This ‘perspective piece’ on the topic of psychopharmacology was requested to be opinion-driven and conceptual in nature, rather than a systematic review or a state-of-the-science article. Recently we (Volkow & Swanson, 2008a) adopted a broad approach to address multiple classes of psychotropic medication used to treat children (stimulants, anti-depressants, and anti-psychotics). We provided examples from traditional clinical pharmacology to discuss their pharamacokinetic (PK) and pharmacodynamic (PD) properties, as well as examples from modern positron emission tomography (PET) brain imaging to characterize the time course of drug effects at the primary cellular sites of action in the brain (transporters, enzymes, and receptors). Rather than repeat this broad approach here, we will provide a narrow, opinion-driven, and conceptual review of one of these classes – stimulant medication – that has been used primarily for the treatment of children with attention deficit hyperactivity disorder (ADHD) and hyperkinetic disorder (HKD) and recently has shown dramatic increases (see Swanson & Volkow, 2008) for the treatment of adolescents and adults. To narrow the scope further, we will focus on established concepts that have been challenged in the literature over the past decade (from 1998 to 2008). As requested, we will focus on personal experiences in research related to these concepts to highlight the historical context and some changes in clinical psychopharmacology over the past decade.
The literature on effects of the stimulant medications amphetamine (AMP) and methylphenidate (MPH) for the treatment of ADHD and HKD is enormous and increasing. However, the fundamental clinical effects of AMP were well described initially by Bradley (1937, 1950) over a half century ago and later by many investigators (including by Weiss, Werry, Minde, Douglas, & Sykes, 1968 in this journal), and the fundamental behavioral and cognitive effects of MPH were described initially by Conners and Eisenberg (1963) over 40 years ago and later by many investigators including by Taylor et al. (1977) and in this journal by Douglas et al. (1986).
Many reviews have been published to summarize the plethora of studies that followed, including influential early reviews in this journal (see Barkley, 1977) and from the European perspective by Taylor (1979) and in this journal by Bramble (2003). All seem to reach about the same basic conclusions about the effects of AMP and MPH that were reported in these initial studies. Fifteen years ago these were summarized by Swanson et al. (1993) in a ‘review of reviews’ that suggested what should be expected (e.g., short-term reduction in symptoms of ADHD and associated features of opposition and aggression) and what should not be expected (long-term benefits, absence of side effects, paradoxical response, large effect on higher-order processes). Almost a decade later, this was reinforced by Conners (2002), who concluded that the ‘effects of stimulants are consistent over time despite changes in diagnosis, assessment instrument, and research methodology’ (p. S29). So, what new concepts and controversial questions will be addressed here?
Over the past decade there have been some major changes in how the stimulants are used in clinical practice, as well as some major controversies about the fundamental pharmacological and neurochemical processes underlying the action of stimulant medications. For our opinion-driven article we selected five controversial questions to address: (1) How has clinical pharmacology been used to direct major changes in clinical practice? (2) How have new findings from PET imaging studies changed the understanding of the neural effects of stimulant medications and the brain-basis for ADHD? (3) How have long-term outcomes in large-scale clinical trials changed the rationale for treatment with stimulant medications? (4) How has the continued increase in use of stimulants for treatment altered concern about misuse of stimulant medication? (5) How has industry-sponsored research altered the clinical practice of treatment of individuals with stimulant medication?
After addressing these five concepts, we will update expectations about the use of stimulant medications in 2008, discuss the impact of current expectations of the rationale for and clinical practice of using stimulant medications in the treatment of ADHD and HKD, and offer some conclusions based on personal experiences in these areas of research on psychopharmacology.
Controversial concepts and questions
1. How has clinical pharmacology been used to direct major changes in clinical practice?
Changes have occurred in clinical practice since the beginning. The initial clinical practice described by Bradley in 1937 was based on the use of the racemic formulation of AMP (Benzedrine®), which was marketed by Smith, Kline and French in 1936, but by 1950 this shifted to the use of the pure d-isomer of AMP (Dexedrine®) that could be used at lower doses, which was marketed in 1949. By the 1970s, clinical practice had shifted again to the use of a different drug, MPH (Ritalin®), which was developed by CIBA pharmaceutical and received FDA approval in 1960. In 1994, there was an attempt to revive of use of AMP, but this was not successful initially. Richwood Pharmaceuticals tried to market a formulation of AMP developed by Rexar Pharmaceuticals and approved for appetite control in 1960 (Obetrol®, a racemic 75:25 mixture of the d-AMP and l-AMP optical isomers), with a new name (Adderall®). One of the claims was that Adderall® was a unique alternative and long-acting stimulant that could be given once a day and thus avoid in-school dosing (see full page advertisement in the Journal of the American Academy of Child and Adolescent Psychiatry, November, 1994). The evidence for this was apparently based on ‘some physician’s testimony as to special benefit in a segment of ADHD patients’ (see FDA Minutes of Meeting, NDA 11–522, 1995), which was challenged by the FDA. An earlier FDA review (see Federal Register, 1973) found insufficient evidence of efficacy and safety of this drug despite the approval before modern guidelines were in place. However, after negotiation with the FDA, Richwood Pharmaceutical received re-approval in 1996 to market Adderall® for the treatment of ADHD, even though there were no controlled trials of the effects on children with ADHD.
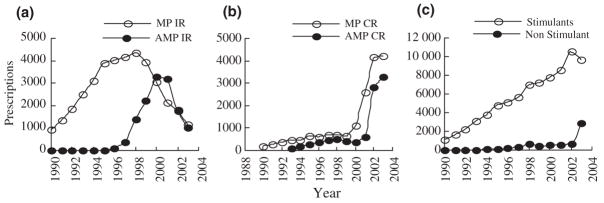
This called for clinical pharmacological studies to document under double-blind conditions the PK and PD effects of Adderall®. Richwood Pharmaceuticals funded the first controlled studies, which utilized the laboratory school paradigm and surrogate measures of response to compare the duration of action of immediate release (IR) formulations of AMP (Adderall®) and MPH (Ritalin®) in small groups of children with ADHD. One of these studies confirmed the claim of equal efficacy (maximum effect after an acute dose) and different PD half-lives for Adderall® (6 hours) and Ritalin® (4 hours) (Swanson et al., 1998). The other with just 21 children confirmed equivalence of efficacy of comparable multiple dose regimes for IR formulations with different PD half-lives (i.e., BID Adderall® and TID Ritalin® regimes) (Pelham et al., 1999). Additional controlled research in naturalistic settings of the home and school confirmed these laboratory studies. As shown in Figure 1a, there was a dramatic increase in prescriptions for IR AMP starting in 1998 that by 2000 remarkably equaled the number of prescriptions for IR MPH. In 2000, Richwood Pharmaceuticals was acquired by Shire Pharmaceuticals, which had a larger sales force and increased the marketing of Adderall®.
Figure 1.
Estimates of Prescriptions for IR and CR MPH and AMP in the USA
The second major change in clinical practice was a shift from IR to controlled release (CR) formulations. One common limitation of Adderall® and Ritalin® was the relatively short duration of action of these IR formulations that required multiple doses to maintain full efficacy across the day. In the 1980s, first-generation CR formulations of AMP (Dexedrine Spansules®) and MPH (Ritalin SR®) were available, but they were considered to have lower efficacy than multiple-dose regimes of the IR formulations and thus were not widely adopted in clinical practice. The consensus opinion was that the stimulant drugs required bolus doses and a PK profile with peaks and valleys to produce and maintain clinical efficacy, which implied an inherent limitation on CR formulations.
This also called for studies based on principles and techniques from clinical pharmacology. In a series of small studies funded by Alza Pharmaceuticals, Swanson et al. (1999) tested the bolus-dose assumption using the ‘sipping study’ methodology in a small proof of concept study to consider another possible explanation for reduced efficacy of CR formulations – acute tolerance to stimulant medication. A laboratory school study of 29 children with ADHD showed that a zero-order smooth (flat) drug delivery profile was insufficient to maintain efficacy across the day compared to the standard BID regime of IR MPH, but that a first-order smooth (ascending) PK profile without a bolus could achieve the full efficacy of the bolus dose regime. PK/PD modeling (see Levy, 1994; Park et al., 1998) suggested that acute tolerance to MPH could account for this pattern of PD effects. This discovery led to the design of a new commercial product (Concerta®) based on the osmotic release oral system (OROSR), which was modified to achieve the proposed optimum first-order (ascending) drug delivery profile. Concerta® was tested in proof of product studies in the laboratory classroom to document onset and duration of efficacy (see Pelham et al., 2002; Swanson et al., 2003). This was followed by typical multi-site clinical trials with much larger groups of subjects (see Swanson et al., 2000; Wolraich et al., 2001) considered necessary for submission to the FDA in order to document efficacy and safety and gain approval, which was granted in 2000. As shown in Figure 1b, Concerta® had almost immediate acceptance in clinical practice when it was introduced and marketed in 2000. Prescriptions for CR MPH starting increasing then, and by 2002 the use of CR MPH virtually replaced IR MPH in clinical practice. In 2002, Alza Pharmaceuticals was acquired by Johnson & Johnson, which had a larger sales force and increased the marketing of Concerta®.
To maintain competitiveness in the rapidly increasing market for stimulant drugs, Shire Pharmaceutical initiated a drug development program for CR AMP to match the predominant clinical regime of IR AMP (i.e., BID doses of Adderall®) and achieve full efficacy across the day with once-a-day administration. PK studies in adults (see Tulloch et al., 2002) and children (see Greenhill et al., 2003) were conducted to guide this development, which revealed a 6-hour PK half-life of a single dose of IR AMP and an ascending drug delivery profile associated with the BID regime of Adderall® with the doses given 4 hours apart. A dual-beaded drug delivery system was designed to match this ascending drug delivery profile, which was developed as a CR formulation called Adderall XR®. Proof-of-product PK/PD studies confirmed efficacy and duration of action (see McCracken et al., 2003). Upon approval granted by the FDA in 2002, Adderall XR® also gained almost immediate acceptance in clinical practice, as reflected by the rapid increase in prescriptions shown in Figure 1b.
In summary, two major changes in clinical practice occurred over the past decade in the USA (see Figure 1): the dramatic revival of AMP starting in 1998 and widespread acceptance of second-generation CR formulations of MPH and AMP starting in 2000. Both of these changes were stimulated by small studies based on principles of clinical pharmacology, with the latter based on PK/PD modeling and the hypothesis that predicted that smooth ascending PK profiles for once-a-day CR formulations would counteract acute tolerance and maintain full efficacy across the day.
2. How have new findings from PET imaging changed the understanding of brain-basis for ADHD and the neural effects of stimulant medications?
One of the first biochemical theories of ADHD was based on speculation about the neurochemical effects of the stimulants that produced rapid reduction of symptoms. Wender (1971) proposed the catecholamine deficit theory based in part on the belief that stimulants were catecholamine agonists that produced enhancement of NE and DA signals in the brain (see Solanto, 1998 for the history and early elaborations of this biochemical theory).
One question about the neural mechanism of action of MPH revolved around its similarity to cocaine in site and primary mechanism of action, blockade of dopamine transporters (DAT) in the striatum, but without similar euphoric effects. The early studies by Volkow et al. (1995) clarified this by using PET imaging with radiolabeled MPH to document the PK properties of the drug in the human brain. MPH had a much longer brain PK half-life than cocaine, which resulted in persistence of high brain levels of MPH and thus prolonged high exposure after the peak concentration was achieved. Apparently this produced acute tolerance to the brain levels of MPH that initially produced euphoric effects after intravenous dosing. However, questions remained about oral doses of MPH, which historically had been considered to produce a weak stimulant effect, which was assumed to be because rapid peripheral metabolism prevented high brain concentrations of the drug. Volkow et al. (1998, 2002) performed PET studies to estimate the neural effects of oral MPH doses on occupancy of DAT, and documented that on the average 80% of transporters in the striatum were blocked in adults by oral dose less than 1.0 mg/kg. This level of DAT blockade by an oral dose in the clinical range was as great as for intravenous doses of MPH or cocaine. This supported the hypothesis that differences in the euphoric effects of these two drugs were due to differences in their brain PK properties (and the presence of acute tolerance related to the extended presence of high concentrations of MPH in the brain), rather than to low concentrations of MPH at the neural site of action.
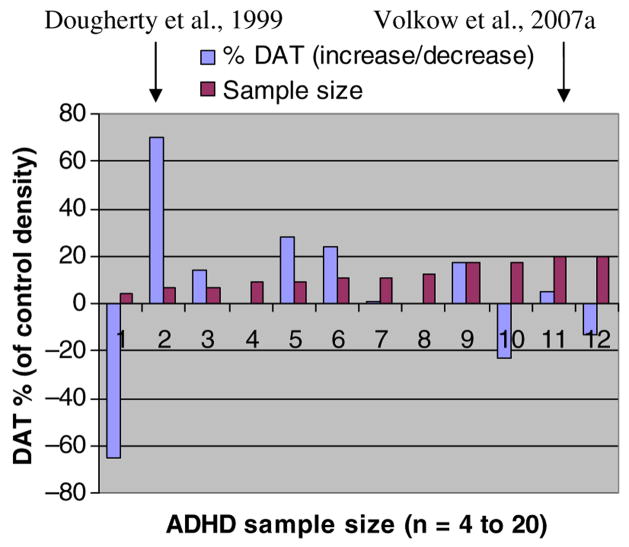
PET methods have also been used to investigate possible biological markers for ADHD. An exceedingly influential study by Dougherty et al. (1999) was based on the use of Single Photon Emission Computed Tomography (SPECT), a low resolution alternative to PET, and a new radioligand (iodine-23-labeled altropane) to estimate the density of DAT in the basal ganglia of the brain. A study of 6 adults with ADHD suggested that DAT density was 70% higher than expected by historical norms for the SPECT-altropane method. Some studies by another group have partially replicated the effect in sub-groups of ADHD subjects with different SPECT methods (see Krause et al., 2000). This theory was appealing since high DAT density could account for an ADHD-related DA deficit (i.e., this would produce an increased reuptake of DA released into the synapse), as well as the beneficial response to MPH (i.e., the blockade of DAT would reduce DA uptake and act to correct the DA deficit).
The hypothesis of high DAT density as a brain-basis of ADHD was accepted for over a decade, and is now typically cited as one of the primary biological bases of ADHD. To test this hypothesis, Volkow et al. (2007a) evaluated a larger sample (20 stimulant-naïve adults with ADHD and 25 controls matches for sex and ethnicity) and a more sensitive method of estimating DAT density (using PET rather than SPECT and radiolabeled cocaine rather than altropane as the ligand). Surprisingly, this study was unable to document lower DAT density in the caudate nucleus or in any basal ganglia region, and in fact observed a trend in the opposite direction. As shown in Figure 2, some of the other subsequent studies (see Volkow et al., 2007 for specific references) using PET methods with higher resolution and larger samples of ADHD and control subjects have also reported failure to replicate the finding of dramatically increased DAT density associated with ADHD.
Figure 2.
Funnel Plot of Effects from Studies of DAT Density in ADHD (The references for studies 1 to 12 are provided in Volkow et al., 2007a)
Based on this selected literature review, we believe that modern PET studies have confirmed the DA-agonist theory of stimulant drugs and have challenged the DAT-density theory of the brain-basis of ADHD. The recent findings from these studies are not universally accepted, so references to the old and long-accepted theories still permeate the literature.
3. How have long-term outcomes in large-scale clinical trials changed the rationale for treatment with stimulant medications?
Despite extensive and accumulating evidence of short-term efficacy of stimulant medication, in 1990 there was a glaring lack of evidence documenting long-term benefits. Several early follow-up studies in the literature suggested that clinical effectiveness could be maintained for years (see Satterfield et al., 2007 for a review), but controlled studies had not been conducted to provide solid evidence of long-term benefit. The Multimodal Treatment study of ADHD (MTA) was initiated in 1993 to evaluate the long-term effects of treatments using the ‘gold standard’ for evidence-based medicine – a randomized clinical trial (RCT) – to contrast the long-term effects of state-of-the-art pharmacological treatment (MedMgt), psychosocial treatment (Beh), and the combination of these two treatment modalities (Comb). As with most RCTs, relative rather than absolute effects were evaluated by comparing outcomes of these treatments to each other, and (in lieu of a no-treatment control group) to treatment-as-usual in the community (CC). After a 14-month treatment-by-protocol phase, the MTA became an observational follow-up that is still in progress. Elsewhere, the MTA Group has provided summaries and detailed accounts of the main findings, interpretations, and qualifications from the 14-month, 24-month, and 36-month assessments of outcomes (see Arnold et al., 2008; Swanson et al., 2008a, 2008b), so only a brief summary will be presented here. Despite initial evidence of long-term relative benefits over the first two years of treatment, when the definition of long-term was extended to 3 years, the secondary analyses of the MTA follow-up were not able to document any long-term relative benefits of prior or current treatment with stimulant medication. However, post-hoc analyses of growth in MTA revised the once-discredited (see Spencer et al., 1996) hypothesis of stimulant-related growth suppression. By the third year of the study when the participants were between the ages of 10 and 12 years of age, an accumulated reduction in height gain of about 2 cm and a reduction in weight gain by about 2 kg was observed in the newly treated subgroups compared to the subgroup of cases never treated with stimulant medication. The clinical significance of this finding has been questioned by some (see Faraone et al., 2008).
One of the greatest concerns about the long-term clinical use of stimulant medication in childhood has been the possibility that this might increase the risk for drug abuse (see Volkow & Swanson, 2003). However, over the past decade, the opposite was suggested, with claims that childhood treatment with stimulant medication decreased risk (see Wilens et al., 2003). In the 36-month follow-up of the MTA, this hypothesis was evaluated (see Molina et al., 2007). Increased substance use in the ADHD group compared to a non-ADHD classmate control group was documented, but this emergence of early substance use in the ADHD group was not significantly reduced by treatment with stimulant medication. Also, recent publications of long-term follow-up of cohorts that were included in the Wilens et al. (2003) review suggest that by adulthood there was no evidence of the long-term effects of childhood treatment with stimulants – beneficial or harmful – on later substance use or abuse (see Volkow & Swanson, 2008c).
In summary, it is surprising and disappointing that the current literature does not support two of the most fervent expectations – the absence of stimulant-related growth suppression (Spencer et al., 1996) and the presence of protection from substance use (Wilens et al., 2003) – that had been used for over a decade as part of the rationale and justification for the use of childhood treatment with stimulant medication.
4. How has the continued increase in use of stimulants for treatment altered concern about misuse of stimulant medication?
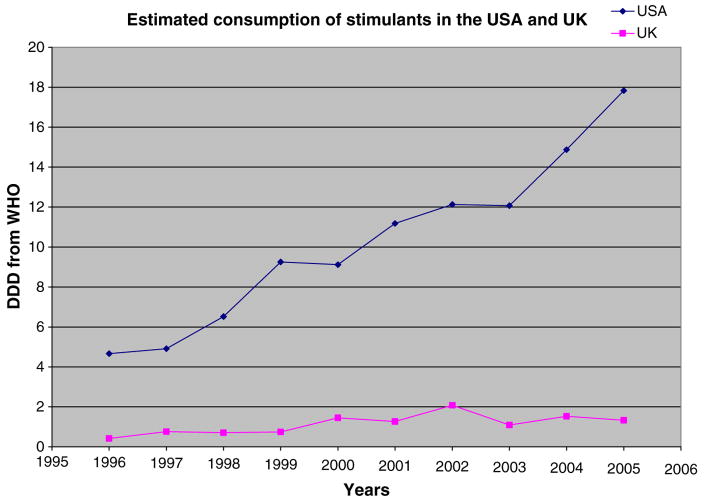
Three decades ago, Taylor (1979) observed that despite similarities of prevalence of ADHD in different countries, stimulant medications ‘…are used in treatment with frighteningly different frequency in different places’. Overmeyer and Taylor (1999) speculated that there was under-recognition and under-treatment of even HKD (the severe form of ADHD) in the UK compared to the USA, even though ADHD cases with HKD may be more responsive to stimulant medication that ADHD cases that do not meet the criteria for HKD (see Santosh et al, 2005; Taylor et al, 2004). The years of undertreatment may have been partially due to the unavailability of stimulant medications, which were voluntarily with-drawn from the UK market in 1980. Availability was changed in 1995 when MPH was re-licensed in the UK, and over the next 5 years there was a dramatic 10-fold increase in prescriptions from about 20,000 to nearly 200,000 (see Bramble, 2003). However, Jick et al. (2004) pointed out that a large UK–USA difference remained over the period from 1999 to 2001: the percentage of the 5- to 14-year-old children treated in the UK estimated from the General Practice Research Database (0.5%) was about 20-fold lower than that estimated by a health maintenance organization from the west coast of the USA (9.3%).
Estimates of national supplies of stimulants provide another way to characterize cross-national differences and to extend the comparison through 2005. The UN provides annual reports of supply of stimulant drugs (along with other drugs with abuse potential) by countries, stated in terms of defined daily dose (DDD) per 1,000 in the population, with DDD = 30 mg for MPH and 15 mg for AMP. The UK was not listed in these reports before 1996, but reports since 1996 provide data for a UK–USA comparison of the national supplies of stimulants. As shown in Figure 3, the combined MPH–AMP DDD estimate increased by a factor of 3.17 for the UK (from 0.42 in 1996 to 1.33 in 2005), but increased by an even greater factor of 3.83 for the USA over the same time period (from 4.66 to 17.83).
Figure 3.
UN Supply Estimates (combined DDD for MPH and AMP) for UK and USA
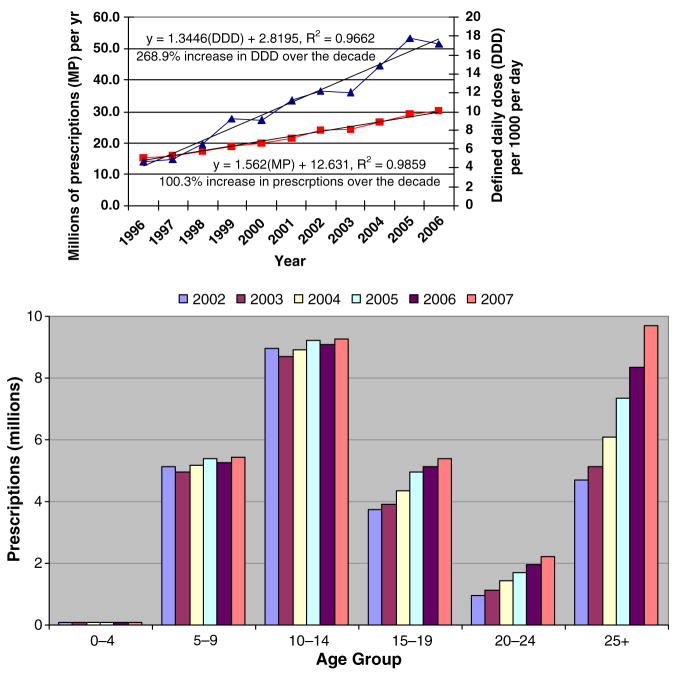
How has the continued increase in the USA been used or misused? In the 1980s through the 2000s, the use of stimulants showed regular linear increases (Safer & Krager, 1988; Zito et al., 2003). Dramatic increases in the early 1990s were attributed to correction of prior under-recognition and under-treatment of ADHD. A survey of use of stimulants in children in the USA suggested a leveling off of the number of children treated with stimulants by 2002 to about 2.2 million (4.8% of 6–12-year-olds, 3.2% of 13–19-year-olds, and .3% of children under the age of 6 year (see Zuvekas et al., 2006). However, findings from subjective survey method should be confirmed by more objective methods, such as by inspection of the trend in UN estimates of the national supply. Swanson and Volkow (2008) noted that the national supply in the USA continued to increase linearly after 2002 (see Figure 4a). By 2007, the annual supply (stated in terms of UN estimate of DDD) was about 17, which for the population of the USA is sufficient to treat about 5 million individuals per day. However, the supply estimates do not provide age-specific trends. Since 2000, prescription records have been provided separately for age groups. While the total increase in prescriptions remained linear from 2000 to 2007 and reached about 30 million by 2007 (see Swanson & Volkow, 2008b and Figure 4a), there was no increase over this time period in prescriptions for the 0–5, 6–10, or 10–14 age groups (confirming the report provided by Zuvekas et al., 2006). However, in the 15–19, 20–24, and over-25 age groups, the increase in prescriptions did not asymptote, but rather continued to increase linearly (see Figure 4b).
Figure 4.
Estimates of Supply and Prescriptions in the USA from International Narcotics Control Board (1999–2006) and Veripan(R)-One National.
Of course, the recent increases in prescriptions for adolescents and adults may just reflect a correction of prior under-recognition and under-treatment of those age groups. However, another contributing factor should be considered: when stimulants are prescribed for adolescent and adults who are seeking treatment for themselves, there may be a higher rate of diversion for non-medical use than for children whose parents are seeking treatment for them. For example, in a series of publications based on school-based surveys, McCabe et al. (2004, 2006) reported that about 8% of non-ADHD students in middle school, high school, and college engaged in nonmedical use of stimulants. Others have confirmed this pattern in adults as well as in children (see Wilens et al., 2008 for a review). A primary source for non-medical use is apparently from prescriptions for medical use diverted by sale or other means.
Increased diversion may be related to the provocative suggestion that stimulant medications may be ‘cognitive enhancers’ for the general population (Sahakian & Morein-Zamir, 2007). This commentary was presented to stimulate discussion, and it generated pro and con points of view on a special website (see http://www.nature.com). In an Internet survey of adults (18 to 49 years of age), Novak et al. (2007) found that in a majority of the participants the primary motivation given for non-medical use was to increase productivity (i.e., for cognitive enhancement). In a survey of college students, Teter et al. (2006) also found that diversion in a majority of cases was for cognitive enhancement.
In summary, over the past decade, the linear increase in supply and prescriptions for stimulants has continued, fueled recently by an increase in use of stimulants by adolescents and adults. This age-specific increase in groups seeking treatment should increased concern about diversion from medical to non-medical use.
5. How has industry-sponsored research altered the clinical practice of treatment of individuals with stimulant medication?
Before the mid-1990s, the pharmaceutical industry provided little support for studies of the stimulant medications, and there was little marketing of the approved stimulant medications then available – MPH (Ritalin®), AMP (Dexedrine® and Obetrol ®), and pemoline (Cylert®). Apparently, this was partially due to a general lack of incentives to conduct research with children (see DeVaugh-Geiss et al., 2006). This state of affairs required the acceptance of the practice of conducting clinical trials and gaining approval for a new drug for use in adults and then prescribing it ‘off label’ to treat children. This changed in the USA in 1997, when the US Congress enacted Public Law 105–115, the Food and Drug Modernization Act (FDAMA). Also in 1997, the European Medicines Agency (EMEA) organized a round table (http://emea.europa.eu/pdfs/human/paediatrics/1796704en.pdf) to discuss pediatric medicines, and by 2007 new Pediatric Regulations in the Europe Union were approved to facilitate the development and availability of new medicines for children.
The FDAMA provided provisions to encourage the evaluation of new drugs in children. The ‘pediatric exclusivity rule’ provided an extension of the life of patents, which introduced a lucrative financial incentive to conduct clinical trials in children. The FDA website (http://www.fda.gov/cder/pediatric/index.htm) provides a list of manufacturers who have been granted patent extension based on the Pediatric Exclusivity provision. Both of the primary stimulant medications now in use qualified, and Johnson & Johnson (or its subsidiary, McNeil Consumer Health Care) received an extension of the patent for its CR formulation of MPH (Concerta®) and Shire Pharmaceutical received an extension for its patent of its CR formulation of AMP (Adderall XR®).
In addition to the drug development programs that led to Concerta® and Adderall XR® (see section 1 above), several others have been initiated. This has resulted in additional new CR formulations of stimulant medication commerical products that have been approved for the treatment of ADHD (see Table 1). Studies that compare drugs or formulations with slightly different drug delivery profiles are complex (see Pelham et al., 1999; Swanson et al., 2004), and may be considered unfair by design (see Adesman, 2004), although perhaps unfairly (see Swanson, 2004).
Table 1.
New CR Formulations of MPH
| Drug | Product | Approval | Development | Marketing |
|---|---|---|---|---|
| d,l-AMP | Benezedrine® | 1937 | S, K&F | S, K&F |
| d-AMP | Dexedrine® | 1940 | S, K&F | S, K&F |
| d,l-MPH | Benezedrine Spansules® | 1950 | S, K&F | S, K&F |
| d-AMP | Dexedrine Spansules® | 1950 | S, K&F | S, K&F |
| d,l-MPH | Ritalin® | 1963 | CIBA | CIBA |
| d,l-AMP | Obetrol® | 1950 | Rexar | Rexar |
| pemoline | Cylert® | 1975 | Abbott | Abbott |
| d.l-MPH | Ritalin SR® | 1980 | CIBA | CIBA |
| d,l-MPH | Metadate® | 1982 | MD | Medeva |
| d,l-AMP | Adderall® | 1996 | Richwood | Shire |
| d,l-MPH | Concerta® | 2000 | Alza | J&J |
| d,l-MPh | Ritalin LA® | 2002 | Norvartis | Novartis |
| atomoxetine | Straterra® | 2002 | Lilly | Lilly |
| d,l-MPH | Metadate CD® | 2003 | Medeva | UCB |
| d-MPH | Focalin® | 2004 | Celgene | Novartis |
| d,l-MPH | Focalin LA® | 2005 | Novartis | Novartis |
| d,l-MPH | Bifentin® | 2006 | PurdueCanada | PurdueCanada |
| d-l,MPH | Daytrana® | 2006 | Noven | Shire |
| AMP | Vyvance® | 2007 | New River | Shire |
Two non-stimulants have been evaluated as alternative pharmacological treatments for ADHD. First, the anti-depressant atomoxetine (Strattera®) was developed by Lilly Pharmaceuticals with claims of specific targeting of the norepinephrine transporter, but it had lower efficacy than Adderall XR® (see Wigal et al., 2005) or Concerta® (see Newcorn et al., 2007), and it never displaced the standard treatments with stimulants (see Figure 1). Second, the narcolepsy drug modafinil was evaluated by Cephalon Pharmaceuticals for use in the treatment of ADHD. Based on multiple clinical trials that documented clear efficacy with an effect size only slightly lower than the stimulants (see Biederman et al., 2005; Greenhill et al., 2006; Swanson et al., 2007), a submission was made for FDA approval. However, the presence of dermatological side effects resulted in a decision of non-approval, this led to the and eventual withdrawal of the application (see http://www.FDA.gov).
Two of the CR formulations of MPH developed in the USA have been approved for use in Europe (Concerta® and Metadate CD®, which is labeled Equasym®), and other dual-beaded formulations were developed for use in Europe (Medikinet XL®). One of the non-stimulants was approved for use in Europe (Strattera®). Guidelines for the pharmacological treatment of ADHD and HKD have been published (e.g., NICE Clinical Guideline 72, 2008 see www.nice.org.uk) and discussed in detail (see Taylor et al., 2004) elsewhere, and since these guidelines are widely available they need not be repeated here.
Based on this highly selected review of the literature, it is our opinion that the primary pharmaceutical treatments of ADHD and HKD have not changed in any fundamental way since the initial studies of Bradley (1937, 1950) and Conners and Eisenberg (1963). The primary treatment is still with the stimulant medications (AMP and MPH). The primary difference is that stimulants are now delivered in once-a-day CR formulations rather than multiple daily doses of IR formulations.
Revised concepts and answers to controversial questions
We have presented some concepts about stimulant medication, and offered opinions that address the five controversial questions that we posed. We will summarize these here and offer some opinions about how fundamental concepts might need to be revised:
1. How has clinical pharmacology been used to direct major changes in clinical practice?
Dramatic changes have occurred over the past decade, with the revival of AMP and the shift from IR formulation to CR formulations of MPH and AMP (see figure 1). These were due primarily to development programs of pharmaceutical companies, which invested in small proof-of-concept studies (for Concerta® see Swanson et al., 1999; for Adderall XR® see Greenhill et al., 2003). These studies were relatively inexpensive but directed subsequent development of new CR commercial products. Proof-of-product studies were somewhat larger and more expensive and used the laboratory school paradigm (for Concerta® see Pelham et al., 2001 and Swanson et al., 2003, and for Adderall XR® see McCracken et al., 2003). These were followed by large and very expensive clinical trials to evaluate effects in the home and school settings that were necessary to gain approval for use in the treatment of ADHD. Despite many new approved products in the past 5 years (see Table 1), the predominance of the first two new products – Concerta® and Adderall XR® – has not been altered over the last half of the decade.
2. How have new findings from PET imaging changed the understanding of the neural effects of stimulant medications and the brain-basis for ADHD?
PET studies have clearly confirmed the DA-agonist theory of stimulant medication by documenting that blockade of DAT (and the resulting increase in synaptic DA) is a primary mechanism of action of stimulant medication. The DAT-density theory of ADHD proposed by Dougherty et al. (1999), which was based on a small sample of n = 6 cases, mostly female and with exclusion of recent treatment (past month) but not prior history of treatment, has not been confirmed by some recent studies that used larger samples without prior treatment. It is our opinion that the acceptance of this high DAT density theory for almost a decade has been at the expense of consideration of other theories that suggest that the fundamental neural deficits (or abnormalities) of ADHD are manifested in other component of neural circuits that affect attention and activity and result in symptoms of ADHD. We believe that further investigation is needed of other components of brain circuits that may contribute to ADHD-related DA deficits (see Forssberg et al., 2006 for a study of DA synthesis and Volkow et al., 2002 for a study of DA release) and the presence and length of prior treatment effects that may have effects that are long-lasting (see Ludolph et al., 2008 for a study of DA synthesis and turnover). We proposed that reduced release may characterize adults with ADHD (see Volkow et al., 2002), and Ludolph et al. (2008) proposed that adaptation to chronic treatment with stimulants (e.g., down-regulated DA turnover and increased DAT density) may occur. Further clarification of the components of the DA system and their plasticity could lead to improved understanding of ADHD and its treatment.
3. How have long-term outcomes in large-scale randomized clinical trials changed the rationale for treatment with stimulant medications?
The initial large and clear relative benefits of stimulant medication that were present a year after treatment in the treatment-by-protocol phase of the MTA appear to dissipate completely by the third year of treatment. We do not believe that long-term benefits are not necessary to justify the clear short-term effects of a symptomatic treatment (see Kinsbourne & Swanson, 1980). The immediate reduction of impairment, even if temporary, seems sufficient to justify the short-term use of stimulants for a year or two. However, when a clinical recommendation is made to use stimulant medication as a symptomatic treatment, it seems prudent and essential to recognize the limitations (i.e., lack of long-term effects for 3 or more years) and potential side effects (i.e., possible long-term growth-related side effects) and to acknowledge the revised costs and benefits suggested by the current literature.
4. How has the continued increase in use of stimulants for treatment altered concern about misuse of stimulant medication?
The world wide prevalence of stimulant medications has increased dramatically over the past decade, with the largest increases in the USA where prior use was already high. The current literature suggests that the regular (linear) increases observed for decades are continuing, and that at least in part this is due to self-treatment via non-medical use of stimulant medications in adolescents and adults. We believe that the emerging trend of non-medical use stimulants for cognitive enhancement rather than for medical treatment to reduce impairments related to ADHD symptoms should be curtailed until adequate evaluation of costs (long-term as well as short-term) and benefits (actual not perceived) are clarified.
5. How has industry-sponsored research altered the clinical practice of treatment of individuals with stimulant medication?
It appears that the major changes in clinical practice over the past decade (i.e., the revival of AMP and the switch to CR formulation of MPH and AMP) were the result of studies supported by small pharmaceutical companies. From personal experience, it seems that this segment of the pharmaceutical industry can act on hunches (as demonstrated by the revival of Adderall® by Richwood Pharmaceuticals) and conduct studies of innovative theories (as demonstrated by the drug development program of Alza Pharmaceuticals) without pilot data or the usual scrutiny of scientific or financial review committees. However, very large investments are required by the types of clinical trials needed for the FDA approval process, as well as for marketing new products. It appears that when a small pharmaceutical company develops a new product, it is likely that the company or its innovative product might be acquired by a larger pharmaceutical company that excels in marketing. In the narrow area that is addressed here, this occurred with Concerta® (Alza was acquired by Johnson & Johnson), Adderall® (Richwood was acquired by Shire), Metadate CD® (Medeva was acquired by UCB), and Focalin® (the drug developed by Celgene was acquired by Novartis). This increased the approved alternatives for treatment, but it also increased the marketing efforts and advertising. In the example of new stimulants that were developed and approved between 1998 and 2008, advertising and marketing appear to emphasize small differences to distinguish the variety of CR formulation rather than fundamental PK/PD properties of the stimulant medications that characterize all of the new stimulant drugs approved for use in the treatment of ADHD.
The influence of the pharmaceutical industry on clinical research has been a topic of much discussion recently, as exemplified by a commentary of the Editor of the Journal of the American Medical Association (see DeAngelis & Fontanarosa, 2008). One issue is about some practices in clinical trials, when the sponsoring company may take the lead in the design and analysis of the study, and investigators may be ‘little more than hired hands, supplying patients and collecting data according to the company protocol’ (Angell, 2008). Another is about industry influence on literature reviews. In a non-ADHD area of research Jorgensen et al. (2006) addressed this issue with a comparison of ‘Cochrane’ reviews and industry-supported meta-analyses of the same literature. They found major differences, with industry-supported meta-analyses reaching more favorable conclusions, and warned that ‘industry supported reviews of drugs should be read with caution’. DeAngelis and Fontanarosa (2008) describe high-profile examples of how involvement and influence of for-profit companies can go awry. They offer 11 proposals to ensure that ‘primum non nocere’ holds true not just for physicians directly treating patients, but also for ‘all involved in medical research, biomedical publication, and medical education’. Rothman and Chimonas (2008) describe new recommendations for conflict of interest that have been developed and approved by the American Association of Medical Colleges.
Given the major involvement of the pharmaceutical companies in the chosen topic – ‘Psychopharmacology: concepts and opinions about the use of stimulant medications’ – awareness of the conflict-of-interest issue and adherence to recommendations intended to reduce it seems essential to avoid ‘impugning the integrity of medical science’ (DeAngelis & Fontanarosa, 2008).
Summary
Our ‘opinion-driven and conceptual review’ of the past decade of research has identified many differences between the prior and current use and understanding of effects of stimulant medications, which are summarized in Table 2. As requested, we will offer some conclusions based on personal experiences in research related to the five concepts considered here.
Table 2.
Changes in the Use and Understanding of Stimulant Medications
| In the early 1990, it was reasonable to expect the following: |
| About 1 million individuals in the USA (mostly school-aged boys) would be treated with stimulants. |
| With few approved products and quotas imposed, promotion would be limited and advertising was not used to increase sales or to gain market share. |
| In most cases the treatment would be with MPH administered in the IR-formulation given 2 to 3 times a day. |
| Reverse sculpting of IR doses would be used, with an afternoon dose lower than the morning dose, to prevent an ascending drug level across the day. |
| Treatment would be described as producing temporary symptomatic relief without claims of long-term effects. |
| Typically growth suppression would not be discussed, since it was the consensus that it would not occur or later growth rebound and ‘catch-up’ would occur. |
| The general mechanism of action (i.e., DA agonism) would be used to explain the clinical effects of stimulant drugs without detailed understanding of components. |
| Based on the current literature, we believe that in 2008 it is reasonable to expect the following: |
| That about 6 million individuals in the USA will take stimulants each day, and about half will take MPH and half AMP (and others will take non-stimulants). |
| Compared to 1990 this will include more females, more adolescent and adults, and more non-clinical cases (i.e., diversion for non-clinical use) than a decade ago. |
| In most cases, CR formulations for once-a-day administration will be used that delivers an ascending level of medication across the day. |
| With many CR formulation approved for use, pharmaceutical companies will use promotion and advertising to highlight relatively small differences in efficacy and side effects. |
| With state-of-the-art medication regimes, beneficial effects should be expected for the first year or two, but beyond two years these effects are likely to dissipate. |
| Long-term side effects of growth suppression are likely to occur and accumulate over the initial three years of treatment, with little of no rebound before puberty. |
| Treatments in early childhood should not be expected to offer protection from the expected emergence of substance use of abuse. |
| Findings from modern brain imaging studies could provide new knowledge about the DA system and ADHD and the mechanism of action of stimulant medication. |
From personal experience in developing the second-generation CR formulations that are the mainstay of current clinical treatment, it seems that the advances that led to these formulations were initiated by development programs of small pharmaceutical companies (Alza and Richwood), which invested in proof-of-concept studies directed by fundamental principles of modern PK/PD evaluation (see Park et al., 1998). These studies implicated acute tolerance as a factor and recommended ascending drug delivery profiles, which theoretically act to overcome acute tolerance and maintain full efficacy across the day for controlled-release MPH (for Concerta® see Swanson et al., 1999) and AMP (for Adderall XR® see Tulloch et al., 2002 and Greenhill et al., 2003). However, this fundamental principle of acute tolerance is not understood or recognized by all, which is reflected in reviews of the second-generation CR formulations (see the absence of mention of acute tolerance in the reviews by Banaschewski et al., 2006 and Connor & Steingard, 2004) and by some investigators who have participated in the development of new CR formulations without an ascending drug delivery profile (e.g., see Schachar et al., 2008).
From personal experience with PET imaging studies of adults, it seems that the most difficult component of this research is not the high cost of the imaging procedure but instead is the recruitment of cases without prior treatment and comorbid conditions and the accumulation of a sufficient number of both cases and controls to allow for evaluation of other important factors that may affect DAT density (such as sex and ethnicity). For example, the sample of n = 20 cases and n = 25 controls evaluated by Volkow et al. (2007) required several years to identify and test, but this was necessary to exclude the effects of prior treatment and comorbid factors and to evaluate the effects of sex and gender factors.
From personal experience in the analysis of data from the serial follow-ups of the MTA (aMTA Cooperative Group, 2004a, MTA Cooperative Group, 2004b; Swanson et al., 2007a, 2007b), it seems difficult to evaluate long-term effects due to changes in treatment regimes over time. Sophisticated statistical procedures are necessary to test assumptions about mediators and moderators as well as hypotheses about selection bias and subtypes based on outcome trajectories over time (Swanson et al., 2007). Based on analyses utilizing these statistical methods, the expectation of long-term persistence of the initial relative superiority of state-of-the-art treatment with stimulants over other treatments in the MTA has not been confirmed in the naturalistic follow-up (see Swanson et al., 2008a, 2008b).
From personal experience and participation in the current debate about the medical and non-medical use of stimulant drugs (Volkow & Swanson, 2007; Swanson & Volkow, 2008b), it seems necessary to utilize multiple assessments (e.g., household surveys, prescription records, estimates of supply, etc.) to establish national patterns of use of stimulant medication. The linear increase that has persisted for decades has not abated, but logically this must reach an asymptote in the future if treatment is to be restricted to only a percentage of the population.
From personal experience about industry-funded studies (e.g., Swanson et al., 1998, 1999, 2003), it is clear the costs of proof-of-principle or proof-of-concept studies are small compared to the costs of large clinical trials that follow new discoveries and are essential for gaining FDA approval for new and improved commercial products. However, public criticism of potential conflict of interest may inhibit the future funding for all types of studies supported by pharmaceutical companies, which are encouraged under the FDAMA (see Public Law 105-55, 1997) and are usually too expensive for NIH funding. Strict adherence to gudelines is clearly essential to ensure support for the research required to develop new products, with evaluation of safety and efficacy in children, and to gain approval for use in this age group rather than to rely on off-label prescribing of medications evaluated and approved in adults.
Key points
Principles from clinical pharmacology were applied in the 1990s to develop second-generation controlled-release formulations that by 2000 replaced immediate-release formulations of methylphenidate and amphetamine for treatment of children with ADHD.
Applications of positron emission tomography brain imaging to evaluate stimulant naïve adults recently produced new findings that challenge established theory that a neural correlate of ADHD was abnormally high dopamine transporter density in the striatum.
The long-term naturalistic follow-up of the Multimodal Treatment study of ADHD suggests that rigorous childhood treatment with stimulant medication produces initial relative benefits over other treatments that may not persist beyond 2 years.
The overall rate of prescription of stimulant medication has increased worldwide and has continued to increase in the USA, even reaching asymptote for children with ADHD by 2000, due to increases for adults and adolescents and possibly increased diversion for non-medical use.
Industry-sponsored studies of stimulants have increased over the past decade, due to clinical trials for approval of new formulations and studies for promotion and marketing, which may have generated concern about the influence of commercial firms on clinical use.
Footnotes
Conflict of interest statement: James M. Swanson has received honoraria for lectures from J & J Jassen-Ortho, Inc., UCB Pharma Ltd and Convention Likage Inc., and consulting fees from NV Organon.
References
- Adesman A. Flawed attention-deficit/hyperactivity disorder medication comparison. Pediatrics. 2004;114:1132. doi: 10.1542/peds.2004-1310. [DOI] [PubMed] [Google Scholar]
- Angell M. Industry sponsored research: A broken system. Journal of the American Medical Association. 2008;300:1069–1071. doi: 10.1001/jama.300.9.1069. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Swanson JM, Hectman L, Vitiello B, Molina BSG, Jensen P, Hinshaw SP, Wigal T. Understanding the 36-month MTA follow-up findings in context. Attention. 2008;15:14–18. [Google Scholar]
- Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, Danckaerts M, Döpfner M, Faraone SV, Rothenberger A, Sergeant J, Steinhausen HC, Sonuga-Barke EJ, Taylor E. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. European Child and Adolescent Psychiatry. 2006;15:476–495. doi: 10.1007/s00787-006-0549-0. [DOI] [PubMed] [Google Scholar]
- Barkley RA. A review of stimulant drug research with hyperactive children. Journal of Child Psychology and Psychiatry. 1977;18:137–165. doi: 10.1111/j.1469-7610.1977.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Swanson JM, Wigal SB, Kratochvil CJ, Boellner SW, Earl CQ, Jiang J, Greenhill L. Efficacy and safety of modafinil film-coated tablet in children and adolescents with attention-deficit/hyperactivity disorder: Results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005;116:777–784. doi: 10.1542/peds.2005-0617. [DOI] [PubMed] [Google Scholar]
- Bradley C. The behavior of children receiving Benzedrine. American Journal of Psychiatry. 1937;94:577–585. [Google Scholar]
- Bradley C. Benzedrine and Dexedrine in the treatment of children’s behavior disorders. Pediatrics. 1950;5:24–37. [PubMed] [Google Scholar]
- Bramble D. Annotation: The use of psychotropic medications in children: A British view. Journal of Child Psychology and Psychiatry. 2003;44:169–179. doi: 10.1111/1469-7610.00111. [DOI] [PubMed] [Google Scholar]
- Conners CK. Forty years of methylphenidate treatment in attention deficit/hyperactive disorder. Journal of Attention Disorders. 2002;6:S17–S30. doi: 10.1177/070674370200601s04. [DOI] [PubMed] [Google Scholar]
- Conners CK, Eisenberg L. The effects of methylphenidate on symptomatology and learning in disturbed in children. American Journal of Psychiatry. 1963;120:458–464. doi: 10.1176/ajp.120.5.458. [DOI] [PubMed] [Google Scholar]
- Connor DF, Steingard RJ. New formulations of stimulants for attention-deficit hyperactivity disorder: Therapeutic potential. CNS Drugs. 2004;18:101–1030. doi: 10.2165/00023210-200418140-00005. [DOI] [PubMed] [Google Scholar]
- DeAngelis CD, Fontanarosa PB. Impugning the integrity of medical science: The adverse effects of industry influence. Journal of the American Medical Association. 2008;299:1833–1835. doi: 10.1001/jama.299.15.1833. [DOI] [PubMed] [Google Scholar]
- DeVeaugh-Geiss J, March J, Shapiro M, Andreason PJ, Emslie G, Ford LM, Greenhill L, Murphy D, Prentice E, Roberts R, Silva S, Swanson JM, van Zwieten-Boot B, Vitiello B, Wagner KD, Mangum B. Child and adolescent psychopharmacology in the new millennium: a workshop for academia, industry, and government. J Am Acad Child Adolesc Psychiatry. 2006;45:503–11. doi: 10.1097/01.chi.0000194568.70912.ee. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Bonab AA, Fischman AJ, Madras BK, Rauch SL, Spencer TJ. Dopamine transfer density in patient with attention deficit hyperactivity disorder. The Lancet. 1999;354:2132–3233. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- Douglas VI, Barr RG, O’Neill ME, Britton BG. Short-term effects of methylphenidate in the cognitive, learning, and academic performance of children with attention deficit disorder in the laboratory and the classroom. Journal of Child Psychology and Psychiatry. 1986;27:191–211. doi: 10.1111/j.1469-7610.1986.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Fernell E, Waters S, Waters N, Tedroff J. Altered pattern of brain dopamine synthesis in male adolescents with attention deficit hyperactivity disorder. Behavioral and Brain Functions. 2006;2:40–50. doi: 10.1186/1744-9081-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, Biederman J, Boellner SW, Rugino TA, Sangal RB, Earl CQ, Jiang JG, Swanson JM. A randomized, double-blind, placebo-controlled study of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:261–70. doi: 10.1097/01.chi.0000205709.63571.c9. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Steinhoff K, Fried J, Posner K, Lerner M, Wigal S, Clausen SB, Zhang Y, Tulloch S. A pharmacokinetic pharmocodynamic study comparing a single morning dose of Adderall to twice-daily dosing in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;40:1352–1355. doi: 10.1097/00004583-200310000-00015. [DOI] [PubMed] [Google Scholar]
- International Narcotics Control Board. Psychotrophic substances. United Nations Publications; New York: 1999–2006. [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, et al. 3-Year follow-up of the NIMH MTA Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- Jorgensen AW, Hilden J, Gotzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: Systematic review. British Medical Journal. 2006 doi: 10.1136/bmj.38973.444699.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M, Swanson JM. Evaluation of symptomatic treatment of hyperactive behavior by stimulant drugs. In: Knights RM, Bakker D, editors. Treatment of hyperactive and learning disordered children: Current research. Baltimore: University Park Press; 1980. [Google Scholar]
- Levy G. Mechanism-based pharmacodynamic modeling. Clinical Pharmacology and Therapeutics. 1994;56:356–358. doi: 10.1038/clpt.1994.149. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK, et al. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), difference between pharmacologically treated and never treated young adults: A 3,4 – dihdroxy-6-[18F]fluorophenyl-L-alanine PET study. NeuroImage. 2008;41:718–727. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Guthrue SK, Teter CJ. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. Journal of Adolescent Health. 2004;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Teter CJ. Medical use, illicit use and diversion of prescription stimulant medication. Journal of Psychoactive Drugs. 2006;38:43–56. doi: 10.1080/02791072.2006.10399827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, Biederman J, Greenhill LL, Swanson JM, McGough JJ, Spencer TJ, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. Journal of Child and Adolescent Psychiatry. 2003;42:673–683. doi: 10.1097/01.CHI.0000046863.56865.FE. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: Prevalence, course, and treatment effects. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1028–1040. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24 month outcomes of treatment strategies for attention-deficit/hyperactivity disorder (ADHD) Pediatrics. 2004a;113:754–761. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: Changes in effectiveness and growth after the end of treatment. Pediatrics. 2004b;113:762–769. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- Novak SP, Kroutil LA, William RL, Van Brunt DL. The nonmedical use of prescription ADHD medication: Results from a national Internet panel. Substance Abuse Treatment, Prevention, and Policy. 2007;2:1–17. doi: 10.1186/1747-597X-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeyer S, Taylor E. Annotation: Principles of treatment for hyperkinetic disorder: Practice approaches for the UK. Journal of Child Psychology and Psychiatry. 1999;40:1147–1157. [PubMed] [Google Scholar]
- Park K, Verotta D, Gupta SK, Sheiner LB. Use of pharmacokinetic/pharmacodynamic model to design an optimal dose input profile. Journal of Pharmacokinetics and Biopharmacology. 1998;26:471–492. doi: 10.1023/a:1021068202606. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Aronoff HR, Midlam JK, Shapiro CJ, Gnagy EM, Chronis AM, Onyango AN, Forehand G, Nguyen A, Waxmonsky J. A comparison of Ritalin and Adderall: Efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;103:1–14. doi: 10.1542/peds.103.4.e43. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:1–15. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- Rothman DJ, Chimonas S. New developments in managing physician–industry relationships. Journal of the American Medical Association. 2008;300:1067–1069. doi: 10.1001/jama.300.9.1067. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Krager JM. A survey of medication treatment for hyperactive/inattentive students. Journal of the American Medical Association. 1988;260:2256–2258. [PubMed] [Google Scholar]
- Sahakian B, Morein-Zamir S. Professor’s little helper. Nature Publishing Group. 2007;450:1157–1159. doi: 10.1038/4501157a. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Taylor E, Swanson J, Wigal T, Chuang S, Davies M, et al. Refining the diagnosis of inattention and overactivity disorders: Reanalysis of the Multimodal Treatment Study of attention-deficit/hyperactivity disorder (ADHD) based on ICD-10 criteria for hyper kinetic disorder (HD) Clinical Neuroscience Research. 2005;5:307–314. [Google Scholar]
- Satterfield JH, Faller KJ, Crinella FM, Schell AM, Swanson JM, Homer LD. A 30-year prospective follow-up study of hyperactive boys with conduct problems: Adult criminality. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:601–609. doi: 10.1097/chi.0b013e318033ff59. [DOI] [PubMed] [Google Scholar]
- Schachar R, Ickowicz A, Crosbie J, Donnelly GAE, Reiz JL, Miceli PC, Harsanyi Z, Darke AC. Cognitive and behavioral effect of multi-layer-release methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18:11–24. doi: 10.1089/cap.2007.0039. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: A review and integration. Behavioral Brain Research. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Harding M, O’Donnell D, Faraone SV, Wilens TE. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996;35:1460–1469. doi: 10.1097/00004583-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Gupta S, Lam A, Shoulson I, Lerner M, Modi N, Lindemulder E, Wigal S. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: Proof-of-concept and proof-of-product studies. Archives of General Psychiatry. 2003;60:204–211. doi: 10.1001/archpsyc.60.2.204. [DOI] [PubMed] [Google Scholar]
- Swanson JM, McBurnett K, Wigal T, Pfiffner LJ, Lerner MA, Williams L, Christian D, Tamm L, Willcutt E, Crowley K, Clevenger W, Khouzam N, Woo C, Crinella FM, Fisher TD. Effect of stimulant medication on children with attention deficit disorder: A “Review of Reviews”. Exceptional Children. 1993;60:154–162. [Google Scholar]
- Swanson J, Wigal S, Greenhill L, Browne R, Waslik B, Lerner M, Williams L, Flynn D, Agler D, Crowley K, Fineberg E, Baren M, Cantwell D. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37:519–526. [PubMed] [Google Scholar]
- Swanson JM. Flawed attention-deficit/hyperactivity disorder medication comparison: In reply. Pediatrics. 2004;114:1132–1133. doi: 10.1542/peds.2004-1310. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2007a;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Hinshaw SP, Arnold LE, Gibbons R, Marcus S, Hur K, et al. Secondary evaluations of MTA 36-month outcomes: Propensity score and growth mixture model analyses. Journal of the American Academy of Child and Adolescent Psychiatry. 2007b;46:1002–1013. doi: 10.1097/CHI.0b013e3180686d63. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Agler D, Flynn D, Guinta D, Gupta S, Lerner M, Shoulson I, Wigal S, Williams L. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clinical Pharmacology Therapy. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw S, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA): Part I: Executive study. Journal of Attentional Disorders. 2008a;12:4–14. doi: 10.1177/1087054708319345. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw S, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA): Part II: Supporting details. Journal of Attentional Disorders. 2008b;12:15–43. doi: 10.1177/1087054708319525. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Greenhill LL, Lopez FA, Sedillo A, Earl CQ, Jiang JG, Biederman J. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: Results of a randomized double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. Journal of Clinical Psychiatry. 2006;67:137–147. doi: 10.4088/jcp.v67n0120. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, Kollins S, Nguyen AS, DeCory HH, Hirshey-Dirksen SJ, Hatch SJ COMACS Study Group. comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (The Comacs Study) Pediatrics. 2004;113:206–216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature. 2008;453:586. doi: 10.1038/453586a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. The use of drugs in hyperkinetic states: Clinical issues. Neuropharmacology. 1979;18:951–958. doi: 10.1016/0028-3908(79)90158-8. [DOI] [PubMed] [Google Scholar]
- Taylor E, Döpfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European clinical guidelines for hyperkinetic disorder – first upgrade. European Child and Adolescent Psychiatry. 2004;13(Suppl 1):1–30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- Taylor E, Schachar R, Thorley G, Wieselberg HM, Everitt B, Rutter M. Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behavior. Psychosomatic Medicine. 1977;17:121–143. doi: 10.1017/s0033291700013039. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: Prevalence, motives and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch SJ, Zhang Y, McLean A, Wolf KN. SLI381 (Adderall XR), a two-component, extended-release formulation of mixed amphetamine salts. Pharmacotherapy. 2002;22:1405–1415. doi: 10.1592/phco.22.16.1405.33687. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Archives of General Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Gatley JS, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. American Journal of Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Basic neuropsychopharmacology. In: Rutter M, editor. Rutter’s child and adolescent psychiatry. 5. Oxford: Blackwell; 2008a. [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. American Journal of Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. The action of enhancers can lead to addiction – Comment on Sahakian and Morein-Zamir, 2007, Nature, 450, 1157–1158. Nature. 2007b;451:520. doi: 10.1038/451520a. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM, Schulz K, Pradhan K. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. NeuroImage. 2007a;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Weiss G, Werry JS, Minde K, Douglas VL, Sykes D. Studies on the hyperactive child V: The effects of dextroamphetamine and chlor-promazine on behavior and intellectual functioning. Journal of Child Psychology and Psychiatry. 1968;9:145–156. doi: 10.1111/j.1469-7610.1968.tb02219.x. [DOI] [PubMed] [Google Scholar]
- Wender PH. Minimal brain dysfunction in children. New York: Wiley-Interscience; 1971. [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: A systemic review of the literature. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Faraone SV, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Greenhill LL, Pelham W, Swanson J, Wilens T, Palumbo D, Atkins M, McBurnett K, Bukstein O, August G. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:883–892. doi: 10.1542/peds.108.4.883. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Magder L, Soeken K, Boles M, Lynch F, Riddle MA. Psychotropic practice pattern for youth: A 10 year perspective. Archives of Pediatric Adolescent Medicine. 2003;157:17–25. doi: 10.1001/archpedi.157.1.17. [DOI] [PubMed] [Google Scholar]