Abstract
The expression patterns of (bone morphogenetic proteins) BMPs during fracture repair and pre-natal bone development suggests that these processes are regulated through the coordinated actions of multiple BMPs. Murine bone marrow stromal cells (MSCs) in culture provide a well recognized ex vivo system of mesenchymal stem cell differentiation in which the effects of BMPs can be examined. Studies were performed to determine if MSC differentiation is dependent on the endogenous expression of multiple BMPs and to characterize their interactions. MSCs were harvested from the bone marrow of tibiae and femora of 8 to 10 week old male C57/B6 mice and prepared by standard methods. Osteogenic differentiation was assessed by histological assays, alkaline phosphatase enzyme activity and assays for the expression of multiple mRNAs for BMPs and osteogenic development. The role of autogenously expressed BMPs in controlling the osteogenic differentiation of marrow stromal cells in vitro was assessed in both gain-of-function and loss-of-function experiments. Gain of function experiments were carried out in the presence of exogenously added BMP-2 or 7 and loss of function experiments were carried out by BMP antagonism with noggin and BMP-2 antibody blockade. Osteogenic differentiation was concurrent with and proportional to increases in the expression of BMPs 2, 3, 4, 5, 6, and 8A. BMP antagonism with either noggin or BMP-2 antibody blockade inhibited osteogenic differentiation by 50% to 80% respectively and reduced the expression of endogenous levels of BMPs 2, 3, 5, and 8A. In contrast, antagonism induced the expression of BMP-4 and 6. The addition of rhBMP-2 or 7 enhanced osteogenic differentiation and produced a reciprocal expression profile in the endogenous BMPs expression as compared to BMP antagonism. BMP antagonism could be rescued through the competitive addition of rhBMP-2. These studies demonstrated that osteogenic differentiation was regulated by a complex network of multiple BMPs that showed selective increased and decreased expression during differentiation. They further demonstrated that BMP-2 was a central regulator in this network.
Keywords: Marrow Stromal Stem Cells, Bone Morphogenetic Proteins, BMP, Osteoinduction, Noggin
INTRODUCTION
The principle of osteoinduction was discovered when subcutaneously implanted demineralized bone matrix was shown to induce de novo formation of bone and cartilage in vivo [1]. Although originally thought to be the result of implantation of a single protein named bone morphogenetic protein, this seminal observation eventually led to the purification and characterization of a family of bone morphogenetic proteins (BMPs) present within the extracellular matrix of bone [2–4]. Studies of fracture repair [5] and pre-natal bone development [6, 7] have demonstrated the expression of multiple BMP genes which show both temporal and spatial variability. These data suggest that repair and developmental processes are regulated through the coordinated actions of multiple BMPs instead of a single master BMP. Early experiments using recombinant human BMP-2, BMP-4 or BMP-7, demonstrated that these proteins have the ability to individually stimulate osteoblastic or chondrogenic phenotypic expression in various mesenchymal precursor cell lines [4, 8–10]. However, unlike the in vitro investigations, in vivo studies suggest that these factors function in a coordinated manner [7, 11].
The post-natal bone marrow compartment is viewed as an organ composed of two separate cell populations; the hematopoietic cell lineages and the associated supporting mesenchymal stromal cells. The stromal cell population contains a reservoir of stem cells with the potential to differentiate into the various terminal mesenchymal cell lineages. Tissue cultures of bone marrow aspirates provide an ideal ex vivo model system in which to investigate the functional role that BMPs may have, in directing mesenchymal stem cell commitment and differentiation into mature bone-forming osteoblasts. Numerous studies have now demonstrated that the stromal cells have significant proliferative capacity and cells within this population contribute to the endothelial reticulum and possess the ability to differentiate into multiple cell types including osteoblasts, chondrocytes, and adipocytes. Previous studies have demonstrated that exogenous addition of multiple individual BMPs can stimulate osteogenic differentiation in mesenchymal cells [4, 8, 9]. However, these effects have not been fully characterized at a molecular level. Furthermore, the presence and importance of endogenous BMPs produced by a population of MSCs during their natural differentiation in culture has not been fully investigated.
To elucidate the interactions of BMPs during MSC differentiation we performed both gain and loss-of-function experiments and demonstrated a network of BMPs in which BMP-2 plays a central role in its regulation.
MATERIALS AND METHODS
Primary Bone Marrow Cell Cultures
Research was conducted in conformity with all federal and USDA guidelines under an IACUC approved protocol. C57BL/6J (B6) male mice of 8–10 weeks of age (Jackson Laboratories, Bar Harbor, ME) were used for these studies. Cell preparations were made using the basic protocol developed by Kuznetsov et al for human cells [12] and subsequently modified for mice by Kalajzic et al [13]. Briefly after euthanasia hind limbs were aseptically removed and bones dissected free of soft tissues, marrow cavities were flushed, and the cell suspension filtered through a 70 μm nylon strainer. Cells were plated at 12–15 × 106 cells / 33mm diameter well and left undisturbed for four days. On day four half of the media was replaced with fresh αMEM. At day six all cultures were treated with osteoinductive media consisting of αMEM supplemented with 10% FBS, dexamethasone 10−8 M, L-Ascorbic acid 70ng/ml, and β-glycerol phosphate, disodium salt 8 mmol [14–15]. Media was changed every two days. Recombinant BMP-2, noggin, monoclonal anti-human BMP-2 antibody, and non specific mouse IgG1 isotype were all purchased from R&D Systems (Minneapolis, MN). Noggin, blocking antibodies, or BMP-2 were added with each media change at 300ng/ml, 2.5μg/ml, or 100ng/ml respectively. BMP-7 was provided through a materials transfer agreement with Stryker Biotech (Hopkinton, MA). BMP-7 was added at a concentration of 100ng/ml following the same protocol as was used for BMP-2.
Nodule Counting and Area Assessments
At defined time points MSCs were washed three times with warmed PBS and fixed with 2% paraformaldehyde in 0.2 mol/L cacodylic buffer for 10min at room temperature. Fixed cells were washed several times with deionized water and stained for one hour with 1ml of filtered von Kossa solution (5% silver nitrate in ddH2O). Digital photographs of stained culture wells were taken and nodule numbers and stained areas were quantitatively measured using a computerized imaging system (Image-Pro Plus 4.1; Media-Cybernetics; Silver Spring, MD). All data are expressed as the mean ± SEM, calculated from a minimum of 3 wells at each condition. Each experiment was reconfirmed at least four times using cells from different marrow stromal preparations.
Molecular Analysis
RNA was isolated from cell cultures at the specified times by direct Trizol extraction and purified total RNA was quantified using its OD260 nm. The integrity of the RNA was visualized by a denaturing RNA gel electrophoresis. Ribonuclease Protection Assay (RPA) was carried out to quantitatively determine the steady state mRNA expression of various BMPs and extracellular matrix proteins [5, 16]. Numerous autoradiographic exposures were made and the hybridization of individual probes was determined by direct counting on flat bed β-counter to determine cpm/band for each nuclease protected product. All gene expression values were normalized relative to the expression of L32.
RESULTS
The first series of studies were carried out in order to identify the temporal relationship between the mRNA expressions of the various BMP isotypes and the progression of MSC osteogenic differentiation as assessed by the expression of specific ECM proteins (Figures 1 and 2). By 14 days in culture, or 9 days after placement into osteoinductive media, areas of mineralized nodules were detectable (Figure 1). Both the number of nodules per culture well and the total area of the culture surface that was covered by nodules continued to linearly increase throughout the time course of the experiments which ended at 21 days after harvest. The increase in both numbers and size of the nodules therefore suggests that there is both continual recruitment of the marrow stromal stem cells to differentiate into osteogenic cells and an expansion of those cells that have committed to the osteogenic lineage within each nodule. Enzymatic activity of alkaline phosphatase (APase), a well-defined marker of osteoblastic activity, was detectable at 10 days after harvest and also demonstrated a fairly linear rise in activity throughout the time course (Figure 1).
Figure 1.

Temporal progression of bone marrow stromal stem cell selection and osteogenic differentiation over a 21 day period in growth culture. (A) Representative von Kossa staining of mineralized nodules seen in 6 well culture dishes 14, 16, and 21 days after plating. (B) Graphical representation of the average number of mineralized nodules per 0.33cm well at each time point. (C) Graphical representation of the percent area von Kossa stained mineral per 0.33 cm well. (D) Graphical representation of alkaline phosphatase enzymatic activity during MSC osteogenic differentiation. Values are presented as total OD410 per 33mm well per 1 minute. Days after plating in culture are denoted in figure. Average values were derived from four independent cell preparations. For all figures, error bars represent the standard deviation (S.D.) of the inter-experimental mean of a minimum of four separate replicates.
Figure 2.
Relationship of osteogenic differentiation to endogenous BMP expression in bone marrow stromal cell cultures. Quantitative analysis of steady state mRNA expression for specific extracellular matrix proteins (ECM), transcription factors (TF), and bone morphogenetic proteins (BMP) during osteogenic differentiation in vitro. (A) Representative autoradiographic images of the RPA products as resolved on a 6% PAGE sequencing gel. Days after culture and the labeled positions of the various protected mRNA products are denoted in the figures. The autoradiograph in the farthest right panel shows a comparison of protected mRNA species seen in 21 day marrow stromal cell cultures (MSC), those found in the 21 day post fracture callus tissues (F) and the labeled undigest probe that is used as a marker (M). Five to ten micrograms of total RNA were assayed per lane. (B) Graphic representation of the mean relative steady state mRNA expression levels through the time course of in vitro osteogenic differentiation. Several different autoradiographic exposures were utilized to determine band densities which were subsequently normalized to the L32 gene. The mean relative steady state mRNA expression levels were determined from RPAs from at least two individual experimental analyses. C) Representative autoradiographic images of the RPA products as resolved on a 6% PAGE sequencing gel. The nature of the set of protected products and the positions of the various protected mRNA products are denoted in the figures. (FM= FBS and αMEM, C= complete media as defined in the materials and methods). Five to ten micrograms of total RNA were assayed per lane.
Characterization of the osteogenic differentiation by ribonuclease protection assays demonstrated the sequential expression of mRNAs for multiple extracellular matrix proteins temporally matched the progression of differentiation of the cultures as assessed by the development of the nodules and the APase enzyme activity (Figure 2). As shown by numerous other studies [14, 17, 18] the temporal pattern of osteogenic differentiation was defined by the sequential expression of osteopontin (OPN), bone sialoprotein (BSP) and osteocalcin (OC). This later mRNA being the most specific gene product of osteogenic cells, and was expressed when these cells had completed their differentiation. Collagen type I was expressed at a relatively high levels throughout the time course. During this same period there were associated changes in the pattern of transcription factors that are expressed by osteogenic cells (increased Runx2 and Dlx5 with decreasing levels of Msx2), which were also consistent with commitment of mesenchymal cells to the osteogenic lineage (Figure 2 Left middle Panel) [19].
It was of particular interest that multiple members of the BMP family were expressed by the marrow stromal cell culture system (Figure 2, Right middle Panel). Within this culture model only a subset of BMPs that were assayed were expressed with BMPs 2, 3, 3b, 4, 5 and 8a being the most prevalent while BMPs 6 and 8b were expressed at very low to almost undetectable levels. It was surprising to note that BMP-7 was undetectable in these cultures (MSCs) even though it is expressed at comparable levels to BMP 8a during late stages of fracture healing (F) in vivo (Panel A Figure 2 far right lanes) [5]. The expressed BMPs demonstrated a reproducible temporal and quantitatively distinct profile, with BMP4 expressed early and showing diminished expression at the later time points. In contrast, BMPs 2, 3 and 8a demonstrated increasing levels of expression with their highest levels of induction being observed during the period when the osteogenic mRNA expression was peaking. It is also interesting to note that tolloid enzyme (BMP-1), which proteolytically processes the BMPs was expressed at very high levels throughout the entire time course of MSC growth in vitro.
In order to verify that the increased expression of endogenous BMPs was a function of the differentiation of the cultures and not a function of the outgrowth of either a selective population of cells or time of growth in the cultures, the expression of differentiated function was compared to cultures grown in either osteogenic media or just αMEM supplemented with 10% FBS. In these studies the expression of osteoblast associated extracellular matrix protein and the endogenous BMP mRNAs were examined at the end of 21 days of growth in culture (Figure 2C). In the absence of growth in the osteoinductive media, only small increases in both the expression of differentiated function and the expression of BMPs were observed. No mineralized nodules were observed in the non osteoinductive media (data not shown).
The selective changes in the expression of the various BMPs over the time course of differentiation led us to hypothesize that the endogenous expression of the various BMPs might drive osteogenic differentiation through the autoregulation of their own expression. Given the complexity and number of BMPs that were expressed, we conducted gain and loss-of-function experiments to determine if changes in the endogenous BMP expression profiles would coordinately change with inhibition or enhancement of osteogenic differentiation of cultured MSCs. In the first series of experiments, recombinant BMPs-2 or BMP-7 (OP-1) were exogenously added to the MSC cultures each at a concentration of 100ng/ml (Figure 3). The exogenous supplementation of the cultures with either BMP resulted in increased numbers of osteogenic nodules and an enhancement of alkaline phosphatase activity (Panels A & B). A comparison of the expression of the specific extracellular matrix protein mRNAs between days 14 to 21 in both BMP-treated cultures, as compared to control cultures, showed quantitatively greater induction of extracellular matrix mRNAs by approximately 200% to 400% of control steady state levels. It was apparent that the induction of the various mRNAs was also temporally shifted by at least two days in the treated cultures (Figure 3C & D).
Figure 3.
Gain of function of marrow stromal stem cell selection and osteogenic differentiation over a 21 day growth culture period in response to exogenously added BMPs 2 or 7. (A) Graphical representation of the average number of nodules per 0.33cm well in control and rhBMP-2 and rhBMP-7 treated cultures. (B) Graphical representation of alkaline phosphatase activity observed at 10, 14, 16, and 21 days compared to cultures supplemented with rhBMP-2 and rhBMP-7. Alkaline phosphatase values are presented as total OD410 per 33mm well per 1minute. All data points represent inter-experimental means derived from four independent cell preparations. Single experimental group is an average derived from a minimum of triplicate wells. Error bars represent the standard deviation (S.D.) of the inter-experimental mean. (C) Quantitative analysis of steady state mRNA expression was made by ribonuclease protection assay for specific extracellular matrix proteins (ECM). Representative autoradiographic images of the RPA products as resolved on a 6% PAGE sequencing gel are depicted. Days after culture and the labeled positions of the various protected mRNA products are denoted in the figures. (D) Graphic representation of the percentage difference of steady state mRNA expression levels from that of control averaged across the time course of in vitro osteogenic differentiation. Several different autoradiographic exposures were utilized to determine band densities which were subsequently normalized to the L32 gene. The mean relative steady state mRNA expression levels were determined from RPAs from at least two individual experimental analyses.
The exogenous addition of either BMP-2 or BMP-7 also lead to very specific differences in the expression of the endogenously produced BMPs, with earlier and more sustained level of BMP 2, 3 and 8a expression while BMPs 3b, 4 and 6 showed diminished expression over the time course of differentiation relative to control cultures (Figure 4A & B). A comparison between BMP-2 and BMP-7(OP-1) demonstrated that the exogenous addition of either BMP had the same effect on the endogenously expressed BMP profiles (Figure 4B).
Figure 4.
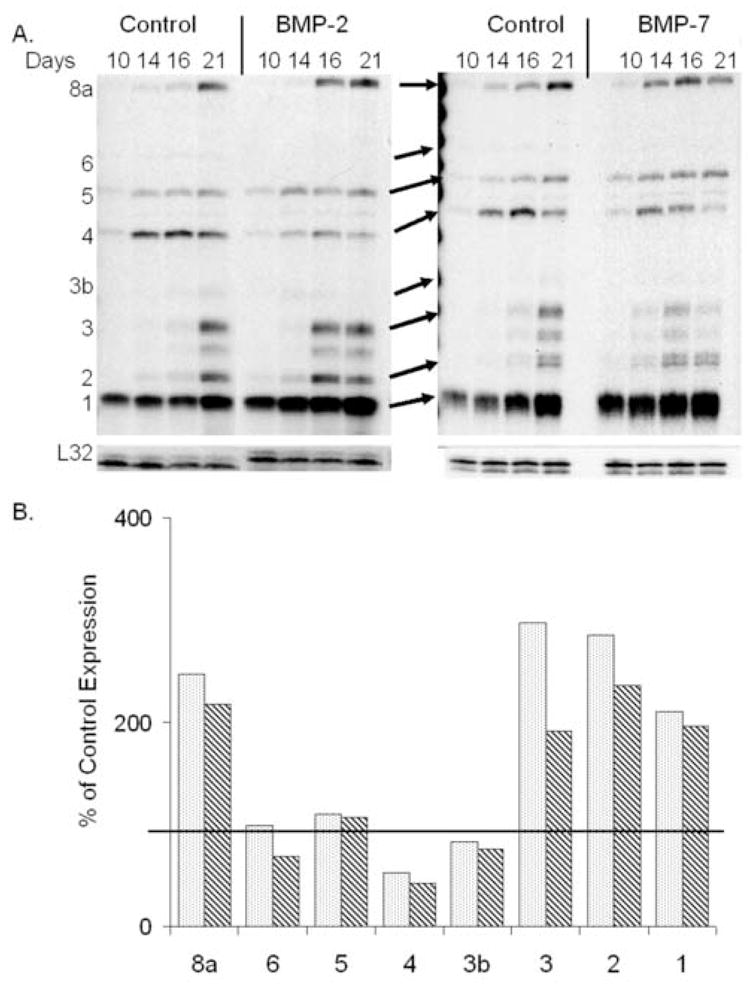
Response of autogenous BMP expression in bone marrow stromal cell cultures to exogenously added BMPs 2 or 7. (A) Quantitative analysis of steady state mRNA expression was made by ribonuclease protection assay for specific BMPs. Representative autoradiographic images of the RPA products as resolved on a 6% PAGE sequencing gel are depicted. Days after culture and the labeled positions of the various protected mRNA products are denoted in the figures. Ten micrograms of total RNA were assayed per lane. (B) Graphic representation of the percentage difference of steady state mRNA expression levels from that of control averaged across the time course of in vitro osteogenic differentiation. The control 100% level is denoted in the figure by heavy line in the graph and the bar shading is consistent as denoted in figure 3. Several different autoradiographic exposures were utilized to determine band densities which were subsequently normalized to the L32 gene. The mean relative steady state mRNA expression levels were determined from RPAs from at least two individual experimental analyses.
In order to determine if the autogenous expression of BMPs by marrow stromal cells was necessary to drive osteogenic differentiation, two separate loss-of-function experiments were carried out. In the first of these experiments, noggin, a naturally produced non-competitive antagonist of BMPs 2, 4 and 7, was supplemented to cultures at 300ng/ml [20]. These cultures showed an inhibition in the average number of osteogenic nodules formed per well and the total area per well of stainable mineral, suggesting an inhibition in both the initial selection of marrow stromal stem cells into the osteogenic lineage as well as their subsequent expansion. The assessment of osteogenic differentiation in response to antagonism by noggin was further validated by measures of both APase and extracellular matrix protein mRNA expression (Figure 5C–E). Culture supplementation with rhNoggin protein clearly blocked the differentiation of the marrow stromal cells in culture.
Figure 5.
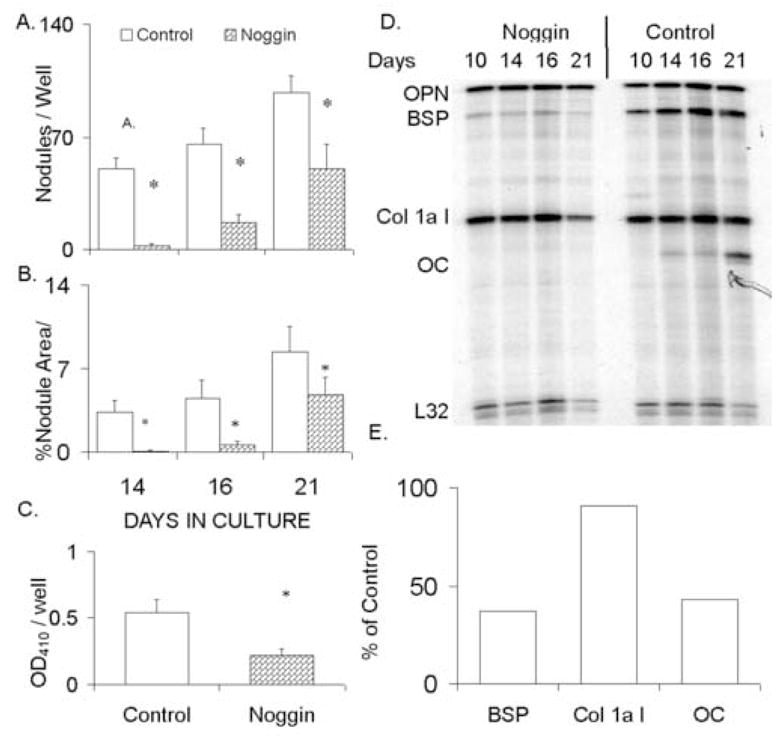
Inhibition of marrow stromal cell osteogenic differentiation in response to BMP antagonism with rhNoggin. (A&B) Graphical analysis of the average number of von Kossa stained nodules and percent area stained mineral per 0.33cm well. (C) Alkaline phosphatase activity observed in control cultures compared to rhNoggin supplementation. Alkaline phosphatase activity was compared at 16 days in culture. All data points represent inter-experimental means derived from four independent cell preparations with error bars representing the standard deviation (S.D.) of the inter-experimental mean. Statistical significance at (<0.05) between the inter-experimental means as determined by 2-tailed t-test assuming equal variance is denoted (*). (D) Representative RPA analysis of steady state mRNA expression for specific extracellular matrix proteins expressed in the marrow stromal cultures in control and rhNoggin treated cultures. Graphical analysis of the relative steady state mRNA levels at sixteen days in of the rhNoggin treated cultures relative to the control cultures is shown. Data points were normalized to the detected expression of the L32 gene.
While noggin is known to show varying degrees of antagonism against specific BMPs such that it has the highest affinity against BMP-2, BMP-4, and lesser activity towards BMP-7 [20] the inhibition of individual BMPs in this culture system provide information regarding the molecular hierarchy of BMPs as they drive osteogenic differentiation. Since BMP-7 expression was not detectable in this marrow system and BMP-4 expression demonstrated diminishing levels of expression as osteogenic differentiation proceeded and appear to be repressed in response to exogenous BMP treatment, we speculated that the autogenous expression of BMP-2 was perhaps the primary morphogen driving the differentiation of these cells. In order to test this hypothesis, BMP-2 activity was inhibited by neutralizing antibody blockade. The results of these experiments are shown in Figure 6. Several control experiments were first carried out to ensure that the BMP-2 neutralization activity of this antibody was specific. In these control experiments the number of nodules was assayed. Cultures were allowed to differentiate under standard culture conditions, in the presence of a pre-immune isotype specific control serum or 2.5μg/ml of anti-BMP-2 antibody. A competition or add-back experiment was also performed in which rhBMP-2 was added back to cultures treated with anti-BMP-2 antibody such that the exogenously added BMP-2 would be in excess to the binding capacity of the antibody. In this way we could determine if the inhibitory effect of the neutralizing antibody would be rescued. As demonstrated in Figure 6A, the competition assay and isotype pre-immune serum were equivalent to control cultures and produced significantly more nodules at 16 days compared to neutralizing antibody supplemented cultures. Figure 6B and C show that the blockade of BMP-2 activity by the neutralizing antibody lead to an immediate 50–75% diminishment in both the number of nodules per well and the APase activity in the cultures compared to controls. Furthermore, extracellular matrix protein mRNA expression demonstrated a lack of induction in cultures treated with neutralizing antibody to a level that was only ~25% of control culture mRNAs. (Figure 6D & E).
Figure 6.
Inhibition of bone marrow stromal cell osteogenic differentiation in response to BMP-2 antagonism with BMP-2 selective blocking antibody. (A) Graphical comparisons of the average number of nodules per 0.33cm well at 16 days within MSCs differentiated in the presence of 2.5ug/ml anti-BMP-2 monoclonal antibody (Anti BMP-2), 300ng/ml BMP-2 and 2.5ug/ml anti-BMP-2 monoclonal antibody Competition Assay (Comp Assay), 2.5ug/ml IgG1 isotype control antibody (IsoType), un-supplemented cultures (control). (B&C) Graphical comparison of average nodules per 0.33cm well and alkaline phosphatase activity in marrow stromal cultured cells with or without 2.5ug/ml anti-BMP-2 neutralizing antibody throughout the time course of their differentiation. Enzymatic values are presented as total OD410 per 33mm well per minute. Total days after plating are denoted in the figure. A single experimental result consists of the average value derived from those measures in a minimum of triplicate wells. All data points represent inter-experimental means derived from four independent cell preparations with error bars representing the standard deviation (S.D.) of the inter-experimental mean. Statistical significance at (<0.05) between the inter-experimental means as determined by 2-tailed t-test assuming equal variance is denoted (*). (D) Representative RPA analysis of steady state mRNA expression for specific extracellular matrix proteins expressed in bone marrow stromal cultures with or without anti-BMP-2 neutralizing antibody supplementation. Graphical analysis of the relative steady state mRNA levels at sixteen days in of the rhNoggin treated cultures relative to the control cultures is shown. Data points were normalized to the detected expression of the L32 gene.
Gross microscopic examination of cellular densities in cultures treated with either BMP-2 neutralizing antibody or stimulated with rhBMP-2 protein showed no differences compared to controls. The number of cells in the cultures was quantitatively assayed by measuring mitochondrial dehydrogenase activity (MTT). These analysis showed that at 16 days in culture there were no significant differences in viable cells per well between control cultures and those treated with either human BMP-2 (100ng/ml) or following BMP removal with anti-BMP-2 antibody (2.5ug/ml) (Data not shown). These results suggest that the main action of BMP-2 is to selectively stimulate mesenchymal stem cell differentiation and not alter cell replication or survival. Thus, the inhibition of BMP-2 activity does not appear to be neither overtly cytotoxic nor does BMP-2 addition appear to be overtly mitogenic to cells within the cultures.
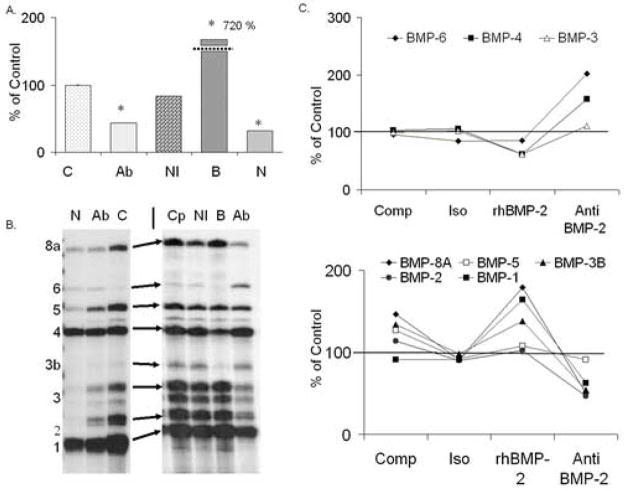
The final experiment that was performed, examined the effects on the endogenously expressed BMPs following MSC treatment with anti-BMP-2 neutralizing antibody or rhNoggin, as compared to exogenous addition of rhBMP-2. In these studies APase levels were followed to verify the inhibitory effects of the various treatments which were compared concurrently. Supplementation of marrow stromal cells with either rhNoggin protein or neutralizing antibody to BMP-2 again showed a significant repression of osteogenic differentiation as compared to controls while the addition of BMP-2 significantly enhanced APase levels (Figure 7A). The effect of BMP inhibition on the endogenous expression of BMPs by bone marrow stromal cells was next determined under each of the different experimental conditions. Following removal of BMP-2 activity from the system using either noggin or antibody blockade to BMP-2, the expression of selective BMPs over the time course of MSC growth in culture was inhibited (Figure 7B & C). Interestingly, while almost all of the endogenously produced BMPs showed decreased expression in response to BMP antagonism, expression of BMPs 4 and 6 was increased. BMP 6 expression was 238% and 191% of the control respectively in the noggin or antiBMP-2 treated cultures while a lesser effect on BMP-4 was seen with 140% and 127 % levels of the control cultures (Figure 7B). With the exception of BMP-1, which showed mRNA levels that were approximately the same as the controls, BMPs 2, 3, and 5 were all expressed at approximately 60% of control levels, and BMP-3b (GDF-10) and 8a were detected at 50–25% of control in both noggin and anti-BMP-2 treated cultures respectively (Table 1). MSC cultures treated with either IgG isotype control or receiving BMP-2 added back in the presence of anti-BMP-2 demonstrated an endogenous BMP expression profile similar to controls (Figure 7B&C). For comparison purposes the effects of BMP-2 addition are also presented in this figure. In the context of this comparison, it is interesting to note that those BMPs that showed inhibited expression in the presence of either noggin or the inhibitory antibody showed an inverse level of expression when stimulated with BMP-2 (Figure 7C). A summary of the results of the three replicate experiments (N=12 cell preparations) is presented in Table 1. These results show that there was a consistent and reciprocal effect on endogenous BMP expression when BMPs were either exogenously added or the endogenous levels of expression were blocked.
Figure 7.
Response of endogenous BMP expression profiles following rhNoggin and selective BMP-2 antagonism by anti-BMP-2 neutralizing antibody treatment. (A) Graphical comparison of alkaline phosphatase activity at 16 days in MSCs cultured in the presence anti-BMP-2 neutralizing antibody (Anti-B2), IgG1 isotype control antibody (IsoType), rhNoggin (Noggin), rhBMP-2 (BMP-2), or media alone (control). One experiment represents the average OD from a minimum of 6 wells. All data points represent inter-experimental means derived from three independent cell preparations. All data points represent inter-experimental means derived from four independent cell preparations with error bars representing the standard deviation (S.D.) of the inter-experimental mean. Statistical significance at (<0.05) between the inter-experimental means as determined by 2-tailed t-test assuming equal variance is denoted (*). (B) Representative RPA analysis of steady state mRNA expression for specific BMPs in MSC cultures collected at 16 days after plating. RPA gels are labeled as follows; (N) rhNoggin, (Ab) anti-BMP-2 neutralizing antibody, (Cp) Competition assay, (NI) Non immune Isotype IgG control, (B) 100ng/ml rhBMP-2. (C) Graphical representation of percentage inhibition or elevation in response to various culture perturbations. Top panel depicts that group that showed elevated expression is response to BMP-2 blockade while the bottom panel shows that group that shows elevation in response to exogenous BMP-2 addition. The reciprocal pattern in the two panels to various treatments may be noted by visual comparison of the top and bottom panels.
Table 1.
Effect of Gain and Loss of BMP Function on Endogenous BMP Expression+
| BMP-8A | BMP-6 | BMP-5 | BMP-4 | GDF-10 | BMP-3 | BMP-2 | BMP-1 | |
|---|---|---|---|---|---|---|---|---|
| Control | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Anti-BMP-2 | 53 | 182 | 88 | 149 | 102 | 54 | 46 | 65 |
| Noggin | 37 | 238 | 24 | 140 | ND | 24 | 11 | 103 |
| rhBMP-2 | 170 | 80 | 105 | 61 | 58 | 132 | 160 | 166 |
Composite set of measurements made from multiple experimental determinations. Values are expressed as the percent of the control.
DISCUSSION
Numerous experiments in a variety of in vitro models have demonstrated that multiple BMPs, used individually or in various combinations can induce the differentiation of a wide variety of tissues including bone, cartilage, adipose tissue, and muscle [4, 8, 21, 22]. BMP-2 and BMP-7 have further been demonstrated to enhance bone healing in vivo in animals and humans in settings such as spinal fusion, fracture repair and distraction osteogenesis [23–25]. Although there appears to be some benefit from the use of BMPs clinically there continues to be uncertainty about the BMP potential since animal and in vitro models have demonstrated much more potent responses than results observed in cultured human cells or in clinical studies. Such data lead to questions regarding the specificity of the individual BMPs, the functional role and interactions of the various BMP receptors, and/or how each of the BMPs individually can promote their specific actions in different tissues.
Previous studies from our laboratory have shown that multiple BMPs, as well as other members of the larger TGF-β gene family, are expressed at varying temporal and quantitative levels across skeletal healing [5]. In the current study, we observed that multiple BMPs were expressed during osteogenic differentiation in bone marrow stromal cell cultures. Interestingly, within this culture model system, there was also both temporal and quantitative variation in the steady state mRNA levels of multiple BMPs. These data suggest that that multiple, BMPs are important to sustain osteogenic recruitment and/or to maintain and expand lineage progression. The current study further supports the hypothesis that when individual BMPs, such as BMP-2 or BMP-7 are added exogenously that they have the capacity to augment differentiation through their actions on the expression of other endogenously produced BMPs.
In order to determine the influence of the actions of the endogenously produced BMPs on the osteogenic differentiation within bone marrow stromal cell cultures, specific studies were directed at assessing how the network of endogenously expressed BMPs would respond to both exogenous BMP addition or antagonism by either noggin or the more selective blockade of BMP-2 activity. Noggin is naturally expressed during developmental processes and is known to act as non-competitive inhibitor of BMPs 2, 4 and 7 with a low (Kd 1.0×10−10) disassociation constant [20]. Interestingly we were unable to detect BMP-7 in this system, despite being present in control fracture callus tissue specimens at levels equivalent to BMP-2 and 3 in other mesenchymal cell populations [5, 9]. Since BMP-7 expression is not detectable, its role in the differentiation of these cultures is considered negligible. It is presumed therefore that the actions of adding rhNoggin to these cultures are most likely directed at BMP-2 and 4 antagonisms within the marrow stromal system, assuming these are the only other BMPs that Noggin antagonizes. Our results for the actions of Noggin are also similar to two previous reports. In one study, marrow stromal cells prepared from mice that had been engineered to overexpress noggin under the control of the osteocalcin promoter failed to undergo differentiation in culture [26]. In the other study showed that when noggin was added exogenously to normal marrow stromal cultures that both osteoblast and osteoclast differentiation was blocked [27].
While these previous studies and our own studies using Noggin can not make the distinction between BMP-2 and BMP-4, our studies using the BMP-2 the neutralizing antibody produced a similar and more potent repression of biochemical and genetic markers of osteoblastic differentiation in MSCs cultures as compared to Noggin. Since the commercially obtained BMP-2 antibody that was used in these studies had been determined to be100 fold more specific for BMP-2 versus BMP-4, and titrating the levels of antibody over several logs from those that are reported in our studies did not lead to differing qualitative effects than those that we have presented (data not shown), we have concluded that the primary effects with the blocking antibody were due to its selective actions on the activity of BMP-2. The current studies also do not support a positive regulatory role for BMP-4 in osteogenous since: a) as MSC differentiation progresses there is less BMP-4 expressed; b) both Noggin and BMP-2 antagonism lead to increased BMP-4 expression; c) both BMP-2 or BMP-7 induction of osteogenic differentiation leads to down regulation of endogenous BMP-4 expression. Finally, previous data from our laboratory had also shown that C3H10T½ cells in their undifferentiated state expressed high endogenous levels of BMP-4 whereas in a similar fashion as seen in this study, the exogenous addition of BMP-2 and BMP-7 downregualted its expression during skeletal cell differentiation [9].
While both noggin and the blocking antibody produced a potent inhibition and disruption of the endogenous BMP signaling, Noggin was more potent in its selective inhibitory effects on the endogenous expression of specific BMPs (Table 1). In contrast, BMP-2 antibody blockade was more effective in its inhibition of osteogenesis. (Compare data in figures 5 and 6). These differences in inhibitory effects on osteogenic phenotype versus endogenous BMP expression however may reflect how either the multiple BMPs interacts to drive differentatiation or how Noggin selectively interacts with different BMPs. Clarity to the differences in the two observations will only be provided when the exact nature of the relationships between the interactions of the various endogenously expressed BMPs as to their antagonism or synergism in promoting osteogenous is further defined.
The ability to block differentiation by selective BMP-2 blockade supports the hypothesis that BMP-2 is an early acting morphogenetic signal for osteogenic differentiation. Specifically, these data suggests that BMP-2 regulates its own expression in conjunction with several other BMPs including 3, 3a (GDF-10), 5, and 8a, while repressing BMPs 4 and 6. It is interesting to note that BMP-2 has been associated with the differentiation processes of a number of different tissues, and it may be speculated that BMP-2 is of central importance to the survival or differentiation of multiple stem cell lineages since in vivo studies in transgenic mice in which BMP-2 has been globally ablated show very early embryonic death [27]. Such results indicate the very early developmental role that this BMP plays relative to many other BMPs that do not produce early lethality when they are ablated. Other data that support the central role of BMP-2 in promoting mesenchymal stem cell differentiation comes from the studies of Phimpphilai et al [28] that have shown that Runx2 transfected C3H10T ½ mesenchymal stem cells where unable to undergo osteogenic differentiation when their endogenous BMP expression was inhibited with noggin. Such results place BMP-2 activity either temporally concurrent or preceding the activation of RUNX-2. Finally in a recent study BMP2 was shown to be a necessary component of the signaling cascade that governs fracture repair and mice lacking the ability to produce BMP2 in their limb bones had spontaneous fractures that did not resolve with time. More germane to the observations and conclusions that we have drawn from our studies was the demonstration that bones lacking BMP2 showed that the earliest steps of fracture healing seem to be blocked. These results lead these authors to the central conclusion that although other osteogenic stimuli were still present in the limb skeleton of these BMP2-deficient mice, that they cannot compensate for the absence of BMP2 [30].
The last point to comment on is the equal response of the endogenous BMP expression following BMP-2 or BMP-7 treatment, because BMP-2 is expressed by the MSC system while BMP-7 is undetectable. These results suggest that the MSC receptor affinity for BMP-7 was present and that it may substitute in function for one of the BMPs that are endogenously produced by the cells. It is interesting to note that BMPs 2,4,6 and 7 are all able to induce osteogenic differentiation when added exogenously to human bone marrow mesenchymal stem cells [31–33]. Such results suggest that subgroups of BMPs may share selective activities through common interactions with BMP receptors. In this regard the concept that the BMPs might work in networks to produce specific biological results is consistent with recent findings the BMP-3 interferes with activin and BMP-4 binding to type II activin receptors but does not activate R-Smads [33]. In this way it modulates activin receptor II activity and modifies the activities or activating BMPs. Thus, the mix of exogenously expressed BMPs and differing interactive BMP antagonists may produce a wide spectrum of biological activities.
In summary, the results reported here support the hypothesis that BMPs mediate the differentiation of MSCs through an autogenously-driven signaling network of other BMPs. This network demonstrates variability in response to BMP stimulation or inhibition such that the expression of the endogenous BMPs exhibit both selective repression and stimulation. The data support a second hypothesis that BMP-2 is an early mediator of stem cell differentiation and it has the ability to control the regulation of other endogenous BMPs, including its own expression.
Acknowledgments
This work has been partially supported with a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P01 AR049920). Institutional support was provided by the Department of Orthopaedic Surgery, Boston University School of Medicine. Portions of this paper where presented by CME in partial fulfillment of the requirements for his PhD thesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Rosen V, Thies RS. The BMP proteins in bone formation and repair. Trends Genet. 1992;8:97–102. doi: 10.1016/0168-9525(92)90197-c. [DOI] [PubMed] [Google Scholar]
- 3.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 4.Valcourt U, Ronziere MC, Winkler P, Rosen V, Herbage D, Mallein-Gerin F. Different effects of bone morphogenetic proteins 2,4,12, and 13 on the expression of cartilage and bone markers in the MC615 chondrocyte cell line. Exp Cell Res. 1999;251:264–74. doi: 10.1006/excr.1999.4584. [DOI] [PubMed] [Google Scholar]
- 5.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor-b superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–20. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 6.Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- 7.Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996;57:145–57. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- 8.Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 9.Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90:1112–27. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- 10.Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Bio. 1994;161:218–228. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 11.Macias D, Ganan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development. 1997;124:1109–17. doi: 10.1242/dev.124.6.1109. [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsov SA, Friendenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–70. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalajzic I, Kalajzic Z, Hurley MM, Lichtler AC, Rowe DW. Stage specific inhibition of osteoblast lineage differentiation by FGF2 and noggin. J Cell Biochem. 2003;88:1168–76. doi: 10.1002/jcb.10459. [DOI] [PubMed] [Google Scholar]
- 14.Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987;122:49–60. doi: 10.1016/0012-1606(87)90331-9. [DOI] [PubMed] [Google Scholar]
- 15.Aubin J. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999;72:396–410. [PubMed] [Google Scholar]
- 16.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 17.Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143:213–21. doi: 10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- 18.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;1:81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Karsenty G. Transcription factors in bone: developmental and pathological. Trends Mol Med. 2002;8:340–5. doi: 10.1016/s1471-4914(02)02340-7. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 21.Miyazono K. Bone morphogenetic protein receptors and actions (Chapter 51) In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 22.Angley C, Kumar M, Dinsio KJ, Hall AK, Siegel RE. Signaling by bone morphogenetic proteins and Smad1 modulates the postnatal differentiation of cerebellar cells. J Neurosci. 2003;23:260–8. doi: 10.1523/JNEUROSCI.23-01-00260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A:1425–35. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Welch RD, Jones AL, Bucholz RW, Reinert CM, Tjia JS, Pierce WA, Wozney JM, Li XJ. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res. 1998;13:1483–1490. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 25.Cook SD, Wolfe MW, Salkeld SL, Rueger DC. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J Bone Joint Surg Am. 1995;77:734–50. doi: 10.2106/00004623-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Gazzerro E, Du Z, Devlin RD, Rydziel S, Priest L, Economides AN, Canalis E. Noggin arrests stromal cell differentiation in vitro. Bone. 2003;32:111–19. doi: 10.1016/s8756-3282(02)00948-1. [DOI] [PubMed] [Google Scholar]
- 27.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15(4):663–73. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 29.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–46. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genetics. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 31.Dosch R, Gawantka V, Delius H, Blumenstock C, Niehrs C. BMP-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325–34. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- 32.Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–54. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- 33.Gamer LW, Nove J, Michael Levin M, Rosen V. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Bio. 2005;285:156–68. doi: 10.1016/j.ydbio.2005.06.012. [DOI] [PubMed] [Google Scholar]