Abstract
Obesity has been associated with a higher risk for impaired cognitive function, which most likely reflects associated medical complications (i.e., cerebrovascular pathology). However, there is also evidence that in healthy individuals excess weight may adversely affect cognition (executive function, attention, and memory). Here, we measured regional brain glucose metabolism (using positron emission tomography (PET) and 2-deoxy-2[18F]fluoro-D-glucose (FDG)) to assess the relationship between BMI and brain metabolism (marker of brain function) in 21 healthy controls (BMI range 19–37 kg/m2) studied during baseline (no stimulation) and during cognitive stimulation (numerical calculations). Statistical parametric mapping (SPM) revealed a significant negative correlation between BMI and metabolic activity in prefrontal cortex (Brodmann areas 8, 9, 10, 11, 44) and cingulate gyrus (Brodmann area 32) but not in other regions. Moreover, baseline metabolism in these prefrontal regions was positively associated with performance on tests of memory (California Verbal Learning Test) and executive function (Stroop Interference and Symbol Digit Modality tests). In contrast, the regional brain changes during cognitive stimulation were not associated with BMI nor with neuropsychological performance. The observed association between higher BMI and lower baseline prefrontal metabolism may underlie the impaired performance reported in healthy obese individuals on some cognitive tests of executive function. On the other hand, the lack of an association between BMI and brain metabolic activation during cognitive stimulation indicates that BMI does not influence brain glucose utilization during cognitive performance. These results further highlight the urgency to institute public health interventions to prevent obesity.
INTRODUCTION
There is growing evidence that obesity (BMI >30 kg/m2 (1)) is associated with impaired cognitive function including executive function, attention, and memory (2–4). The adverse influence of obesity on cognitive performance is also evidenced by higher rates of attention-deficit hyperactivity disorder (5) and of Alzheimer disease, cortical atrophy, and white matter disease in obese subjects (6–8). Comorbid medical conditions that occur with obesity (i.e., cerebrovascular pathology, hypertension, diabetes) are likely to contribute because they adversely affect cognition. However, studies have also documented adverse effects of high BMI on cognitive performance in healthy subjects, which suggests that excessive weight may be deleterious to cognition (specifically executive function) (4). The mechanisms underlying this effect are not understood. Inasmuch as prefrontal regions are involved with executive function, we hypothesized that the influence of BMI on cognitive performance in healthy individuals would be mediated in part by its effects on prefrontal activity.
To test our hypothesis, we measured the association between BMI and regional brain glucose metabolism (using positron emission tomography (PET) and 2-deoxy-2[18F] fluoro-D-glucose (FDG) (9)) in healthy individuals at baseline and during cognitive stimulation (numerical calculations). Baseline regional brain metabolic measures have been shown to be a sensitive indicator of brain function/dysfunction that is associated with neurocognitive performance (10). We also measured brain metabolism during cognitive stimulation (numerical calculations) to determine whether BMI affected brain glucose utilization during cognitive performance. In parallel, we measured performance in neuropsychological tests that involve prefrontal processing including executive function and verbal memory. We hypothesized an inverse correlation between BMI and baseline metabolism in prefrontal regions and a positive association between prefrontal metabolism and performance on tests of executive function and of memory.
METHODS AND PROCEDURES
Subjects
A total of 21 right-handed healthy subjects (12 M and 9 F; 34 ± 7 years of age) were studied. Subjects were evaluated by a physician (for exclusion criteria, which included (i) current or past neuropsychiatric disorder or drug abuse, (ii) significant medical illness, (iii) current treatment with medication including over-the-counter drugs, and (iv) pregnancy. Normal physical examination and laboratory tests were required for entry. Prescan urine tests ensured the absence of any psychoactive drugs in all subjects and of pregnancy in females. Subjects were monetarily compensated for their participation in the study. Written informed consent was obtained in all subjects.
Neuropsychological evaluation
Subjects were tested within 2 weeks of the PET studies with a battery that included tests for memory (California Verbal Learning Test) and of executive function (Wisconsin Card Sorting Test, Symbol Digit Modalities Test, Trail Making Test and Stroop Interference Test; we also used the digit span and matrix reasoning subtests of the Wechsler Adult Intelligence Scale (WAIS)) (11). As estimates of verbal IQ we used the scaled scores on the Wide Range Achievement Test Revised III reading subtest, and, for nonverbal IQ, we used the scaled scores on the Wechsler Adult Abbreviated Scale of Intelligence Scale III matrix reasoning subtest (11). As control tasks we used motor tests (finger tapping, pegboard, and the gait) (11).
Scans
PET scans were obtained with a whole-body, high-resolution tomograph (Siemens/CTI ECAT HR+, with 4.6 × 4.6 × 4.2 mm resolution at center of field of view and 63 slices) using FDG as ligand. Subjects were scanned on 1 day during baseline (passively viewing nature cards) and on another during cognitive activation (additions, subtractions, multiplications, or divisions). The order of the scans was randomized. The difficulty of the numerical problems was controlled to achieve 80% accuracy, and the problems were presented on colored cards (one card per min) and correct responses were remunerated. Details about the methods for the scanning are published elsewhere (12). Briefly, a 20-min emission scan was started 35 min after injection of 4–6 mCi of FDG. Arterialized blood sampling was used to measure FDG in plasma. During the study, subjects were positioned supine in the PET camera with their eyes open; the room was dimly lit and noise was kept to a minimum.
Image and data analysis
Correlation analyses were performed between the baseline brain metabolic measures and the BMI and between the difference image (baseline–cognitive stimulation) and BMI using statistical parametric mapping (SPM) (13). In addition, significant findings detected with SPM were corroborated with independently drawn predefined regions of interest (ROIs).
For the SPM analyses the images were spatially normalized using the template provided in the SPM 99 package and subsequently smoothed with a 16 mm isotropic Gaussian kernel. A voxel-by-voxel correlation was done with the BMI as seed value, and voxels, where correlations were significant at P < 0.005 (uncorrected, cluster size >100 pixels), were backprojected into a structural image of magnetic resonance imaging.
For the ROI analysis, we extracted independently drawn ROI using an automated extraction method that is based on the standard brain template from the Talairach atlas (14). First, to eliminate variations across individuals’ brains, the FDG images were mapped into the Talairach brain using SPM’s normalization package. The inverse-mapping procedure was used to extract the Talairach coordinates of all voxels for a given anatomical region using the stereotaxic coordinates in the Talairach Daemon database (15,16). Pearson product moment correlations were done between BMI and the regional metabolic measures on these ROIs and also between the baseline metabolic measures and the task-induced changes in metabolism, which was done to assess whether baseline measures predicted activation responses.
To assess whether the regions that varied as a function of BMI predicted cognitive performance, we performed correlation analyses between the scores on the neuropsychological tests and both the baseline metabolic measures and the changes in metabolism during cognitive stimulation. For a priori hypotheses BMI would be negatively associated with prefrontal metabolism and prefrontal metabolism would be positively associated with executive function and memory, with significance set at P < 0.05. For exploratory tests, we set significance at P < 0.005 and report findings at P < 0.05 as trends. We also report on the correlations between the measures of IQ and the scores on the other neuropsychological tests.
RESULTS
Average BMI in our subjects corresponded to 25 ± 4 kg/m2 (range 19–37 kg/m2); average age to 34 ± 7 (range 22–45 years); and average education to 14 ± 2 years (range 11–18 years). Three of the subjects fitted criteria for obesity (BMI ≥30 kg/m2). Average brain glucose metabolism for the group corresponded to 36.4 ± 6 μmol/100 g/min (range 27 to 50). There were no significant correlations between BMI and age (r = 0.29, P = 0.21) or education (r = 0.20, P = 0.37) or between age and regional brain metabolism (prefrontal cortex r = 0.05 P = 0.84; cingulate gyrus r = 0.08 P = 0.73).
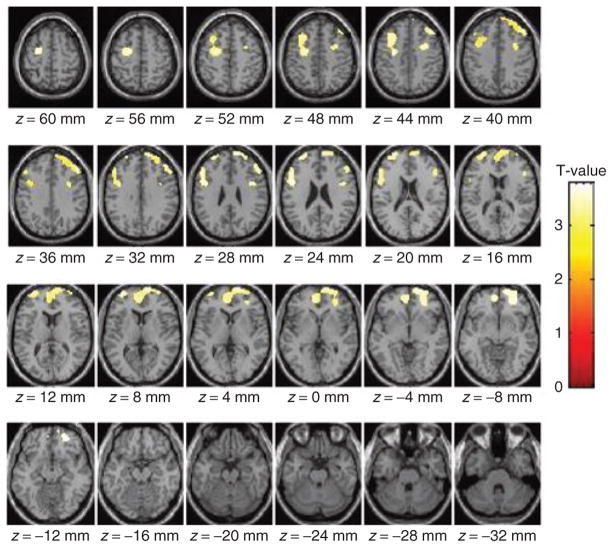
The SPM analysis on the baseline metabolic images revealed a negative association between BMI and metabolism in four clusters that included left and right prefrontal regions (Brodmann area (BA) 8, 9, 10) and anterior cingulate gyrus (BA 32) (Figure 1). The clusters comprised: (i) right prefrontal regions (coordinates 50, 34, 38, size 3,601 pixels and t = 3.8); (ii) left prefrontal and anterior cingulate regions (coordinates −26, −8, 52, size 1,972 pixels, t = 3.6); (iii) left prefrontal regions (coordinates −34, 60, 14, size 470 pixels, t = 3.6); and (iv) right prefrontal regions (coordinates 32, 4, 38, size 372 pixels, t = 3.4).
Figure 1.
Images showing the areas where BMI correlated negatively with absolute metabolic measures (statistical parametric mapping (SPM), P < 0.005, uncorrected). There were no regions where BMI showed a positive correlation with metabolism.
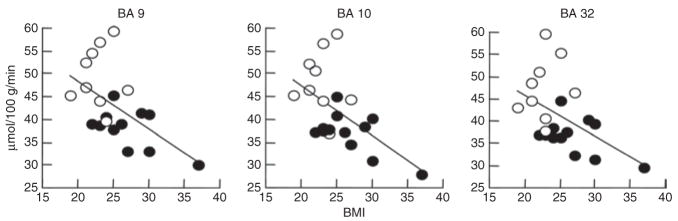
The metabolic measures extracted from the ROI corroborated a significant negative correlation between BMI and the left and right prefrontal regions identified by SPM as well as for BA 11 (r = −0.53; P ≤0.01). Table 1 provides the regional metabolic values for these prefrontal regions (BA 8, 9, 10, 32) and for the other cortical, cerebellar, and subcortical regions. Figure 2 shows the regression slopes for the averaged (left and right) measures in BA 9, 10, and 32, and BMI. These analyses also revealed trends with the left caudate (P < 0.01), putamen (P < 0.05), temporal (P < 0.05), and right hippocampus (P < 0.05) (Table 1).
Table 1.
Correlations between baseline glucose metabolism and BMI and mean and standard deviations for the baseline metabolic measures and range
| Brain Region | Left | Right | |
|---|---|---|---|
| Frontal | R = −0.52, P < 0.02 | R = −0.53, P < 0.01 | 38 ± 7 μmol/100 g/min (28–51) |
| BA 8 | R = −0.54, P < 0.01 | R = −0.54, P < 0.01 | 46 ± 9 μmol/100 g/min (32–64) |
| BA 9 | R =−0.56, P < 0.008 | R = −0.55, P < 0.009 | 43 ± 8 μmol/100 g/min (30–59) |
| BA 10 | R = −0.59, P < 0.005 | R = −0.57, P < 0.007 | 42 ± 8 μmol/100 g/min (28–58) |
| BA 32 | R = −0.52, P < 0.02 | R = −0.47, P < 0.03 | 41 ± 8 μmol/100 g/min (30–60) |
| Parietal | NS | NS | 39 ± 7 μmol/100 g/min (31–56) |
| Temporal | R = −0.44, P < 0.05 | NS | 35 ± 5 μmol/100 g/min (28–48) |
| Occipital | NS | NS | 43 ± 8 μmol/100 g/min (31–61) |
| Caudate | R = −0.54, P <0.01 | NS | 37 ± 8 μmol/100 g/min (21–57) |
| Putamen | R = −0.45, P < 0.05 | NS | 41 ± 7 μmol/100 g/min (31–59) |
| Thalamus | NS | NS | 42 ± 10 μmol/100 g/min (25–62) |
| Hippocampus | NS | R = −0.45, P < 0.04 | 31 ± 7 μmol/100 g/min (21–46) |
| Cerebellum | NS | NS | 34 ± 5 μmol/100 g/min (26–47) |
The prefrontal regions were those where SPM showed a significant correlation with BMI; values for the other cortical and subcortical regions are shown as comparisons.
BA, Brodmann area; NS, not significant.
Figure 2.
Regression slopes between BMI and baseline metabolism in prefrontal regions (Brodmann area (BA) 9 and 10) and anterior cingulate gyrus (BA 32). Regional metabolic values correspond to averaged left and right regions. Females are identified as open circles and males as closed circles.
Table 2 shows the scores on the neuropsychological measures and the correlations with BMI and IQ. BMI was negatively correlated with performance on the Wisconsin Card Sorting Test and selected subtests of the WAIS (Table 2). As expected, many of the tests correlated with the IQ measures (Table 2).
Table 2.
Scores on the neuropsychological tests and results for the correlations with verbal IQ (WRAT-R reading subtest scaled score) and nonverbal IQ (matrix reasoning scaled score) and with BMI
| CVLT | Scores | Verbal IQ | Nonverbal IQ | BMI |
|---|---|---|---|---|
| Trials 1–5: total learning | 45.8 ± 12 | 0.001 | 0.02 | NS |
| Short delay free recall | 9.7 ± 3 | 0.004 | 0.05 | NS |
| Short delay cued recall | 9.8 ± 4 | 0.003 | NS | NS |
| Long delay free recall | 9.3 ± 4 | 0.03 | NS | NS |
| Long delay cued recall | 10.0 ± 4 | 0.003 | NS | NS |
| Recognition hits: accuracy | 14.8 ± 1 | NS | NS | NS |
| WCST | ||||
| % Correct | 74.5 ± 12 | NS | 0.003 | 0.03 |
| % Perseverative errors | 13.4 ± 9 | NS | 0.04 | 0.05 |
| Stroop interference (scaled) | 1.7 ± 9 | 0.05 | NS | NS |
| Trail A (s) | 41.1 ± 20 | 0.04 | 0.03 | NS |
| Trail B (s) | 104.1 ± 78 | NS | 0.008 | NS |
| Symbol digit modality: | ||||
| Written | 46.0 ± 12 | 0.009 | NS | NS |
| WAIS-R | ||||
| Digits forward | 6.5 ± 1 | NS | 0.009 | 0.05 |
| Digits backward | 4.7 ± 2 | 0.03 | NS | 0.05 |
| WRAT-R | ||||
| Reading subtest (scaled scores) | 92.1 ± 17 | not applicable | 0.01 | NS |
| WASI | ||||
| Matrices (scaled scores) | 9.7 ± 4 | NS | not applicable | 0.04 |
| Motor tests | ||||
| Finger tapping (left hand) | 44.3 ± 6 | NS | NS | NS |
| Pegboard (left hand) | 96.3 ± 23 | NS | NS | NS |
| Gait | 10.7 ± 1 | NS | NS | NS |
Test scores correspond to averages and standard deviations and reflect raw values (unless otherwise specified).
CVLT, California Verbal Learning Test; WAIS, Wechsler Adult Intelligence Scale; WASI, Wechsler Adult Abbreviated Scale of Intelligence; WCST, Wisconsin Card Sorting Test; WRAT, Wide Range Achievement Test.
The correlations between neuropsychological measures and regional brain metabolism were similar for left and right regions, so we report on the averaged left and right measures (Table 3). The significant correlations were restricted to prefrontal regions (BA 9 and BA 11) and to cingulate gyrus (BA 32), which were also regions that showed significant correlations with BMI. These regions were significantly correlated with scores on the California Verbal Learning Test, Stroop Interference, Trail Making Test, Symbol Digit Modalities Test, and selected subtests of the WAIS (Table 3). Scores on the WAIS (digits forward and backward, matrix reasoning) were associated both with prefrontal metabolism and with BMI. None of the correlations between metabolism and motor tests were significant.
Table 3.
Correlations between performance in cognitive (CVLT, WCST, stroop Interference, symbol digit Modality test, WAIS) and motor tests (finger tapping, pegboard, and gait) and metabolism in prefrontal (BA 9, 10, 11) and cingulate (BA 32) regions
| CVLT | BA 9 | BA 10 | BA 32 | BA 11 |
|---|---|---|---|---|
| Trials 1–5: total learning | 0.001 | 0.002 | 0.001 | 0.002 |
| Short delay free recall | 0.004 | 0.005 | 0.003 | 0.003 |
| Short delay cued recall | 0.003 | 0.004 | 0.002 | 0.002 |
| Long delay free recall | 0.03 | 0.03 | 0.02 | 0.02 |
| Long delay cued recall | 0.003 | 0.004 | 0.002 | 0.002 |
| Recognition hits: accuracy | NS | NS | NS | 0.04 |
| WCST | ||||
| % Correct | NS | NS | NS | NS |
| % Perseverative errors | NS | NS | NS | NS |
| Stroop interference | 0.05 | NS | 0.05 | NS |
| Trail AS | 0.04 | 0.03 | 0.05 | NS |
| Trail BS | NS | NS | NS | NS |
| Symbol digit modality: | ||||
| Written | 0.009 | 0.02 | 0.02 | 0.04 |
| WAIS-R | ||||
| Digits forward | NS | NS | NS | 0.05 |
| Digits backward | 0.03 | 0.03 | NS | NS |
| Matrix reasoning | NS | NS | NS | NS |
| Motor tests | ||||
| Finger tapping | NS | NS | NS | NS |
| Pegboard | NS | NS | NS | NS |
| Gait | NS | NS | NS | NS |
Motor tests were used as control tests to assess the specificity of the correlations with cognition.
CVLT, California Verbal Learning Test; BA, Brodmann area; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test.
Performance during the cognitive task (numerical calculations) increased brain metabolism, an effect that was most pronounced in frontal, parietal, and occipital cortices (Table 4). For most brain regions the increases in metabolism during the cognitive task were negatively associated with the baseline metabolic measures (Table 4). Despite this, the SPM analysis between BMI and the changes in brain metabolism during the cognitive task were not significant. The correlations between the changes in metabolism and the neuropsychological measures were also not significant.
Table 4.
Percent increases in brain metabolism (averaged values in left and right regions) with the cognitive task, significance level for the comparison with the baseline metabolic measures (paired t-tests) and correlations between the changes in metabolism with the task and the baseline metabolic measures
| Brain region | % Increase in metabolism with task | Paired t-test Baseline vs. task | Correlations with baseline metabolism |
|---|---|---|---|
| Frontal | 19 ± 26 | T = 3.2 P < 0.005 | R = 0.64 P < 0.002 |
| Parietal | 21 ± 26 | T = 3.7 P < 0.002 | R = 0.54 P < 0.01 |
| Temporal | 16 ± 25 | T = 2.5 P < 0.05 | R = 0.53 P < 0.01 |
| Occipital | 22 ± 26 | T = 3.7 P < 0.002 | R = 0.53 P < P 0.05 |
| Caudate | 16 ± 28 | T = 2.3 P < 0.05 | R = 0.43 P < 0.06 |
| Putamen | 16 ± 27 | T = 2.4 P < 0.05 | R = 0.67 P < 0.001 |
| Thalamus | 26 ± 35 | T = 3.3 P < 0.004 | R = 0.63 P < 0.003 |
| Hippocampus | 21 ± 27 | T = 2.2 P < 0.05 | R = 0.73 P < 0.0002 |
| Cerebellum | 21 ± 30 | T = 2.9 P < 0.008 | R = 0.67 P < 0.001 |
The values for the percent increases correspond to mean and standard deviations.
DISCUSSION
Here, we show in healthy subjects a significant negative correlation between BMI and baseline brain glucose metabolism in prefrontal regions and in the anterior cingulate gyrus. We also document a negative association between prefrontal metabolism and performance on tasks of executive function and verbal learning. In contrast, regional brain activation during cognitive stimulations was not associated with BMI nor with the neuuropsychological measures.
The negative association between BMI and prefrontal metabolism (BA 9, 10, 32) is consistent with the findings of reduced gray mater volumes in prefrontal cortex in obese subjects when compared to lean individuals (17). They are also consistent with those from a large study done in Japanese healthy individuals (n =1,428) that showed a negative correlation in males (not in females) between BMI and gray matter volumes in a small region within the prefrontal cortex (medial BA 9) (18). However, the latter study also reported that males showed a positive correlation between BMI and gray volumes in ventral areas of the prefrontal cortices (medial areas BA 10 and BA 11 at z coordinates 0 and −10). This is different from our results because we found no brain areas that exhibited a positive correlation with BMI. This discrepancy could reflect the differences between volumetric and metabolic measures, but it could also reflect the relatively small sample of our study.
In this study, we also show an association between prefrontal and cingulate metabolism and cognitive function. In contrast, none of the correlations with motor tests were significant, which indicates that these associations were specific for cognitive tasks. For example, metabolic activity in prefrontal regions and in cingulate gyrus were significantly correlated with performance in tests of memory (California Verbal Learning Test) and of executive function (Symbol Digit Modalities Test, Stroop Interference, Trail A, and the WAIS subtests for digits backward). These findings are consistent with prior studies showing an association between metabolic activity in prefrontal regions and performance in cognitive tests of executive function (10).
BMI was associated with performance on the WAIS (selected subtests) and the Wisconsin Card Sorting Test in this group of healthy subjects. This result corroborates prior findings of a negative association between BMI and cognitive performance in healthy adults (4). Similar to the prior report, in our study the tests that correlated with BMI were tests of executive function indicating that this may be one of the most sensitive to the deleterious effects of excessive weight. Similarly, in obese subjects, cognitive impairment includes disruption in executive function and memory. Though in obese subjects comorbid medical complications (i.e., hypertension, diabetes) are likely to contribute to cognitive impairment, the fact that we show a similar association in healthy subjects suggests that high BMI may have a direct negative impact on certain aspects of cognition. The association between prefrontal metabolism and BMI suggests that decreased prefrontal activity may under-lie this effect. Though the mechanism underlying the relationship between BMI and prefrontal metabolism is unclear, we postulate that it reflects in part disrupted dopaminergic activity. Dopamine (DA) is one of the neurotransmitters that modulates prefrontal activity thus regulating executive function (19,20). Indeed, studies in healthy subjects have shown a correlation between DA synthesis (assessed with PET and [18F]-fluoro-L-DOPA) in striatum and cognitive performance in tasks linked to the prefrontal cortex (21). Similarly studies of normal aging have reported that the age-related loss of DA markers (D2 receptors and DA transporters) is associated with decreases in prefrontal metabolism (22) and with performance on tests of executive function (23,24). The same associations have been documented by studies of patients with damage of the DA system (i.e., Parkinson’s Disease, methamphetamine abuse) in whom impairments in executive function have been shown to be associated with markers of DA damage (25,26).
In obesity, both preclinical and clinical studies have documented impairments in brain DA activity (27–31). Studies in obese subjects and in rodent models of obesity have reported an inverse relationship between BMI (humans) and weight (rodents) and D2 receptors (28,30,31). In obese subjects, we have also observed a negative relationship between D2 receptor availability and prefrontal metabolism (32). Thus, the impaired cognitive performance in obese subjects could reflect not only medical complications but also the detrimental effects of obesity on brain DA activity with a consequent disruption of prefrontal activity.
In interpreting these results one needs to consider the possibility that it is not BMI that is detrimental for prefrontal activity but that decreased prefrontal activity and reduced executive function may increase the risk to overeat. In fact, the prefrontal cortex is involved in regulating impulse control, self-monitoring, and goal-directed behaviors (33), all of which could influence the ability of an individual to self-regulate his/her eating behavior. Indeed, it has been postulated that the decline in executive function that occurs with aging (34) could contribute to the increased prevalence of overweight and obesity with age (4).
The cognitive task increased brain metabolism and the largest effects were in prefrontal, parietal (regions involved in processing numerical calculations) (35), and occipital regions. The regional increases were negatively associated with baseline metabolism; subjects with lower baseline metabolism had the largest increases with activation. Even though one would have, therefore, predicted that increases in prefrontal metabolism would have been larger in subjects with higher BMI who have lower baseline prefrontal metabolism; however, that was not the case. Indeed, the correlations between BMI and metabolic activation during cognitive performance were not significant. This suggests that BMI does not affect the amount of energy (glucose) required by the brain to perform the cognitive task. However, it is also possible that failure to see a correlation reflects the fact that the activation pattern is specific to the cognitive processes measured by the task. Thus, we cannot rule out the possibility that a different cognitive task may have yielded a significant association with BMI.
In our subjects, we did not find an association between brain metabolism and age. This differs from prior studies showing reductions in baseline prefrontal metabolism with age (22). The reason for this discrepancy is likely to reflect the limited age range of subjects (22–44 years of age) because the steepest decline in prefrontal metabolism occurs in late middle age (36). We also did not find an association between age and BMI. However, to the extent that BMI increases with age it would be relevant to assess the contribution of BMI to the age-related decreases in prefrontal metabolism.
A limitation for this study was that we did not measure physical activity; thus, we can not rule out its contribution, particularly because physical activity has been shown to have a beneficial effect on cognition (37).
In summary, here we document a negative association between the BMI and the baseline prefrontal metabolic activity that is likely to contribute to the impairment in cognitive performance reported in healthy overweight/obese subjects. This further highlights the urgency to institute public health interventions to promote a healthy weight in individuals.
Acknowledgments
We thank David Schlyer, David Alexoff, Paul Vaska, Millard Jayne, Colleen Shea, Youwen Xu, Lisa Muench, Pauline Carter, Karen Apelskog, Barbara Hubbard, Thomas Maloney, Patricia Woicik, and Linda Thomas for their contributions. Research supported by NIH’s Intramural Research Program (NIAAA), by DOE (DE-AC01-76CH00016), and NIDA (DA06278-15).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Pi-Sunyer FX. NHLBI Obesity education initiative expert panel of the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 2.Elias M, Elias P, Sullivan L, Wolf P, D’Agostino R. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 3.Chelune G, Ortega D, Linton J, Boustany M. Personality and cognitive findings among patients electing gastroplasty for morbid obesity. Int J Eat Disord. 1986;5:701–712. [Google Scholar]
- 4.Gunstad J, Paul RH, Cohen RA, et al. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Altfas J. Prevalence of attention deficit/hyperactivity disorder among adults in obesity treatment. BMC Psychiatry. 2002;2:9. doi: 10.1186/1471-244X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson D, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriat. 2004;16:327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 9.Reivich M, Alavi A, Wolf A, et al. Glucose metabolic rate kinetic model parameter determination in humans: The lumped constants and rate constants for 18F-fluorodeoxyglucose and 11C-deoxyglucose. J Cereb Blood Flow Metab. 1985;5:179–192. doi: 10.1038/jcbfm.1985.24. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RZ, Leskovjan AC, Hoff AL, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Lezak MD. Neuropsychological Assessment. 3. Oxford University Press; New York: 1995. [Google Scholar]
- 12.Wang GJ, Volkow ND, Roque CT, et al. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- 13.Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 14.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- 15.Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 16.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pannacciulli N, Del Parigi A, Chen K, et al. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 18.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 19.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 20.Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharm. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- 21.Vernaleken I, Buchholz HG, Kumakura Y, et al. Prefrontal” cognitive performance of healthy subjects positively correlates with cerebral FDOPA influx: an exploratory [18F]-fluoro-L-DOPA-PET investigation. Hum Brain Mapp. 2007;28:931–939. doi: 10.1002/hbm.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Logan J, Fowler JS, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 24.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 25.Marie RM, Barre L, Dupuy B, et al. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson’s disease. Neurosci Lett. 1999;260:77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 27.Hamdi A, Porter J, Chandan P. Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 1992;589:338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- 28.Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 29.Huang XF, Zavitsanou K, Huang X, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 31.Haltia LT, Rinne JO, Merisaari H, et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse. 2007;61:748–756. doi: 10.1002/syn.20418. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Park H, O’Connell J, Thomson R. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry. 2003;18:1121–1134. doi: 10.1002/gps.1023. [DOI] [PubMed] [Google Scholar]
- 35.Dehaene S, Molko N, Cohen L, Wilson AJ. Arithmetic and the brain. Curr Opin Neurobiol. 2004;14:218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Mielke R, Kessler J, Szelies B, et al. Normal and pathological aging—findings of positron-emission-tomography. J Neural Transm. 1998;105:821–837. doi: 10.1007/s007020050097. [DOI] [PubMed] [Google Scholar]
- 37.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]