Abstract
Chronic hypoxia (CH) present in infants with cyanotic congenital heart disease may be responsible for subsequent cognitive deficits seen in these children. In a rat model of CH [10% O2 between postnatal day (P) 3 and 28], we have demonstrated significant alterations in energy metabolism and excitatory neurotransmission in the developing hippocampus. These alterations may adversely affect dendritic morphology, which is a highly energy-dependent and excitatory neurotransmitter-mediated event, and hippocampus-mediated behaviors. We measured the apical segment length of dendrites in pyramidal neurons of the CA1 region of the hippocampus using microtubule-associated protein-2 (MAP-2) histochemistry on P28 while the animals were hypoxic (n=8 in CH and n=6 in control), and on P56 after the animals had been normoxic for 4 weeks (n= 8/group). We also compared dorsal hippocampus-dependent trace fear conditioning and dorsal hippocampus-independent delay fear conditioning on P56. Developmental trajectory of the apical segment length was similar in CH and controls, decreasing between P28 and P56. However, when compared with the controls, the apical segment length was longer in the CH group on both P28 [55.11± 2.30 μM (CH) vs 40.52 ±1.20μM (control), p < 0.001] and P56 [44.01± 1.56 μM (CH) vs 31.75 ± 1.31 μM (control), p < 0.001], suggesting the persistence of an immature dendritic architecture. Both trace and delay fear conditioning were decreased in the CH group, suggesting functional abnormality beyond the dorsal hippocampus. These structural and functional alterations may contribute to the cognitive deficits seen in infants at risk for CH.
Keywords: chronic hypoxia, dendritic morphology, developing rat, fear potentiated startle, hippocampus, microtubule-associated protein-2
1. Introduction
The incidence of cyanotic congenital heart disease is approximately 2 per 1000 live births (Hoffman, 2000). Advances in the techniques of cardiopulmonary bypass and hypothermia have improved the survival rates of these infants (Mayer, 1998). Central nervous system injury is a major cause of neurological morbidity in infants with cyanotic congenital heart disease (Wray and Sensky, 1999). These infants are exposed to multiple risk factors, such as ischemia, infection and acute and chronic hypoxia, of which chronic hypoxia (CH) may play a significant role in the neurodevelopmental deficits.
Children with cyanotic congenital heart disease are in a state of CH during the first 2–3 years of life prior to the repair of their cardiac lesion. This period of CH encompasses the phase of rapid hippocampal growth, where neuronal proliferation, migration, glial cell proliferation, synaptogenesis and myelination are ongoing (Nelson et al., 2006). CH may alter these processes in the hippocampus, a structure important in acquisition of long term memory (Nelson, 1995) and vulnerable to injury from adverse conditions during the perinatal period (Rao et al., 2003; Towfighi et al., 1997).
Using in vivo 1H NMR spectroscopy, we have previously demonstrated that CH has an adverse effect on the developing rat hippocampus. CH caused significant alterations in energy metabolism and glutamatergic neurotransmission, as suggested by increased phosphocreatine to creatine (PCr/Cr) ratio and altered glutamate to glutamine ratio (Raman et al., 2005). The increased PCr/Cr ratio in the presence of decreased oxidative phosphorylation that has been demonstrated in a similar animal model (LaManna et al., 1996; Caceda et al., 2001), suggests reduced energy utilization in the hippocampus, presumably as an adaptive response to CH. The altered glutamate-glutamine ratio in the CH hippocampus suggests that the decreased energy utilization may occur through suppression of glutamate-mediated excitatory neurotransmission, a metabolically expensive process (Attwell and Laughlin, 2001). While such adaptation may be beneficial in the short term, it may have deleterious effects in the long-term, since glutamate plays an important role in synaptogenesis through stimulation of NMDA receptors in the developing brain (Monnerie et al., 2003). Based on this, we hypothesized that CH would alter dendritic morphology in the hippocampus.
Dendritic growth is essential for synaptogenesis, and variations in dendritic morphology exert a critical influence on neuronal information processing (Hausser et al., 2000). Dendritic growth and development are highly dynamic processes. Peak neurite formation and extension within the hippocampus occurs in the first postnatal month in rats (Pokorny and Yamamoto, 1981). After the establishment of the initial segment length, branching occurs, and as the animal matures, dendritic segment length decreases with increasing branching (Pokorny and Yamamoto, 1981).
The first objective of our study was to demonstrate acute and chronic alterations in the apical segment length of the dendrites in the hippocampus following CH using microtubule-associated protein-2 (MAP-2) histochemistry. We studied the apical segment length of dendrites on P28 (i.e., while rats were hypoxic) and on P56 after the rats had been normoxic for 4 weeks.
Expression of MAP-2 has been used to study dendritic morphology in the hippocampus (Jorgenson et al., 2003). MAP-2 is the most abundant MAP in the brain and is highly compartmentalized in the cell body and dendrites while being excluded from the axons (Bernhardt and Matus, 1984). MAP-2 is a highly energy-sensitive protein (Avila et al., 1994). There is established correlation between increase in phosphorylation of MAP-2 and increase in dendritic branching (Diez-Guerra and Avila, 1993). Disruption of MAP-2 immunostaining following an insult to the brain is postulated to be the result of a cascade of biochemical events. This cascade results in calcium influx into the cell with activation of Ca2+/Calmodulin kinase II (CAMKII), which may result in abnormal phosphorylation and impaired dendritic structure and function (Fineman et al., 1993). Altered apical segment in the CA1 subarea has been demonstrated in perinatal iron deficiency, an energy-compromised condition (deUngria et al., 2000) similar to CH (Jorgenson et al, 2003).
Altered hippocampal structure and neurochemistry due to CH may be expected to affect hippocampus-dependent memory. Hence, the second objective was to assess long-term hippocampus-based cognitive impairments due to CH using auditory trace and delay Pavlovian fear conditioning on P56.
In Pavlovian fear conditioning, fear is acquired to a conditioned stimulus (CS), such as an auditory tone after it has been paired with an unconditioned stimulus (US), such as an electric shock (Gewirtz and Davis, 2000). In the delay fear conditioning paradigm the CS and the US overlap in time, whereas in a trace fear conditioning paradigm the US is delivered at a given time interval after the end of the CS. Trace, but not delay, fear conditioning is susceptible to lesions of the dorsal hippocampus in rats (Gewirtz and Davis, 2000; Quinn et al, 2005).
2. Results
The body weight of the rats in the CH group was decreased by 60% on P28 and 49% on P56, when compared with the normoxic controls (Table 1). The brain weight was also decreased by 56% on P28 and 17% on P56. The hematocrit was elevated in the CH group by 67% on P28 and by 14% on P56. The brain iron concentration was not different from the controls on P56 (Table 1).
Table 1.
Effect of chronic hypoxia on body weight, brain weight, hematocrit and brain iron concentration of rats on postnatal days 28 and 56
| Age (d) | Chronic hypoxia | Normoxia | P-value | |
|---|---|---|---|---|
| Body weight(g) | 28 | 61.1 ± 2.14 | 97.5 ± 3.91 | 0.00 |
| 56 | 226 ± 1.34 | 337.5 ± 5.06 | 0.00 | |
| Brain weight*(g) | 28 | 0.73 ± 0.01 | 1.14 ± 0.01 | 0.00 |
| 56 | 1.73 ± 0.08 | 2.03 ± 0.01 | 0.03 | |
| Hematocrit (%) | 28 | 65.1 ± 1.10 | 39.3 ± 0.49 | 0.00 |
| 56 | 50.75 ± 1.59 | 44.5 ± 1.24 | 0.05 | |
| Brain Iron*(μg/g) | 56 | 54.68 ± 3.15 | 51.8 ± 4.80 | 0.64 |
Values: mean ± SEM (n = 8/group at each age except * n = 4). Significance by two-tailed unpaired t test.
2.1. Imunohistochemical analysis
Dendritic morphology in the hippocampal subarea of CA1 (Fig 1) followed the normal developmental trajectory of decreasing apical segment length in both groups. Overall, the length was increased in the CH group when compared with the controls on both P28 and P56 (Fig 2). Mean length of the apical segment on P28 was 55.11±2.30 μm (CH) and 40.52±1.20μm (control), p < 0.001, and on P56 was 44.01±1.56 μm (CH) and 31.75±1.31 μm (control), p < 0.001. Quantification showed that the mean apical segment length was increased by 36% in the CH group on P28 and 39% on P56.
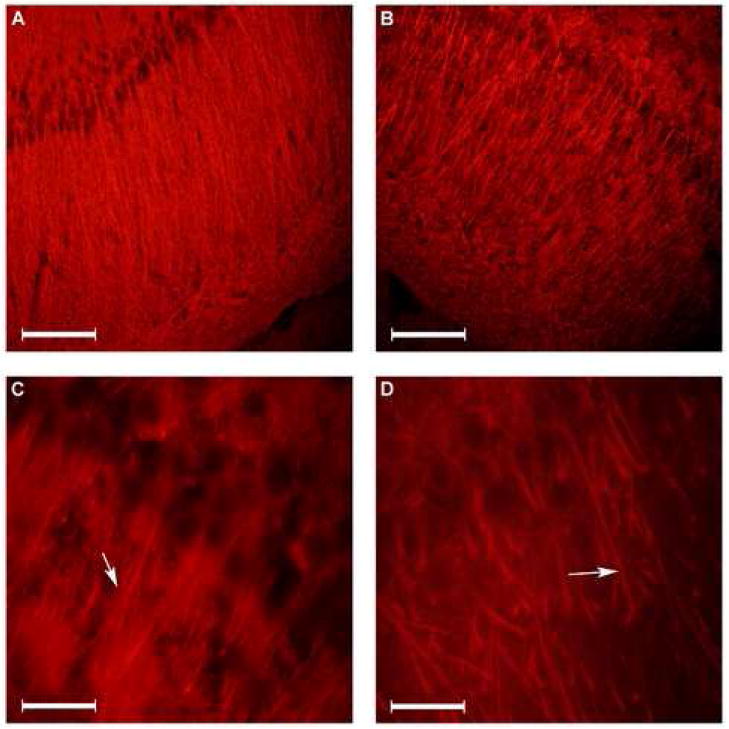
Figure 1.
Microtubule Associated Protein (MAP) -2 immunofluorescence staining of hippocampal CA1 subarea. The dendritic morphology in the hippocampal CA1 subarea in normoxic (panels A and C) and chronic hypoxic (panels B and D) rats using MAP-2 immunofluorescence is shown. Arrows point to the origin of initial branching. (20μm coronal sections of the dorsal hippocampus. Bar in panels A and B = 100 μm. Bar in panels C and D = 20 μm).
Figure 2.
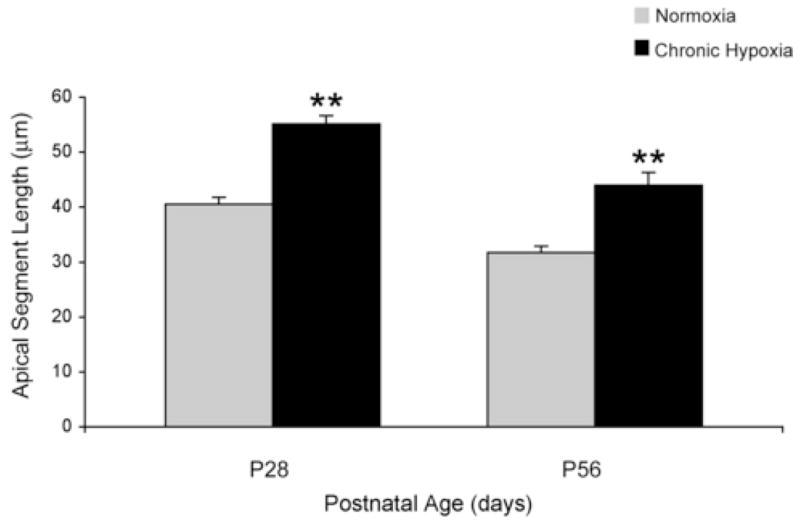
Apical segment length on postnatal day (P) 28 and P56. The effect of chronic hypoxia on the apical segment length in the CA1 region of the hippocampus on P28 and P56 (n = 8 per group except on P28 control where n = 6) is demonstrated. Values are mean ± SEM. ** p < 0.001 (ANOVA).
2.2. Behavioral study
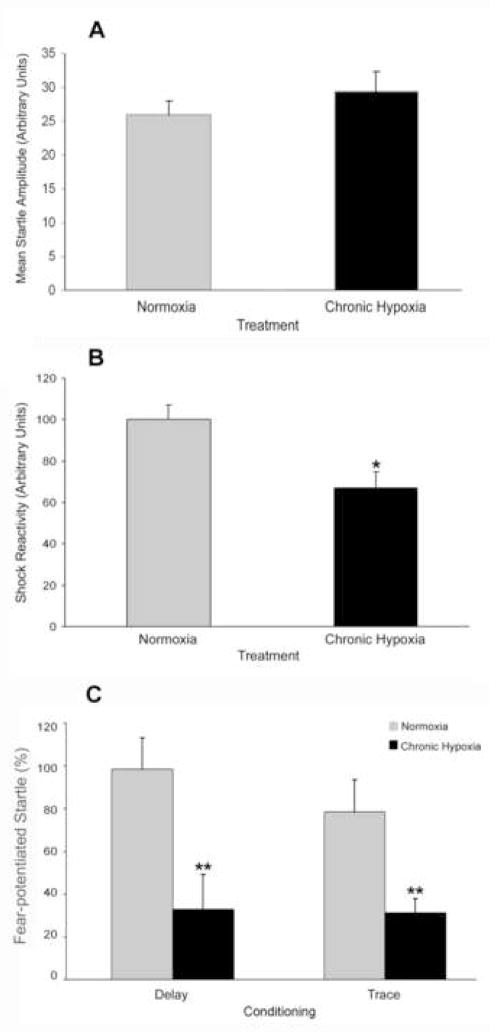
Prior to fear conditioning, baseline startle was not different between previously hypoxic and normoxic rats (Fig 3A). However, reactivity to footshock was reduced in all CH rats (Fig 3B), regardless of type of conditioning, F(1,18) = 9.54, p < 0.01. There was no main effect of conditioning and no treatment × conditioning interaction.
Figure 3.
Effect of chronic hypoxia on behavioral measures. Baseline startle amplitude (A), shock reactivity (B) and fear-potentiated trace and delay conditioning (C) on postnatal day 56. Values are mean ± SEM, n = 5 hypoxic litters and 6 normoxic litters/conditioning group. * p < 0.05; ** p < 0.01 (ANOVA).
CH treatment reduced both trace and delay fear-potentiated startle, F(1, 18) = 15.61, p < 0.005 (Fig 3C). There was no main effect of conditioning paradigm and no treatment × conditioning paradigm interaction. Trace conditioning resulted in CH rats’ fear-potentiated startle being 40% of controls. Following delay conditioning, CH rats displayed fear-potentiated startle that was 33% of controls.
ANCOVA was used to determine if the effects of CH on both forms of fear-potentiated startle were related to the reduced levels of footshock reactivity in the CH animals. When controlling for shock reactivity, there was still a main effect of CH treatment on fear-potentiated startle, F(1, 17) = 7.68, p < 0.05.
3. Discussion
The current studies used a model of CH that is well described and known to produce a degree of hypoxia similar to that seen in infants with cyanotic congenital heart disease (Nakanishi et al., 1997). Using MAP-2 immunofluorescence, we found that CH caused significant alteration in apical dendritic segment length during hypoxia, and that this pattern persisted even after the rats had been in normoxia for 4 weeks. While the typical developmental decrease in dendritic segment length with postnatal age was seen, the apical dendritic segment length was increased in the CH group, suggesting persistence of an immature pattern.
MAP-2 is a highly energy-sensitive protein (Avila et al., 1994). In vivo, MAP-2 phosphorylation is a dendrite-specific event, and its regulation is important in neuronal plasticity (Diaz-Nido et al., 1990). Phosphorylation of MAP-2 and dendritic branching occur in parallel (Diez-Guerra and Avila, 1993). Phosphorylation of MAP-2 results in inhibition of microtubule assembly, thereby resulting in cytoskeletal rearrangement and thus, determining the final dendritic branching pattern (Jameson et al., 1980; Murthy and Flavin 1983). The immature dendritic pattern in the CH group may suggest decreased phosphorylation of MAP-2 due to decreased energy availability. Similar, immature dendritic morphology demonstrated in perinatal iron deficiency (Jorgenson et al., 2003), a condition characterized by compromised energy production in the hippocampus (deUngria et al., 2000; Rao et al., 2003), supports our contention.
The longer apical length seen in our study suggests decreased dendritic branching. Studies correlating synaptic efficacy with dendritic branching have shown that increased number of branches in the dendritic segments are associated with increased synaptic efficacy (Groc et al., 2002). Hence, we can conclude that the longer apical segments observed in this study after CH exposure, are likely associated with reduced synaptic efficacy. Consistent with this possibility, an increase in the apical segment length in the CA1 pyramidal neurons of the hippocampus (measured by MAP-2 immunohistochemistry) following perinatal iron deficiency was associated with alteration in long-term potentiation in the hippocampus (Jorgenson et al., 2003; Jorgenson et al., 2005). Hence, the persistence of an immature pattern of dendritic morphology may at least partially explain the impairment in fear conditioning seen in the adult rat in the present study.
In contrast to our original hypothesis, both trace and delay fear conditioning were reduced in the young adult after early postnatal exposure to CH. Both trace and delay fear conditioning remained altered, even after controlling for reduced shock reactivity in the CH rats This suggests that the deficit in fear conditioning was not caused by impaired nociception. Furthermore, the fact that startle reactivity was unaffected in the CH animals suggests that the deficit in trace and delay fear conditioning was not caused by impaired processing of the auditory CS. Therefore our data suggest that postnatal CH affects both forms of fear conditioning, long after the animals have returned to a normoxic state.
One possible mechanism underlying the impairment in both forms of fear conditioning is that the amygdala may be affected in CH. Both trace and delay fear conditioning require participation of the amygdala (Walker and Davis, 2002). Therefore, it is possible that any underlying differences in hippocampus-dependent learning were masked by an overriding dysfunction of the amygdala. Further research is necessary to investigate the effect of CH on the structure and function of the amygdala.
A second possible explanation for the altered trace and delay fear conditioning may be related to the extent of hippocampal injury present. Recent evidence suggests that trace fear conditioning relies on the dorsal hippocampus, but both trace and delay conditioning require the ventral hippocampus, which projects directly to the amygdala (Burman et al., 2006). Hence, the abnormal delay and trace fear conditioning seen in our model may reflect damage to the hippocampus throughout its longitudinal axis.
Although the behavioral and morphological effects associated with the CH rats may result directly from CH, it is also possible that they are caused indirectly, via one or more mediating variables. For example, the body weight was reduced in the CH rats perhaps indicative of malnutrition. Although studies of dendritic morphology in models of malnutrition show alterations of dendritic spine density and terminal dendritic segment lengths in granule cells, cells in the CA1 region are spared (Andrade et al., 1996). Moreover, although deficits in hippocampus-dependent behavior following perinatal malnutrition have been reported, these changes were transient and had normalized by adulthood (Castro and Rudy, 1987; Castro and Rudy, 1989). In the current study, in contrast, early-life hypoxia induced cytoarchitectural abnormalities in CA1 region of the hippocampus, and impairments in fear conditioning in adulthood.
An alternative possibility is that the observed effects were a direct result of brain iron deficiency in the CH group. Even though brain iron concentration of CH rats was similar to that of controls by P56, our previous study has demonstrated 20% brain iron deficiency during the period of CH (Raman et al, 2005). Early-life brain iron deficiency can produce long-term cognitive sequelae (Lozoff et al, 2006). However, the pattern of fear conditioning deficits was not consistent with those previously reported in rats exposed to perinatal iron deficiency (McEchron et al., 2005).
Finally since the dams were also chronically hypoxic as their pups, the observed cytological and behavioral effects could be mediated, at least in part, by changes in the mothers’ behavior towards their pups. Differences in maternal behavior can have profound long-term neuronal morphological, neurochemical, neuroendocrine, and behavioral consequences in the adult offspring (Huot et al., 2002; Meaney, 2001). Although worthy of further investigation, this possibility cannot be excluded definitively because, the CH model does not permit hypoxic pups to be cross-fostered to normoxic dams.
In summary, CH had a significant effect on the dendritic morphology of the CA1 region of the hippocampus, as well as on both delay and trace fear conditioning measured four weeks after correction of hypoxia. Because delay and trace fear conditioning were both impaired, disruption of neural circuitry beyond the dorsal hippocampus may be affected. It remains to be seen whether similar anatomical abnormalities are also present in brain regions implicated in fear conditioning such as the amygdala. Nevertheless, in view of the importance of the hippocampus in memory, the persistent morphological abnormalities in CA1 observed in our study may contribute to the cognitive deficits that follow exposure to hypoxia early in life.
4. Experimental Procedure
4.1. Animal preparation
The study was conducted according to the guidelines of the Institutional Animal Care and Use Committee at the University of Minnesota. Pregnant Sprague-Dawley rats were purchased (Harlan Sprague-Dawley; Indianapolis, IN) and housed individually with free access to food and water in the local animal care facility. The dams and pups were maintained on an ad libitum diet (Teklad 4% Mouse/Rat diet 7001, Harlan-Teklad; Madison, WI). On P3, the pups were culled randomly to eight per litter to ensure adequate nutrition. Fourteen rats (male and female), eight in the CH group and six in the control group, were used for apical segment measurements on P28. Sixteen rats (male and female), eight each in the CH and control groups, were used for apical segment measurements on P56. One to three male rats from each of the 6 normoxic litters and one to two male rats from each of the 5 previously hypoxic litters were used for trace and delay fear conditioning at P56. Across all treatments and training conditions, 35 rats were used for the behavioral experiments. A separate set of animals from a single litter (n=4/group) was used for brain weight and brain iron measurement at P56.
4.2. Induction of Chronic Hypoxia
Dams with their pups were randomly allotted to control and CH groups. The dams and pups in the CH group were housed in a plexiglass cage placed within a larger, airtight plexiglass chamber (Scientific Apparatus; University of Minnesota; Minneapolis, MN) from P3 to P28. The oxygen concentration was maintained at 10% ± 1% by mixing air and nitrogen and was continuously monitored using an oxygen analyzer (Miniox II; Catalyst Research Corporation; Owings Mills, MD). The degree of hypoxia was based on our previous experience (Raman et al., 2005) and existing literature (Ment et al.,1998; Xia et al., 1995). Excess humidity from the chamber was removed using calcium bisulphate (Drierite; W.A. Hammond Drierite Company; Xenia, OH). The chamber was opened twice a week for approximately 10 minutes for maintenance. The chamber was kept in a temperature-controlled room with a 12-hour light:dark cycle, with lights on at 0600. The animals were removed from the chamber on P29 and were maintained in normoxia (room air) thereafter. Control animals were maintained in normoxia throughout. They were housed and maintained in a manner that was identical to the hypoxic animals, except that their cages were not placed within an airtight outer chamber.
4.3. Tissue Preparation
Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg i.p). They were transcardially perfused with normal saline, followed by 5% formalin and 5% sucrose in 0.01% phosphate buffer saline (PBS; pH = 7.4). The brains were removed and postfixed with sucrose-formalin solution at 4°C for 24 hours and then transferred to serial sucrose solutions of 20%, 30% and 40% over three days for cryoprotection. Brains were embedded in tissue medium (Triangle Biomedical Sciences, Durham, NC) and stored at −80°C until ready for sectioning. Twenty-μm coronal sections were obtained using a cryostat (Model CM 1900, Leica Instruments GmbH, Nussloch; Germany) at −23°C to −25°C mounted onto a poly-L-lysine coated slide and stored at −20°C until immunohistochemistry. Rats that were used for iron estimation were perfused with normal saline, and brains were dissected, flash-frozen and stored at −80° C. Brain iron assay was performed by atomic absorption spectroscopy and expressed in μg of elemental iron/g of wet tissue weight (Rao et al., 1999).
4.4. Immunohistochemistry
Dendritic morphology was assessed in the control and CH group on P28 and P56 using MAP-2 histochemistry (Jorgenson et al., 2003). The slides were treated with 3% hydrogen peroxide for 30 min to remove any endogenous peroxidase activity, followed by incubation in blocking solution with 1% donkey serum for 30 min. After gently tapping off the excess blocking solution, the slides were incubated with the primary antibody for MAP-2 (1:100 dilution; Abcam International, Cambridge, MA) for 2 hours at room temperature. Control sections were treated similarly without the incubation of the primary antibody against MAP-2. After the slides were washed with PBS, they were incubated with secondary antimouse IgG tagged with fluorophore Cy3 (1:800, Jackson Immuno, West Grove, PA) for 45 min. The sections were then rinsed in PBS twice for 5 min and mounted with fluorescence mounting media (Vector Laboratories, Burlingame, CA).
4.5. Histochemical analysis
Slides stained with MAP-2 were visualized using a Nikon E600 microscope with CF169 infinity optics utilizing a TRITC cube ( Nikon, Tokyo, Japan) at a magnification of × 200–1000. Digital microscopic images were obtained using a mounted digital camera (Nikon, Tokyo, Japan) and projected on to computer screen using the ACT-1 software program (Nikon, Tokyo, Japan). A total of 17 sections in the control group and 20 sections in the CH group on P28, and 29 sections in the control group and 25 sections in the CH group on P56 were used for analyses (2–4 sections per animal). Only sections that included the entire cross-sectional dorsal hippocampus (subareas CA1, CA3 and dentate gyrus) were used for quantification. Hippocampal sections from 0.8 mm to 2.6 mm anterior to the interaural line on P28, and 0.8 mm to 3.0 mm anterior to the interaural line on P56, were used for visualization (Sherwood and Timirus, 1970). This area was chosen because it corresponded to the placement of the volume of interest in our previous 1H NMR study (Raman et al., 2005). The length of the dendritic segment extending from the pyramidal soma in CA1 to the first proximal branch was measured (Fig 1). Only dendrites in which the connection to the pyramidal soma and a clear presence of lateral branching in the field plane could be seen were used for quantification. A total of 196 dendrites were analyzed on P28 (94-control, 102-CH) and a total of 234 dendrites were analyzed on P56 (109-control, 125-CH).
4.6. Behavioral study
Male rats from 6 normoxic and 5 hypoxic litters were assessed for trace and delay fear conditioning at P56. Following CH, rats were transferred to another laboratory for behavioral testing. The light:dark cycle in the second lab was also for 12 hours, with lights on at 0800. Rats were left undisturbed for 3 weeks prior to behavioral assessment. Two days prior to behavioral testing, rats were handled for approximately 5 min on each day.
4.6.1. Apparatus
Animals were tested in four identical 7.5-cm × 9.0-cm × 17.0-cm stabilimeter devices. Each stabilimeter consisted of a plexiglass cage that rested on 4 compression springs and was located within a ventilated sound-attenuating chamber. Cage movement resulted in the displacement of a Type 338B35 accelerometer (PCB Piezotronics, Depew, NY) attached to the top of the cage. The accelerometer voltage was amplified by a signal processor (Model 482A20; PCB Piezotronics) and was proportional to the velocity of cage displacement. An Instrunet 100b board (GW Instruments, Somerville, MA) interfaced to a Macintosh G3 microcomputer digitized the analog output of the accelerometer on a scale of 0–1000 units. Startle amplitude was defined as the peak accelerometer voltage that occurred during the first 200 ms after the onset of the startle stimulus. High-frequency speakers (Radio Shack Supertweeters, range 5–40 kHz) located 5 cm from the cage delivered the 50-ms (rise-decay: 5-ms) bursts of white noise (low pass, 22 kHz) at 95 and 105 dB. White noise through the high-frequency speakers, together with the ventilation system, elevated the background noise to 58 dB. The footshock was a 0.5-s, 0.6-mA constant current scrambled shock delivered by a shock generator (SGS-004, by BRS-LVE) through the four bars that made up the bottom of the stabilimeter device. Shock intensity was measured with a 1-kΩ resistor across a differential channel of an oscilloscope in series with a 100-kΩ resistor connected between two floor bars in each cage. Current was defined as the root mean square voltage across the kΩ resistor, where mA = 0.707 × 0.5 peak-to-peak voltage. The CS was a 4-s or a 7.5-s, 65-dB band pass-filtered noise, with high and low cut-offs set at 4 kHz and 24 dB per octave attenuation. The noise was generated by the computer and delivered through a low-frequency speaker (Radio Shack woofer, model 40-1024A) situated 15 cm from the cage.
4.6.2. Baseline Assessment
We assessed the response to the acoustic startle stimuli across two days to habituate the rats to the chambers and stimuli, and to establish a stable startle baseline (Harris and Gewirtz, 2004). Animals were grouped into trace and delay conditions according to their startle responses on the second day of testing to ensure similar mean basal startle in each group. A test session consisted of a 5-min habituation period followed by 20 min of testing. During testing, an acoustic startle stimulus was presented every 30 s, with 20 stimuli of each intensity (95 dB and 105 dB) presented in a semi-random order during the session. After the testing session was over, the chambers were cleaned with 70% ethanol.
4.6.3. Trace and Delay Fear-Conditioning
On each of 3 days, rats were exposed to 16 pairings of a conditioned stimulus (tone) with an unconditioned stimulus (footshock) after a 5-min acclimation period. Delay conditioning consisted of a 7.5-s tone coterminating with a 0.5-s footshock. Trace conditioning consisted of a 4-s tone followed 3 s later by the 0.5-s footshock. Hence, the interval between CS and US onset was the same in the two conditions. The interval between shocks was variable, with a mean of 2.75 min (range 2.0–3.5 min) (Burman and Gewirtz, 2004).
4.6.4. Assessment of fear-potentiated startle
Twenty-four hours after the third training session, rats were assessed for the presence of fear-potentiated startle. After a 5-min acclimation period, 30 startle stimuli (15 at each of 95 and 105 dB) were delivered in a pseudorandom order every 30 s. This was followed by three types of test trials, in which the startle stimulus was presented: 1) 3.5 s after the onset of CS; 2) 7 s after the onset of CS; and 3) in the absence of CS. CS duration in these test trials was the same as during training (i.e., 4 s in the trace conditioning groups and 7.5 s in the delay conditioning groups.) There were 5 presentations of each type of trial, and the interval between successive startle stimuli was 30 s throughout the session.
4.7. Statistical analyses
Apical dendritic segment length was measured in μm and mean apical length was determined in each group. The effects of age and CH and age on apical segment length were compared using univariate ANOVA. The effect of CH at a particular age was compared using unpaired two-tailed t-tests.
Baseline startle was analyzed as the mean startle response to each stimulus over the second baseline startle session (Harris and Gewirtz 2004). Shock reactivity was measured as the average cage displacement upon application of the footshock during the fear conditioning procedure. Fear-potentiated startle was calculated by comparing mean startle amplitude obtained on each CS trial type to mean startle amplitude obtained on startle-alone trials [i.e., (CS-startle - Startle alone)/startle alone × 100%] (Burman and Gewirtz, 2004). In some cases, two or three rats from a litter were exposed to the same experimental condition. In such cases, the values from all rats from the same litter were averaged prior to statistical analysis. ANOVA was used to analyze baseline startle, shock reactivity and fear-potentiated startle. Because of a significant effect of CH on shock reactivity, an analysis of covariance (ANCOVA) was used to determine if effects of CH on fear-potentiated startle were the result of differences in shock reactivity. The data are presented as mean ± SEM. Statistical significance was set at P < 0.05.
Acknowledgments
This work was supported by in part by grants from Vikings Children’s Fund (GIA09-04), the Center for Neurobehavioral Development, the Minneapolis Medical Research Foundation, and the National Institutes of Health (HD33692 and T32 DA09079). The technical assistance of Feng Wang, Kathleen Ennis, Jane Wobken, and Eric Reese with manuscript preparation is gratefully acknowledged.
Abbreviations
- CAMK-II
calcium calmodullin kinase II
- CH
chronic hypoxia
- Cr
creatine
- MAP-2
microtubule-associated protein-2
- PCr
phosphocreatine
- P
postnatal day
- CS
conditioned stimulus
- US
unconditioned stimulus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade JP, Castanheira-Vale AJ, Paz-Dias PG, Madeira MD, Paula-Barbosa MM. The dendritic trees of neurons from the hippocampal formation of protein-deprived adult rats. A quantitative Golgi study. Exp Brain Res. 1996;109:419–433. doi: 10.1007/BF00229626. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Avila J, Dominguez J, Diaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol. 1994;38:13–25. [PubMed] [Google Scholar]
- Bernhardt R, Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984;226:203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC. Timing of fear expression in trace and delay conditioning measured by fear-potentiated startle in rats. Learn Mem. 2004;11:205–212. doi: 10.1101/lm.66004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Caceda R, Gamboa JL, Boero JA, Monge CC, Arregui A. Energetic metabolism in mouse cerebral cortex during chronic hypoxia. Neurosci Lett. 2001;301:171–174. doi: 10.1016/s0304-3940(01)01630-5. [DOI] [PubMed] [Google Scholar]
- Castro CA, Rudy JW. Early-life malnutrition selectively retards the development of distal- but not proximal-cue navigation. Dev Psychobiol. 1987;20:521–537. doi: 10.1002/dev.420200506. [DOI] [PubMed] [Google Scholar]
- Castro CA, Rudy JW. Early-life malnutrition impairs the performance of both young and adult rats on visual discrimination learning tasks. Dev Psychobiol. 1989;22:15–28. doi: 10.1002/dev.420220103. [DOI] [PubMed] [Google Scholar]
- deUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgeiff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Diaz-Nido J, Serrano L, Hernandez MA, Avila J. Phosphorylation of microtubule proteins in rat brain at different developmental stages: comparison with that found in neuronal cultures. J Neurochem. 1990;54:211–222. doi: 10.1111/j.1471-4159.1990.tb13303.x. [DOI] [PubMed] [Google Scholar]
- Diez-Guerra FJ, Avila J. MAP2 phosphorylation parallels dendrite arborization in hippocampal neurones in culture. Neuroreport. 1993;4:419–422. doi: 10.1097/00001756-199304000-00020. [DOI] [PubMed] [Google Scholar]
- Fineman I, Hovda DA, Smith M, Yoshino A, Becker DP. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Using pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learn Mem. 2000;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- Groc L, Petanjek Z, Gustafsson B, Ben-Ari Y, Hanse E, Khazipov R. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. Eur J Neurosci. 2002;16:1931–1938. doi: 10.1046/j.1460-9568.2002.02264.x. [DOI] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Hoffman JIE. Incidence, prevalence, and inheritance of congenital heart disease. In: Moller JH, Hoffman JIE, editors. Pediatric Cardiovascular Medicine. 1. Churchill Livingstone; Philadelphia: 2000. pp. 257–262. [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Jameson L, Frey T, Zeeberg B, Dalldorf F, Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980;19:2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Kutina-Nelson KL, Hritz MA, Huang Z, Wong-Riley MT. Decreased rat brain cytochrome oxidase activity after prolonged hypoxia. Brain Res. 1996;720:1–6. doi: 10.1016/0006-8993(95)01495-0. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Barbara F, Georgieff Mk, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JE. Cardiopulmonary bypass. In: Chang AC, Hanley FL, Wernovsky DLG, editors. Pediatric Cardiac Intensive Care. Williams and Wilkins; Baltimore: 1998. pp. 189–199. [Google Scholar]
- McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci. 2005;8:195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 1998;111:197–203. doi: 10.1016/s0165-3806(98)00139-4. [DOI] [PubMed] [Google Scholar]
- Monnerie H, Shashidhara S, Le Roux PD. Effect of excess extracellular glutamate on dendrite growth from cerebral cortical neurons at 3 days in vitro: Involvement of NMDA receptors. J Neurosci Res. 2003;74:688–700. doi: 10.1002/jnr.10797. [DOI] [PubMed] [Google Scholar]
- Murthy AS, Flavin M. Microtubule assembly using the microtubule-associated protein MAP-2 prepared in defined states of phosphorylation with protein kinase and phosphatase. Eur J Biochem. 1983;137:37–46. doi: 10.1111/j.1432-1033.1983.tb07792.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi k, Inoue M, Sugawara E, Sano S. Ischemic and reperfusion injury of the cyanotic myocardium in chronic hypoxia rat model: changes in cyanotic myocardial antioxidant system. J Thorac Cardiovasc Surg. 1997;114:1088–1096. doi: 10.1016/S0022-5223(97)70024-2. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The ontogeny of human memory: A cognitive neuroscience perspective. Developmental Psychology. 1995;31:723–738. [Google Scholar]
- Nelson CA, Thomas KM, de Haan M. Neural bases of cognitive development. In: Damon W, Lerner R, Kuhn D, Siegler R, editors. Handbook of Child psychology, Cognitive, perception and language. Vol. 2. John Wiley and Sons; New Jersey: 2006. pp. 3–57. [Google Scholar]
- Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull. 1981;7:113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Trojan S. Chronic changes in the receptive field of the pyramidal cells of the rat hippocampus after intermittent postnatal hypoxia. Physiol Bohemoslov. 1983;32:393–402. [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res Dev Brain Res. 2005;156:202–209. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Rao R, deUngria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal Iron deficiency increases the vulnerability of rat hippocampus to hypoxia ischemic insult. J Nutr. 1999;129:199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timirus PS. A Stereotaxic Atlas of The Developing Rat Brain. Berkeley, Los Angeles, London; University of California Press: 1970. [Google Scholar]
- Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in a immature rat model of cerebral hypoxia-ischemia a light microscopic study. Brain Res Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Wray J, Sensky T. Controlled study of preschool development after surgery for congenital heart disease. Arch Dis Child. 1999;80:511–516. doi: 10.1136/adc.80.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Warshaw JB, Haddad GG. Chronic hypoxia causes opposite effects on glucose transporter1 mRNA in mature versus immature rat brain. Brain Res. 1995;675:224–230. doi: 10.1016/0006-8993(95)00079-6. [DOI] [PubMed] [Google Scholar]