Abstract
The phototransduction cascade is perhaps the best understood model system for G protein-coupled receptor (GPCR) signaling. Phototransduction links the absorption of a single photon of light to a decrease in cytosolic cGMP. Depletion of the cGMP pool induces closure of cGMP-gated cation channels resulting in the hyperpolarization of photoreceptor cells and consequently a neuronal response. Many biochemical and both low- and highresolution structural approaches have been utilized to increase our understanding of rhodopsin, the key molecule of this signaling cascade. Rhodopsin, a member of the GPCR or seven-transmembrane spanning receptor superfamily, is composed of a chromophore, 11-cis-retinal that is covalently bound by a protonated Schiff base linkage to the apo-protein opsin at Lys296 (in bovine opsin). Upon absorption of a photon, isomerization of the chromophore to an all-trans-retinylidene conformation induces changes in the rhodopsin structure, ultimately converting it from an inactive to an activated state. This state allows it to activate the heterotrimeric G protein, transducin, by triggering nucleotide exchange. To fully understand the structural and functional aspects of rhodopsin it is necessary to critically examine crystal structures of its different photointermediates. In this review we summarize recent progress on the structure and activation of rhodopsin in the context of other GPCR structures.
INTRODUCTION
The process of phototransduction couples the absorption of light to a structural change which initiates a signaling cascade that culminates in visual perception. Briefly, phototransduction is initiated with absorption of a photon by the chromophore 11-cis-retinal that is covalently linked to the G protein-coupled receptor (GPCR) known as opsin. Isomerization of 11-cis-retinal to all-trans-retinal converts an inverse agonist to full agonist and produces a conformational change in the opsin and consequent activation of the coupled heterotrimeric G protein, transducin (1). The Gtα subunit of transducin binds to and activates a phosphodiesterase which depletes levels of cGMP. This depletion induces closure of cGMP-gated cation channels and hyperpolarization of photo-receptor cells, leading to a decrease in glutamate neurotransmitter release (2). This decrease in glutamate signaling is detected by secondary neurons and ultimately results in the perception of a visual signal within the brain.
Beginning with the determination of the complete primary sequence of bovine rhodopsin in 1983, it was possible to assign secondary structural elements to the protein based on hydrophobicity plots (3,4). From this early work, a 2-D model of rhodopsin with seven transmembrane helices was derived (3,4). Later work involving 2-D crystals further extended these models into three dimensions (5-7). As early as 1990, there were reports of rhodopsin crystals, but technological limitations precluded data collection and structure determination (8,9). With the advent of cryo-crystallography, the availability of synchrotron X-rays and the growth and analysis of new rhodopsin crystals, it became possible to determine the crystal structure of rhodopsin (10). This advance permitted the placement of a huge volume of biochemical and biophysical data into a structural context (11-13). The initial rhodopsin structure was critical for the determination of all subsequent rhodopsin and adrenergic receptor structures as the structure or a derivative thereof served as a molecular replacement model used to solve these structures (10,14-17).
Structural studies of rhodopsin and the fit of prior biochemical and biophysical studies within this structural framework have been extensively reviewed (1,18-20). For example, after the determination of the rhodopsin structure it was possible to place the EPR studies of rhodopsin activation into context (21). While initial EPR studies suggested that “rigid body movements” of helix-VI on the order of >10 Å occurred upon activation of rhodopsin, this movement has since been revised to ~6 Å (22,23). It must be noted that these measurements involving EPR or DEER spectroscopy estimate single pair wise distances between covalently attached spin labels in heterologously expressed proteins and that a rotation of an EPR probe by as little as 30° can increase the measured distance between spin labels by 6-10 Å, which is within the revised measurements for activation of rhodopsin.
STRUCTURAL ANALYSIS BY X-RAY CRYSTALLOGRAPHY
While great progress has been made in the field of NMR spectroscopy, the only technology currently available for the analysis of GPCRs that simultaneously reveals all atomic positions within the protein is X-ray crystallography. The protein within these crystals is fully hydrated and surrounded by detergent and/or lipid as a membrane mimetic. The environment in which the protein exists in these crystals may approximate some aspects of the protein within the physiological membrane. For example, the protein density within rhodopsin crystal structures is only twice that of rhodopsin in its physiological membrane and crystal contacts seen in crystal structures may recapitulate the crystalline arrays seen in atomic force microscopy (AFM) images of the rod outer segment membrane (15,24,25).
Any detailed examination of crystal structures should not ignore the fact that such structures by their very nature “freeze” a thermodynamic minima which may not fully represent the state(s) present in solution. This is not to say that crystal structures are not representative of actual states in which proteins exist, just that crystal structures must be considered in the context of other biochemical and biophysical evidence to understand their biological relevance. When making conclusions about any data, it is important to consider the precision of the measurement; this holds true for both crystallography as well as for EPR/DEER spectroscopy. While EPR and DEER experiments can give pairwise distances which are precise to within ±3 Å, X-ray crystal structures can yield more precise measurements (Table 1) (26,27). Furthermore, the fact that structural factors now are deposited along with atomic coordinates allows independent analysis of published structures and this can yield alternate interpretations that may improve the quality of protein models (28,29).
Table 1.
Diffraction precision indexes (dpi)* for selected GPCR structures.
| PDB ID | DPI‡ |
|---|---|
| 1U19 | 0.15 |
| 1GZM | 0.83 |
| 2I37 | 1.43 |
| 3CAP | 0.25 |
| 2Z73 | 0.28 |
| 2VT4 | 0.79 |
| 2RH1 | 0.30 |
| 3D4S | 0.42 |
DPI is a stringent measure of the certainty of atomic positions within an X-ray structure and indicates the minimal significant difference between two structures.
DPI is calculated as follows: dpi = 1*(Ni/Nobs)1/2C-1/3Rfreedmin where Ni is the number of fully occupied sites within the asymetric unit, Nobs is the number of observations, and C is the completeness of the dataset.
Structural precision (DPI) was calculated using the electron density server (EDS) if possible (27). For 3CAP and 2I37 the program SFC HECK was used because the EDS fails to calculate maps for 2I37 and 3CAP. For 1U19, the program ESCET was used as there are no structure factors deposited (53). Interestingly, the re-refinement of the 1GZM structure in the higher symmetry space group p64 was able to significantly increase the certainty of atomic positions (from 0.83 to 0.26). These numbers should be taken into account when making hypotheses based on distance measurements of the structures.
ANALYSIS OF RHODOPSIN CRYSTAL STRUCTURES
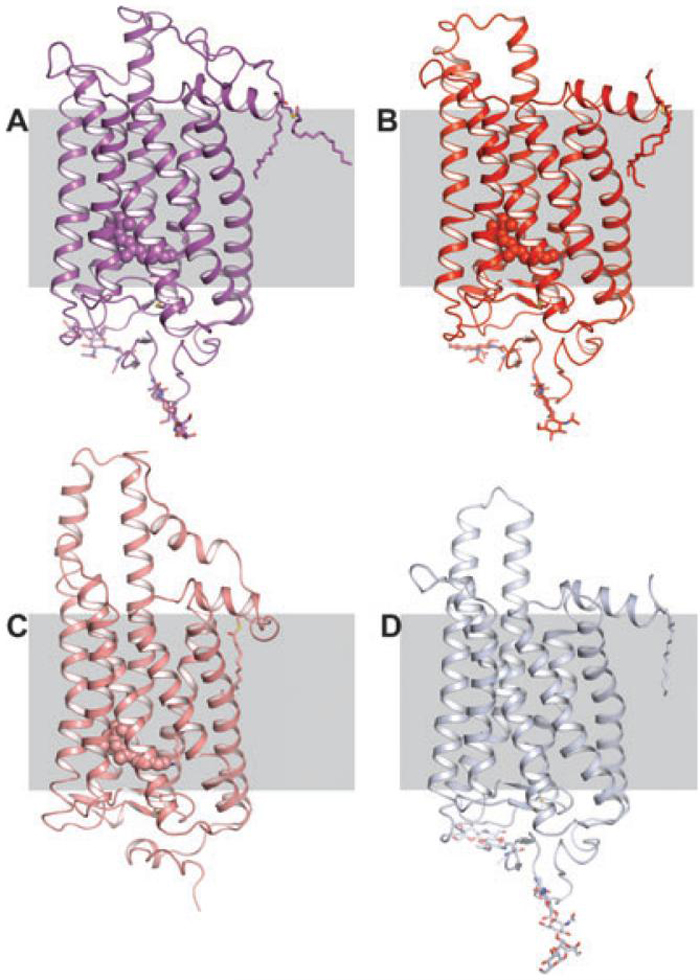
Fifteen bovine rhodopsin (or opsin) crystal structures now have been determined and published. These include rhodopsin in the ground state, rhodopsin containing the non-native chromophore 9-cis-retinylidene (isorhodopsin) in the ground state, two early photointermediates, regenerated mutant rhodopsin produced in cell culture, a photoactivated structure as well as the apo-protein opsin (Fig. 1) (10,15,28,30-35). In addition to the above bovine rhodopsin structures, the crystal structure of squid rhodopsin has been determined independently by two groups (36,37). Comparisons between these structures reveal large differences in the C-III loop but these differences do not correlate with activation state (Fig. 2). In fact, this loop has some of the highest temperature factors in the ground state structures indicating that it is a mobile element. When compared with the opsin and ground state squid rhodopsin structures and considering biochemical data regarding G protein binding, it is likely that this loop must move in order for transducin (or Gαq in case of squid rhodopsin) to couple to the receptor (38-40). These structural differences could also explain the large differences in Gt activation rate seen between meta II and opsin (41).
Figure 1.
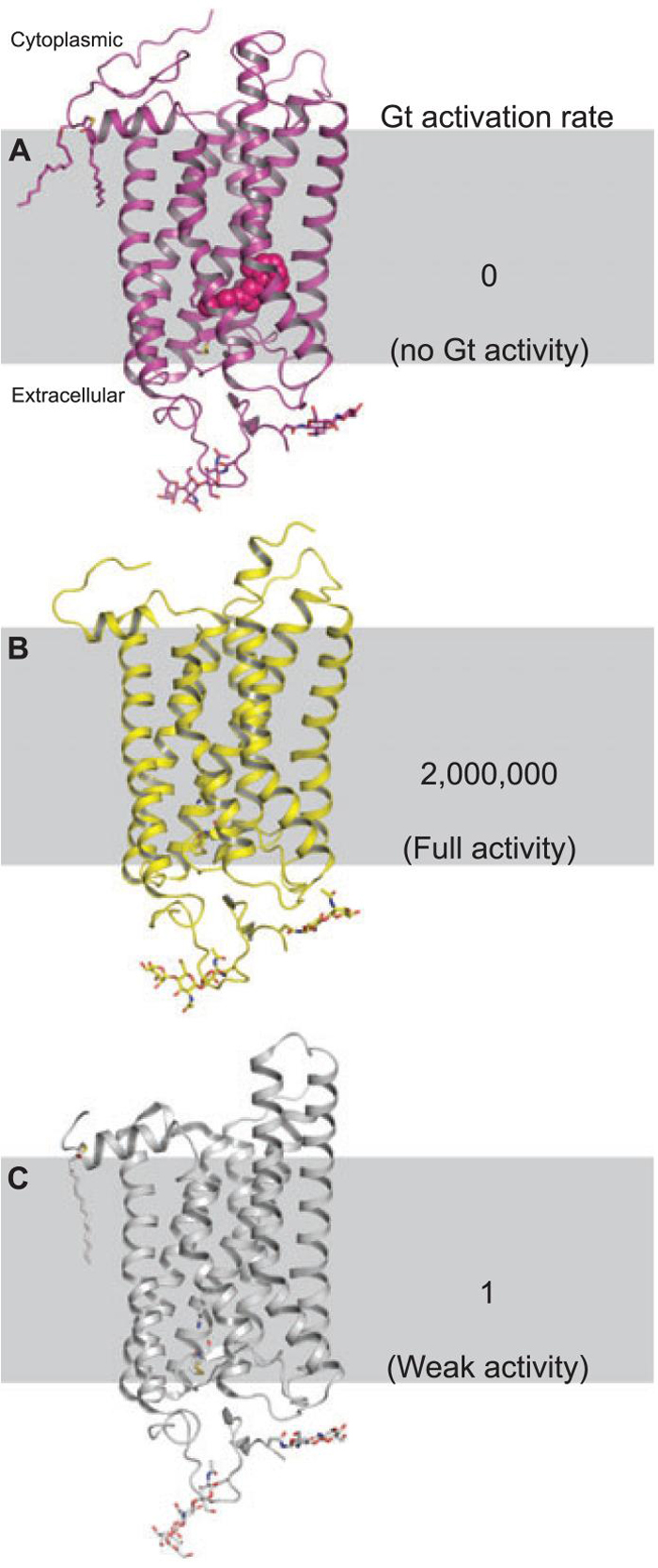
Structures of rhodopsin, photoactivated rhodopsin and opsin and their relative rates of transducin activation. (A) Rhodopsin which has no constitutive activity in the ground state. Rhodopsin is unique in this as to the best of our knowledge all GPCRs exhibit some amount of constitutive activity. (B) The Meta II state which activates G protein at a rate from 4000-2 000 000 times greater than (C), the apo-protein, opsin (41,52).
Figure 2.
Extension of the C-III loop of rhodopsins when placed in the context of a hypothetical membrane. (A) Rhodopsin structure solved in the tetragonal space group P41 (PDB ID:1U19). (B) Rhodopsin solved in trigonal space group P31 and re-refined in the higher symmetry space group P64 (PDB IDs:1GZM and 3C9L respectively). (C) Squid rhodopsin (PDB ID:2Z73). (D) Opsin solved in the trigonal space group, H3 (PDB ID:3CAP). A large protrusion of the C-III loop is evident in B, C and D and this region has higher temperature factors than the surrounding residues and is disordered in the photoactivated structure (PDB ID:2I37). It is rigidified in the opsin structure by interaction with C-I on a symmetry-related molecule. It is likely that this region must move or become less ordered upon activation in order for Gt to interact with and become activated by the receptor.
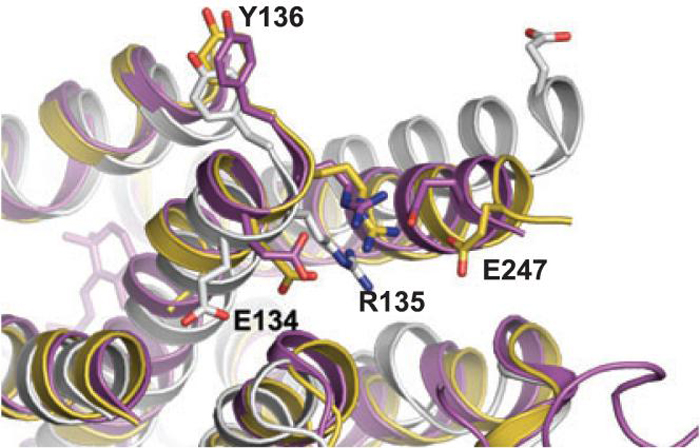
There are several benchmarks for attainment of the activated state (meta II) of rhodopsin. One is the spectral shift of the absorbance maxima from 500 nm to 378 nm which is indicative of the deprotonation of the Schiff-base linking the all-trans-retinylidene to Lys296, an event that coincides with proton transfer to the counter ion Glu113 (42,43). The structure of photoactivated rhodopsin, solved at 4.1 Å resolution, has this spectral shift characteristic of the meta II state as well as isomerization of the chromophore from 11-cis to all-trans (15). A second characteristic involves the breaking of hydrogen bond interactions between the between Arg135 of the (D/E)RY motif and Glu247. This motif is highly conserved (>66%) in GPCRs and is thought to play an integral role in activation (44).
The superposition of the ground state rhodopsin (1U19), the (deprotonated Schiff-base) DSB (2I37) and opsin (3CAP) structures reveals that upon activation there is a disruption of the interactions that Arg135 makes with Glu247 in the “ionic lock” motif. The distance between Arg135 and Glu247 changes from 2.7 Å in the ground state receptor to 4.1 Å in the photoactived intermediate and increases to 15.3 Å in the structure of the apo-protein as shown in Fig. 3. This change in distance in the apo-protein is in part due to the clockwise rotation of helix-VI consistent with previously predicted measurements in the receptor following photoactivation (22,23). While the precision of this distance measurement (Table 1) in the DSB structure precludes the unambiguous determination of a disruption of this motif, the fact that the side chains of the interacting residues are disordered and that the distance is 1.4 Å greater than that of the same structure in ground state rhodopsin it is reasonable to conclude that the ionic lock is broken in the DSB structure.
Figure 3.
Differences in the D/ERY motif based on the photostate of rhodopsin. Super position of crystal structures of bovine rhodopsin in the ground state (magenta), deprotonated Schiff base (DSB) (yellow), and the apo-protein opsin (white). The CII and CIII loops have been removed for clarity. The distance between the nitrogen of Arg135 to the oxygen of Glu247 is 2.7 Å in ground state rhodopsin, 4.1 Å in the DSB structure, and 15.3 Å in the opsin structure.
COMPARISONS BETWEEN RHODOPSIN AND OTHER GPCR STRUCTURES
The initial rhodopsin structure and subsequent improvements in resolution have provided a template for the creation of homology models (45,46). While GPCRs share many hallmark motifs and structural homologies, their primary sequences are quite divergent (44). With the publication of the crystal structures of the β1- and β2-adrenergic receptors it is now possible to both utilize these alternative templates for the creation of homology models as well as to validate the previous rhodopsin-based homology models. Recent homology studies show that in some cases the adrenergic receptor may better serve as a basis for homology model generation (47). Comparisons of the transmembrane regions of all GPCRs of known structure reveal that although most rhodopsin structures superpose quite well (Table 2), the squid rhodopsin structure superposes much more poorly on ground state rhodopsin and opsin. Additionally, although they are much closer in sequence, the transmembrane regions of the β1- and β2-adrenergic receptor structures superpose better onto rhodopsin than they do on one another.
Table 2.
Structural similarity of the transmembrane regions of selected GPCR structures.*
| PDB ID | IU19 | 1GZM | 2I37 | 3CAP | 2Z73 | 2VT4 | 2RH1 | 3D4S | 3EML |
|---|---|---|---|---|---|---|---|---|---|
| 1U19 | 0.39 | 0.9 | 1.7 | 1.4 | 4.1 | 3.4 | 3.3 | 2.7 | |
| 1GZM | 0.9 | 1.7 | 1.3 | 4.2 | 3.4 | 3.3 | 2.7 | ||
| 2I37 | 1.6 | 1.5 | 4.1 | 3.5 | 3.5 | 2.8 | |||
| 3CAP | 1.8 | 4.4 | 3.4 | 3.4 | 3.1 | ||||
| 2Z73 | 4.1 | 3.2 | 3.2 | 3.1 | |||||
| 2VT4† | 5.8 | 5.8 | 4.2 | ||||||
| 2RH1 | 0.36 | 4.4 | |||||||
| 3D4S | 4.3 | ||||||||
| 3EML |
The pairwise root mean squared deviations (RMSDs) of the transmembrane regions of rhodopsin, opsin and adrenergic receptors were calculated. Transmembrane regions were determined by homology to the transmembrane regions of bovine rhodopsin in the cases of the adrenergic receptors and squid rhodopsin. While RMSDs of all rhodopsin transmembrane regions are within 1.8 Å, the adrenergic receptors superpose more poorly (3.3-3.5 Å for β2 and 4.1-4.4 for β1). Surprisingly, β1-adrenergic receptor and β2-adrenergic receptor superpose better onto rhodopsin than they do onto one another. PDB codes for the structure superpositions: ground state rhodopsin, 1U19 and 1GZM (30,33); photoactivated rhodopsin, 2I37 (15); opsin, 3CAP(16); squid rhodopsin, 2Z73 (36); β1-adrenergic receptor, 2VT4(48); β2-adrenergic receptor, 2RH1 and 3D4S (17,49); A2A-adenosine receptor, 3EML(54). RMSDs were calculated with the program SUPERPOSE, using the following transmembrane segments of rhodopsin and homologous regions of the other GPCRs: H-I; 36-64, H-II; 72-100, H-III; 107-139, H-IV; 149-173, H-V 200-228, H-VI; 246-276, H-VII; 286-306.
In structures containing more than one GPCR, monomer A was utilized for comparisons, with the exception of the β1-adrenergic receptor monomer B was used because monomer A contains a bend in H-I which is not expected to exist physiologically.
ACTIVATION AND ACTIVATED STATES IN GPCR STRUCTURES
The recent determination of the structures of opsin and the β1- and β2-adrenergic receptors requires us to critically evaluate the ensembles of these ligand-bound and apo-protein structures (16,17,48). It is unclear whether the β2-adrenergic receptor structure is in an active conformation; while partial inverse agonist is bound, the T4 lysozyme which was fused into the C-III loop contributes an Arg that disrupts the (D/E)RY motif. The T4 lysozyme receptor fusion protein shows an increased affinity for agonist relative to the wild-type demonstrating functional perturbations which may explain structural features such as the disruption of the ionic lock (17,49). The opsin structure poorly approximates the activated state of the receptor because opsin is barely capable of activating transducin (Gt). Opsin only activates Gt at 4 × 103-2 × 106 of the rate exhibited by the meta II state (16,41,50). Obviously, some rearrangement of the transmembrane and/or cytoplasmic regions must transpire for the activation of Gt to occur given the extremely low level of constitutive activity of this receptor. The attribution of a single “activated state” to a static crystal structure is probably not appropriate anyhow as most GPCRs exhibit some degree of constitutive activity that blurs the line in between the active and inactive states (18).
Upon examination of the (D/E)RY region in the β1- and β2-adrenergic receptor structures, it is not clear why or if binding of an inverse agonist should break the ionic lock. In the case of the β2-adrenergic receptor structures, the T4 lysozyme fusion makes direct contact with the Arg residue, thereby disrupting the ionic lock. The β1-adrenergic receptor structure also exhibits a disruption of the ionic lock, however, in this case the inverse agonist, cyanoproindolol, is bound. It is unclear as to why a compound that reduces the basal activity of a receptor should disrupt this region, a region shown to be important for maintaining the receptor in an inactive state (51). Progress in determining the atomic structures of the adrenergic receptors is remarkable, but these are structures of heterologously expressed mutant receptors which exhibit ligand-binding affinities that differ from that of wild-type receptor. Furthermore, ligand binding is necessary but not sufficient for attainment of the active state. The most appropriate measure for determining if a GPCR is in its active state is G protein activation rather than ligand binding.
CONCLUSION
Phototransduction serves as a model system for a multitude of G protein-mediated signal transduction pathways initiated by GPCR activation. Thus, a more rigorous understanding of this process will have broad application to other signal transduction cascades. In the last decade, the progress in understanding how rhodopsin signals utilizing high-resolution structural methods has placed a huge volume of GPCR biochemistry within a structural framework. Further structural work on complexes of the individual components will be required to gain a more comprehensive understanding of phototransduction and the activated state as well as other GPCR signaling processes. The questions as to the active conformation of a GPCR will ultimately have to be answered by solving the crystal structure of an activated GPCR in complex with its cognate G protein.
Acknowledgements
This work was supported in part by NIH grants EY09339, GM 079191 and R01EY008061 (K.P) and T32EY007157 (D.T.L. and T.E.A.) as well as an unrestricted grant from Amgen, Inc. We thank Dr. Leslie Webster Jr. for critical reading of the manuscript.
Footnotes
This paper is part of the Proceedings of the 13th International Conference on Retinal Proteins, Barcelona, Spain, 15-19 June 2008.
REFERENCES
- 1.Palczewski K. G protein-coupled receptor rhodopsin. Ann. Rev. Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belgum JH, Copenhagen DR. Synaptic transfer of rod signals to horizontal and bipolar cells in the retina of the toad (Bufo marinus) J. Physiol. 1988;396:225–245. doi: 10.1113/jphysiol.1988.sp016960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargrave PA, McDowell JH, Curtis DR, Wang JK, Juszczak E, Fong SL, Rao JK, Argos P. The structure of bovine rhodopsin. Biophys. Struct. Mech. 1983;9:235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- 4.Ovchinnikov Iu A, Abdulaev NG, Feigina M, Artamonov ID, Bogachuk AS. Visual rhodopsin III. Complete amino acid sequence and topography in a membrane. Bioorg. Khim. 1983;9:1331–1340. [PubMed] [Google Scholar]
- 5.Dratz EA, Van Breemen JF, Kamps KM, Keegstra W, Van Bruggen EF. Two-dimensional crystallization of bovine rhodopsin. Biochim. Biophys. Acta. 1985;832:337–342. doi: 10.1016/0167-4838(85)90268-7. [DOI] [PubMed] [Google Scholar]
- 6.Schertler GF, Villa C, Henderson R. Projection structure of rhodopsin. Nature. 1993;362:770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin JM. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993;12:1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yurkova EV, Demin VV, Abdulaev NG. Crystallization of membrane proteins: Bovine rhodopsin. Biomed. Sci. 1990;1:585–590. [PubMed] [Google Scholar]
- 9.de Grip WJ, Oostrum van. J., de Caluwe GLJ. Studies towards the crystallization of the rod visual pigment rhodopsin. J. Cryst. Growth. 1992;122:375–384. [Google Scholar]
- 10.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 11.Okada T, Palczewski K. Crystal structure of rhodopsin: Implications for vision and beyond. Curr. Opin. Struct. Biol. 2001;11:420–426. doi: 10.1016/s0959-440x(00)00227-x. [DOI] [PubMed] [Google Scholar]
- 12.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: New insights from structural and biochemical studies. Trends Biochem. Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 13.Teller DC, Okada T, Behnke CA, Palczewski K, Stenkamp RE. Advances in determination of a high-resolution three-dimensional structure of rhodopsin, a model of G-protein-coupled receptors (GPCRs) Biochemistry. 2001;40:7761–7772. doi: 10.1021/bi0155091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schertler GF. Structure of rhodopsin and the metarhodopsin I photointermediate. Curr. Opin. Struct. Biol. 2005;15:408–415. doi: 10.1016/j.sbi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc. Natl Acad. Sci. USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 17.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park PS, Lodowski DT, Palczewski K. Activation of g protein-coupled receptors: Beyond two-state models and tertiary conformational changes. Ann. Rev. Pharmacol. Toxicol. 2008;48:107–141. doi: 10.1146/annurev.pharmtox.48.113006.094630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakmar TP, Menon ST, Marin EP, Awad ES. Rhodopsin: Insights from recent structural studies. Annu. Rev. Biophys. Biomol. Struct. 2002;31:443–484. doi: 10.1146/annurev.biophys.31.082901.134348. [DOI] [PubMed] [Google Scholar]
- 20.Filipek S, Stenkamp RE, Teller DC, Palczewski K. G protein-coupled receptor rhodopsin: A prospectus. Annu. Rev. Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: A perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv. Protein Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- 22.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 23.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc. Natl Acad. Sci. USA. 2008;105:7439–7444. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodowski DT, Salom D, Le Trong I, Teller DC, Ballesteros JA, Palczewski K, Stenkamp RE. Crystal packing analysis of rhodopsin crystals. J. Struct. Biol. 2007;158:455–462. doi: 10.1016/j.jsb.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sale K, Song L, Liu YS, Perozo E, Fajer P. Explicit treatment of spin labels in modeling of distance constraints from dipolar EPR and DEER. J. Am. Chem. Soc. 2005;127:9334–9335. doi: 10.1021/ja051652w. [DOI] [PubMed] [Google Scholar]
- 27.Cruickshank D. Remarks about protein structure precision. Acta Crystallogr. D. 1999;55:583–601. doi: 10.1107/s0907444998012645. [DOI] [PubMed] [Google Scholar]
- 28.Stenkamp RE. Alternative models for two crystal structures of bovine rhodopsin. Acta Crystallogr. D. 2008;64:902–904. doi: 10.1107/S0907444908017162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwart PH, Grosse-Kunstleve RW, Lebedev AA, Murshudov GN, Adams PD. Surprises and pitfalls arising from (pseudo)symmetry. Acta Crystallogr. D. 2008;64:99–107. doi: 10.1107/S090744490705531X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J. Mol. Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Nakamichi H, Buss V, Okada T. Photoisomerization mechanism of rhodopsin and 9-cis-rhodopsin revealed by x-ray crystallography. Biophys. J. 2007;92:L106–108. doi: 10.1529/biophysj.107.108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamichi H, Okada T. Local peptide movement in the photoreaction intermediate of rhodopsin. Proc. Natl Acad. Sci. USA. 2006;103:12729–12734. doi: 10.1073/pnas.0601765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 34.Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GF. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23:3609–3620. doi: 10.1038/sj.emboj.7600374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian DD, Schertler GF. Crystal structure of a thermally stable rhodopsin mutant. J. Mol. Biol. 2007;372:1179–1188. doi: 10.1016/j.jmb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami M, Kouyama T. Crystal structure of squid rhodopsin. Nature. 2008;453:363–367. doi: 10.1038/nature06925. [DOI] [PubMed] [Google Scholar]
- 37.Shimamura T, Hiraki K, Takahashi N, Hori T, Ago H, Masuda K, Takio K, Ishiguro M, Miyano M. Crystal structure of squid rhodopsin with intracellularly extended cytoplasmic region. J. Biol. Chem. 2008;283:17753–17756. doi: 10.1074/jbc.C800040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acharya S, Saad Y, Karnik SS. Transducin-alpha C-terminal peptide binding site consists of C-D and E-F loops of rhodopsin. J. Biol. Chem. 1997;272:6519–6524. doi: 10.1074/jbc.272.10.6519. [DOI] [PubMed] [Google Scholar]
- 39.Janz JM, Farrens DL. Rhodopsin activation exposes a key hydrophobic binding site for the transducin alpha-subunit C terminus. J. Biol. Chem. 2004;279:29767–29773. doi: 10.1074/jbc.M402567200. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Kim SH, Ablonczy Z, Crouch RK, Knapp DR. Probing rhodopsin-transducin interactions by surface modification and mass spectrometry. Biochemistry. 2004;43:11153–11162. doi: 10.1021/bi049642f. [DOI] [PubMed] [Google Scholar]
- 41.Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys. J. 1997;73:3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakmar TP, Franke RR, Khorana HG. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc. Natl Acad. Sci. USA. 1989;86:8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnis S, Fahmy K, Hofmann KP, Sakmar TP. A conserved carboxylic acid group mediates light-dependent proton uptake and signaling by rhodopsin. J. Biol. Chem. 1994;269:23879–23881. [PubMed] [Google Scholar]
- 44.Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: Similarities to rhodopsin. Biochemistry. 2003;42:2759–2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput. Biol. 2006;2:e13. doi: 10.1371/journal.pcbi.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filipek S, Teller DC, Palczewski K, Stenkamp R. The crystallographic model of rhodopsin and its use in studies of other G protein-coupled receptors. Annu. Rev. Biophys. Biomol. Struct. 2003;32:375–397. doi: 10.1146/annurev.biophys.32.110601.142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costanzi S. On the applicability of GPCR homology models to computer-aided drug discovery: A comparison between in silico and crystal structures of the beta2-adrenergic receptor. J. Med. Chem. 2008;51:2907–2914. doi: 10.1021/jm800044k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jager S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- 51.Audet M, Bouvier M. Insights into signaling from the beta2-adrenergic receptor structure. Nat. Chem. Biol. 2008;4:397–403. doi: 10.1038/nchembio.97. [DOI] [PubMed] [Google Scholar]
- 52.Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: Substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 53.Schneider TR. Domain identification by iterative analysis of error-scaled difference distance matrices. Acta Crystallogr. 2004;60:2269–2275. doi: 10.1107/S0907444904023492. [DOI] [PubMed] [Google Scholar]
- 54.Jaakola V-P, Griffith MT, Hanson MA, Cherezov V, Chien EYT, Lane JR, Ijzerman AP, Stevens RC. The 2.6 Angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]