7.6), membranes were incubated with rabbit polyclonal anti-HSP70 (1:2000) and anti-β-actin antibodies (sc-33575, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Following this, they were washed and incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody, incubated in SuperSignal® West Pico Chemiluminescent Substrate (Pierce), exposed to CL-Xposure Film (Pierce), and developed using an All-Pro 100 Plus automated x-ray film processor (All-Pro Imaging Corporation, Hicksville, NY, USA). The resultant bands were quantified using AlphaEaseFC software (AlphaInnotech, San Leandro, CA, USA).
Apoptosis analysis
The Annexin V-FITC kit (BD Biosciences, San Diego, CA, USA) was used to identify apoptotic and viable cells, with slight modifications of manufacturer’s instructions. After exposed to 4 Gy radiation, cells were harvested by trypsinization (Invitrogen), washed with cold PBS, and resuspended in 100 µL of binding buffer. A total of 5 µL of Annexin V-FITC and 10 µL of propidium iodide (PI) were added and the mixture was incubated for 30 minutes in the dark. Finally, 400 µL of binding buffer was added to the cells, the mixture was analyzed with a flow cytometer, using FITC signal detector and PI staining with a phycoerythrin emission signal detector. The percentage of apoptotic cells in 10 000 cells was determined, after which all the experiments were performed 3 times. The data were analyzed using WinMDI 2.8 software (Scripps Institute, La Jolla, CA, USA) for calculation of percentage of apoptotic cells per group.
In vitro invasion assays
Invasion assays were done using a modified Transwell chamber system as described previously (13). Cells (2 × 105) were seeded on Matrigel-coated membrane inserts with a pore size of 8 µm (BD Bioscience, Heidelberg, Germany) in the presence of DMEM supplemented with 10% FBS. The same medium of 750 µL was placed in the lower wells of the chamber system. Thereafter, the cells were irradiated and incubated for 24 hours. The cells on the upper side were scraped off with a rubber policeman and the cells that had migrated into the lower compartment were fixed (4% paraformaldehyde in phosphate buffered saline, PBS), stained with hematoxylin and eosin, and counted from 5 random high power fields at 200 magnification in each well.
Statistical analysis
Data are shown as means ± standard deviation of replicate samples in single experiments or replicate experiments as described in the figure legends. t test was used to test the comparisons between two groups. Multiple group comparisons were performed by one-way ANOVA, followed by the Tukey-Kramer multiple group comparisons test. All statistical analyses were performed using SPSS statistical software, version 10.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Basal and irradiation-induced HSP70 expression
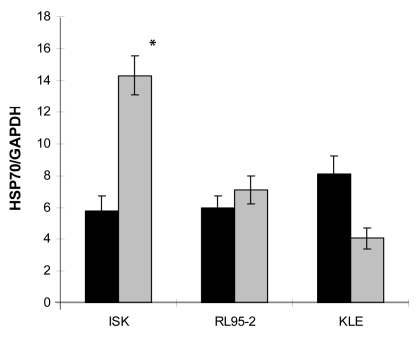
Although in our previous studies of the radiosenistivities of ISK, KLE, and RL95-2 endometrial cancer cell lines showed that ISK line was relatively radioresistant (2), high endogenous basal expression HSP70 levels were observed in all cell lines (Figure 1). However, the irradiation-induced overexpression of HSP70 was only observed in ISK cells (14.31 ± 1.24 vs 5.82 ± 0.93, P = 0.014) (Figure 1).
Figure 1.
Basal and irradiation-induced expression of HSP70 mRNA in ISK, RL95-2, and KLE cells. The level of mRNA was determined by quantitative real-time polymerase chain reaction. Bars represent the means and error bars standard deviations from 3 independent experiments. *P = 0.014 vs control.
HSP70 expression in ISK cells transfected with siRNA-HSP70 and pcDNA3-HSP70
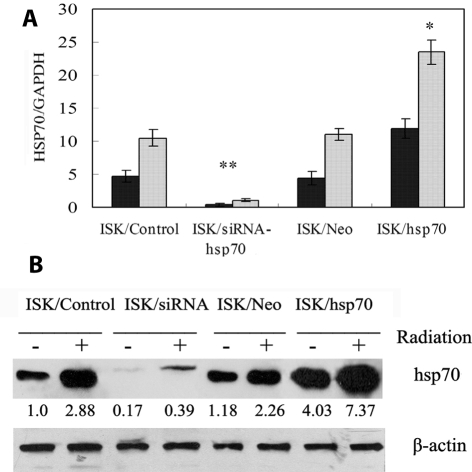
We examined the HSP70 mRNA and HSP70 protein expression in ISK cells transfected with vehicle, siRNA-HSP70, and pcDNA3-HSP70 (Figure 2). Transfection with siRNA-HSP70 resulted in dramatic reduction of endogenous levels of HSP70 compared with the vehicle control (ie, up to 95%, P < 0.001) and in the inhibition of irradiation-mediated HSP70 induction (ie, up to 90%, P < 0.001). Transfection of HSP70 resulted in 2.7-fold increase in ectopic expression of HSP70 compared with the vehicle control (P = 0.018) and 2.1-fold increase in irradiation-mediated HSP70 induction (P = 0.037). These findings were confirmed by western-blot analysis of HSP70 protein expression.
Figure 2.
Expression of HSP70 in ISK cells transfected with siRNA-HSP70 (ISK/siRNA), control siRNA (ISK/Control), pcDNA3-HSP70 (ISK/HSP70), or pcDNA3.1 (ISK/Neo). (A) Quantitive real-time validation confirmed significant differential expression of HSP70 in ISK/siRNA and ISK/HSP70 cells vs control cells. Closed bars – no radiation, open bars – radiation. Asterisk – P < 0.05 vs control; double asterisk – P < 0.01 vs control. (B) Representative Western blot demonstrating expression of HSP70 in cells transfected as shown above.
siRNA-HSP70 radiosensitizes ISK cells
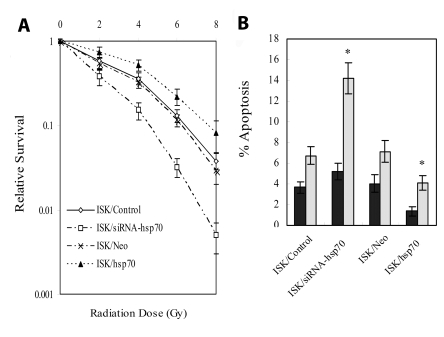
Clonogenic survival assays were used to examine the ability of siRNA-HSP70 to sensitize ISK cells to the effects of radiation. Irradiation alone resulted in a dose-dependent reduction in cell survival and the effect was significantly enhanced in the cells transfected with siRNA-HSP70 before irradiation, compared with control (Figure 3A). Cell killing induced by 4 Gy irradiation in ISK/siRNA-HSP70 was comparable with that observed in ISK/control at a dose of 5.1 Gy irradiation, corresponding to an enhancement factor of 1.27. Furthermore, transfection of cells with small interfering RNA to silence HSP70 abolished the shoulder of radiation dose-response curves, which is considered to reflect repair of sublethal damage (15). Cells transfected with the nonspecific RNA clearly retained the usual shoulder.
Figure 3.
HSP70 enhances resistance of ISK cells to ionizing radiation and silencing the gene radiosensitizes ISK cells. (A) Clonogenic survival curves for cells transfected with siRNA-HSP70 (ISK/siRNA), control siRNA (ISK/Control), pcDNA3-HSP70 (ISK/HSP70), or pcDNA3.1 (ISK/Neo) exposed by x-ray radiation. (B) Flow cytometric analysis of irradiation-induced apoptosis. Error bars, standard deviations of results in 3 independent experiments. *P < 0.05 vs control.
To investigate the effects of irradiation on the induction of apoptosis in ISK/control and ISK/siRNA-HSP70 cells, flow cytometric measurement was used to quantify the percentage of apoptotic cells in the total cell population. After 24-hour irradiation, the percentage of apoptotic cells significantly increased in ISK/siRNA-HSP70 cells compared with control (6.74% vs 14.22%, P = 0.021, Figure 3B). However, there was no significant difference between the two groups before irradiation (3.7 vs 5.2%, P = 0.087). These results indicated that a decrease in HSP70 protein enhanced the ability of irradiation to inhibit the survival and improve apoptosis of ISK cells.
Increased expression of HSP70 improved the survival of ISK cells after exposure to radiation
To determine whether increased expression of HSP70 conferred protection against irradiation, cell survival was examined in irradiated ISK/HSP70 and ISK/Neo cells. We found that overexpression of HSP70 increased cell survival in ISK/HSP70 cells. For example, after 4 Gy irradiation, ISK cell survival fraction was reduced to 0.32 ± 0.04 in comparison with untreated cells (reference value = 1), but the survival fraction of ISK/siRNA-HSP70 cell was increased to 0.51 ± 0.08 (P = 0.026; Figure 3A).
After 24-hour irradiation, a significantly lower percentage of apoptotic cells was observed in ISK/HSP70 than mock transfected ISK/Neo cells (7.15% vs 4.08%, P = 0.043; Figure 3B). These results indicated that the overexpression of HSP70 protein inhibited apoptosis and improves the survival of ISK cells after exposure to radiation.
HSP70 promotes invasive potential in ISK cells
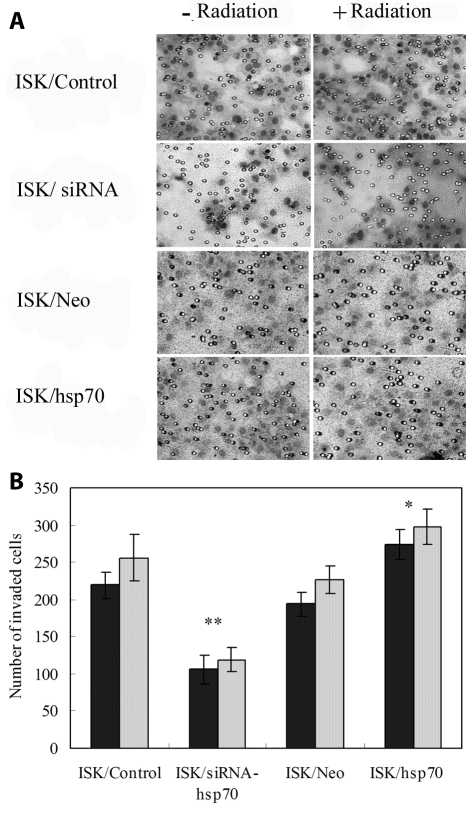
The invasiveness of ISK cells transfected with siRNA and HSP70 was determined using matrigel invasion assay. ISK cells irradiated at doses of 4 Gy had more invasive cells than untreated controls, but the difference was not significant (P = 0.083, Figure 4). Silencing HSP70 significantly inhibited cell invasion before and after irradiation (106 ± 19 vs 219 ± 18 and 119 ± 16 vs 256 ± 31, P = 0.007, Figure 4A, 4B). On the contrary, more ISK/HSP70 cells invaded through the filters than mock transfected ISK/Neo cells before and after irradiation (274 ± 21 vs 194 ± 16 before irradiation, and 298 ± 24 vs 227 ± 19 after irradiation, respectively, P = 0.032). These results indicated that HSP70 promoted invasiveness in ISK cells and abortion of this gene may act as a means to inhibit cell invasion in endometrial cancer.
Figure 4.
Cell invasiveness of ISK/control, ISK/siRNA, ISK/Neo and ISK/HSP70 before or after irradiation exposure. (A) Cells were on the lower surface of the transwell filters (magnification ×200). (B) The number of cells that invaded through the filters was counted under a microscope in 5 views. Columns, mean of three independent experiments; bars, standard deviation. Asterisk – P < 0.05 vs control; double asterisk – P < 0.01 vs control.
Discussion
Our study demonstrated that, although all 3 tested endometrial cancer cell lines had high endogenous basal expression HSP70 levels, the irradiation-induced overexpression of HSP70 was observed only in radioresistant ISK cells, but not in relatively radiosensitive KLE or RL95-2 cells. These results suggest that the effect of treatment by irradiation should not simply be predicted by the basal HSP70 level in endometrial cancer. In fact, irradiation-induced HSP70 expression may play an important role in radioresistance. HSP70 silencing caused a significant increase in irradiation-induced cell killing and in the percentage of apoptotic cells. Moreover, HSP70 silencing significantly inhibited cell invasion before and after irradiation.
HSP70 is present in a variety of malignant cell types and its expression is induced by several stressors, such as hyperthermia, infection, UV radiation, as well as γ-radiation. HSP70 is known to rescue cells from tumor necrosis, factor-induced caspase-independent programmed cell death, heat shock, serum starvation, and oxidative stress. It is associated with increased cell proliferation, inhibition of apoptosis, lymph node metastasis, higher clinical stage, and poor prognosis in endometrial cancer (16,17).
Inducible HSP70 has been suggested to have multiple roles in cytoprotection against irradiation-induced apoptosis. Brondani et al reported that the content of HSP70 was associated with glioblastoma cell radioresistance (9). The overexpression of HSP70 also enhanced radioresistance of mouse embryo fibroblasts and fibrosarcoma (18,19). Several preclinical studies evaluated the antitumor potency of HSP70 inhibitors which inhibit tumor growth and enhance radiation response in vitro as well as in vivo (20-23). HSP70 has been considered as a potential target for cancer therapy in recent years (24). However, little is known about its role in radiosensitivity of endometrial cancer.
Given that a number of potential mechanisms explain the relation between HSP70 and radioresistance, specific overexpression and suppression of the HSP70 gene could help elucidate the precise role of HSP70 in cancer radiotherapy. Our results identified that cells depleted of HSP70 displayed strikingly different radiosensitivity. Cells transfected with siRNA showed enhanced radiosensitivity compared with cells transfected with vehicle or HSP70. We also observed that increased constitutive expression of HSP70 in ISK cells was critical for radioresistance, because specific cells transfected with HSP70 led to a lower apoptosis and greater clonogenicity.
Malignant progression of cancer depends not only on rapid proliferation of tumor cells but also on other biological behaviors including motility, invasiveness, and metastatic potential. The metastasis of cancer cells is one of the major factors deciding the cancer outcome. However, changes in motility or invasive potential after irradiation have been poorly understood. Radiation-induced enhancement in invasiveness was observed in glioblastoma, hepatocellular, breast, and pancreatic carcinoma (25-27). The mechanism may be associated with overexpression of MMP-2 and MMP-9 through the PI3K/Akt/NF-kappaB signal transduction pathway or activation of epidermal growth factor receptor (EGFR). Although we did not find remarkable enhancement of invasiveness after irradiation-exposure in ISK cells, we showed that irradiation promoted invasive potential in ISK/HSP70 cells, whereas cell proliferation was significantly suppressed. However, cells with HSP70 depletion inhibited invasion after irradiation, which suggests that this radiation-enhanced invasiveness may be associated with an increased expression of HSP70 and abortion of this gene may act as a means to inhibit cell invasion in endometrial cancer although its mechanism still awaits investigation.
To the best of our knowledge, this study is the first report suggesting that in vitro radiosensitivity of endometrial cancer cells may not be controlled by the basal expression level of HSP70 but be at least partly dependent on the irradiation-induced HSP70 content. Increased constitutive expression of HSP70 plays a critical role in endometrial cancer cells radioresistance and invasiveness, because specific transfection of HSP70 promotes clonogenicity and increases cell invasion. Furthermore, abrogation of HSP70 induction significantly enhances apoptotic effects of irradiation and reduces cell invasiveness. According to our results, siRNA-HSP70 can be used as a means to increase radiosensitivity and depress invasiveness in the treatment for endometrial cancer.
Acknowledgment
This work was supported by the grant from The Shandong Provincial Natural Science Foundation (No. Y2006C88).
Reference
- 1.Anderson PR. The role of radiation therapy in locally advanced endometrial cancer. Semin Radiat Oncol. 2006;16:152–7. doi: 10.1016/j.semradonc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Du XL, Jiang T, Wen ZQ, Li QS, Gao R, Wang F. Differential expression profiling of gene response to ionizing radiation in two endometrial cancer cell lines with distinct radiosensitivities. Oncol Rep. 2009;21:625–34. [PubMed] [Google Scholar]
- 3.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 4.Noonan E, Giardina C, Hightower L. Hsp70B' and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res. 2008;314:2468–76. doi: 10.1016/j.yexcr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Kalogeraki A, Giannikaki E, Tzardi M, Kafousi M, Ieromonachou P, Dariviannaki K, et al. Correlation of heat shock protein (HSP70) expression with cell proliferation (MIB1), estrogen receptors (ER) and clinicopathological variables in invasive ductal breast carcinomas. J Exp Clin Cancer Res. 2007;26:367–8. [PubMed] [Google Scholar]
- 6.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bausero MA, Page DT, Osinaga E, Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004;25:243–51. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wataba K, Saito T, Fukunaka K, Ashihara K, Nishimura M, Kudo R. Over-expression of heat shock proteins in carcinogenic endometrium. Int J Cancer. 2001;91:448–56. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1077>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, et al. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005;12:38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- 10.Brondani Da Rocha A, Regner A, Grivicich I, Pretto Schunemann D, Diel C, Kovaleski G, et al. Radioresistance is associated to increased Hsp70 content in human glioblastoma cell lines. Int J Oncol. 2004;25:777–85. [PubMed] [Google Scholar]
- 11.Bases R. Clonogenicity of human leukemic cells protected from cell-lethal agents by heat shock protein 70. Cell Stress Chaperones. 2005;10:37–45. doi: 10.1379/CSC-58R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ZG, Ma QZ, Xu CX. Abrogation of heat-shock protein (HSP)70 expression induced cell growth inhibition and apoptosis in human androgen-independent prostate cancer cell line PC-3m. Asian J Androl. 2004;6:319–24. [PubMed] [Google Scholar]
- 13.Lee KJ, Kim YM, Kim DY, Jeoung D, Han K, Lee ST, et al. Release of heat shock protein 70 (Hsp70) and the effects of extracellular Hsp70 on matric metalloproteinase-9 expression in human monocytic U937 cells. Exp Mol Med. 2006;38:364–74. doi: 10.1038/emm.2006.43. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.vanAnkeren SC, Murray D, Stafford PM, Meyn RE. Cell survival and recovery processes in Chinese hamster AA8 cells and in two radiosensitive clones. Radiat Res. 1988;115:223–37. doi: 10.2307/3577160. [DOI] [PubMed] [Google Scholar]
- 16.Bai XX, Chen YQ, Xin XY, Wang WD. Expression and significance of heat shock protein 70, 90 in endometrial carcinomas. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:38–40. [in Chinese] [PubMed] [Google Scholar]
- 17.Wataba K, Saito T, Fukunaka K, Ashihara K, Nishimura M, Kudo R. Over-expression of heat shock proteins in carcinogenic endometrium. Int J Cancer. 2001;91:448–56. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1077>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Lee SJ, Chung HY, Kim TH, Cho CK, Yoo SY, et al. Inducible heat-shock protein 70 is involved in the radioadaptive response. Radiat Res. 2000;153:318–26. doi: 10.1667/0033-7587(2000)153[0318:IHSPII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Kabakov AE, Malyutina YV, Latchman DS. Hsf1-mediated stress response can transiently enhance cellular radioresistance. Radiat Res. 2006;165:410–23. doi: 10.1667/RR3514.1. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi J, Yamashita K, Ishikawa T, Hosokawa H, Sumida K, Nagayama M, et al. The effects inhibiting the proliferation of cancer cells by far-infrared radiation (FIR) are controlled by the basal expression level of heat shock protein (HSP) 70A. Med Oncol. 2008;25:229–37. doi: 10.1007/s12032-007-9020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calini V, Urani C, Camatini M. Overexpression of HSP70 is induced by ionizing radiation in C3H 10T1/2 cells and protects from DNA damage. Toxicol In Vitro. 2003;17:561–6. doi: 10.1016/S0887-2333(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 22.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, et al. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabakov AE, Malyutina YV, Latchman DS. Hsf1-mediated stress response can transiently enhance cellular radioresistance. Radiat Res. 2006;165:410–23. doi: 10.1667/RR3514.1. [DOI] [PubMed] [Google Scholar]
- 24.Havik B, Bramham CR. Additive viability-loss following hsp70/hsc70 double interference and Hsp90 inhibition in two breast cancer cell lines. Oncol Rep. 2007;17:1501–10. [PubMed] [Google Scholar]
- 25.Paquette B, Baptiste C, Therriault H, Arguin G, Plouffe B, Lemay R. In vitro irradiation of basement membrane enhances the invasiveness of breast cancer cells. Br J Cancer. 2007;97:1505–12. doi: 10.1038/sj.bjc.6604072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25:7009–18. doi: 10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 27.Qian LW, Mizumoto K, Urashima T, Nagai E, Maehara N, Sato N, et al. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res. 2002;8:1223–7. [PubMed] [Google Scholar]