Abstract
Auditory filial imprinting in the domestic chicken is accompanied by a dramatic loss of spine synapses in two higher associative forebrain areas, the mediorostral neostriatum/hyperstriatum ventrale (MNH) and the dorsocaudal neostriatum (Ndc). The cellular mechanisms that underlie this learning-induced synaptic reorganization are unclear. We found that local pharmacological blockade of N-methyl-d-aspartate (NMDA) receptors in the MNH, a manipulation that has been shown previously to impair auditory imprinting, suppresses the learning-induced spine reduction in this region. Chicks treated with the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV) during the behavioral training for imprinting (postnatal day 0–2) displayed similar spine frequencies at postnatal day 7 as naive control animals, which, in both groups, were significantly higher than in imprinted animals. Because the average dendritic length did not differ between the experimental groups, the reduced spine frequency can be interpreted as a reduction of the total number of spine synapses per neuron. In the Ndc, which is reciprocally connected with the MNH and not directly influenced by the injected drug, learning-induced spine elimination was partly suppressed. Spine frequencies of the APV-treated, behaviorally trained but nonimprinted animals were higher than in the imprinted animals but lower than in the naive animals. These results provide evidence that NMDA receptor activation is required for the learning-induced selective reduction of spine synapses, which may serve as a mechanism of information storage specific for juvenile emotional learning events.
Spines are considered specialized structures subserving a biochemical compartmentalization to provide a protected microenvironment for calcium and other messengers and, therefore, to play a key role in the expression of synaptic plasticity (1–5). Changes in the numerical density of spine synapses are proposed to represent a principal cellular correlate of learning and memory formation (6–12). Auditory filial imprinting in the domestic chicken is associated with a reduction of spine synapses in two associative forebrain areas, the mediorostral neostriatum/hyperstriatum ventrale (MNH) and the reciprocally connected dorsocaudal neostriatum (Ndc) (8, 12–14), an area that provides indirect auditory input into the MNH (15). Based on anatomical criteria, such as its glutamatergic thalamic afferents and its tegmental dopaminergic inputs, the MNH may be considered as the avian analogue of the mammalian prefrontal cortex, whereas the Ndc seems to correspond to second-order parasensory association areas in the mammalian parietotemporal cortex (15–17).
The cellular and molecular mechanisms that trigger, regulate, and mediate proliferative and regressive changes of synaptic density in the course of this juvenile learning event are unclear. Although there is convincing evidence that activation of N-methyl-d-aspartate (NMDA) receptors is a key step in learning and memory formation (18–28) and that it is involved in the cellular events underlying synaptic plasticity in the hippocampus (29, 30), memory storage in the neocortex (31), and activity-dependent development of neuronal connectivity in sensory systems (32–33), a direct link between NMDA receptor activation and learning-related morphological synaptic changes, in particular, synaptic elimination, has not yet been revealed.
Changes of the glutamate system in relation to filial imprinting have been shown in microdialysis studies, where an enhanced release of glutamate was observed in imprinted animals during presentation of the learned acoustic stimulus (34), which is paralleled by an enhanced neuronal activity (8, 35, 36). The application of the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV) into the MNH was shown to induce a severe impairment of auditory imprinting in a dose-dependent manner (35). In addition, this APV treatment prevents the development of enhanced metabolic excitability in the MNH, which in imprinted chicks can be typically evoked by presentation of the acoustic imprinting stimulus (35). Furthermore, NMDA-dependent long-term potentiation (LTP) has been described in the spine-losing neuron type in the MNH (37).
If the activation of NMDA receptors is required for auditory filial imprinting, is it also involved in the synaptic selection that is associated with this learning process?
To address this question the effect of a local NMDA receptor blockade in the MNH on the learning-induced synaptic elimination was examined. In addition to the analysis of spine frequencies and dendritic length in the injected MNH, these parameters were analyzed in the reciprocally connected Ndc, because previous studies indicated possible transsynaptic effects of the pharmacological treatment (16, 38). As control, spine frequencies in the primary sensory ectostriatum, an equivalent of the mammalian visual cortex, which lies directly adjacent to the injection site and is not involved in auditory imprinting, were analyzed.
MATERIALS AND METHODS
Subjects.
Eggs of White Leghorn chickens were obtained from a local hatchery (Horstmann, Nienburg, Germany) and individually incubated at 37.5 ± 0.3°C in acoustically isolated boxes. After hatching, the chicks were individually reared in these isolation boxes at 28–30°C with free access to food and water. The boxes were illuminated by diffuse light (12-h light/12-h dark cycle), and the animals were kept under a continuous white-noise level to avoid acoustic deprivation. These strictly controlled rearing conditions were chosen to exclude external sensory stimuli that could lead to incidental imprinting.
Behavioral Procedures.
The training and test procedures (Fig. 1) were conducted in a V-shaped arena. On the hatching day (day 0) the chicks were stimulated for 2 × 15 min with a rhythmic tone pulse (frequency modulated with an average frequency of 400 Hz; for details see ref. 36) in the presence of a mother surrogate. On day 1 an approach test consisting of two trials was performed in which the chicks were given the opportunity to approach the tone stimulus. To test whether imprinting had been successful two discrimination tests (on day 1 and 2 posthatch), each consisting of four trials, were performed in which the animals had to show a clear preference for the imprinted tone pulse toward an alternately presented novel acoustic stimulus (frequency-modulated tone pulse with an average frequency of 700 Hz). During the approach and discrimination tests the mother surrogate was not visible. A discrimination trial was scored positive when the chicken approached the imprinting stimulus within 3 min; longer approach times or approaching the unfamiliar tone were scored negative. A complete discrimination test was scored positive if at least three of the four discrimination trials were scored positive, and a chicken was considered imprinted only if the two discrimination tests were scored positive. The probability to incidentally score at least three positive trials out of four total trials is P = 0.11 (binomial distribution). The random incidence that a chicken scores both discrimination tests correct is P = 0.012 (multiplication theorem).
Figure 1.
Experimental design. Bars represent treatment of the experimental groups: N, naive chicks; I, noninjected, imprinted chicks; V, vehicle-injected, imprinted chicks; A, APV-injected, trained but nonimprinted chicks. Solid arrows indicate time points of APV injections; open arrows indicate time points of vehicle injections. See text for further details.
Experimental Groups.
For quantitative spine analysis in Golgi–Cox impregnated neurons, chicks of four experimental groups were analyzed (Fig. 1).
Naive chicks (n = 5).
Chicks of this group were reared in the isolation boxes until postnatal day 7 without any sensory or social contact.
Noninjected, imprinted chicks (n = 3).
These chicks were imprinted as described and had developed a clear preference for the imprinting stimulus.
Vehicle-injected, imprinted chicks (n = 3).
Chicks of this group received bilateral intracerebral microinjections of 1 μl Hanks’ buffered salt solution (HBSS buffer, Gibco) into the MNH region according to the time schedule shown in Fig. 1. The imprinting procedure was performed as described, and the animals displayed a clear preference for the imprinting stimulus.
APV-injected trained, but nonimprinted chicks (n = 5).
These chicks received bilateral intracerebral microinjections of 1 μl APV (Sigma) into the MNH, following the same time schedule as for the vehicle-injected group (Fig. 1). The injected APV dose was 12.5 nmol, a dose that significantly impairs imprinting (35). The chicks of this group were exposed to the same imprinting procedure as described for the other two groups, but none of these chicks developed a preference for the imprinting tone.
Golgi–Cox Staining and Analysis.
All animals were sacrificed on day 7, and the brains were rapidly removed and immersed in 50 ml of Golgi–Cox solution for 14 days. Brains were dehydrated and embedded in 8% Celloidin, and serial transverse 150-μm forebrain sections were collected and mounted on glass slides. The sections were processed according to a modified Golgi–Cox method that allows parallel Golgi impregnation and Nissl staining (39). Spine frequencies and dendritic length of large type I neurons in the MNH and in the Ndc (see refs. 8, 13, and 14) were quantified. As controls, neurons in the ectostriatum, a primary sensory area and equivalent of the mammalian visual cortex, were analyzed. The ectostriatum was chosen because it is located directly adjacent to the MNH. All neurons were analyzed using the image analysis system neurolucida (MicroBrightField, Colchester, VT), which allows quantitative three-dimensional analysis of complete dendritic trees. For each experimental group approximately 80 (MNH, Ndc) or 50 dendrites (ectostriatum), respectively, were analyzed. The entire length of the dendritic trees, which were subdivided into branch orders and numbered consecutively from proximal (branch order 1) to distal (compare Figs. 2 and 3), was measured by tracing the whole dendrite while counting dendritic spines. The mean spine frequencies (number of visible spines per 10 μm) and the average length of each dendritic branch were calculated and tested for significant differences between the groups using a Kruskal–Wallis One-Way ANOVA followed by a two-tailed Mann–Whitney U test. All measurements were made by an experimenter blind to the experimental condition of the animals.
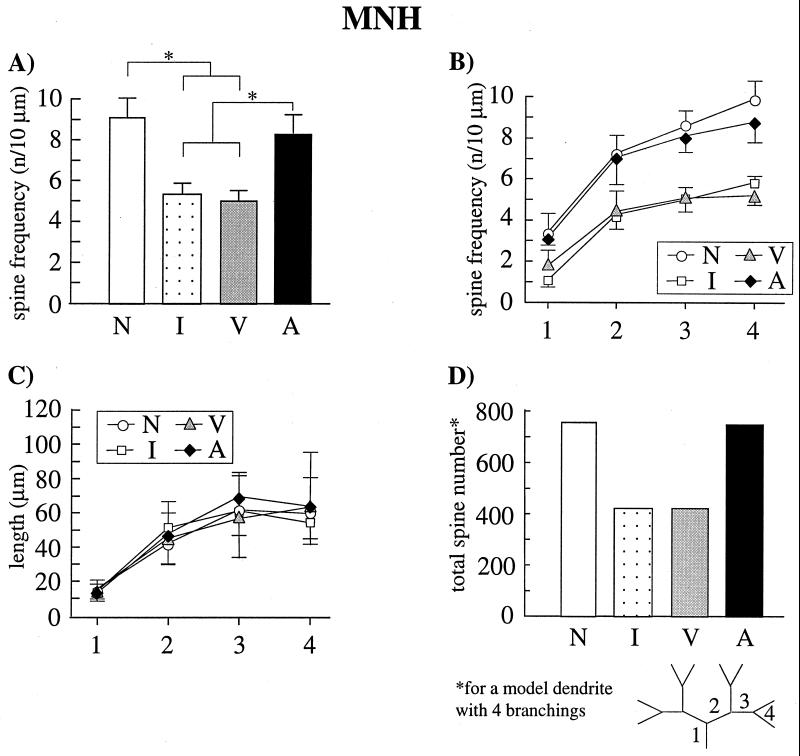
Figure 2.
Spine frequencies, dendritic length, and spine number of type I neurons in the MNH from naive chicks (N), noninjected, imprinted chicks (I), vehicle-injected, imprinted chicks (V), and APV-injected, trained but nonimprinted chicks (A). (A) Mean spine frequencies (±SD) of the pooled 3rd and 4th dendritic branch orders. Spine frequencies of imprinted animals (groups I and V) were significantly lower than those in naive and APV-treated animals (*, P ≤ 0.02). APV-treated animals displayed a similar high spine frequency as the naive control animals. (B) Mean spine frequencies (±SD) for all dendritic segments from proximal (1) to distal (4). Segments were numbered from proximal to distal according to the illustration in D. The results described for the pooled 3rd and 4th branch order (A) were found in all dendritic segments. (C) Mean length (±SD) for all dendritic segments. There were no differences between the four experimental groups. (D) Spine number for a model dendrite with four complete branchings calculated under consideration of the mean spine frequencies and length of dendritic branches. In imprinted chicks spine number was 44% lower than in naive chicks. APV-treated chicks displayed nearly the same spine number as naive control animals.
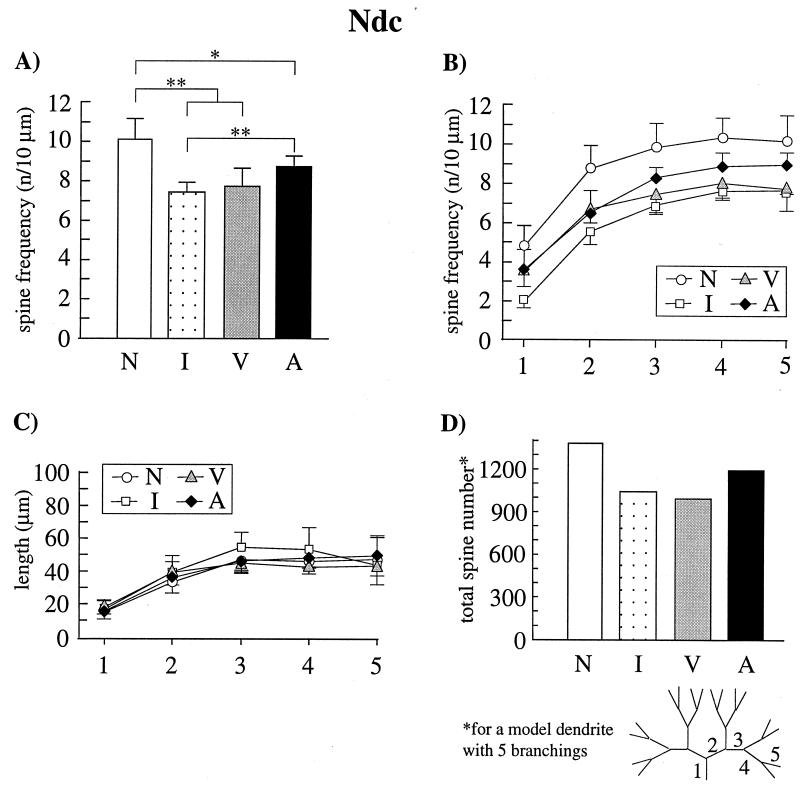
Figure 3.
Spine frequencies, dendritic length, and spine number of neurons in the dorsocaudal neostriatum (Ndc) from naive chicks (N), noninjected, imprinted chicks (I), vehicle-injected, imprinted chicks (V), and APV-injected, trained but nonimprinted chicks (A). (A) Mean spine frequencies (±SD) of the pooled 3rd to 5th dendritic branch orders. Spine frequencies of imprinted animals (groups I and V) were significantly lower than those in naive and APV-treated animals (∗, P ≤ 0.01). APV-treated chicks displayed a significantly higher spine frequency than the imprinted animals (∗, P ≤ 0.01) and a significantly lower spine frequency than the naive controls (*, P ≤ 0.05). (B) Mean spine frequencies (±SD) for all dendritic segments from proximal (1) to distal (5). Segments were numbered according to the illustration in D. The results described for the pooled 3rd to 5th branch orders (A) were found in all dendritic segments with exception of the basal segment in which the noninjected, imprinted animals (I) had a significantly lower spine frequency than the other three groups. (C) Mean length (±SD) for all dendritic segments. There were no differences between the four experimental groups. (D) Spine number for a model dendrite with five complete branchings calculated under consideration of the mean spine frequencies and length of dendritic branches. In imprinted animals spine number was 25% lower than in naive controls. The spine number of APV-injected animals was between the spine number of the naive animals and those of the imprinted groups.
RESULTS
MNH.
Bilateral injections of APV into the MNH during auditory imprinting nearly completely suppressed the elimination of dendritic spines in this area. Pooling the results for the distal dendritic segments (3rd and 4th branch order), on which spine reduction was most prominent (8), a significant difference in the spine frequency between the four experimental conditions was found (P ≤ 0.01, Kruskal–Wallis One-Way ANOVA). Compared with naive control chicks, the untreated, imprinted chicks and the vehicle-injected, imprinted chicks displayed an about 40% lower spine frequency (P ≤ 0.02, Mann–Whitney U test) (Fig. 2A). No differences were detected between naive and APV-injected chicks and between untreated, imprinted and vehicle-injected, imprinted chicks. APV-injected chicks displayed a higher spine frequency than animals of the two imprinted groups (untreated, vehicle-injected) (P ≤ 0.02).
Comparison of the spine frequencies for each dendritic branch order revealed significant differences (P ≤ 0.05, Kruskal–Wallis One-Way ANOVA) between the four experimental conditions in each branch order from proximal (1st order) to distal (4th order) (Fig. 2B). In each branch order the results matched those described for the pooled 3rd and 4th branch order.
The average length of the dendritic segments did not differ between the different experimental groups (Fig. 2C), indicating that the observed differences in spine frequency are due to changes in the total number of dendritic spines. Calculated values for a model dendrite with four complete branchings under consideration of the average spine frequencies and length of dendritic branches revealed a 44% lower total number of spine synapses in imprinted chicks compared with naive controls. APV-treated chicks displayed nearly the same number of spine synapses as naive control animals (Fig. 2D).
Ndc.
In the Ndc, which is reciprocally connected with the MNH and most likely not directly influenced by the injected APV (35), the suppression of spine elimination in the APV-treated animals was only partial (Fig. 3). Pooling the results for the distal dendritic segments (3rd to 5th branch order), significant differences in the spine frequency between the four experimental conditions were found (P ≤ 0.05, Kruskal–Wallis One-Way ANOVA). Ndc neurons of noninjected, imprinted and vehicle-injected, imprinted chicks displayed significantly lower spine frequencies compared with naive control animals (P ≤ 0.01; Fig. 3A). However, in contrast to the MNH neurons, the Ndc neurons of APV-injected chicks displayed significantly lower spine frequencies than those of the naive controls (P ≤ 0.05). No significant difference was found between APV-injected and vehicle-injected, imprinted chicks and between vehicle-injected, imprinted and noninjected, imprinted chicks. A significantly lower spine frequency was seen in the noninjected, imprinted chicks compared with the APV-injected animals (P ≤ 0.01). Comparison of the spine frequencies in each dendritic branch order revealed results similar to those obtained for the pooled 3rd–5th branch order, with exception of the basal segment, in which the noninjected, imprinted animals displayed a significantly lower spine frequency than the other three groups (P ≤ 0.05; Fig. 3B).
The average length of the dendritic segments did not differ between the four experimental groups (Fig. 3C), again indicating that the observed differences in spine frequency are due to changes in the total number of dendritic spines. Calculated values for a model dendrite with five complete branchings revealed an approximately 25% lower number of spine synapses in imprinted chicks compared with naive controls. The spine number of APV-injected animals was between the spine number of the naive animals and the imprinted groups (Fig. 3D).
Ectostriatum.
In the primary visual ectostriatum, which is located directly adjacent to the MNH and to the injection site of the drug, no differences of spine frequency and dendritic length were found between the four experimental conditions (Fig. 4).
Figure 4.
Spine frequencies (±SD) (A) and dendritic length (±SD) (B) of neurons in the ectostriatum from naive chicks (N), noninjected, imprinted chicks (I), vehicle-injected, imprinted chicks (V), and APV-injected, trained but nonimprinted chicks (A). In both parameters there were no differences between the four experimental groups.
DISCUSSION
The results presented here provide evidence that NMDA receptor activation is a crucial step in the elimination and selective survival of spine synapses, which accompanies a juvenile learning process.
The reduction in spine frequencies as a consequence of auditory imprinting found in this study confirms earlier results (8, 12). In extension of these studies we found (i) that the learning-induced spine reduction appears to be specific for higher associative forebrain regions such as the MNH and Ndc, which are directly involved in this learning process, and does not occur in the primary sensory ectostriatum and (ii) that the reduced spine frequencies reflect a reduction of the total number of spine synapses. Because already at birth, spines on type I neurons in the MNH bear the ultrastructural features of synaptic connections, i.e., they are occupied by one or more vesicle-filled axonal boutons (40), the observed changes most likely reflect the elimination of functional supernumerary synaptic connections rather than the pruning of “empty” spines.
Our results indicate further that experience-driven activation of NMDA receptors is critically involved in the learning-induced elimination and selective survival of spine synapses, but it seems not to interfere with the developmental spine proliferation that occurs during the first postnatal week (12, 41). This specific effect of NMDA receptor blockade on spine elimination may be partly due to the fact that we did not chronically block NMDA receptors but applied receptor blockade only during and around the time when the animals were exposed to the imprinting situation. Thus, developmentally regulated, proliferative changes in spine density may have remained more or less unaffected by the pharmacological treatment. Moreover, the finding that naive animals display high spine frequencies similar to APV-treated animals indicates that the high spine frequency in the APV-treated animals is not due to a spine proliferation occurring as a consequence of NMDA receptor blockade (42) but rather a developmental process. APV treatment appears not to induce degenerative changes of neuronal cytoarchitecture and function as described in other studies (43), which is indicated by the observation that APV-treated animals showed the same degree of spine proliferation as naive animals. Furthermore, no histologically detectable lesions or functional disturbances were detected in APV-injected animals (35). The functional properties of the neuronal network in this area appear to remain intact, because APV-treated chicks still could be imprinted a few days after the termination of the APV treatment and they developed the same enhanced metabolic excitability in the MNH as noninjected imprinted chicks (35).
NMDA receptor blockade not only blocked spine elimination in the APV-injected MNH but also partly suppressed the learning-related spine elimination in the reciprocally connected Ndc, which, according to previous control experiments, was not influenced directly by the injected drug (35). This may be indicative of a transsynaptic effect, e.g., via an excitatory synaptic input from NMDA-sensitive neurons in the MNH, whose excitation was blocked by the APV application. These MNH neurons may innervate spines of Ndc neurons, in which, because of the blockade of one of their excitatory inputs, spine elimination was partly suppressed. Some evidence for this presumed NMDA-mediated transsynaptic interaction between MNH and Ndc also is derived from 2-fluorodeoxyglucose studies in which the metabolic activity in the MNH was suppressed after APV injection into the Ndc (16).
Synaptic elimination requires neuronal activation, because it does not occur in our naive control animals that were raised under severe deprivation conditions. The synaptic selection hypothesis of imprinting (8, 13, 41) proposes that during learning only a subset of synapses in the MNH are activated and thereby strengthened and maintained, whereas nonactivated and inappropriate or exuberant synaptic contacts are weakened and finally removed. It is tempting to speculate that the stimulus-evoked glutamate release (34) and the resulting synaptic activation contribute to the potentiation and stabilization of activated synaptic contacts via activation of NMDA receptors located on dendritic spines. In parallel, a sequence of cytoplasmic events may trigger a hitherto unknown molecular signal that leads to the elimination of non- or less-activated spines. The process of synaptic selection requires an association between the acoustic imprinting stimulus and a positive emotional situation (mother), because presentation of the acoustic stimulus alone is not sufficient to induce these regressive synaptic changes (12).
Before being discovered as having a role during learning-induced spine elimination, NMDA receptor activation has been shown to play a role in synaptic elimination during development. In the cerebellum and retina the blockade of NMDA receptors prevents the elimination of climbing-fiber-Purkinje cell synapses and the elimination of dendritic spines on retinal ganglion cells, respectively (44, 45). Also, NMDA receptor activation is critical for the segregation of eye-specific stripes and pruning of retinal axon arbors in the central nervous system of frogs (46, 47) and for the experience-regulated maturation of the mammalian visual cortex (33, 48–50).
The NMDA receptor may be a key player in the initiation of mechanisms underlying learning-induced and developmental synaptic selection, because this receptor can serve as a detector of coincident pre- and postsynaptic activity. The NMDA receptor makes active synapses bidirectionally modifiable by triggering synaptic depression when input activity coincides with low postsynaptic responses and triggering synaptic potentiation when input activity coincides with large postsynaptic responses (50). Such mechanisms for modifying synaptic strength have been demonstrated in the spine-losing type I neurons, where, similar to the mammalian brain, the induction of LTP and LTD is critically dependent on the activation of NMDA receptors (37).
The cellular signaling mechanisms for synaptic elimination are unknown. They may include activity-induced changes of the balance between proteases and protease inhibitors (51), the selective removal of postsynaptic transmitter receptors (52), and competition of innervating terminals for trophic factors (53).
The learning- and experience-induced regressive changes during early postnatal development may be essential for the establishment of functional pathways, which enable the maturing individual to perceive and respond more selectively to behaviorally meaningful environmental stimuli and to acquire adequate behavioral strategies. It has been proposed that mental retardation, personality disorders, and affective psychoses result from disturbances of such perinatal synaptic reorganization processes (54–56).
Acknowledgments
We thank Ute Kreher and Petra Kremz for excellent technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Br1692/4-1 and a grant from the state of Saxony-Anhalt 665B/8427B.
ABBREVIATIONS
- MNH
mediorostral neostriatum/hyperstriatum ventrale
- Ndc
dorsocaudal neostriatum
- NMDA
N-methyl-d-aspartate
- APV
2-amino-5-phosphonovaleric acid
References
- 1.Horner C H. Prog Neurobiol. 1993;41:281–321. doi: 10.1016/0301-0082(93)90002-a. [DOI] [PubMed] [Google Scholar]
- 2.Koch C, Zador A. J Neurosci. 1993;13:413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris K M, Kater S B. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 4.Segal M. Trends Neurosci. 1995;18:468–471. doi: 10.1016/0166-2236(95)92765-i. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd G M. J Neurophys. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- 6.Globus A, Rosenzweig M R, Bennett E L, Diamond M. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig M R, Bennett E L, Diamond M C. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- 8.Wallhäusser E, Scheich H. Dev Brain Res. 1987;31:29–44. doi: 10.1016/0165-3806(87)90080-0. [DOI] [PubMed] [Google Scholar]
- 9.Comery T A, Shah R, Greenough W T. Neurobiol Learn Mem. 1995;63:217–219. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- 10.Stewart M G, Rusakov D A. Behav Brain Res. 1995;66:21–28. doi: 10.1016/0166-4328(94)00119-z. [DOI] [PubMed] [Google Scholar]
- 11.Trommald M, Hulleberg G, Andersen P. Learn Mem. 1996;3:218–228. doi: 10.1101/lm.3.2-3.218. [DOI] [PubMed] [Google Scholar]
- 12.Bock J, Braun K. Neural Plasticity. 1998;6:17–27. doi: 10.1155/NP.1998.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheich H. J Comp Physiol A. 1987;161:605–619. doi: 10.1007/BF00603664. [DOI] [PubMed] [Google Scholar]
- 14.Braun, K., Bock, J., Metzger, M., Jiang, S. & Schnabel, R. (1998) Behav. Brain Res., in press. [DOI] [PubMed]
- 15.Metzger M, Jiang S, Wang J, Braun K. J Comp Neurol. 1996;376:1–27. doi: 10.1002/(SICI)1096-9861(19961202)376:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Bock J, Schnabel R, Braun K. Eur J Neurosci. 1997;9:1262–1272. doi: 10.1111/j.1460-9568.1997.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 17.Metzger M, Jiang S, Braun K. J Comp Neurol. 1998;395:380–404. [PubMed] [Google Scholar]
- 18.Morris R G M. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchuladze R, Rose S P R. Eur J Neurosci. 1992;4:533–538. doi: 10.1111/j.1460-9568.1992.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Butcher S P, Morris R G M. J Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe B J, Davey J E, Horn G. Behav Neurosci. 1992;106:947–953. doi: 10.1037//0735-7044.106.6.947. [DOI] [PubMed] [Google Scholar]
- 22.Burns L H, Everitt B J, Robbins T W. Behav Neural Biol. 1994;61:242–250. doi: 10.1016/s0163-1047(05)80007-x. [DOI] [PubMed] [Google Scholar]
- 23.Riters L V, Bingman V P. Behav Neural Biol. 1994;62:50–59. doi: 10.1016/s0163-1047(05)80058-5. [DOI] [PubMed] [Google Scholar]
- 24.Hatfield T, Gallagher M. Behav Neurosci. 1995;109:663–668. doi: 10.1037//0735-7044.109.4.663. [DOI] [PubMed] [Google Scholar]
- 25.Aamodt S M, Nordeen E J, Nordeen K W. Neurobiol Learn Mem. 1996;65:91–98. doi: 10.1006/nlme.1996.0010. [DOI] [PubMed] [Google Scholar]
- 26.Meehan E F. Behav Neurosci. 1996;110:1487–1419. doi: 10.1037//0735-7044.110.6.1487. [DOI] [PubMed] [Google Scholar]
- 27.Gewirtz J C, Davis M. Nature (London) 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- 28.Packard M G, Teather L A. Neurobiol Learn Mem. 1997;68:42–50. doi: 10.1006/nlme.1996.3762. [DOI] [PubMed] [Google Scholar]
- 29.Bliss T V, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 30.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 31.Bear M F. Proc Natl Acad Sci USA. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shatz C J. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 33.Goodman, C. S. & Shatz, C. J. (1993) Neuron10, Suppl., 77–98.
- 34.Gruss M, Braun K. J Neurochem. 1996;66:1167–1173. doi: 10.1046/j.1471-4159.1996.66031167.x. [DOI] [PubMed] [Google Scholar]
- 35.Bock J, Wolf A, Braun K. Neurobiol Learn Mem. 1996;65:177–188. doi: 10.1006/nlme.1996.0019. [DOI] [PubMed] [Google Scholar]
- 36.Bredenkötter M, Braun K. Neuroscience. 1997;2:355–365. doi: 10.1016/s0306-4522(96)00381-8. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Babinsky R, Scheich H. Neuroscience. 1994;3:689–699. doi: 10.1016/0306-4522(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 38.Bock J. Ph.D. thesis. Magdeburg: Otto von Guericke University; 1998. [Google Scholar]
- 39.Glaser E M, Van der Loos H. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- 40.Faber H. Ph.D. thesis. Darmstadt: Technical University; 1992. [Google Scholar]
- 41.Scheich H, Wallhäusser-Franke, Braun K. In: Memory: Organisation and Locus of Change. Squire L R, Weinberger N M, Lynch G, McGaugh J L, editors. Oxford: Oxford Univ. Press; 1991. pp. 114–159. [Google Scholar]
- 42.Rocha M, Sur M. Proc Natl Acad Sci USA. 1995;92:8026–8030. doi: 10.1073/pnas.92.17.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olney J W, Labruyere J, Wang G, Wozniak D F, Price M T, Sesma M A. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 44.Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- 45.Lau K C, So K-F, Tay D. Brain Res. 1992;595:171–174. doi: 10.1016/0006-8993(92)91471-p. [DOI] [PubMed] [Google Scholar]
- 46.Cline H T, Debski E A, Constantine-Paton M. Proc Natl Acad Sci USA. 1987;84:4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cline H T, Constantine-Paton M. Neuron. 1989;3:413–429. doi: 10.1016/0896-6273(89)90201-8. [DOI] [PubMed] [Google Scholar]
- 48.Kleinschmidt A, Bear M F, Singer W. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- 49.Bear M F, Kleinschmidt A, Gu Q, Singer W. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bear M F. Prog Brain Res. 1996;108:205–218. doi: 10.1016/s0079-6123(08)62541-8. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Fields R D, Fitzgerald S, Festhoff B W, Nelson P G. J Neurobiol. 1994;25:325–335. doi: 10.1002/neu.480250312. [DOI] [PubMed] [Google Scholar]
- 52.Balice-Gordon R J, Lichtman J W. J Neurosci. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dan Y, Poo M M. Curr Opin Neurobiol. 1994;4:95–100. doi: 10.1016/0959-4388(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 54.Feinberg I. J Psychiatry Res. 1982;17:319–330. [Google Scholar]
- 55.Keshavan M S, Anderson S, Pettegrew J W. J Psychiatry Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 56.Comery T A, Harris J B, Willems P J, Oostra B A, Irwin S A, Weiler I J, Greenough W T. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]