Abstract
In the current study, we examined the effect of the selective D3 receptor antagonists SB-277011A and NGB 2904 on operant food self-administration (FSA) in Zucker obese and lean rats. Obese (Ob) and lean (Le) Zucker rats were maintained under a restricted feeding regimen (70% of ad-libitum rat chow) and were trained to lever press for food during daily, 2 hour fixed-ratio 4 (FR4) schedules. Once rats reached a stable baseline for FSA, they were injected with vehicle until a stable FSA criterion was achieved. Animals then received daily injections of different random doses of SB-277011A (3, 10, and 30 mg/kg i.p.), and NGB-2904 (0.3, 1 and 3 mg/kg i.p.). SB-277011A produced a significant decrease in both food intake and active lever responses in both Ob and Le rats. In contrast, NGB-2904 did not decrease food intake levels or lever presses for food in Ob and Le rats. These results suggest that along with its involvement in seeking behavior for drugs of abuse, the D3 dopamine receptor may also be involved in seeking behavior for natural reinforcers such as food.
Keywords: Hunger, Ingestive behavior, Operant conditioning, Anorexia, Anorexigenic
1. Introduction
Food consumption is one of the most widely studied reward survival mechanisms (Fetissov et al., 2002). The overconsumption of food is one of the major factors that has contributed to a significant increase in the incidence of obesity (Erlanson-Albertsson and Zetterstrom, 2005; Isasi et al., 2006; Hedley et al., 2004; Ogden et al., 2002). Although the pathophysiology of obesity remains to be elucidated, it is generally thought to result from a dysregulation in energy homeostasis which is mediated in part through food intake regulation (Cecil et al., 2006). It has been shown that various peptides, hormones and brain neurotransmitters are involved in food intake regulation (Erlanson-Albertsson and Zetterstrom, 2005). Dopamine (DA) is known to regulate a broad range of biological functions such as locomotor activity, cognition, food intake, and hormone secretion (Palmiter, 2007; Pijl, 2003) as well as reinforcement of addictive substances and behaviors (Di Chiara, 2002; Di Chiara and Bassareo, 2007). The dopaminergic perikaryia from the ventral tegmental area (VTA) send projections that innervate a number of limbic and telencephalic structures, including the olfactory tubercle, amygdala, frontal and limbic cortices, medial prefrontal cortex (mPFC), and the nucleus accumbens (NAc), a brain area that plays a significant role in reward-related behavior and reward learning (Day and Carelli, 2007; Di Chiara and Bassareo, 2007; Fenu and Di Chiara, 2003). DA plays an essential role in food-seeking behavior (Wise, 2006a,b), reward prediction (Phillips et al., 2007; Schultz et al., 2000), reward motivation (to seek and obtain reward) (McClure et al., 2003; Phillips et al., 2007) and facilitation of conditioned learning (Fenu and Di Chiara, 2003). Since DA has been shown to mediate food intake through brain reward mechanisms (McQuade et al., 2004), DA modulation may be responsible for regulating the reward or reinforcement necessary to maintain, enhance or attenuate feeding behavior (Berridge, 1996).
The hormone leptin is secreted by adipocytes (Hagan et al., 1999) and is an important signal in the regulation of energy balance (Hagan et al., 1999). Rodents and humans with homozygous mutations in the leptin or leptin receptor genes manifest hyperphagia and severe obesity (Hagan et al., 1999). Zucker obese (fa/fa) rats are widely used as animal models in obesity research due to their phenotypic similarities with obese humans such as metabolic and cardiopulmonary deficits (Brooks-Asplund et al., 2002); type 2 diabetes mellitus (Hunt et al., 1976), hypertension (Alonso-Galicia et al., 1996), upper airway narrowing and poor exercise capacity (Dockstader et al., 2001; Lee et al., 2005). These rats also exhibit hyperphagia, hypertriglycemia, and hyperinsulinemia (Boulange et al., 1979). Their genotype (fa/fa) denotes that they are homozygous for defective leptin receptors, which prevents leptin signaling in these animals (Malcher-Lopes et al., 2006).
It has previously been shown that anorectic concentrations of leptin in the brain reduces the firing of mesolimbic DAergic neurons in vivo and suppresses DA-related motivational aspects of feeding (Krugel et al., 2003) which may indicate decreased DA levels in leptin receptor deficient obese Zucker (fa/fa) rats as one of the causes for obesity (Brunetti et al., 1999). Furthermore, in mice deficient in both DA and leptin, DA was shown to be required for the initiation of food intake in the absence of leptin (Szczypka et al., 2000). Leptin receptors have been found to be extensively co-expressed within DA neurons of the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) (Figlewicz et al., 2003) suggesting that DA release in relation to food intake is modulated by leptin. We recently reported that leptin receptor deficient obese Zucker rats display lower D2 receptor (D2) levels than lean rats and that these levels were modulated by food restriction (Michaelides et al., 2006; Thanos et al., 2007). In addition, microPET assessment of D2 receptor density in these rats suggest that D2 receptor availability is differentially influenced by food restriction in obese as compared to lean rats, (Michaelides et al., 2006; Thanos et al., 2007).
In rats, D3 receptors and D3 receptor mRNA is predominantly located in limbic brain regions such as the NAc, the islands of Calleja, and the VTA and SNc (Booze and Wallace, 1995; Bouthenet et al., 1991; Curran and Watson, 1995; Diaz et al., 1995, 2000; Levant, 1997; Shafer and Levant, 1998; Sokoloff et al., 1990). In humans D3 receptors have been localized in the NAc, internal globus pallidus, ventral pallidum, septum, islands of Calleja, amygdala, and VTA (Landwehrmeyer et al., 1993; Meador-Woodruff et al., 1994; Suzuki et al., 1998). Until recently, it has been difficult to characterize the functional role of D3 receptors in the CNS due to the lack of selective D3 receptor agents. This obstacle has been surmounted by the synthesis of selective D3 receptor antagonists. SB-277011A, a selective D3 receptor antagonist, has an 80- to 100-fold selectivity for the D3 receptor compared to other DA receptors and has a high affinity for human (pKi 7.95) and rat (pKi 7.97) cloned D3 receptors compared to D2 receptors (pKi=5.98). In addition, a number of studies indicated that SB-277011A significantly decreases the rewarding/reinforcing actions of various drugs of abuse as well as drug-seeking behavior. In rats, SB-277011A has been reported to 1) attenuate cue-induced cocaine self-administration behavior (Di Ciano and Everitt, 2003; Vorel et al., 2002); 2) block the expression of cocaine and heroin-induced conditioned place preference (CPP) (Ashby et al., 2003; Vorel et al., 2002); 3) attenuate alcohol intake in ethanol preferring rats (Heidbreder et al., 2007; Thanos et al., 2005) and 4) suppress alcohol self-administration, and prevent reinstatement of alcohol-seeking in mice (Heidbreder et al., 2007; Thanos et al., 2005). In addition, SB-277011A significantly attenuated nicotine-triggered relapse to nicotine seeking (Andreoli et al., 2003; Pak et al., 2006) as well as sucrose-seeking behavior induced by sucrose-associated cue reintroduction (Cervo et al., 2007).
Another selective D3 antagonist is NGB-2904, which has 155-fold selectivity for the primate D3 over the D2 and over 800-fold selectivity for the rat D3 (Newman et al., 2003). In rats, NGB-2904 dose-dependently decreased cocaine self-administration behavior (Xi et al., 2006; Gilbert et al., 2005) and significantly attenuated the cocaine-induced leftward shift in brain stimulation reward paradigm (BSR). Finally, while NGB-2904 pretreatment was shown to decrease cocaine triggered reinstatement of cocaine-seeking behavior, it had no effect on sucrose-plus-sucrose-cue-triggered reinstatement of sucrose-seeking behavior (Xi et al., 2006).
To our knowledge, there are no published reports regarding the effects of highly selective D3 antagonists (such as SB-277011A and NGB-2904) in an operant FSA paradigm in obese rats. The present study consisted of two experiments that aimed at examining the effects of these two selective D3 antagonists SB-277011A (Experiment 1) and NGB-2904 (Experiment 2) in Zucker obese and lean rats in the FSA task. We chose to utilize the obese (fa/fa) Zucker rat based on its severe hyperphagia and weight gain. These characteristics, coupled with the leptin receptor mutation provide an original examination of changes in food-seeking and FSA behavior.
2. Materials and methods
2.1. Experiment 1
2.1.1. Animals
Eight-week old Zucker obese (fa/fa) (n=6; 372±15.4 g) and lean (Fa/?) rats (n =6; 257±12.3 g) were purchased from Charles River Laboratories (Wilmington, MA). Each animal was allowed to acclimate for 7 days after arriving at the Brookhaven National Laboratory Animal Facility. Animals were restricted to 15 g of Purina Rodent Chow per day with ad-libitum water access. Animals were individually housed in standard 9 in.×24 in.×9 in. plastic cages with wire covers. The home cage environment was set at 22±1 °F with approximately 60% humidity and reverse 12 h/12 h light/dark cycle with lights off at 0700 h and on at 1900 h. Ad-libitum access to water was maintained but access to food was restricted to 2 h daily during operant sessions throughout the entire experiment. All animals received food supplementation following each operant session to maintain them on a limited food access diet of 70% of the food consumed by two similarly aged and ad-libitum fed groups of lean and obese rats. On average, lean rats consumed 13.2 g/day and obese rats consumed 22 g/day. Experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals (NAS and NRC, 1996) and Brookhaven National Laboratory Institutional Animal Care and Use Committee protocols.
2.1.2. Drugs
SB-277011A (Reavill et al., 2000; Bull et al., 2000) (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl] cyclohexyl]-4-quinolinecarboxamide) was obtained from GlaxoS-mithKline (Harlow, Essex, U.K.). A 3% methylcellulose w/v (M-7140 Sigma) and 0.9% saline solution was used as the vehicle. All rats were injected with 3, 10 and 30 mg/kg of SB-277011A which was prepared in concentrations of 3, 10, and 30 mg/ml for physiological use in Zucker obese (fa/fa) and Zucker lean (Fa/?) rats.
2.2. Experiment 2
2.2.1. Animals
Eight-week old Zucker obese (Ob) (fA/fA) (n=6; 365±17 g) and lean (Le) (Fa/?) rats (n=6; 243±10 g) (Charles River Laboratories) were used in this study. All other details were the same as stated above for Experiment 1.
2.2.2. Drugs
NGB 2904 N-(4-[4-{2,3-dichlorphenyl}-1piperazinyl] butyl)-2-fluorenylcarboxamide was synthesized in the Medicinal Chemistry Section, NIDA-IRP. All rats were injected with 0.3, 1 and 3 mg/kg of NGB-2904 (0.3, 1 and 3 mg/ml solutions) which was dissolved in 25% 2-hydroxy-propyl-β-cyclodextrin (w/v); (vehicle solution (Sigma/RBI, Saint Louis, MO)).
2.3. Experiments 1 and 2
2.3.1. Apparatus
Both experiments utilized the 45 mg dustless precision food pellets (Prod#: F0021; Bio-Serv Inc.; Frenchtown, NJ) throughout the operant conditioning task. Clear acrylic operant test chambers measuring 32×25×33 cm were used (Coulbourn Instruments, Allentown, PA). Each test cage was enclosed in an environment isolation chamber to keep out any outside environmental stimuli. Cage floors were constructed of stainless steel horizontal bars spaced 2 cm apart. The test cages were equipped with two randomly assigned and counterbalanced response levers; a reinforced (R) and a non-reinforced (NR) lever, with cue lights located above each. R lever presses led to a delivery of a food pellet. A lever press on the NR lever had no consequences. All data from the test chamber was recorded using Graphic State software version 3.0.
2.3.2. Procedures
Sessions were run daily for 2-h per day. Animals were started on a fixed ratio 1 (FR1, 1 lever press/1 pellet dispensed) schedule of reinforcement and were increased by one FR level until reaching FR4. Upon completion of each session, animals were promptly returned to their home cage. This schedule was used to train (the rats to press the lever) and feed the rats until baseline criteria (≤20% change in daily food intake for five consecutive sessions) were met. After completion of the food training protocol, each rat was injected intraperitoneally (i.p.) with approximately 0.3 ml of the vehicle 15 min prior to the operant sessions under the FR4 schedule that included a 30 second time-out period until the vehicle baseline criteria (daily food intake ≤20% for three consecutive sessions) was met. Upon meeting the vehicle baseline criteria, all the rats in Experiment 1 continued on the same protocol using a randomized Latin square design of SB-277011A administration (3 mg/kg; 10 mg/kg and 30 mg/kg for three consecutive sessions at each dose). The doses of SB-277011A were selected based on previous studies (Cervo et al., 2007; Heidbreder et al., 2007; Thanos et al., 2005). For Experiment 2, the similar randomized design was used for NGB-2904 after vehicle criteria was achieved (0.3 mg/kg; 1 mg/kg and 3 mg/kg). The doses of NGB-2904 were based on data indicating that they produced significant effects in other behavioral paradigms (0.1, 1 and 5 mg/kg dose-dependently inhibited cocaine SA) (Xi et al., 2004, 2006). One day after the last drug treatment of SB-277011A for Experiment 1 or NGB-2904 for Experiment 2, rats received vehicle for three days.
2.3.3. Data analysis
Food intake (number of pellets), lever presses, were collected during each experimental session. All data was analyzed using a Two-way Repeated Measures Analysis of Variance (RM ANOVA), followed by pairwise multiple comparison using the Holm–Sidak method. The vehicle data represent the average of the first (baseline), intermittent (between treatments) and last (after the last drug treatment) vehicle sessions.
3. Results
3.1. Experiment 1
3.1.1. Food Intake
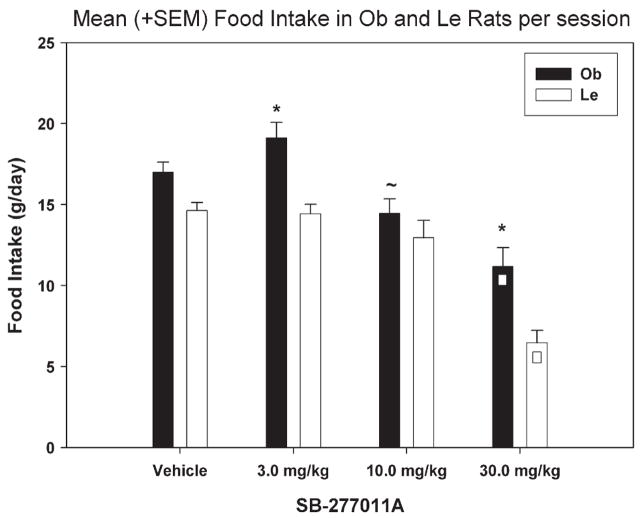
SB-277011A effects on food consumed are shown in Fig. 1. A two-way repeated measures ANOVA revealed significant differences for strain [F (1,143)=27.619; p<0.001] and treatment [F (3, 143)=34.501; p<0.001) but no significant interaction effect. Subsequent multiple pairwise comparisons (Holm–Sidak; p<0.05) revealed that in Le rats food intake was significantly lower at 30 mg/kg SB-277011A treatment compared to all other treatments [vehicle (t=7.191), 3 mg/kg (t=7.010), 10 mg/kg (t=6.497)]. In Ob rats food intake was also significantly lower at 30 mg/kg SB-277011A treatment compared to all other treatments [vehicle (t=5.136), 3 mg/kg (t=7.007), 10 mg/kg (t=2.913)]. Also, 10 mg/kg SB-277011A treatment in Ob rats decreased food intake compared to 3 mg/kg (t=4.094). Finally, Ob rats consumed more food than Le rats at the 3 mg/kg (t=4.697) and 30 mg/kg (t=4.700) SB-277011A doses.
Fig. 1.
Mean (+SEM) number of grams of food intake per 2-h session, following injection of: vehicle, 3, 10 or 30 mg/kg of SB-277011A. □ Denotes significance between specific treatment relative to all others, ~denotes significance between 10 and 3 mg/kg in Ob rats and * denotes significance between Ob and Le rats at specific dose. The vehicle column data represent the average of the first (baseline), intermittent (between treatments) and last (after the last drug treatment) vehicle sessions.
3.1.2. Reinforced (R) and non-reinforced (NR) lever responses
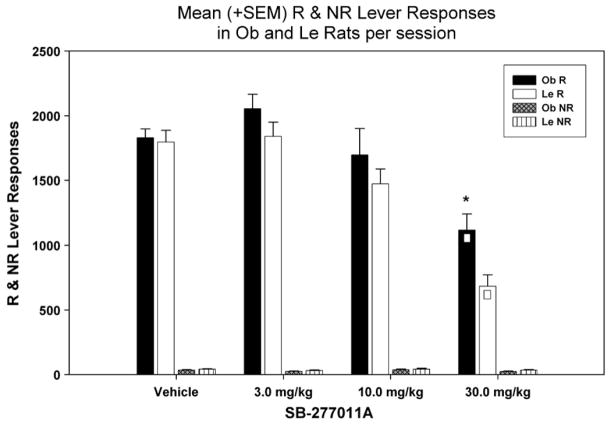
We examined the mean number of R lever responses using a two-way repeated measures ANOVA (Fig. 2) which revealed a significant main effect of strain [F (1, 143)=10.917; p=0.004] and SB-277011A treatment [F (3, 143)=30.288; p<0.001] but no interaction effect. Subsequent multiple pairwise comparisons (Holm–Sidak; p<0.05) revealed that in Le rats R lever responses were significantly lower at 30 mg/kg SB-277011A treatment compared to all other treatments [vehicle (t=7.021, 3 mg/kg (t=7.296), 10 mg/kg (t=4.980)]. In Ob rats R lever responses were also significantly lower at 30 mg/kg SB-277011A treatment compared to all other treatments [vehicle (t=4.479), 3 mg/kg (t=5.898), 10 mg/kg (t=3.639)]. Finally, Ob rats elicited a greater number of R lever responses than Le rats only at the 30 mg/kg dose of SB-277011A (t=3.008).
Fig. 2.
Mean (+SEM) number of R (total and during time-out) and NR lever responses per 2-h session, following injection of: vehicle, 3, 10, or 30 mg/kg of SB-277011A. □ Denotes significance between specific treatment relative to all others, and * denotes significance between Ob and Le rats at specific dose. The vehicle column data represent the average of the first (baseline), intermittent (between treatments) and last (after the last drug treatment) vehicle sessions.
A two-way repeated measures ANOVA did not reveal any significant main effects (strain [F (1, 143)=2.732; p=0.117], treatment [F (3, 143)=3.556; p=0.021], interaction [F (3, 143)= 0.142; p=0.935]) on the number of NR lever responses in response to SB-277011A treatment (Fig. 2).
3.2. Experiment 2
3.2.1. Food intake
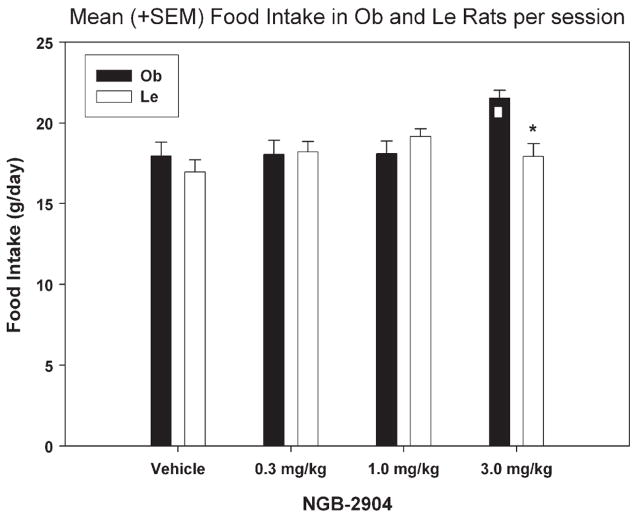
NGB-2904 effects on food intake are shown in Fig. 3. A two-way repeated measures ANOVA revealed significant differences for treatment [F (3, 143) =4.369; p = 0.008], interaction [F (3, 143) = 5.094; p = 0.004] but no significant strain effect. Subsequent multiple pairwise comparisons (Holm–Sidak; p <0.05) revealed that in Ob rats food intake was significantly greater at 3 mg/kg NGB-2904 treatment compared to all other treatments [vehicle (t =3.936), 0.3 mg/kg (3.845), 1 mg/kg (t = 3.802). NGB-2904 did not affect food intake in Le rats since we did not detect any significant differences as a function of NGB-2904 treatment. Finally, at 3 mg/kg Ob rats consumed significantly more food compared to Le rats (t = 3.443).
Fig. 3.
Mean (+SEM) number of grams of food intake per 2-h session, following injection of: vehicle, 0.3, 1, or 3 mg/kg of NGB-2904. □ Denotes significance between specific treatment relative to all others, and * denotes significance between Ob and Le rats at specific dose. The vehicle column data represent the average of the first (baseline), intermittent (between treatments) and last (after the last drug treatment) vehicle sessions.
3.2.2. R and NR lever responses
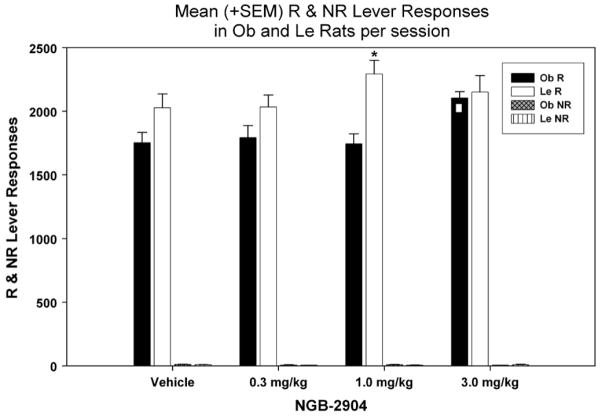
We examined the mean number of R lever responses using a two-way repeated measures ANOVA (Fig. 4) which revealed a significant main effect of strain [F (1,143)=8.662; p=0.009], treatment [F (3, 143)= 4.221; p = 0.01] as well as interaction [F (3, 143) =3.865; p = 0.01] effects. Subsequent multiple pairwise comparisons (Holm–Sidak; p <0.05) revealed that in Ob rats R lever responses were significantly greater at 3 mg/kg NGB-2904 treatment compared to all other treatments [vehicle (t = 3.313), 0.3 mg/kg (t =2.940), 1 mg/kg (t = 3.406)]. NGB-2904 did not affect R lever responses in Le rats since we did not detect any significant differences. Finally, at 1 mg/kg (t = 4.190) Le rats elicited significantly more R lever responses compared to Ob rats.
Fig. 4.
Mean (+SEM) number of R (total and during time-out) and NR lever responses per 2-h session, following injection of: vehicle, 0.3, 1, or 3 mg/kg of NGB-2904. □ Denotes significance between specific treatment relative to all others, and * denotes significance between Ob and Le rats at specific dose. The vehicle column data represent the average of the first (baseline), intermittent (between treatments) and last (after the last drug treatment) vehicle sessions.
A two-way repeated measures ANOVA did not reveal any significant main effects (strain [F (1, 143)=0.391; p=0.540], treatment [F (3, 143)=2.256; p=0.093], interaction [F (3, 143)= 1.997; p=0.126]) on the number of NR lever responses in response to NGB-2904 treatment (Fig. 4).
4. Discussion
4.1. SB-277011A
The role of the D3 receptor in obesity and feeding-related behaviors has not been elucidated. One study found that male D3 receptor deficient mice become obese when fed a high fat diet for a period of three months but did not gain weight when fed regular chow for the same amount of time (McQuade et al., 2004). Quite surprisingly, the increases in weight gain and adiposity were reported independently of increased food intake. The same study also reported that male D3 knockout mice showed increased plasma concentrations of leptin, but not insulin in response to the high fat diet and not the chow. Subsequently, the authors concluded that the D3 receptor is involved in the regulation of body weight and body fat when consuming diets differing in palatability and fat content. Although a gene-knockout model of D3 inactivation does not necessarily parallel pharmacological studies of D3 inactivation, the above finding is very important in providing insight into the potential involvement of the D3 in reward and reward-seeking.
The effect of SB-277011A on food intake in obese animals has not been previously reported. One study in non-obese animals examined the effects of SB-277011A on drinking sucrose solution and showed no effect on R lever responses (Di Ciano et al., 2003). Similarly, a recent experiment (again in non-obese rats) showed that SB-277011A (10 mg/kg) did not modify conditioned reinstatement triggered by sucrose pellet-associated cues (Cervo et al., 2007). SB-27701A was also shown to have no effect on the number of pellets earned or the response rate in a food self-administration experiment conducted under a progressive-ratio (PR) schedule in non-obese rats even though it was reported that two out of eight rats tested showed a greater than 50% reduction in food intake at the 30 mg/kg dose (Ross et al., 2007).
Contrary to these previous studies, the present study is the first to examine the effects of SB-277011A on obese Zucker rats in an operant FSA task. The current results demonstrated that SB-277011A attenuated the amount of food consumed and R lever presses in both Ob and Le Zucker rats. Specifically, both Ob and Le rats exhibited a decrease in the amount of food consumed during the high (30 mg/kg) doses as compared to vehicle (Fig. 1). Both groups also showed significantly lower R lever responses at the highest dose (30 mg/kg) than at any other treatment. At this same dose, Ob rats showed greater R lever responses than their Le counterparts. SB-277011A administration did not interfere with R vs. NR lever response discrimination since NR lever responses comprised only a small percentage of total lever responses.
Discrepancies between this study and previous experiments that have assessed the effect of SB-277011A on food intake include: a) animal strain (Zucker obese rats used here versus non-obese rats in other studies); b) task (operant FR4 FSA task versus other FR and variable-interval tasks); c) age of the animals (adolescent animals used here versus adult animals in other studies); d) solid versus liquid food (solid food pellet used here versus liquid sucrose solution used in other studies); e) palatability of food (regular chow pellets versus sucrose or high fat pellets) and f) caloric intake value. It may be possible that the Zucker Ob rat is more sensitive to D3 receptor antagonism than non-obese rats and that leptin (leptin receptor is deficient in Zucker Ob rats) may regulate this effect. Thus, while the present results support that SB-277011A may be valuable in reducing food intake in obese rats; future experiments assessing D3 levels in these obese rats as well as the role of the D3 should also examine among other things the interaction with leptin on food intake and weight gain.
4.2. NGB-2904
NGB-2904 showed no attenuation of R lever presses or food intake in the Ob or Le rats. Previous studies have shown that NGB-2904 did not affect the reinstatement response to sucrose in rats (Xi et al., 2006) or food-maintained operant responding in rhesus monkeys (Martelle et al., 2007). In our study, NGB-2904 had a minimal effect on operant FSA. Specifically, the only significant differences found was at the high dose (3 mg/kg) where Ob rats consumed more food than the Le rats. This was a similar effect to that observed with 3 mg/kg SB-277011A. While higher doses (>3 mg/kg) of NGB-2904 were not tested in this study future investigation on this is warranted. NGB-2904 did not have an effect on R lever presses except that at the highest dose tested (3 mg/kg) where there was no longer a difference between Ob and Le. No significant differences for NR responses were observed with NGB-2904.
Although both SB-277011A and NGB-2904 are highly selective for the D3, SB-277011A may not be as selective as NGB-2904 in rats (Newman et al., 2003). Nevertheless, such a comparison has not been thoroughly evaluated as the binding profile of SB-277011A has been extensively examined (specifically, it has been shown that SB-277011A has selectivity for the D3 receptor compared to at least 150 other targets) while NGB-2904 has not undergone this degree of testing. Since SB-277011A’s D3/D2 ratio (100) is not as high as that of NGB-2904 (800), it is possible that SB-277011A may be binding to some D2 as well as D3 receptors. This may serve as an explanation for the SB-277011A effect on decreasing operant FSA in Zucker rats as observed in this study even though it has been shown not affect progressive-ratio for food reinforcement and to not modify sucrose-seeking behavior induced by sucrose-associated cues in rats (Ross et al., 2007; Cervo et al., 2007) as well as decreasing drug reward as observed in other studies (for review see Heidbreder et al., 2005), particularly since the D2 is significantly involved in both drug and food consumption (Volkow and Wise, 2005). In contrast to this explanation, SB-277011A (at doses as high as 90 mg/kg) was shown not to exhibit any of the behavioral properties attributed to D2 receptor antagonists (Pak et al., 2006). Therefore, it is not likely that SB-277011A would be interacting significantly with D2 receptors at the doses used in this study.
4.3. Zucker Ob rats and FSA
One important finding of this study was that Ob and Le rats did not show significant differences in operant FSA during the vehicle sessions. Taking the severe hyperphagia that Ob rats exhibit into account, one would expect that these rats would show increased R lever responses and consequently consume a lot more food than their Le counterparts. Although our FSA results indicated that Ob rats had a greater food intake than Le rats following vehicle treatment, it was not statistically significant. There are several possible factors that may explain this finding. Previous studies have examined FSA in both Ob Zucker and diet-induced obese (DIO) rats under an FR schedule and experimental procedures similar to ours (Glass et al., 1999; la Fleur et al., 2007). In these studies, operant lever responses for food did not differ between obese and lean animals (Glass et al., 1999; la Fleur et al., 2007). Nevertheless, operant responding behavior in obese rats seems to be subject to the specific schedule of reinforcement. Indeed, experiments that examined FSA and utilized variable-interval schedules of reinforcement [i.e. progressive-ratio (PR)] demonstrated the opposite: Ob Zucker and DIO rats show increased lever responses compared to lean controls (la Fleur et al., 2007; Vasselli et al., 1980). The increased operant responding in obese rats as demonstrated using PR schedules has been interpreted to reflect the increased incentive value and motivational properties of food in obese rats (la Fleur et al., 2007). However, it has been postulated that obese rats do not show increased lever responses for food under FR schedules because of the influence of satiety signals in responding for food. Under FR schedules, it is believed that rats achieve satiety after a certain number of lever responses, which in turn decreases the motivation for food and eventually the lever response (la Fleur et al., 2007). An exception to this was the Greenwood et al. study (Greenwood et al., 1974) which showed increased lever responses for Ob Zucker rats compared to Le and which assessed operant responding under an FR schedule but over a period of 24 h. A possible explanation for this is that Ob and Le Zucker rats differ in their circadian feeding patterns and therefore variability in circadian rhythms between the two strains may contribute to differences observed in the Greenwood et al. study. Indeed, since then it has been shown that Ob Zucker rats differ from Le in circadian rhythms for temperature, locomotor activity and feeding (Fukagawa et al., 1992; Mistlberger et al., 1998; Murakami et al., 1995).
Zucker Ob rats exhibit differences in food regulatory messengers such as leptin, ghrelin and insulin among others (Michaelides et al., 2006; Thanos et al., 2007). It has been shown that intra-cerebroventricular administration of both leptin and insulin decreased FSA in normal rats (Figlewicz et al., 2006). Differences in concentrations of these messengers may also contribute to the lack of difference observed between Ob and Le rats during vehicle sessions in the operant FSA task.
Recently it was shown that Ob Zucker rats have lower D2 receptor levels than Le rats and that chronic food restriction leads to increases in D2 receptors in both strains (Michaelides et al., 2006; Thanos et al., 2007). The negative correlation between weight and D2 receptor levels was consistent with what was observed clinically between the Body Mass Index (BMI) and D2 receptors (Wang et al., 2001, 2004, 2003). Furthermore, Ob rats show a distinct DA profile that responds uniquely to fasting and food restriction (Michaelides et al., 2006; Thanos et al., 2007). Therefore, differences in DA and its receptors between Ob and Le rats may also contribute to the operant FSA behavior observed, especially since pharmacological manipulation of DA and D2 receptors has been shown to modulate food operant responding (Barrett et al., 2004; Ishiwari et al., 2004). Future experiments measuring D3 receptor levels in Ob and Le Zucker rats may shed light on the involvement of D3 receptors in weight gain and food intake and how these responses may be modulated by leptin.
4.4. Limitations
D3 receptor levels have not been characterized in obese clinical or preclinical studies partly because of the lack of availability of highly selective D3 receptor radioligands. It is possible that there exists a difference in D3 receptor levels with obesity and this of course has not been determined between obese and non-obese rats (this would produce a differential sensitivity to D3 receptor antagonism). This remains to be evaluated in future experiments. Different D3 profiles between Ob and Le Zucker rats may serve as a potential contribution to the Ob Zucker phenotype. This is possible since, it has been recently shown that they have been characterized with different D2 receptors and DA profiles (Hamdi et al., 1992; Michaelides et al., 2006; Thanos et al., 2007) and therefore differences in other receptors and transmitters may exist.
4.5. Conclusion
The findings of this experiment, taken together with evidence that lack of D3 receptors contributes to increased adiposity and disruption of leptin and insulin levels in response to a high fat diet but not regular chow (McQuade et al., 2004) supports a modulatory role for the D3 receptor with respect to obesity. At the doses examined, SB-277011A significantly inhibited operant FSA behavior while NGB-2904 did not in Zucker Ob rats. While both SB-277011A and NGB-2904 have been previously shown to decrease drug intake in rodents, and may be valuable pharmacotherapeutic agents for drug addiction; SB-277011A displayed properties in the Zucker obese rat that warrant further investigation with respect to food-seeking and weight loss in obesity. Furthermore, higher doses of NGB-2904 need to be examined. Our findings thus as well as other reports support the further investigation of the contribution and interaction of the D3 receptors with several key signaling messengers (like leptin, insulin and ghrelin) in modulating reward and satiety for natural reinforcers such as food. Furthermore, these results may indicate that the interaction and involvement of these signals may function differently for drug reward versus natural rewards like food.
Acknowledgments
Support contributed by NIAAA Intramural Research Program, Laboratory of Neuroimaging (LNI), the NIDA Intramural Research Program, and by the U.S. Department of Energy under contract DEAC02-98CH10886. For more information about our research see http://www.bnl.gov/thanoslab.
References
- Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–54. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–80. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse (New York NY) 2003;48:154–6. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Booze RM, Wallace DR. Dopamine D2 and D3 receptors in the rat striatum and nucleus accumbens: use of 7-OH-DPAT and [125I]-iodosulpride. Synapse. 1995;19:1–13. doi: 10.1002/syn.890190102. [DOI] [PubMed] [Google Scholar]
- Boulange A, Planche E, de Gasquet P. Onset of genetic obesity in the absence of hyperphagia during the first week of life in the Zucker rat (fa/fa) J Lipid Res. 1979;20:857–64. [PubMed] [Google Scholar]
- Bouthenet M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–19. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brooks-Asplund EM, Shoukas AA, Kim SY, Burke SA, Berkowitz DE. Estrogen has opposing effects on vascular reactivity in obese, insulin-resistant male Zucker rats. J Appl Physiol. 2002;92:2035–44. doi: 10.1152/japplphysiol.00559.2001. [DOI] [PubMed] [Google Scholar]
- Brunetti L, Michelotto B, Orlando G, Vacca M. Leptin inhibits norepinephrine and dopamine release from rat hypothalamic neuronal endings. Eur J Pharmacol. 1999;372:237–40. doi: 10.1016/s0014-2999(99)00255-1. [DOI] [PubMed] [Google Scholar]
- Bull E, Reavill C, Hagan JJ, Overend P, Jones DN. Evaluation of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder: acquisition and performance of the DRL-60s test. Behav Brain Res. 2000;109:27–35. doi: 10.1016/s0166-4328(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Cecil JE, Watt P, Palmer CN, Hetherington M. Energy balance and food intake: the role of PPARgamma gene polymorphisms. Physiol Behav. 2006;88:227–33. doi: 10.1016/j.physbeh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol. 2007;10:167–81. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Curran EJ, Watson SJ., Jr Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. J Comp Neurol. 1995;361:57–76. doi: 10.1002/cne.903610106. [DOI] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13:148–59. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. From rats to humans and return: testing addiction hypotheses by combined PET imaging and self-reported measures of psychostimulant effects. Commentary on Volkow et al. ‘Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies’. Behav Pharmacol. 2002;13:371–7. doi: 10.1097/00008877-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117:952–60. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–38. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C, et al. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–45. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz J-C, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–84. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockstader CL, Rubinstein M, Grandy DK, Low MJ, van der Kooy D. The D3 receptor is critical in mediating opiate motivation only in opiate-dependent and withdrawn mice. Eur J Neurosci. 2001;13:995–1001. doi: 10.1046/j.1460-9568.2001.01455.x. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C, Zetterström R. The global obesity epidemic: snacking and obesity may start with free meals during infant feeding. Acta Paediatr. 2005;94:1523–31. doi: 10.1080/08035250500323780. [DOI] [PubMed] [Google Scholar]
- Fenu S, Di Chiara G. Facilitation of conditioned taste aversion learning by systemic amphetamine: role of nucleus accumbens shell dopamine D1 receptors. Eur J Neurosci. 2003;18:2025–30. doi: 10.1046/j.1460-9568.2003.02899.x. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol. 2002;283:R905–10. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–15. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–6. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Fukagawa K, Sakata T, Yoshimatsu H, Fujimoto K, Uchimura K, Asano C. Advance shift of feeding circadian rhythm induced by obesity progression in Zucker rats. Am J Physiol. 1992;263:R1169–75. doi: 10.1152/ajpregu.1992.263.6.R1169. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, et al. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, O’Hare E, Cleary JP, Billington CJ, Levine AS. The effect of naloxone on food-motivated behavior in the obese Zucker rat. Psychopharmacology. 1999;141:378–84. doi: 10.1007/s002130050847. [DOI] [PubMed] [Google Scholar]
- Greenwood MR, Quartermain D, Johnson PR, Cruce JA, Hirsch J. Food motivated behavior in genetically obese and hypothalamic-hyperphagic rats and mice. Physiol Behav. 1974;13:687–92. doi: 10.1016/0031-9384(74)90241-8. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Havel PJ, Seeley RJ, Woods SC, Ekhator NN, Baker DG, et al. Cerebrospinal fluid and plasma leptin measurements: covariability with dopamine and cortisol in fasting humans. J Clin Endocrinol Metab. 1999;84:3579–85. doi: 10.1210/jcem.84.10.6034. [DOI] [PubMed] [Google Scholar]
- Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 1992;589:338–40. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR., Jr Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict Biol. 2007;12:35–50. doi: 10.1111/j.1369-1600.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- Hunt CE, Lindsey JR, Walkley SU. Animal models of diabetes and obesity, including the PBB/Ld mouse. Fed Proc. 1976;35:1206–17. [PubMed] [Google Scholar]
- Isasi CR, Soroudi N, Wylie-Rosett J. Youth WAVE Screener: addressing weight-related behaviors with school-age children. Diabetes Educ. 2006;32:415–22. doi: 10.1177/0145721706288763. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–7. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes. 2007 Aug;31(8):1286–94. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res Mol Brain Res. 1993;18:187–92. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–40. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–52. [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–50. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl) benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–82. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26:423–8. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- McQuade JA, Benoit SC, Xu M, Woods SC, Seeley RJ. High-fat diet induced adiposity in mice with targeted disruption of the dopamine-3 receptor gene. Behav Brain Res. 2004;151:313–9. doi: 10.1016/j.bbr.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, et al. Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–48. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Wang G, Thanos P, Volkow N. Age and diet-related changes of striatal dopamine D2 receptor (D2R) binding in obese (Ob) (fa/fa) and lean (Le) Zucker rats revealed by positron emission tomography and autoradiography. Obesity. 2006;14:A130. [Google Scholar]
- Mistlberger RE, Lukman H, Nadeau BG. Circadian rhythms in the Zucker obese rat: assessment and intervention. Appetite. 1998;30:255–67. doi: 10.1006/appe.1997.0134. [DOI] [PubMed] [Google Scholar]
- Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol. 1995;269:R1038–43. doi: 10.1152/ajpregu.1995.269.5.R1038. [DOI] [PubMed] [Google Scholar]
- NAS, NRC. Guide for the Care and Use of Laboratory Animals. Washington D.C: National Academy Press; 1996. [Google Scholar]
- Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-(4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl)arylcarboxamides as novel dopamine D3 receptor antagonists. Bioorganic Med Chem Letters. 2003;13:2179–83. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, et al. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–95. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol. 2003;480:125–31. doi: 10.1016/j.ejphar.2003.08.100. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–65. [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol. 2007;559:173–9. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shafer RA, Levant B. The D3 dopamine receptor in cellular and organismal function. Psychopharmacology. 1998;135:1–16. doi: 10.1007/s002130050479. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature. 1990;347:146–51. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz J-C, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA, Palmiter RD. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet. 2000;25:102–4. doi: 10.1038/75484. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Katana JM, Ashby CR, Jr, Michaelides M, Gardner EL, Heidbreder CA, et al. The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav. 2005;81:190–7. doi: 10.1016/j.pbb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides MM, Piyis YK, Wang G-J, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in striatum in a rat model of obesity. Synapse. 2008;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Vasselli JR, Cleary MP, Jen KL, Greenwood MR. Development of food motivated behavior in free feeding and food restricted Zucker fatty (fa/fa) rats. Physiol Behav. 1980;25:565–73. doi: 10.1016/0031-9384(80)90123-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Thanos PK, JS F. Positron emission tomographic evidence of similarity between obesity and drug addiction. Psychiatr Ann. 2003;33:104–11. [Google Scholar]
- Wang G-J, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wise RA. The parsing of food reward. Am J Physiol. 2006a;291:R1234–5. doi: 10.1152/ajpregu.00443.2006. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006b;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, et al. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, et al. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]