Abstract
We tested the hypothesis that obese individuals experience greater reward from food consumption (consummatory food reward) and anticipated consumption (anticipatory food reward) than lean individuals using functional magnetic resonance imaging (fMRI) with 33 adolescent girls (M age = 15.7 SD = 0.9). Obese relative to lean adolescent girls showed greater activation bilaterally in the gustatory cortex (anterior and mid insula, frontal operculum) and in somatosensory regions (parietal operculum and Rolandic operculum) in response to anticipated intake of chocolate milkshake (versus a tasteless solution) and to actual consumption of milkshake (versus a tasteless solution); these brain regions encode the sensory and hedonic aspects of food. However, obese relative to lean adolescent girls also showed decreased activation in the caudate nucleus in response to consumption of milkshake versus a tasteless solution, potentially because they have reduced dopamine receptor availability. Results suggest that individuals who show greater activation in the gustatory cortex and somatosensory regions in response to anticipation and consumption of food, but who show weaker activation in the striatum during food intake, may be at risk for overeating and consequent weight gain.
Keywords: obesity, anticipatory food reward, consummatory food reward, fMRI
Obesity is a chronic disease that is credited with over 111,000 deaths annually in the US, which largely result from atherosclerotic cerebrovascular disease, coronary heart disease, colorectal cancer, hyperlipidemia, hypertension, gallbladder disease, and diabetes mellitus (Flegal, Graubard, Williamson, & Gail, 2005). Regrettably, the treatment of choice for obesity only results in transitory weight loss (Jeffery et al., 2000) and most obesity prevention programs do not reduce risk for future weight gain (Stice, Shaw, & Marti, 2006). These interventions may have limited efficacy because our understanding of the etiologic processes is still incomplete. Although it has been established that obesity is the result of a positive energy balance, it is unclear why some individuals have such a difficult time balancing caloric intake with expenditure.
One possible explanation is that some individuals have abnormalities in subjective reward from food intake or anticipated intake that increase risk for obesity. Some scholars hypothesize that obese individuals experience greater activation of the meso-limbic reward system in response to food intake (consummatory food reward), which may increase risk for overeating (Davis, Strachan, & Berkson, 2004; Dawe & Loxton, 2004). This is akin to the reinforcement sensitivity model of substance abuse, which posits that certain people show greater reactivity of reward circuitry to psychoactive drugs (Dawe & Loxton, 2004). In contrast, others hypothesize that obese individuals experience less activation of the meso-limbic reward system in response to food intake, which leads them to overeat to compensate for this deficiency (Comings & Blum, 2000; Wang, Volkow, & Fowler, 2002). This is similar to the reward deficiency syndrome thesis, which suggests that people turn to alcohol and drug use to stimulate sluggish reward circuitry (Comings & Blum, 2000). A third hypothesis is that greater anticipated reward from food intake (anticipatory food reward) increases risk for overeating (Pelchat, Johnson, Chan, Valdez, & Ragland, 2004; Roefs, Herman, MacLeod, Smulders, & Jansen, 2005).
Two lines of evidence imply it may be useful to conceptually distinguish between consummatory food reward and anticipatory food reward. First, animal studies suggest that the reward value of food shifts from the consumption of food to the anticipated consumption of food after conditioning, wherein cues associated with food consumption begin to elicit anticipatory food reward. Naive monkeys that had not experienced rewards in a setting showed activation of mesotelencephalic dopamine neurons only in response to food taste; however, after conditioning, dopaminergic activity began to precede reward delivery and eventually maximal activity was elicited by the conditioned stimuli that predicted the impending reward rather than by actual food receipt (Schultz, Apicella, & Ljungberg, 1993; Schultz, & Romo, 1990). Kiyatkin and Gratton (1994) found that the greatest dopaminergic activation occurred in an anticipatory fashion as rats approached and pressed the bar that produced food reward and activation actually decreased as the rat received and ate the food. Indeed, Blackburn, Phillips, Jakubovic, and Fibiger (1989) found that dopamine activity was greater in the nucleus accumbens of rats after presentation of a conditioned stimulus that usually signaled food receipt than after delivery of an unexpected meal. Second, how hard participants work to earn snack food in an operant task (which they are later permitted to consume) is a stronger predictor of ad lib caloric intake than are pleasantness ratings of tastes of the snack foods (Epstein, Temple et al., 2007; Epstein et al., 2004a). These data also seem to imply that anticipated reward from food intake is a stronger determinant of caloric intake than the reward experienced when the food is actually consumed. Collectively, these data imply that it may be useful to distinguish between consummatory food reward and anticipatory food reward when examining potential risk factors for obesity.
Brain imaging studies have identified regions that appear to encode consummatory food reward in normal weight individuals. Consumption of palatable foods, relative to consumption of unpalatable foods or tasteless foods, results in greater activation of the orbitofrontal cortex (OFC) and frontal operculum/insula, as well as greater release of dopamine in the dorsal striatum (O’Doherty, Deichmann, Critchley, & Dolan, 2002; Small, Jones-Gotman, & Dagher, 2003; Volkow et al., 2003). Other brain imaging studies have identified regions that appear to encode anticipatory food reward in normal weight humans. Anticipated receipt of a palatable food, versus anticipated receipt of unpalatable food or a tasteless food, results in greater activation in the OFC, amygdala, cingulate gyrus, striatum (caudate nucleus and putamen), ventral tegmental area, midbrain, parahippocampal gyrus, and fusiform gyrus (O’Doherty et al., 2002; Pelchat et al., 2004). These studies suggest that somewhat distinct brain regions are implicated in anticipatory and consummatory food reward, but that there is some overlap (OFC and striatum). To date only two studies have directly compared activation in response to anticipatory and consummatory food reward to isolate regions that show greater activation in response to one phase of food reward versus the other. Anticipation of a pleasant taste, versus actual taste, resulted in greater activation in the dopaminergic midbrain, nucleus accumbens, and the posterior right amygdala (O’Doherty et al., 2002). Another study found that anticipation of a pleasant drink resulted in greater activation in the amygdala and mediodorsal thalamus, whereas the receipt of the drink resulted in greater activation in the left insula/operculum (Small et al, 2008). These two studies suggest that the amygdala, midbrain, nucleus accumbens, and mediodorsal thalamus are more responsive to anticipated consumption versus consumption of food, whereas the frontal operculum/insula is more responsive to consumption versus anticipated consumption of food. Thus, available evidence seems to suggest that distinct brain regions have been implicated in encoding anticipatory and consummatory food reward, although more research will be necessary before firm conclusions are possible.
Certain findings appear to be consistent with the thesis that obese individuals experience greater food reward, though it is not clear whether findings are reflective of disturbances in consummatory versus anticipatory food reward. Obese relative to lean individuals recall that high-fat and high-sugar foods are more pleasant tasting and report that eating is more reinforcing (Rissanen et al., 2002; Saelens & Epstein, 1996; Westenhoefer & Pudel, 1993). Children at risk for obesity by virtue of parental obesity rate tastes of high-fat food as more pleasant and show a more avid feeding style than children of lean parents (Stunkard, Berkowitz, Stallings, & Schoeller, 1999; Wardle, Guthrie, Sanderson, Birch, & Plomin, 2001). Obese children are more likely to eat in the absence of hunger (Fisher & Birch, 2002) and work harder for food than lean children (Temple et al., in press). Self-reported food cravings correlated positively with body mass and objectively measured caloric intake (Delahanty, Meigs, Hayden, Williamson, & Nathan, 2002; Forman et al., 2007; Franken & Muris, 2005; Nederkoorn, Smulders, & Jansen, 2000). Obese adults report stronger craving of high-fat, high-sugar foods (Drewnowski, Kurth, Holden-Wiltse, & Saari, 1992; White, Whisenhunt, Williamson, Greenway, & Netemeyer, 2002) and work for more food than lean adults (Epstein et al., 2007; Saelens & Epstein, 1996). Morbidly obese relative to lean individuals showed greater resting metabolic activity in the oral somatosensory cortex, a region associated with sensation in the mouth, lips, and tongue (Wang, Volkow, Felder et al., 2002), which may render the former more sensitive to the rewarding properties of food intake and increase risk for overeating.
To date, few brain imaging studies have compared the brain activation in response to presentation of pictured food or actual food among obese verse lean individuals. One study found increased activation in the right parietal and temporal cortices after exposure to pictured food in obese but not lean women and that this activation correlated positively with hunger ratings (Karhunen, Lappalainen, Vanninen, Kuikka, & Uusitupa, 1997). Rothemund and associates (2007) found greater dorsal striatum response to pictures of high-calorie foods in obese verse lean adults and that body mass correlated positively with response in insula, claustrum, cingulate, somatosensory cortex, and lateral OFC. Stoeckel and associates (2008) found greater activation in the medial and lateral OFC, amygdala, ventral striatum, medial prefrontal cortex, insula, anterior cingulate cortex, ventral pallidum, caudate, and hippocampus response to pictures of high-calorie foods (versus low-calorie foods) for obese relative to lean individuals. However, activation of the OFC and cingulate in response to viewing pictures of palatable foods correlated negatively with BMI among normal weight women (Killgore & Yargelun-Todd, 2005). Del Parigi et al (2004) found that the dorsal insula and posterior hippocampus remain abnormally responsive to consumption of food in previously obese compared to lean individuals, leading to the conclusion that these abnormal responses may increase risk for obesity.
Other findings are more consistent with the notion that obese individuals may experience less food reward. Wang et al. (2001) found that D2 receptors are reduced in the striatum in morbidly obese individuals in proportion to their body mass, suggesting that they exhibit decreased dopamine receptor binding in the meso-limbic system. Although it has yet to be determined whether obese individuals show reduced D2 receptor density relative to lean individuals, obese rats have lower basal dopamine levels and reduced D2 receptor expression than lean rats (Fetissov, Meguid, Sato, & Zhang, 2002; Hamdi, Porter, & Prasad, 1992; Orosco, Rouch, & Nicolaidis, 1996), yet obese rats show more phasic release of dopamine during feeding than lean rats (Yang & Meguid (1995). Furthermore, lean and obese adults with the TaqI A1 allele, which is associated with reduced D2 receptors and weaker dopamine signaling, work more to earn food in operant paradigms (Epstein et al., 2004b, 2007). These results echo evidence that addictive behaviors such as alcohol, nicotine, marijuana, cocaine, and heroin abuse are associated with reduced D2 receptor density and blunted sensitivity of mesolimbic circuitry to reward (Comings & Blum, 2000; Martinez et al., 2005). Wang, Volkow, and Fowler (2002) posit that deficits in D2 receptors may predispose individuals to use psychoactive drugs or overeat to boost a sluggish dopamine reward system. However, it is possible that overeating high-fat and high-sugar food leads to down-regulation of D2 receptors (Davis et al., 2004), paralleling neural response to chronic use of psychoactive drugs (Volkow, Fowler, & Wang, 2002). Indeed, animal studies suggest that repeated intake of sweet and fatty foods results in down-regulation of D2 receptors and decreased D2 sensitivity (Bello, Lucas, & Hajnal, 2002; Kelley, Will, Steininger, Xhang, & Haber, 2003); changes that occurs in response to substance abuse.
In sum, there is emerging evidence that obese individuals may show general abnormalities in food reward relative to lean individuals. Specifically, obese relative to lean individuals report greater craving for high-fat/high-sugar foods, find eating more reinforcing, show greater resting activation of the somatosensory cortex, and show greater reactivity of the gustatory cortex to food intake and presentation of food or pictured food. Yet, there is also evidence that obese individuals show a hypofunctioning striatum, which may prompt them to overeat to boost a sluggish reward network or may be a result of receptor down-regulation. One factor that might have contributed to the mixed findings is that many studies used self-report measures, which could be misleading because those who struggle with overeating may assume that food is more rewarding to them, which influences how they complete the scales. Furthermore, self-report scales likely tap anticipated reward from food intake, or the memory of reward from food intake, rather than reward experienced during food consumption, as the studies did not measure perceived reward during food intake. In addition, findings from self-report and behavioral measures are vulnerable to social desirability biases. In addition, few studies have actually involved food intake or exposure to real food, which may limit the ecological validity of the findings. Perhaps most importantly, previous studies have not used paradigms that were specifically designed to assess individual differences in consummatory and anticipatory food reward when comparing obese to lean individuals. Thus, we think it may be useful to use objective brain imaging paradigms that directly measure activation of reward circuitry in response to food intake and anticipated food intake. To our knowledge, studies have not used brain-imaging to test whether obese individuals show differential activation of food reward circuitry during food consumption or anticipated consumption relative to lean individuals.
The present study sought to more fully characterize the nature of individual differences in neural response to food using objective brain imaging methodology, with the hope that an improved understanding of neurological substrates that increase risk for obesity will advance etiologic models and the design of more effective preventive and treatment interventions. We extended previous findings by examining activation in response to receipt of chocolate milkshake versus tasteless solution (consummatory food reward) and in response to cues signaling impending delivery of chocolate milkshake versus tasteless solution (anticipatory food reward) among obese and lean individuals. We hypothesized that obese relative to lean individuals would show greater activation in the gustatory cortex and the somatosensory cortex, and less activation in the striatum, in response to the anticipation and consumption of milkshake. We also hypothesized that the body mass of participants would show linear relations to activation in these brain regions. We studied adolescents because we wanted to reduce the risk that a long history of obesity might result in receptor down-regulation secondary to a chronically rich diet. We studied females because the primary goal of this study was to test whether food reward abnormalities correlate with bulimic pathology, which is rare in males.
Method
Participants
Participants were 44 healthy adolescent girls (M age = 15.7; SD = 0.93); 2% Asian/Pacific Islanders, 2% African Americans, 86% European Americans, 5% Native Americans, and 5% mixed racial heritage. Participants from a larger study of female high schools students who appeared to meet the inclusion criteria for the present imaging study were asked if they were interested in participating in a study on the neural response to presentation of food. Those who reported binge eating or compensatory behaviors in the past 3 months, any use of psychotropic medications or illicit drugs, head injury with a loss of consciousness, or current Axis I psychiatric disorder were excluded. Data from 11 participants were not analyzed because they showed excessive head movement during the scans; 4 showed such pronounced head movement that the scans were terminated and head movement for another 7 exceeded 2 mm (M = 2.8 mm, range 2–8 mm). Because experience indicates that including participants who show head movement greater than 1 mm introduces excessive error variance, we always exclude such participants from our studies (e.g., Small et al., 2001, 2003; Small, Gerber, Mak, & Hummel, 2005). This resulted in a final sample of 33 participants (body mass index range = 17.3–38.9). The local Institutional Review Board approved this project. All participants and parents provided written consent.
Measures
Body Mass
The body mass index (BMI = kg/m2) was used to reflect adiposity (Dietz & Robinson, 1998). After removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. Two measures of height and weight were obtained and averaged. The BMI correlates with direct measures of total body fat such as dual energy x-ray absorptiometry (r = .80 to .90) and with health measures including blood pressure, adverse lipoprotein profiles, atherosclerotic lesions, serum insulin levels, and diabetes mellitus in adolescent samples (Dietz & Robinson, 1998). Per convention (Barlow & Dietz, 1998), obesity was defined using the 95th percentiles of BMI for age and sex, based on historical nationally representative data because this definition corresponds closely to the BMI cut-point that is associated with increased risk for weight-related health problems (Cole, Bellizzi, Flegal, & Dietz, 2000). Adolescents with BMI scores below the 50th percentile using these historical norms were defined as lean. Among the 33 participants who provided usable fMRI data, 7 were classified as obese, 11 were classified as lean, and the remaining 15 participants fell in between these two extremes.
fMRI paradigm
Participants were asked to consume their regular meals, but to refrain from eating or drinking (including caffeinated beverages) for 4–6 hours immediately preceding their imaging session for standardization purposes. We selected this deprivation period to capture the hunger state that most individuals experience as they approach their next meal, which is a time when individual differences in food reward would logically impact caloric intake. Most participants completed the paradigm between 16:00 and 18:00, but a subset completed scans between 11:00 and 13:00. Before the imaging session, participants were familiarized with the fMRI paradigm through practice on a separate computer.
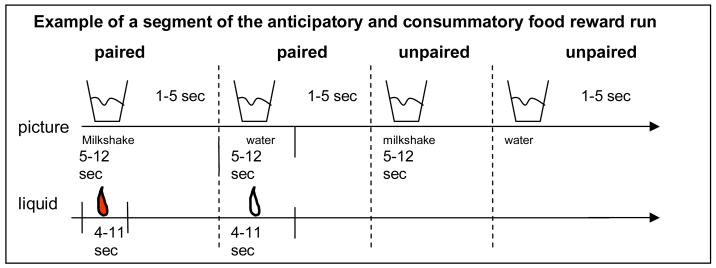
The milkshake paradigm was designed to examine consummatory and anticipatory food reward. Stimuli were presented in 4 separate scanning runs. The stimuli consisted of 3 black shapes (diamond, square, circle) that signaled the delivery of either 0.5 ml of a chocolate milkshake (4 scoops of Haagen-Daz vanilla ice cream, 1.5 cups of 2% milk, and 2 tablespoons of Hershey’s chocolate syrup), a tasteless solution, or no solution. Although the pairing of cues with stimuli and duration of stimulus presentation was randomly determined across participants, we did not randomize order of presentation across participants. The tasteless solution, which was designed to mimic the natural taste of saliva, consisted of 25 mM KCl and 2.5 mM NaHCO3 (O’Doherty et al., 2001). We used artificial saliva because water has a taste that activates the taste cortex (Zald & Pardo, 2000). On 50% of the chocolate and tasteless solution trials the taste was not delivered as expected to allow the investigation of the neural response to anticipation of a taste that was not confounded with actual receipt of the taste (unpaired trials) (Figure 1). There were six events of interest in the paradigm: (1) chocolate milkshake cue followed by milkshake taste (paired milkshake cue), (2) receipt of milkshake taste (milkshake delivery), (3) chocolate milkshake cue followed by no milkshake taste (unpaired milkshake cue), (4) tasteless solution cue followed by tasteless solution (paired tasteless cue), (5) receipt of tasteless solution (tasteless delivery), and (6) tasteless solution cue followed by no tasteless solution (unpaired tasteless cue). The images were presented for 5–12 seconds (M = 7) using MATLAB run from Windows. Taste delivery occurred 4 to 11 seconds (M = 7) after onset of the cue. As a result, each event lasted between 4–12 seconds. Each run consisted of 16 events. Tastes were delivered using two programmable syringe pumps (Braintree Scientific BS-8000) controlled by MATLAB to ensure consistent volume, rate, and timing of taste delivery. Sixty ml syringes filled with the chocolate milkshake and the tasteless solution were connected via Tygon tubing through a wave guide to a manifold attached to the birdcage head coil in the MRI scanner. The manifold fit into the participants’ mouths and delivered the taste to a consistent segment of the tongue. This procedure has been successfully used in the past to deliver liquids in the scanner and has been described in detail elsewhere (e.g., Veldhuizen, Bender, Constable, & Small, 2007). The taste cue remained on the screen for 8.5 seconds after the taste was delivered, and participants were instructed to swallow when the shape disappeared. The next cue appeared 1 to 5 seconds after the prior cue went off. Images were presented with a digital projector/reverse screen display system to a screen at the back end of the MRI scanner bore and were visible via a mirror mounted on the head coil.
Figure 1.
Example of timing and ordering of presentation of pictures and drinks during the run.
Five lines of evidence from an ongoing fMRI study that used this paradigm with adolescent girls (N = 46) suggest that it is a valid measure of individual differences in anticipatory and consummatory food reward. First, participants rated the milkshake as significantly (t = 9.79, df = 45, r = .68, p < .0001) more pleasant than the tasteless solution per a visual analogue scale, confirming that the milkshake was more rewarding to participants than the tasteless solution. Second, pleasantness ratings of the milkshake correlated with activation in the anterior insula (r = .70) in response to milkshake cues and with activation in the parahippocampal gyrus in response to milkshake receipt (r = .72). Third, activation in regions representing anticipatory and consummatory food reward (LaBar et al., 2001; O’Doherty et al., 2002; Small et al., 2001) in response to anticipation and receipt of milkshake in this fMRI paradigm correlated (r = .84 to .91) with self-reported liking and cravings for a variety of foods, as assessed with an adapted version of the Food Craving Inventory (White et al., 2002).1 Fourth, activation in response to anticipatory and consummatory food reward in this fMRI paradigm correlates (r = .82 to .95) with how hard participants work for food and how much food they work for in an operant behavioral task that assesses individual differences in food reinforcement (Saelens & Epstein, 1996). Fifth, participants who show relatively greater activation in response to anticipatory and consummatory food reward in this fMRI paradigm showed significantly (p < .05) more weight gain over a 1-year follow-up than participants who show less activation in this paradigm (r = .54 to .65). Collectively, these findings provide evidence for the validity of this fMRI food reward paradigm.
Imaging and statistical analysis
Scanning was performed by a Siemens Allegra 3 Tesla head-only MRI scanner. A standard birdcage coil was used to acquire data from the entire brain. A thermo foam vacuum pillow and additional padding was used to restrict head motion. In total, 152 scans were collected during each of four functional runs. Functional scans used a T2* weighted gradient single-shot echo planar imaging (EPI) sequence (TE=30 ms, TR = 2000 ms, flip angle=80°) with an in plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 4mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse, oblique plane as determined by the midsagittal section. Structural scans were collected using an inversion recovery T1 weighted sequence (MP-RAGE) in the same orientation as the functional sequences to provide detailed anatomic images aligned to the functional scans. High-resolution structural MRI sequences (FOV = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, slice number ≈ 160) were acquired.
Data were pre-processed and analyzed using SPM5 software (Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB (Mathworks, Inc., Sherborn, MA) (Friston et al., 1994; Worsley & Friston, 1995). The images were time-acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the mean. The images (anatomical and functional) were normalized to the standard MNI template brain implemented in SPM5 (ICBM152, based on an average of 152 normal MRI scans). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel.
To identify brain regions activated in response to consummatory reward we contrasted BOLD response during receipt of milkshake versus during receipt of tasteless solution. We considered the arrival of a taste in the mouth to be consummatory reward, rather than when the taste was swallowed, however, we acknowledge that post-ingestive effects also contribute to the reward value of food (O’Doherty et al., 2002). To identify brain regions activated in response to anticipatory reward in the milkshake paradigm, BOLD response during presentation of the cue signaling impending delivery of the milkshake was contrasted with response during presentation of the cue signaling impending delivery of the tasteless solution. We analyzed data from unpaired cue presentation in which the tastes were not actually delivered to ensure that receipt of the actual tastes would not influence our operational definition of anticipatory brain activation. Condition-specific effects at each voxel were estimated using general linear models. Vectors of the onsets for each event of interest were compiled and entered into the design matrix so that event-related responses could be modeled by the canonical hemodynamic response function (HRF), as implemented in SPM5, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 seconds and the subsequent undershoot. To account for the variance induced by swallowing the solutions, we included the time of disappearance of the cue (subjects were trained to swallow at this time) as a variable of no interest. We also included temporal derivatives of the hemodynamic function to obtain a better model of the data (Henson et al. 2002). A 128 second high-pass filter (per SPM5 convention) was used to remove low-frequency noise and slow drifts in the signal.
Individual contrast maps were constructed to compare the activations within each participant for the aforementioned contrasts in SPM5. Between-group comparisons were then performed using random effect models to account for inter-participant variability. For analysis of consummatory food reward, parameter estimate images from milkshake - tasteless contrast were entered into a second-level 2×2 ANOVA (obese vs. lean) by (milkshake receipt – tasteless receipt). For analysis of anticipatory food reward, the parameter estimate images from unpaired milkshake – unpaired tasteless contrast (i.e., milkshake cue not followed by milkshake receipt – tasteless cue not followed by tasteless receipt) were entered into the second level 2×2 ANOVA (obese vs. lean) by (unpaired milkshake – unpaired tasteless). Thus, we used ANOVA models to specifically test whether obese participants showed significantly greater food reward abnormalities than lean participants.
Individual SPM contrast maps were also entered into regression models with BMI scores entered as a covariate. This model tested whether participants with higher BMI scores showed greater activation believed to reflect consummatory and anticipator food reward relative to participants with lower BMI scores. We estimated these regression models to provide a more sensitive test of these relations using data from all participants in the sample (the ANOVA models only included the obese and lean participants).
The significance of BOLD activation is determined by considering both the maximum intensity of a response as well as the extent of the response. SPM relies primarily upon the maximum intensity to determine significance, setting a strict intensity criterion with t-maps thresholded at p <0.001 (uncorrected) per voxel and a more liberal extent criterion (cluster criterion of 3 voxels). Following convention we used this criterion to determine significance for our activations for both the regression models and the ANOVA models. Activation clusters were considered significant at p < .05 (with respect to clusters) corrected for multiple comparisons across the entire brain. Based upon previous studies we performed directed searches in areas activated by consummatory and anticipatory food reward: striatum, amygdala, midbrain regions, orbitofrontal cortex, dorsolateral prefrontal cortex, insula, anterior cingulate gyrus, parahippocampal gyrus, and fusiform gyrus.
Results
Test of whether obese participants showed differences in anticipatory food reward relative to lean participants (milkshake cue versus tasteless cue)
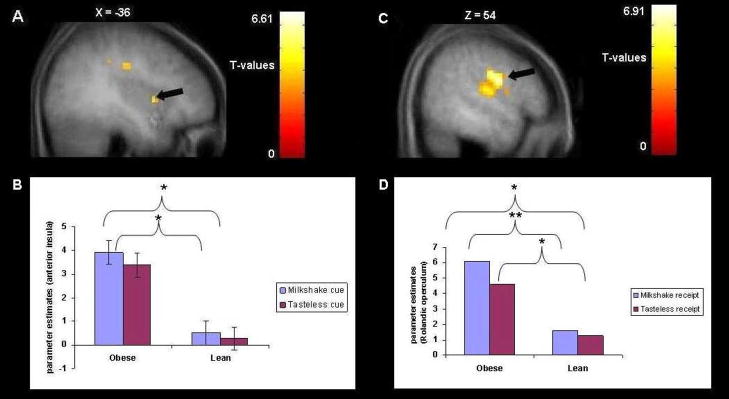
We conducted analyses that compared the brain responses in obese adolescent girls (N = 7, M BMI = 33, SD = 4.25) to lean adolescent girls (N = 11, M BMI = 19.6, SD = 1.08) using a group ANOVA model. A total of 13 activation clusters were located within the insula, the Rolandic region, and the temporal, frontal and parietal opercular regions; obese participants showed greater activation in these areas compared to lean participants (Figure 2A–B and Table 1). Of these 13 activation clusters, 9 fell in the left and 4 in the right hemisphere. Obese participants also showed greater activation in the left anterior cingulate cortex (ventral Brodmann area (BA) 24) than lean participants. Table 1 reports coordinates, voxel size, uncorrected p-values, and effect sizes (η2). Several p-values were significant at p < .05 whole brain corrected at the cluster level. The effect sizes from these analyses ranged from small (η2 = .01) to large (η2 = .17), with an average effect of .05, which represents a medium effect size per Cohen’s criteria (1988).2
Figure 2.
A. Saggital section of greater activation in the left anterior insula (−36, 6, 6, Z = 3.92, P uncorrected <.001) in response to anticipatory food reward in obese compared to lean subjects with B. the bar graphs of parameter estimates from that region. C. Sagittal section of greater activation in the Rolandic operculum (54, −12, 33, Z = 5.95, P uncorrected <.001) in response to consummatory food reward in obese compared to lean subjects with D. the bar graphs of parameter estimates from that region. Note: *p<.05; **p<.001.
Table 1.
Regions Showing Increased Activation during Anticipatory Food Reward and Consummatory Food Reward in Obese Adolescent Girls (N = 7) compared to Lean Adolescent Girls (N = 11)
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Number of voxels in cluster | Activation cluster Z | p value uncorrected | Effect size η2 | |
| Anticipatory food reward | |||||||
| Rolandic operculum | 54 | −3 | 27 | 142 | 5.75 | <.001* | .06 |
| −54 | −9 | 30 | 168 | 4.71 | <.001 | .01 | |
| −39 | −18 | 39 | 168 | 4.60 | <.001 | .10 | |
| 51 | −12 | 33 | 142 | 4.36 | <.001 | .01 | |
| Temporal operculum | −48 | −6 | 3 | 168 | 5.19 | <.001* | .06 |
| Frontal operculum | 51 | 3 | 18 | 17 | 4.47 | <.001* | .01 |
| −54 | −3 | 18 | 42 | 3.92 | <.001 | .02 | |
| Parietal operculum | −60 | −42 | 30 | 6 | 3.91 | <.001 | .08 |
| 60 | −36 | 42 | 7 | 3.56 | <.001 | .01 | |
| Anterior insula | −36 | 6 | 6 | 6 | 3.92 | <.001 | .01 |
| Posterior insula | −39 | −15 | 18 | 4 | 3.79 | <.001 | .07 |
| Ventral anterior cingulate (BA 24) | −6 | 3 | 45 | 8 | 3.65 | <.001* | .17 |
| Consummatory food reward | |||||||
| Rolandic operculum | 54 | −6 | 27 | 284 | 5.95 | <.001* | .06 |
| 54 | −12 | 33 | 284 | 5.68 | <.001 | .08 | |
| −48 | −12 | 33 | 216 | 5.29 | <.001 | .03 | |
| Frontal operculum | −48 | −12 | 12 | 216 | 4.01 | <.001 | .05 |
| 51 | 3 | 18 | 6 | 3.33 | <.001 | .07 | |
Note. p-values significant at p<.05 whole brain corrected at the cluster levels.
Test of whether participants BMI showed linear relations to anticipatory food reward
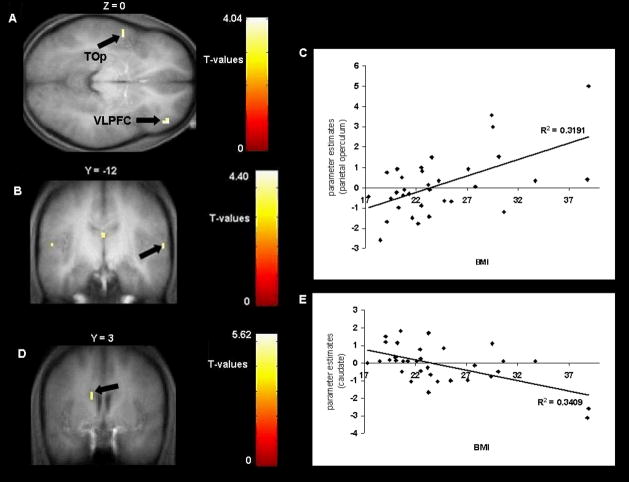
Individual SPM contrasts maps were entered into regression models with BMI scores as a covariate to test whether BMI is linearly related to activation in response to anticipatory food reward. These analyses were more sensitive because they involved all participants, rather than just obese and lean participants. We found a positive correlation of BMI with activations in the ventral lateral and dorsal lateral prefrontal cortex and temporal operculum in response to anticipatory food reward (Figure 3A and Table 2). However, none of the effects were significant at p < .05 whole brain corrected at the cluster level. The effect sizes from these analyses were all large per Cohen’s (1988) criteria (range r = .48 to .68), with a mean r = .56.
Figure 3.
A. Axial section of greater activation in the left temporal operculum (TOp; −54, −3, 3, Z = 3.41, P uncorrected <.001) and in the right ventrolateral prefrontal cortex (VLPFC; 45, 45, 0, Z = 3.57, P uncorrected <.001) in response to anticipatory food reward as a function of BMI.
B. Coronal section of greater activation in the parietal operculum (−54, −3, 3, Z = 3.49, P uncorrected <.001) in response to consummatory food reward as a function of BMI with C. the graph of estimates from that region. D. less activation in the caudate nucleus (−12, 3, 27, Z = 3.57, P uncorrected <.001) in response to food receipt as a function of BMI with E. the graph of parameter estimates from that region.
Table 2.
Regions Responding during Anticipatory Food Reward and Consummatory Food Reward as a Function of Body Mass Index (N =33)
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Number of voxels in cluster | Activation cluster Z | P value (uncorrected) | Effect size r | |
| Anticipatory food reward | |||||||
| Ventrolateral prefrontal cortex | 45 | 45 | 0 | 5 | 3.57 | <.001 | .59 |
| Dorsal lateral prefrontal cortex | −39 | 30 | 36 | 10 | 3.59 | <.001 | .68 |
| −45 | 33 | 30 | 10 | 3.57 | <.001 | .54 | |
| Temporal operculum | −54 | −3 | 3 | 12 | 3.51 | <.001 | .48 |
| −57 | −6 | 6 | 12 | 3.41 | <.001 | .49 | |
| Consummatory food reward | |||||||
| Insula/frontoparietal operculum | −54 | −9 | 12 | 4 | 3.33 | <.001 | .35 |
| Parietal operculum | 63 | −12 | 12 | 6 | 3.43 | <.001 | .51 |
| Caudate nucleus | −12 | 3 | 27 | 4 | −3.57 | <.001 | .58 |
Test of whether obese participants showed differences in consummatory food reward relative to lean participants (receipt of milkshake versus receipt of tasteless)
Comparable to the results with regard to anticipatory food reward, we found that obese adolescent girls showed greater activation in the Rolandic operculum and left frontal operculum in response to consummatory food reward compared to lean participants (Figure 2C–D and Table 1). The activation cluster in the Rolandic operculum was significant at p < .05 whole brain corrected at the cluster level (see Table 1). The effect sizes from these analyses ranged from small (η2 = .03) to medium (η2 = .08), with an average effect of .06, which represents a medium effect size per Cohen’s (1988) criteria.
Test of whether participants BMI showed linear relations to consummatory food reward
Individual SPM contrasts maps were also entered into regression models with BMI scores as a covariate to test whether BMI is linearly related to activation in response to consummatory food reward. A positive relation was found between BMI and activation in the insula and several regions of the operculum (Figure 3B–C and Table 2). BMI was also negatively correlated with activation in the caudate nucleus in response to consummatory food reward in this more sensitive model, indicating that high BMI participants showed decreased response in this area compared to low BMI participants (Figure 3D–E and Table 2). None of the p-values were significant at p < .05 whole brain corrected at the cluster level. The effect sizes from these analyses were medium (r = .35) to large (r = .58) per Cohen’s (1988) criteria, with an average effect that was large (r = .48).
Discussion
This study tested the hypothesis that obese adolescent girls would show differential activation in reward circuitry in response to food consumption and anticipated consumption relative to lean adolescent girls and that activation would be linearly related to the BMI of participants. Brain responses were examined during the receipt of chocolate milkshake versus tasteless solution (consummatory food reward) and in response to cues signaling impending delivery of chocolate milkshake versus tasteless solution (anticipatory food reward). Based on findings of previous studies (e.g., Wang, Volkow, Felder et al., 2002), we expected abnormalities in consummatory and anticipatory food reward among obese participants relative to their lean counterparts.
As hypothesized, the responses to consummatory and anticipatory food reward in the predicted regions were different in the obese adolescent girls compared to their lean counterparts. Obese participants showed greater activation in the primary gustatory cortex (anterior insula/frontal operculum) and in the somatosensory cortex (the Rolandic operculum, temporal operculum, parietal operculum, and posterior insula) and anterior cingulate in response to our measure of anticipatory food reward compared to lean participants. These effect sizes were small to large in magnitude, with an average effect size that was medium. The insula has been shown to play a role in anticipatory food reward (Gottfried, O’Doherty, & Dolan, 2003; Small et al., 2005; Yamamoto, 2006) and food craving (Pelchat et al., 2004). Furthermore, Balleine and Dickenson (2001) showed that animals with resection of the insula fail to learn that behavior responding to food is devalued, also suggesting a role of the insula in anticipatory food reward. The ventral anterior cingulate region has been found to be involved in the coding of energy content and palatability of foods (Araujo & Rolls, 2004). As a result, our findings may suggest that the obese individuals experienced increased anticipation of the palatability of the milkshake compared to lean individuals. It will be important for future studies to rule out the possibility that conditioning that occurs as a result of overeating high-fat and high-sugar foods does not contribute to the elevated anticipatory food reward shown by obese participants.
Also as hypothesized, there was evidence that obese participants showed differential activation in response to consummatory food reward relative to lean participants. The former showed increased activation in the Rolandic operculum, frontal operculum, posterior insula, and cingulate gyrus in response to consummatory food reward compared to the latter. Effect sizes were small to medium in magnitude, with an average effect size that was medium. These results converge with those from previous studies; Del Parigi et al. (2004) found that percentage of body fat correlated with increased activation in the insula during the sensory experience of a meal and Wang, Volkow, Felder et al. (2002) found greater activation in the somatosensory cortex while resting as a function of BMI. Given that the insula and overlying operculum have been associated with subjective reward from food intake (Small et al., 2003; Yamamoto, 2006), these findings may imply that obese individuals experience greater food reward relative to lean individuals, which might correspond to behavioral data from other studies as outlined in the introduction.
We also tested whether BMI is linearly related to activation in response to anticipatory and consummatory food reward with regression models to provide a more sensitive test of the hypothesized relations. Comparable to the results found in the ANOVA models, we found increased activation in the temporal operculum to anticipatory food reward as a function of BMI. Further, greater responses were found in the dorsolateral prefrontal cortex in response to anticipatory food reward as a function of BMI. Also comparable to the findings from the ANOVA models was the increased activation in the insula/frontoparietal operculum in response to consummatory food reward as a function of BMI. Overall, the results of the regression models generally converged with the findings from the ANOVA models, even though the latter analyses only involved obese and lean participants, providing further findings consistent with our hypotheses. The relations identified in the regression models were typically large effects.
Interestingly, the regression models suggested that BMI was inversely related to activation in the caudate nucleus in response to consummatory food reward, as hypothesized based on earlier findings (Wang et al., 2001). This was a large effect size. Our functional finding corroborates and extends the results reported in the study conducted by Wang et al. (2001), in which they found that morbidly obese showed decreased D2 receptor availability at rest in the putamen in proportion to their BMI. These findings may reflect lower dopamine receptor availability. It is possible that individuals overeat to stimulate a sluggish and long-standing dopamine-based reward system (Wang et al., 2001). Alternatively, elevated intake of high-fat and high-sugar foods may result in receptor down-regulation, as has been observed among substance users (Davis et al., 2004). As noted, animal studies suggest that repeated intake of sweet and fatty foods results in down-regulation of D2 receptors and decreased D2 sensitivity (Bello et al., 2002; Kelley et al., 2003). Another possible interpretation is that obese individuals show hypofunctioning of food reward circuitry while resting, but hyperfunctioning when exposed to food or food cues. This interpretation accords with the evidence that obese and post-obese individuals show greater responsivity in the dorsal insula and posterior hippocampus after food intake relative to lean individuals (Del Parigi et al., 2004), that exposure to food cues results in greater activation in the right parietal and temporal cortices in obese but not lean individuals (Karhunen et al., 1997; Stoeckel et al., 2008), that obese individuals show greater activation in dorsal striatum, insula, claustrum, and somatosensory cortex in response to food cues than lean individuals (Rothemund et al., 2007), that obese rats have lower basal dopamine levels and reduced D2 receptor expression than lean rats (Fetissov et al., 2002; Hamdi et al., 1992; Orosco et al., 1996) and that obese rats show more phasic release of dopamine during feeding than do lean rats (Yang & Meguid, 1995). However, this interpretation does not accord with the evidence that obese relative to lean individuals showed greater resting metabolic activity in the oral somatosensory cortex (Wang, Volkow, Felder et al., 2002) and that activation of the OFC and cingulate in response to viewing pictures of palatable foods correlated negatively with BMI among normal weight women (Killgore & Yurgelun-Todd, 2005). It will be useful for future research to determine which interpretation explains the seemingly inconsistent findings, as it would significantly advance our understanding of etiologic and maintenance processes that contribute to obesity.
Collectively, the present findings suggest that different brain regions are activated by anticipatory versus consummatory food reward, which is an important contribution because only a few studies have attempted to identify the neural substrates of anticipatory and consummatory food reward. In the ANOVA models comparing obese to lean participants (Table 1), the Rolandic operculum and frontal operculum was activated by both anticipation and consumption of milkshake, but the temporal operculum, parietal operculum, anterior insula, posterior insula, and ventral anterior cingulate were activated only in response to anticipated receipt of milkshake. In the regression models that examined the relation of BMI to regions of activation (Table 2), there was no overlap in activated regions: whereas the ventrolateral prefrontal cortex, dorsal lateral prefrontal cortex and temporal operculum were activated in response to anticipated receipt of milkshake, the insula, frontoparietal operculum, parietal operculum, and caudate nucleus were activated in response to receipt of milkshake. These findings largely converge with those from previous studies that have investigated brain regions specific to consummatory and anticipatory food reward (O’Doherty et al., 2002; Pelchat et al., 2004; Small et al., 2003; Small et al., 2008; Volkow et al., 2003).
This study is novel in that it is one of the first to test relations between BMI and neural response to anticipatory and consummatory food reward using a paradigm involving delivery of food in the scanner. However, this study had several limitations that should be noted. First, we had a moderate sample size to test between group effects, although it was larger than most previously published fMRI studies of food reward published to date. Second, we used only one palatable flavor. Perhaps other tastes are more rewarding to participants and would have resulted in greater reward response in the brain. Third, since the receipt of the milkshake was always preceded by the cue (i.e., never delivered without the cue), participants always knew about the taste before it was delivered. Past studies (e.g., Berns, McClure, Pagoni, & Montague, 2001) have found differential response to taste and flavors as a function of whether they are expected or unexpected. Therefore, investigators should consider including a measure of response to the receipt of unexpected food reward in future studies. Fourth, the cues used for the milkshake paradigm were geometric shapes, which might not hold enough reward meaning to participants and therefore may have produced blunted anticipatory sensations and brain activation. Fifth, we collected limited behavioral data to validate the fMRI paradigm with participants in our study. Nonetheless, validity data from ongoing studies using this paradigm suggest it is a valid measure of individual differences in food reward.
In conclusion, our results suggest differential neural response during anticipatory and consummatory food reward as a function of obesity status and BMI, although it will be important to replicate these relations in independent samples. Since there was greater response in many regions that have been shown to encode food reward in obese participants, the pattern of response is consistent with behavioral studies suggesting that obese individuals anticipate more reward from food intake and experience greater sensory pleasure when eating. However, we also found that participants with a higher BMI showed less activation in the striatum in response to food consumption relative to those with a lower BMI, which is consistent with the proposal that obese individuals may experience less phasic dopamine release when consuming food relative to lean individuals. It is biologically possible that individuals may anticipate more reward from food intake and experience greater somatosensory pleasure when eating, yet experience less phasic release of dopamine when food is consumed, as each involves separate neural circuitry. However, it is also possible that some of these abnormalities predate obesity whereas others are a consequence of overeating. For example, the former two effects may increase risk for hyperphagia that results in a positive energy balance, and the latter effect may be a product of receptor down-regulation secondary to consumption of a high-fat and high-sugar diet. Alternatively, hypofunctioning of dopamine-mediated reward circuitry may causes individuals to overeat to compensate for this reward deficit, which through conditioning produces greater anticipatory food reward and heightened development of the somatosensotry cortex. It will be vital for prospective studies to investigate which of these abnormalities precedes obesity onset and which are a product of chronic overeating. It is our hope that a systematic study of the abnormalities that predate obesity onset may allow the design of more effective prevention and treatment interventions.
Acknowledgments
This study was supported by research grant (R1MH64560A) from the National Institute of Health.
Thanks go to project research assistant, Keely Muscatell and the participants who made this study possible.
Footnotes
The Food Craving Inventory (FCI, White et al., 2002) assesses the degree of craving for a variety of foods. We adapted this scale by also requesting ratings of how palatable participants find each food. The original FCI has shown internal consistency (α = .93), 2-week test-retest reliability (r = .86), and sensitivity to detecting intervention effects (Martin, O’Neil, & Pawlow, 2006; White et al., 2002). In a pilot study (n = 27) the craving scale and the palatability scale showed internal consistency (α = .91 and .89 respectively).
Whereas some software packages, like AFNI (Analysis of Functional NeuroImages), focus primarily on volume and thus use a larger cluster criterion, SPM focuses primarily on intensity and uses a smaller cluster criterion (but higher intensity requirements). Using a an intensity requirement of t < 0.001 and a contiguous 3-voxel minimum cluster criterion to threshold t-maps is standard for SPM and is the approach we have used in prior studies. Within this context it is important to note that all of the clusters we report are larger than 3 voxels (Tables 1 and 2).
Based on the evidence that reward-related neural function in women is heightened during the mid-follicular phase (Dreher et al., 2007), we created a dichotomous variable that reflected whether participants completed the fMRI scans during the midfollicular phase (days 4–8 after onset of menses; n = 2) or not (n = 31). When we controlled for this variable in all analyses, the activation in the reported regions remained significant.
References
- Balleine B, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: Evidence for a role in incentive learning. Journal of Neuroscience. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SE, Dietz WH. Obesity evaluation and treatment: Expert committee recommendations. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1557–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagoni G, Montague PR. Predictability modulates human brain response to reward. Journal of Neuroscience. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior: A neurochemical analysis. Behavioral Neuroscience. 1989;103:15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal K, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. British Medical Journal. 2000;320:1–6. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in brain research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: Implications for overeating and obesity. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Review. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. Journal of Neuroscience. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM. Psychological and behavioral correlates of baseline BMI in the diabetes prevention program. Diabetes Care. 2002;25:1992–1998. doi: 10.2337/diacare.25.11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Parigi A, Chen K, Hill DO, Wing RR, Reiman E, Tataranni PA. Persistence of abnormal neural responses to a meal in potobese individuals. International Journal of Obesity. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Robinson TN. Use of body mass index (BMI) as a measure of overweight in children and adolescents. The Journal of Pediatrics. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: Carbohydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jaroni JL, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiology and Behaivor. 2004a;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jaroni JL, et al. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. American Journal of Clinical Nutrition. 2004b;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rates and food intake. American Journal of Physiology. 2002;283:R905–910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 years of age. American Journal of Clinical Nutrition. 2002;76:226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K, Graubard B, Williamson D, Gail M. Excess deaths associated with under-weight, overweight, and obesity. Journal of the American Medical Association. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Forman EM, Hoffman KL, McGrath KB, Herbert JD, Brandsma LL, Lowe MR. A comparison of acceptance- and control-based strategies for coping with food cravings: An analog study. Behavior Research and Therapy. 2007;45:2372–2386. doi: 10.1016/j.brat.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy weight women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: Changes during aging. Brain Research. 1992;589:338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords an initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Jeffery R, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill D. Longterm maintenance of weight loss: Current status. Health Psychology. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain. 1997;120:1675–1684. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure) alters striatal enkephalin gene expression. European Journal of Neuroscience. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. NeuroReport. 2005;16:859–863. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Research. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Martin CK, O’Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity. 2006;14:115–121. doi: 10.1038/oby.2006.14. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Jansen A. Cephalic phase responses, cravings and food intake in normal subjects. Appetite. 2000;35:45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Orosco M, Rouch C, Nicolaidis S. Rostromedial hypothalamic monoamine changes in response to intravenous infusions of insulin and glucose in freely feeding obese Zucker Rats: A microdialysis study. Appetite. 1996;26:1–20. doi: 10.1006/appe.1996.0001. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Rissanen A, Hakala P, Lissner L, Mattlar CE, Koskenvuo M, Ronnemaa T. Acquired preference especially for dietary fat and obesity: A study of weight-discordant monozygotic twin pairs. International Journal of Obesity. 2002;26:973–977. doi: 10.1038/sj.ijo.0802014. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Roefs A, Herman CP, MacLeod CM, Smulders FT, Jansen A. At first sight: how do restrained eaters evaluate high-fat palatable foods? Appetite. 2005;44:103–114. doi: 10.1016/j.appet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. The reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to rewarded and conditioned stimuli during successive steps of learning a delayed response task. Journal of Neuroscience. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R. Dopamine neurons of the monkey midbrain: Contingencies of responses to stimuli eliciting immediate behavioral reactions. Journal of Neurophysiology. 1990;63:607–624. doi: 10.1152/jn.1990.63.3.607. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber J, Mak YE, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: The skinny on interventions that work. Psychological Bulletin. 2006;132:667–691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JF. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. American Journal of Clinical Nutrition. 1999;69:524–530. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- Temple JL, Legerski C, Giacomelli AM, Epstein LH. Food is more reinforcing for overweight than lean children. American Journal of Clinical Nutrition In Press. [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Tasting in the absence of taste: Modulation of early gustatory cortex by attention to taste. Chemical Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behavioral Pharmacology. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, et al. Brain dopamine is associated with eating behavior in humans. International Journal of Eating Disorders. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Felder C, Fowler J, Levy A, Pappas N, et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport. 2002;13:1151–1155. doi: 10.1097/00001756-200207020-00016. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opinion On Therapeutic Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wardle J, Guthrie C, Sanderson S, Birch D, Plomin R. Food and activity preferences in children of lean and obese parents. International Journal of Obesity. 2001;25:971–977. doi: 10.1038/sj.ijo.0801661. [DOI] [PubMed] [Google Scholar]
- Westenhoefer J, Pudel V. Pleasure from food: Importance for food choice and consequences of deliberate restriction. Appetite. 1993;20:246–249. doi: 10.1006/appe.1993.1029. [DOI] [PubMed] [Google Scholar]
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the Food-Craving Inventory. Obesity Research. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. [letter; comment] Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Neural substraits for the processing of cognitive and affective aspects of taste in the brain. Archives of Histology and Cytology. 2006;69:243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Meguid MM. The dopaminergic activity in obese and lean zucker rats. Neuroreport. 1995;6:1191–1194. doi: 10.1097/00001756-199505300-00029. [DOI] [PubMed] [Google Scholar]
- Zald DH, Parvo JV. Cortical activation induced by intraoral stimulation with water in humans. Chemical Senses. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]