Abstract
The dorsal striatum plays a role in consummatory food reward, and striatal dopamine receptors are reduced in obese relative to lean individuals, suggesting that the striatum and dopaminergic signaling in the striatum may contribute to development of obesity. Thus, we tested whether striatal activation in response to food intake is related to current and future increases in body mass and whether these relations are moderated by presence of the A1 allele of the TaqlA1 gene, which is associated with compromised striatal dopamine signaling. Cross-sectional and prospective data from two functional magnetic resonance imaging studies support these hypotheses, suggesting that individuals may overeat to compensate for a hypofunctioning dorsal striatum, particularly those with genetic polymorphisms thought to attenuate dopamine signaling in this region.
Although twin studies suggest that biological factors play a major role in the etiology of obesity, few prospective studies have identified biological factors that increase risk for future weight gain. Dopamine is involved in the reinforcing effects of food (1). Feeding is associated with dopamine release in the dorsal striatum and the degree of pleasure from eating correlates with amount of dopamine release (2,3). The dorsal striatum responds to ingestion of chocolate in lean humans and is sensitive to its devaluation by feeding beyond satiety (4). In contrast, the ventral striatum appears to respond to food receipt only if it is unexpected (5) and plays a preferential role in encoding the value of cues associated with food receipt, responding preferentially to cues versus receipt (6) and showing sensitivity to the devaluation of food cues, but not food receipt (4,7). Thus, the dorsal and ventral striatum may serve distinct roles in encoding food reward, with the former playing a more prominent role in encoding consummatory food reward. Dopamine antagonists increase appetite, energy intake, and weight gain, whereas dopamine agonists reduce energy intake and produce weight loss (8,9). Dopamine D2 receptors are reduced in obese relative to lean individuals (10,11). Obese rats have lower basal dopamine levels and reduced D2 receptor expression than lean rats (12,13). It has thus been postulated that obese individuals have hypofunctioning reward circuitry, which leads them to overeat to compensate for a hypofunctioning dopamine reward system (14).
We used blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) to test whether obese relative to lean individuals show abnormal activation of the dorsal striatum, which encodes consummatory food reward (2,4) in response to receipt of a highly palatable food. Although BOLD response reflects blood flow and not dopamine signaling, it has been argued that BOLD signal in dopamine source and target regions probably reflects dopaminergic activity (15–17). In addition, in genetically homogeneous and heterogeneous samples, individuals with an A1 allele are more likely to be obese than those without this allele (18–20). Furthermore, six post mortem and PET studies have found that individuals with at lease one A1 allele of the TaqlA1 DRD2 gene evidenced 30–40% fewer D2 receptors than those with the A2/A2 allele (21–26), suggesting that reduced D2 receptor availability in obese individuals may be related to this polymorphism. The one study in which this effect did not emerge used SPECT (27), implying that it may not be sufficiently sensitive to detect this difference (28). Thus, we further hypothesized that any evidence of abnormal striatal activation in response to food receipt for obese relative to lean individuals would be amplified among those with the A1 allele.
In two fMRI studies, we investigated striatal activation in response to receipt of chocolate milkshake versus a tasteless solution (29). Tastes were delivered using programmable syringe pumps to ensure consistent volume, rate, and timing of taste delivery. This procedure has been used successfully in previous studies (6).
In Study 1, 43 female college students (M age = 20.4, range = 18–22; M BMI = 28.60; range = 23.8–33.2) were scanned while viewing pictures of a glass of chocolate milkshake and a glass of water that predicted taste delivery and while they tasted the milkshake and tasteless solution. The paradigm used in Study 2 was similar, but the cues were geometric shapes (diamond, square, circle) rather than pictures of glasses of milkshake or water. Study 2 involved 33 adolescent girls (M age = 15.7, range = 14–18 years; M BMI = 24.3; range = 17.5–38.9). Genetic data were obtained from 27 of these 33 participants. Because our hypothesis focused on dorsal striatal involvement in consummatory food reward, analyses focused on response to receipt of milkshake and tasteless solution, not on response to cues signaling impending receipt of these tastes.
Individual SPM contrast maps were entered into regression models with BMI scores as a covariate. In all analyses, t-maps were thresholded at p <0.005 with a minimum cluster criterion of 3. We then performed region of interest searches using peaks in the dorsal striatum identified previously (2,4) as centroids to define 10mm diameter spheres. Peaks within these regions were considered significant at p <0.05, False Discovery Rate (FDR) corrected across the small volume.
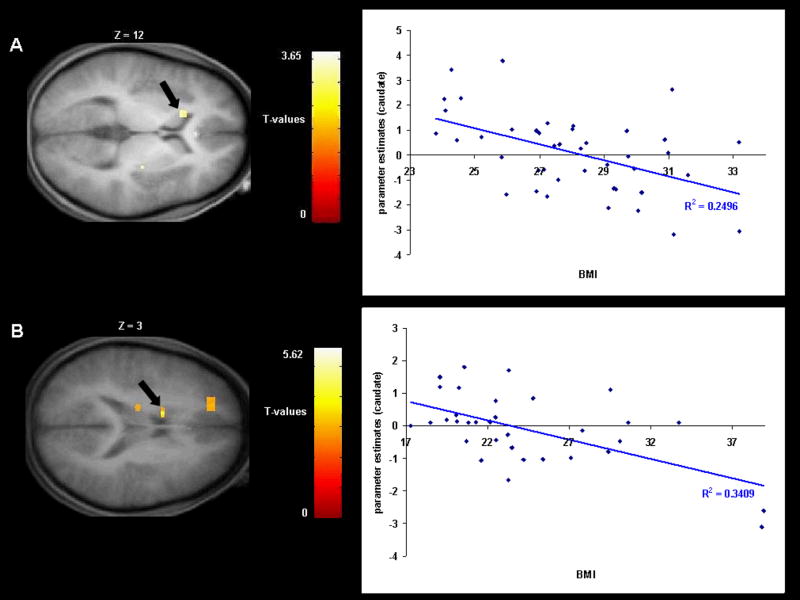
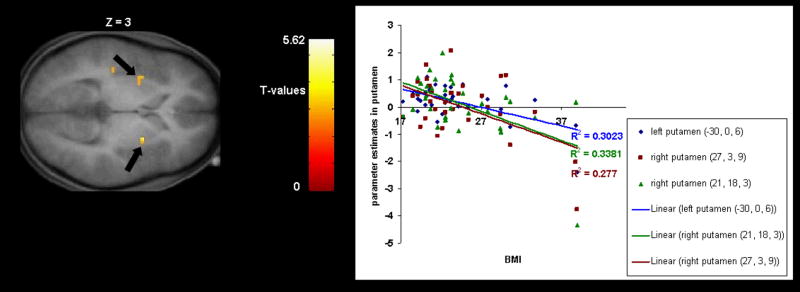
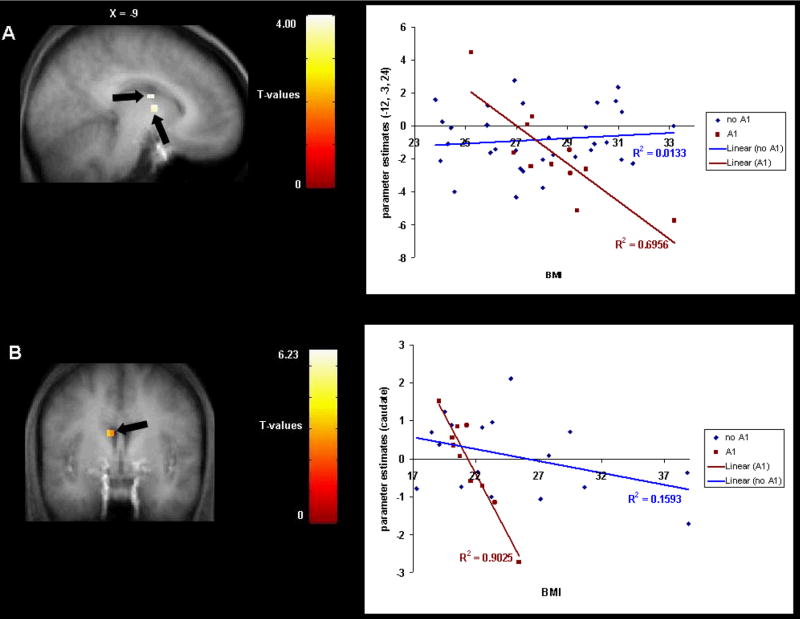
We found a negative correlation between BMI and response in the left caudate nucleus to milkshake receipt versus tasteless solution receipt in Study 1 (r = −.50; Fig. 1A) and in Study 2 (r = −.58; Fig. 1B). We also found a negative correlation between BMI and response bilaterally in the putamen to milkshake receipt versus tasteless solution receipt in Study 2 (r = −.53, −.58; Fig. 2). In Study 1, presence of the A1 allele significantly moderated the negative relation between BMI and activation in the left caudate during receipt of milkshake versus tasteless solution (r = −54, p <.001); activation in this region showed a strong inverse relation (r = −.83) to BMI for those with the A1 allele, but a weak relation (r = .12) to BMI for those without this allele (Fig. 3A). In Study 2, the A1 allele significantly moderated the negative relation between BMI and activation in the left caudate nucleus during receipt of milkshake versus tasteless solution (r = −.68, p<.001); activation in this region showed a strong inverse relation (r = −.95) to BMI for those with the A1 allele, but a weaker relation (r = −.40) to BMI for those without this allele (Fig. 3B). Interestingly, participants with versus without the A1 allele did not differ on milkshake pleasantness ratings. Thus, as hypothesized, in both studies obese relative to lean individuals showed a blunted striatal response to milkshake receipt and this effect was amplified in those with the A1 allele.
Fig 1.
A. Coronal section of weaker activation in the left caudate nucleus (−15, 18, 12, T = 3.65, p <0.05 FDR corrected) in response to milkshake receipt versus tasteless solution receipt as a function of BMI with the graph of parameter estimates from that region (Study 1). B. Coronal section of weaker activation in the left caudate nucleus (−12, 3, 27, T = 4.00, p <0.05 FDR corrected) in response to milkshake receipt versus tasteless solution receipt as a function of BMI with the graph of parameter estimates from that region (Study 2).
Fig 2.
Coronal section of weaker activation bilaterally in the putamen (−30, 0, 6, T = 3.98, p <0.05 FDR corrected; 27, 3, 9, T = 3.45, p <0.05 FDR corrected) in response to milkshake receipt versus tasteless solution receipt as a function of BMI with the graph of parameter estimates from that region (Study 2).
Fig 3.
A. Sagittal section of weaker activation in the left caudate nucleus (−12, −3, 24, T = 4.00, p <0.05 FDR corrected; −9, 0, 15. T = 4.00, p <0.05 FDR corrected) during milkshake receipt versus tasteless solution receipt as a function of BMI depending upon A1 allele status. The graph shows the parameter estimates of the contrast (milkshake receipt versus tasteless solution receipt) across BMI scores for each DRD2 allele type (Study 1). B. Coronal section of weaker activation in the left caudate nucleus (−9, 0, 24, T = 3.81, p <0.05 FDR corrected) during milkshake receipt versus tasteless solution receipt across BMI scores for each DRD2 allele type, with the graph showing the parameter estimates of the contrast (milkshake receipt versus tasteless solution receipt) versus BMI for each allele type (Study 2).
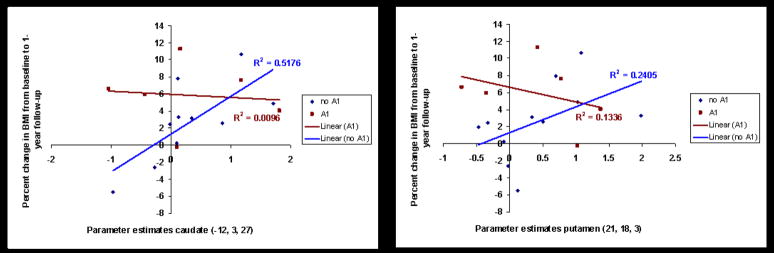
In Study 2, multiple regression models in SPSS tested whether presence of the A1 allele moderated the relation between blunted dorsal striatal activation and future increases in BMI (from an increased positive energy balance) over a 1-year follow-up (N = 17, M BMI percent change = 3.63, range = −5.5–11.3). We controlled for initial BMI, A1 allele status, and dorsal striatal activation. Analyses were performed using the parameter estimates from the most significant peaks from the cross-sectional analyses of Study 2. The interaction between A1 status and activation in the right putamen (r = −.45, p = .01) and activation in the left caudate (r = −.42, p =.02) during milkshake receipt versus tasteless solution receipt in relation to change in BMI were significant and medium in magnitude (Fig 4). Activation in the putamen (r = .19) and caudate (r = .26) and A1 allele status (r = .30) did not show main effects in the prediction of increases in BMI over follow-up.
Fig 4.
A. Activation in the caudate nucleus was negatively related to future weight gain for participants with the A1 allele, but positively related to future weight gain for participants without the A1 allele (Study 1). B. Activation in the putamen was negatively related to future weight gain for participants with the A1 allele, but positively related to future weight gain for participants without the A1 allele (Study 2).
Collectively, results from the present two studies are consistent with the hypothesis that the dorsal striatum is less responsive to food reward in obese relative to lean individuals, potentially because the former have reduced D2 receptor density and compromised dopamine signaling, which may prompt them to overeat in an effort to compensate for this reward deficit. Importantly, we did not observe effects (positive or negative) in the ventral striatum or midbrain, even when reducing the significance threshold. Because we measured BOLD response, we can only speculate that the effects reflect reduced dopaminergic signaling. However, this interpretation seems reasonable because the presence of the A1 allele, which has been associated with reduced dopaminergic signaling in six studies (21–26), significantly moderated the observed BOLD effects, and because prior work has found that this region shows increased blood flow and increased dopamine release in response to ingestion of palatable food (2,4). Our findings converge with evidence that obese relative to lean humans have fewer D2 receptors in the striatum (10,11) and obese relative to lean rats have lower basal dopamine levels and reduced D2 receptor density (12,13). Our findings extend these results by showing that response in the dorsal striatum is blunted during ingestion of palatable food. Our findings also extend work implicating the A1 allele in obesity (30) by providing evidence that the negative relation between striatal response to food receipt and BMI was significantly stronger for individuals with the A1 allele, presumably because these individuals have reduced dopamine signaling capacity in the striatum. Most importantly, our results provide evidence that blunted dorsal striatal response to food intake temporally precedes future weight gain. This finding is consistent with the theory that it represents a vulnerability factor for obesity (32). However, an important alternative explanation to consider is that the hypofunctioning dopamine system results from down-regulation of reward circuitry secondary to overconsumption of high-fat and high-sugar foods (32–33). Indeed, animal studies indicate that chronic excessive intake of such foods results in down-regulation of post-synaptic D2 receptors, increased D1 receptor binding, and decreased D2 sensitivity and μ-opioid receptor binding (33–35), changes that also occur in response to chronic substance use. Although we controlled for initial BMI in our prospective analyses, which reduces the risk that a history of overeating explains the prospective effects, we cannot rule out the possibility that the blunted striatal response is caused by overeating, particularly among individuals with the A1 allele. Paradoxically, such an adaptation may further increase the risk for the persistence of overeating.
One cautionary note is that although studies suggesting that obesity is related to striatal hypofunctioning have included both women and men (10–11, 14) and obesity is equally prevalent for the two genders, our result should be generalized to males with care because we only studied females. Moreover, the evidence that hypofunctioning of the striatum and the A1 allele of TaqlA1 are associated with both obesity and substance abuse (1), implies that individual difference factors, such as affect regulation expectancies, modeling of overeating versus substance abuse, or environmental exposure (to high-fat foods versus psychoactive substances), interact with these general vulnerability factors to determine whether an at-risk individual develops obesity, substance abuse, or neither adverse outcome.
In conclusion, the present results strongly suggest that individuals who show blunted striatal activation during food intake are at risk for obesity, particularly those at genetic risk for compromised dopamine signaling in brain regions implicated in food reward. Thus, behavioral or pharmacologic interventions that remedy striatal hypofunctioning may assist in the prevention and treatment of this pernicious health problem.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Comings DE, Blum K. Prog Brain Res. 2000;126:325. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 2.Small DM, Jones-Gotman M, Dagher A. Neuroimage. 2003;19:1709. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 3.Szczypka M, et al. Neuron. 2001;30:819. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 4.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Brain. 2001;124:1720. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 5.Pagnoni G, Zink CF, Montague PR, Berns GS. Nature Neuroscience. 2002;5:97. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 6.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Neuron. 2008 doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottfried JA, O’Doherty J, Dolan RJ. Science. 2003;301:1104. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 8.de Leon J, et al. J Clin Psychopharmacol. 2007;27:22. doi: 10.1097/JCP.0b013e31802e513a. [DOI] [PubMed] [Google Scholar]
- 9.Leddy JJ, et al. Obes Res. 2004;12:224. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- 10.Wang GJ, et al. Lancet. 2001;357:354. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, et al. Neuroimage. in press. [Google Scholar]
- 12.Fetissov SO, Meguid MM, Sato T, Zhang LH. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- 13.Orosco M, Rouch C, Nicolaidis S. Appetite. 1996;26:1. doi: 10.1006/appe.1996.0001. [DOI] [PubMed] [Google Scholar]
- 14.Wang GJ, Volkow ND, Fowler JS. Expert Opin Ther Targets. 2002;6:601. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 15.D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. Science. 2008;319:1264. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 16.Schultz W, Dayan P, Montague PR. Science. 1997;275:1593. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 17.Kuntson B, Gibbs SEB. Psychopharmacology. 2007;191:813. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 18.Blum K, et al. Pharmacogenetics. 1996;6:297. doi: 10.1097/00008571-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Spitz MR, et al. Nutr Res. 2000;20:371. [Google Scholar]
- 20.Tataranni PA, et al. Diabetes. 2001;50:901. doi: 10.2337/diabetes.50.4.901. [DOI] [PubMed] [Google Scholar]
- 21.Noble EP, et al. Arch Gen Psychiatry. 1991;48:648. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 22.Thompson J, et al. Pharmacogenetics. 1997;7:479. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Pohjalainen T, et al. Mol Psychiatry. 1998;3:256. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson EG, et al. Mol Psychiatry. 1999;4:290. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie T, Noble EP. Neurochem Res. 2003;28:73. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- 26.Tupala E, et al. Neuroimage. 2003;19:145. doi: 10.1016/s1053-8119(03)00060-0. [DOI] [PubMed] [Google Scholar]
- 27.Laruelle M, Gelernter J, Innis R. Molecular Psychiatry. 1998;3:261. doi: 10.1038/sj.mp.4000343. [DOI] [PubMed] [Google Scholar]
- 28.Noble EP. Am J of Medical Genetics. 2003;116B:103. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 29.Materials and methods are available as supporting material on Science Online.
- 30.Epstein LJ, et al. Behav Neuroscience. 2007;121:877. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkow ND, Fowler JS, Wang GJ. Behav Pharmacology. 2002;13:355. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Bello NT, Lucas LR, Hajnal A. Neuroreport. 2002;13:1557. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colantuoni C, et al. Neuroreport. 2001;12:3549. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 35.Kelley AE, et al. Eur J Neurosci. 2003;18:2592. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]