Abstract
AIM: To investigate the effect of a vaccine with recombinant adenovirus interleukin-12 (AdVIL-12) transduced dendritic cells (DCs) against colon cancer in mice.
METHODS: DCs and AdVIL-12 were incubated together at different time intervals and at different doses. Supernatant was collected and tested for IL-12 by enzyme-linked immunosorbent assay (ELISA). In order to determine whether tumor cell lysate-pulsed (TP) AdVIL-12/DCs enhance therapeutic potential in the established tumor model, CT26 colon tumor cells were implanted subcutaneously (s.c.) in the midflank of naïve BALB/c mice. Tumor-bearing mice were injected with a vaccination of CT26 TP AdVIL-12/DCs on d 3 and 10. As a protective colon tumor model, naïve BALB/c mice were immunized s.c. in their abdomens with CT26 TP AdVIL-12/DCs twice at seven day intervals. After the immunization on d 7, the mice were challenged with a lethal dose of CT26 tumor cells and survival times were evaluated. Subsequently, cytotoxic T lymphocyte (CTL) activity and interferon gamma (IFNγ) secretion was evaluated in the immunized mice, and assayed CTL ex vivo.
RESULTS: Murine DCs were retrovirally transduced with AdVIL-12 efficiency, and the AdVIL-12 transduced DCs secreted a high level of IL-12 (AdVIL-12/DCs, 615.27 ± 42.3 pg/mL vs DCs, 46.32 ± 7.29 pg/mL, P < 0.05). Vaccination with CT26 TP AdVIL-12/DCs could enhance anti-tumor immunity against CT26 colon tumor in murine therapeutic models (tumor volume on d 19: CT26 TP AdVIL-12/DCs 107 ± 42 mm3 vs CT26 TP DCs 383 ± 65 mm3, P < 0.05) and protective models. Moreover, the CT26 TP AdVIL-12/DC vaccination enhances tumor-specific CTL activity, producing high levels of IFNγ in immunized mice. Ex vivo primed T cells with AdVIL-12/DCs were able to induce more effective CTL activity than in primed T cells with CT26 TP/DCs (E:T = 100:1, 69.49% ± 6.11% specific lysis vs 37.44% ± 4.32% specific lysis, P < 0.05).
CONCLUSION: Vaccination with recombinant AdVIL-12 transduced DC pulsed tumor cell lysate enhance anti-tumor immunity specific to colon cancer in mice.
Keywords: Vaccination, Dendritic cells, Cytokine, Interleukin-12, Colon cancer, Immunotherapy
INTRODUCTION
Colon cancer is one of the most common cancers[1]. Although there has been great progress in colon cancer therapy, it is still difficult to treat advanced colon cancer because it has usually metastasized to lymph glands and other organs. Currently, radical surgery represents the standard method of therapy. Adjuvant therapy such as chemotherapy and radiation therapy have been widely applied, but colon cancer control at the advanced stage remains difficult[2,3]. A new adjuvant therapeutic method is needed in order to improve the 5-year survival rate of patients with colon cancer. Today, tumor immunotherapy for colon cancer is extremely appealing[4,5].
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that both initiate and modulate the immune response[6]. DCs are cells in the pathway of antigen capture and presentation to T cells, with the unique ability to directly prime naïve CD4+ and CD8+ T cells. They posses the ability to efficiently uptake, process, and present antigens on major histocompatibility complex (MHC) classIand II molecules, together with co-stimulatory molecules such as B7 and CD40[7]. Therefore, DCs are believed to be essential for stimulating tumor-specific cytotoxic T lymphocytes (CTL) and inducing the protective and therapeutic anti-tumor immunity against cancer cells. There have been attempts to produce vaccines based on DCs, including immunizations by DCs pulsed with tumor lysates or apoptotic tumor cells or tumor RNA. DCs have been fused with tumor cells, transduced with peptides to elicit specific antitumor T cells and enhance anti-tumor immunity[8]. Now, DCs can be pulsed with synthetic peptides or proteins derived from known tumor associated antigens (TAA), such as MUC1, Her-2/neu, tyrosinase, CEA or Melan-A/MART. Primary clinical studies were performed on melanoma patients using DCs pulsed with peptides or loaded with tumor cell lysates[9]. This data revealed that such vaccines could be safely applied without significant side effects and induce antigen-specific CTL responses in vivo.
Interleukin (IL)-12 is a heterodimeric, proinflammatory cytokine produced by macrophages, DCs, and B cells, which stimulates T lymphocytes and natural killer cells to release interferon gamma (IFNγ) and promote an anti-tumor response[10,11]. Some studies have shown that IL-12 has powerful adjuvant properties and the immune-modulating characteristics of IL-12 are considered to be responsible for these effects. Therapeutic activity with IL-12 has been observed in various murine tumor models[12,13]. Transduction of vaccine cells with IL-12 genes can substantially improve their immunogenicity[10]. Trials with IL-12 gene-modified melanoma cells have also been initiated in humans[14]. Saika T et al[15] reported that DCs engineered to express murine IL-12 and pulsed with a tumor lysate, induce specific T cell responses and anti-tumor immunity against a mouse prostate cancer model. This work suggests that adenovirus-mediated IL-12 gene transduction enhanced the immune responses.
In this study, we demonstrated that vaccination with DCs engineered to express IL-12 by adenovirus-mediated gene transfer and pulsed with CT26 colon tumor cell lysate, enhanced tumor-specific CTL activity and anti-tumor immunity in CT26 colon tumor models, suggesting promising strategy for colon cancer immunotherapy.
MATERIALS AND METHODS
Animals
Female BALB/c (H-2d) and C57BL/6 (H-2b) mice (8-10 wk old) were purchased from the Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). All mice were kept under pathogen-free conditions in the animal center of the Shanghai Second Medical University (Shanghai, China).
Cell
CT26 (H-2b), a mouse colon adenocarcinoma cell line, and SGC-7901 (H-2b), the gastric cancer cell line were purchased from the Shanghai Cell Biology Institutes, Chinese Academy of Sciences (Shanghai, China). CT26 cell lines grow in BALB/c mice. Both cell lines were cultured in RPMI (Roswell Park Memorial Institute) medium 1640 (GIBCO, USA) containing 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L glutamine, penicillin G (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified incubator supplemented with 5% CO2.
Major
Murine granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, IL-2, and IL-7 were purchased from Becton Dickinson (New Jersey, USA). Phenotypic analysis, fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled monoclonal antibodies against murine cell surface molecules, such as MHCI(H-2Kd), MHC II (I-Ab), CD11b, CD11c, CD40, CD80, CD86, and CD8α, were provided by Pharmigen (CA, USA). Mitomycin C (MMC) was purchased by Jingmei Biothe (Shenzhen, China).
Generation of bone marrow-derived DCs
Primary bone marrow DCs (BM-DCs) was obtained from mouse bone marrow precursor according to a protocol by Zhang et al[16]. BALB/c mice bone marrow was obtained from tibia and femurs by flushing with the media. Tissue was minced in a single-cell suspension through a nylon mesh. BALB/c mice bone marrow cells were stained sequentially with biotinylated anti-c-kit MAb and Streptavidm MicroBeads (Dynal, Norway). c-kit+ hematopoietic progenitor cells were magnetically isolated with a MiniMACS separator (Milteyi Biotec, Auburn, CA) from the of BALB/c mice bone marrow cells. The cells (6 × 106) were cultured for 7 d in fresh RPMI medium 1640 containing 10% FCS, GM-CSF (4 ng/mL), and IL-4 (10 ng/mL).
Immunofluorescence staining
DC immunofluorescence analysis was performed as previously described[16,17]. In brief, DCs prepared and isolated from BALB/c mice, as described above (2 × 105-4 × 105 cells), were incubated with FITC-labeled MAbs against CD40, CD11b, CD11c, or CD80 and PE-labeled MAbs against MHCI, MHC II, CD86 or CD8α followed by FACScan analysis (Becton Dickinson, USA). Instrument compensation was set in each experiment using two-color stained samples.
Recombinant adenovirus vector
Recombinant adenoviruses IL-12 (AdVIL-12) ecoding the murine IL-12 gene were donated by Dr. Y. Y. Zhang (Health Science Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China). These recombinant adenoviruses were propagated in 293 cells and purified on a CsCl density gradient. Their titres were determined using a plaque assay on 293 cells. Aliquots of the adenovirus solutions were stored at -80°C.
Adenoviral transduction of DCs and secretion of IL-12
DCs generated on d 8 were plated at 2 × 106 cells/well in 1ml of RPMI 1640 medium containing 10% FCS with AdVIL-12 at a multiplicity of infection (MOI) of 100. After 2 h incubation at 37°C in a humidified incubator supplemented with 5% CO2 with gentle agitation every 20 min, the DCs culture medium was replaced with 2 mL of RPMI 1640 medium supplemented with 10% FCS, and the cells were incubated for another 48 h. AdVIL-12 transduced DCs (AdVIL-12/DCs) were harvested, washed twice with PBS, and used for the tumor cell lysate pulsing in vitro. Immunofluorescence analysis of AdVIL-12/DCs was performed as described above.
DCs (1 × 106) were infected with AdVIL-12 at MOI 100. Viral supernatant was replaced after 2 h with the cell culture medium. The supernatant was collected and tested for mIL-12 by enzyme-linked immunosorbent assay (ELISA) kit (Rockford, IL, USA) at different time intervals (0, 12, 24, 48, 72, 96 h). Moreover, DCs were infected with AdVIL-12 at different MOI (50, 100, 200, 300, 500). The supernatant was collected 48 h later and tested for mIL-12 by ELISA.
Pulsing DCs with tumor cell lysates
DCs were pulsed with freeze-thawed tumor lysate at a 1:3 DCs: tumor ratio. CT26 tumor (6 × 106) were lysed by rapid freezing (liquid nitrogen) and thawing in a 37°C bath three times. The AdVIL-12 transduced DCs (AdVIL-12/DCs, 2 × 106 cells/mL) or bone marrow-derived DCs (2 × 106 cells/mL) were incubated in six-well plates in the presence of CT26 tumor lysates (6 × 106 cell equivalents/mL) in RPMI Medium 1640 containing 10% FCS, GM-CSF (4 ng/mL), and IL-4 (10 ng/mL) for one day at 37°C and 5% CO2. These CT26 tumor cell lysate-pulsed (TP) AdVIL-12/DCs or DCs were used for vaccination after one day.
Tumor model and DC-based vaccination
In the established tumor model, CT26 tumor cells (5 × 105 cells per mouse) were implanted subcutaneously (s.c.) in the midflank of naïve BALB/c mice (10/each group). Tumor-bearing mice were injected with 1 × 106 DCs vaccination: CT26 TP AdVIL-12/DCs or CT26 TP DCs on d 3 and 10. As a positive control, tumor bearing mice were treated in parallel with unstimulated DCs, CT26 tumor lysate alone, or PBS alone. Tumor growth was assessed by calipers (mean of 2 perpendicular diameters) every 2-3 d. Tumor volume was estimated by the formula 4/3 πr3.
In the protective anti-tumor experiment, each group consisting of 10 naïve BALB/c mice were injected s.c. in their abdominal walls with 1 × 106 DCs vaccination: CT26 TP AdVIL-12/DCs or CT26 TP DCs on d 0, 7, and 14. Control mice were injected with unstimulated DCs, CT26 tumor lysate alone, and PBS alone. Each mouse was challenged s.c. with a lethal dose of 2 × 106 CT26 cells on d 21. Survival differences among the groups receiving different vaccinations were evaluated following the primary challenge with CT26 cells.
Assays for cytotoxic T lymphocyte activity and interferon gamma secretion in the immunized mice
To confirm that tumor-specific cytotoxic T lymphocytes (CTLs) had been generated in the immunized mice, splenic CD3+ T cells from tumor-free mice that survived the CT26 tumor challenge through d 61 following DC vaccination, were magnetically isolated using CD3 MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany). T cells (1 × 106 cells) from surviving mice were restimulated ex vivo by culturing in IMDM containing 10% FCS and MMC-treated CT26 tumor cells (1 × 105). Five days later, the MMC-treated CT26 tumor cells and the T cells were collected for measuring CTL activity and IFNγ secretion. The restimulated effector T cells (2 × 105 in 100 μL per well) were added to the wells containing target CT26 or SGC-7901 tumor cells (5 × 104 in 100 μL per well) in 96-well plates. Supernatant from each well was collected after 20 h and was measured cytolytic activity against target CT26 tumor cells and SGC-7901 tumor cells with a Cytotoxicity Detection Kit (LDH; Boehringer Mannheim, Mannheim, Germany), IFNγ production was determined with the mouse IFNγ ELISA kit (Endogen, Woburn, MA, USA). In some experiments, the target CT26 tumor cells (1.5 × 105) were incubated with a MAb to MHC classImolecules (anti-H2Db/H2Kb; 50 g/mL) or with control antibody (anti-H2Dd; 50 g/mL) at 37°C for 30 min before the addition of restimulated effector T cells to evaluate the specificity of CTL activity.
Assays for cytotoxic T lymphocyte activity ex vivo
To confirm that tumor-specific cytotoxic T lymphocytes (CTLs) can be generated ex vivo, splenic CD3+ T cells (1 × 106 cells/mL) were magnetically isolated from naïve BALB/c mice using CD3 microBeads. These cells were cultured in RPMI medium 1640 containing 10% FCS, then primed ex vivo in the presence of cytokines including IL-2 and IL-7 (5 ng/mL per) at d 0, 7, and 14 with CT26 TP AdVIL-12/DCs or CT26 TP DCs at a 1:20 stimulator to responder cell ratio. Unpulsed DCs and AdVIL-12/DCs were used as controls. Fresh medium containing IL-2 and IL-7 was exchanged every four days. The primed T cells were effector cells, CT26 or SGC-7901 tumor cells were target cells. On d 21, target cell suspension was added into 96 well plates, and effector cells were titrated to the dilutions target cells by serial dilutions (E-T mix, E:T, 1:1, 5:1, 10:1, 20:1, 50:1, 100:1). Supernatant from each well was collected after 20 h in order to measured cytolytic activity against target CT26 and SGC-7901 tumor cells, which was done with a cytotoxicity detection kit as mentioned above.
Statistical analysis
Differences were evaluated using Statistical Package for Social Science 11.0 (SPSS11.0). Statistical analysis was performed using a Student’s t test. Survival differences among mice groups were evaluated with a log-rank test of the Laplan-Meier survival curves. Statistical tests were two-tailed. P values < 0.05 were considered to be statistically significant.
RESULTS
Phenotypic markers of DC analysis by FACScan
The phenotypic profile of a representative population of bone marrow DCs was first determined. These DCs were expressed in moderate levels of MHC classI, CD11b and CD11c, high levels of MHC class II, moderate levels of the costimulatory molecules CD40, CD80, and CD86, and very low levels of CD8α. Phenotypic changes within the DCs induced by AdVIL-12 transduction were examined by phenotypic analysis. Expression levels of CD80, CD86, and MHC class II were increased in AdVIL-12/DCs compared with the DCs (Table 1).
Table 1.
Phenotypic characteristics of AdVIL-12/DCs
| DCs1 |
Mean fluorescence intensity |
|||||||
| MHCI | MHCII | CD40 | CD11b | CD11c | CD80 | CD86 | CD8α | |
| Nontransduced | 35 | 92 | 19 | 48 | 26 | 112 | 69 | 10 |
| AdVIL-12 | 49 | 304a | 23 | 56 | 30 | 271a | 229a | 9 |
Nontransduced DCs and AdVIL-12 transduced DCs were harvested 6 d after the culture initiation and stained with the listed antibodies. Background intensities = 4-6.
P < 0.05 vs those of nontransduced DCs.
Secretion of IL-12by transduced DCs in vitro
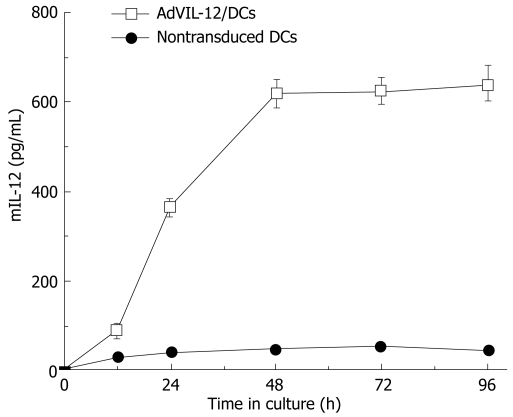
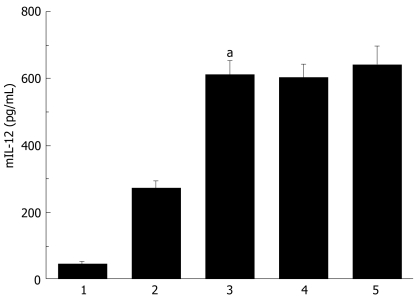
Secretion analysis of AdVIL-12/DCs, DCs and AdVIL-12 was determined by incubation at different cultured time intervals and at different MOI, and supernatant was collected and tested for mIL-12 by ELISA. Figure 1 illustrated that AdVIL-12/DCs secrete high levels of IL-12 at 48 h intervals. However, the levels of IL-12 did not increase with time. In addition, the DCs transfected with AdVIL-12 at a MOI of 100 showed a significant increase in the IL-12 level against the control DCs. This was statistically significant (AdVIL-12/DCs, 615.27 ± 42.3 pg/mL vs DCs, 46.32 ± 7.29 pg/mL, aP < 0.05, Figure 2).
Figure 1.
IL-12 production by the DCs transduced with AdVIL-12. DCs and AdVIL-12 were incubated together at different time intervals (0 h, 12 h, 24 h, 48 h, 72 h, 96 h). Supernatant was collected and tested for mIL-12 by ELISA, with nontransduced DCs as control. Data is representative of two independent experiments, with a given as mean ± SE.
Figure 2.
IL-12 production by the DCs transduced with AdVIL-12. BM-DCs was transduced with AdVIL-12 at various doses. DCs supernatant was examined 48 hrs later for the IL-12 production by ELISA, with nontransduced DCs was as control. Statistical analysis used the paired Student’s t test. Data are given as mean ± SE. aP < 0.05 AdVIL-12/DCs (MOI 100) compared with nontransduced DCs or AdVIL-12/DCs (MOI 50). 1: Nontransduced DCs. 2: AdVIL-12/DCs (MOI 50). 3: AdVIL-12/DCs (MOI 100). 4: AdVIL-12/DCs (MOI 300). 5: AdVIL-12/DCs (MOI 500).
Vaccination with CT26 TP AdVIL-12/DCs enhances anti-tumor immunity in therapeutic or protective models
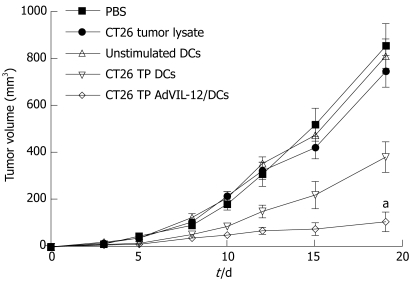
In order to determine whether or not CT26 TP AdVIL-12/DCs enhance therapeutic potential in the established tumor model, CT26 tumor cells (1 × 106) were implanted s.c. in the midflank of naïve BALB/c mice (10/each group). Tumor-bearing mice were injected with different vaccinations on d 3 and 10. Figure 3 shows that tumor growth was significantly inhibited in mice vaccinated with CT26 TP AdVIL-12/DCs. Vaccination with CT26 TP AdVIL-12/DCs significantly retarded tumor growth, and exerted a greater anti-tumor effect than CT26 TP DCs (tumor volume on d 19: PBS control 856 ± 95 mm3, CT26 tumor lysate control 748 ± 67 mm3, unstimulated DCs control 813 ± 73 mm3, CT26 TP DCs 383 ± 65 mm3, and CT26 TP AdVIL-12/DCs 107 ± 42 mm3, CT26 TP AdVIL-12/DCs vs CT26 TP DCs, aP < 0.05). Differences between CT26 TP AdVIL-12/DCs and CT26 TP DCs were statistically significant.
Figure 3.
Vaccination with CT26 TP AdVIL-12/DCs enhances anti-tumor immunity in therapeutic tumor models. CT26 tumor cells (5 × 105 cells per mouse) were implanted s.c. in the midflank of naïve BALB/c mice. On d 3 and 10, tumor-bearing mice were injected with 1 × 106 DCs vaccination with CT26 TP AdVIL-12/DCs. As control, tumor bearing mice were treated in parallel with CT26 TP DCs, unstimulated DCs, CT26 tumor lysate alone, or PBS alone. Tumor growth was assessed by calipers every 2-3 d thereafter. Tumor volume was estimated by the formula 4/3 πr3. Statistical analysis used the paired Student’s t test. Data are given as mean ± SE (n = 10 mice in each group). aP < 0.05, CT26 TP AdVIL-12/DCs vs CT26 TP DCs or the other control groups (d 19).
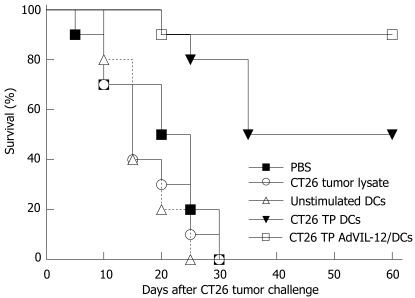
CT26 TP AdVIL-12/DCs were used in vaccinations to see whether there were increases in anti-tumor immunity. Naïve BALB/c mice were immunized s.c. twice at 7 d intervals in abdominal walls with CT26 TP AdVIL-12/DCs, CT26 TP/DCs, unpulsed DCs, tumor lysates alone, and PBS, respectively. On d 7 after secondary immunization, these mice were challenged with 2 × 106 CT26 tumor cells. All mice vaccinated with unstimulated DCs, tumor lysates alone, and PBS died within 30 d after CT26 tumor cell challenge (Figure 4). However, mice immunized with CT26 TP AdVIL-12/DCs had significantly prolonged survival compared with the control or other vaccination groups. During the 60-d observation period, the mice vaccinated with CT26 TP AdVIL-12/DCs had a 90% survival rate and the mice vaccinated with CT26 TP/DCs had a 50% survival rate, respectively, compared with the mice vaccinated with unpulsed DCs (0%), tumor lysates alone (0%), and PBS (0%). Survival advantage of CT26 TP AdVIL-12/DC was statistically significant compared with the CT26 TP DC group or other control groups (Kaplan-Meier, P < 0.05).
Figure 4.
Protective effect of CT26 TP AdVIL-12/DCs in CT26 tumor models. Naïve BALB/c mice were injected s.c. in their abdominal walls on d 0, 7, and 14 with 1 × 106 DCs vaccination: CT26 TP AdVIL-12/DCs or CT26 TP DCs. Mice injected with unstimulated DCs, CT26 tumor lysate alone, and PBS alone were used as controls. On d 21, each mouse was challenged s.c. with a lethal dose of 2 × 106 CT26 cells. Survival was observed after the challenge with tumor cells. Survival rate was compared with a log-rank test of Kaplan-Meier curves. The survival advantage conferred by the CT26 TP AdVIL-12/DC was statistically significant compared with CT26 TP DCs group and other control groups (Kaplan-Meier, P < 0.05).
Enhancement of tumor-specific CTL responses
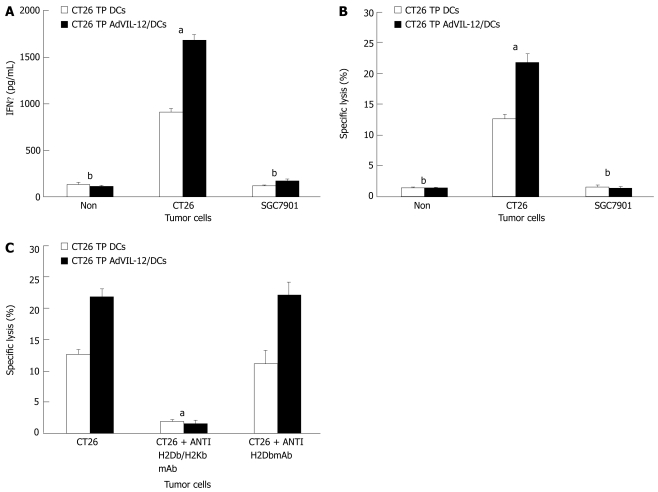
In order to determine whether vaccination with CT26 TP AdVIL-12/DCs enhances tumor-specific CTL responses and to see if the response was tumor specific, we isolated splentic CD3+ T cells obtained from mice still alive 60 d (in CT26 TP AdVIL-12/DCs or CT26 TP/DCs group) after the CT26 tumor cells challenge. Assayed levels of IFNγ were determined, which is a reflection of immune activation and cytotoxic activity against CT26 tumor cells or SGC-7901 tumor cells. Results demonstrated that the splenic mice CD3+ T cells vaccinated with CT26 TP AdVIL-12/DCs produce higher levels of IFNγ in vitro when stimulated with CT26 tumor cells than T cells obtained from mice vaccinated with CT26 TP/DCs (1685.88 ± 61.54 pg/mL in CT26 TP AdVIL-12/DCs group vs 912.58 ± 38.33 pg/mL in CT26 TP DCs, P < 0.05, Figure 5A). At the same time they showed substantially enhanced cytotoxic activity (21.86% ± 1.29% specific lysis in CT26 TP AdVIL-12/DCs group vs 12.67% ± 0.76% specific lysis in CT26 TP DCs, P < 0.05, Figure 5B). In addition, all splenic CD3+ T cells obtained from mice vaccinated with CT26 TP AdVIL-12/DCs and vaccinated with CT26 TP DCs produced high levels of IFNγ and showed specific cytotoxic activity against CT26 tumor cells, but no cytotoxic activity when stimulated with SGC-7901 tumor cells (Figure 5A and B). Furthermore, antibodies to MHC classImolecules abrogated the cytolytic activity against CT26 tumor cells. Vaccination with CT26 TP AdVIL-12/DCs was shown to induce a MHC-I restricted CTL response (Figure 5C).
Figure 5.
Assays for IFNγ secretion and tumor-specific CTL activity in the immunized mice. Splenic CD3+ T cells from mice that survived the CT26 tumor challenge until d 61 in protective models were magnetically isolated. CD3+ T cells were restimulated ex vivo by culturing with the MMC-treated CT26 tumor cells. Then these restimulated effector T cells were added to the wells containing target CT26 or SGC-7901 tumor cells in 96-well plates. After 20 h, supernatant from each well was collected for measuring IFNγ production with the mouse IFNγ ELISA kit (A), and for measuring cytolytic activity with a Cytotoxicity Detection Kit (B). In some experiments, the target CT26 tumor cells were incubated with a MAb to MHC classImolecules (anti-H2Db/H2Kb) or with control antibody (anti-H2Dd) before the addition of restimulated effector T cells to evaluate the specificity of CTL activity. Statistical analysis used the paired Student’s t test. Data are given as mean ± SE. A, B aP < 0.05, CT26 TP AdVIL-12/DCs compared with CT26 TP DCs in the CT26 groups, bP < 0.01 CT26 vs SGC7901 or Non, respectively. (C), aP < 0.05, CT26 + antiH-2kb/DbmAb vs CT26 or CT26 + antiH-2DbmAb, respectively.
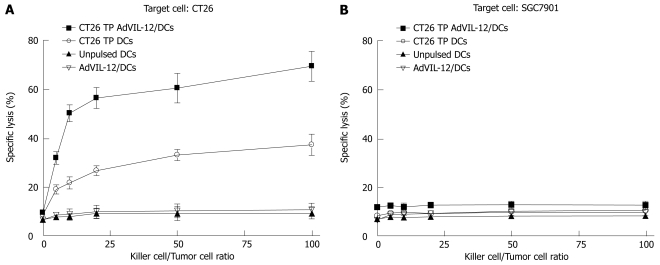
Finally, vaccination with CT26 TP AdVIL-12/DCs enhances tumor-specific CTL responses ex vivo. Naïve mouse splenic T cells were primed ex vivo with CT26 TP AdVIL-12/DCs or CT26 TP/DCs in the presence of IL-2 and IL-7 to elicit cytolytic reactivity against tumor cells. Unpulsed DCs and AdVIL-12/DCs were used as controls. Results demonstrated that primed T cells in vitro with AdVIL-12/DCs or CT26 TP/DCs were able to lyse parental CT26 tumor cells specifically, but not SGC-7901 tumor cells. Also, T cells primed with unpulsed DCs or AdVIL-12/DCs did not induce specific CTL against CT26 tumor cells or SGC-7901 tumor cells (Figure 6A and B). Furthermore primed T cells with AdVIL-12/DCs are able to induce effective CTL activity more than primed T cells with CT26 TP/DCs (E:T = 100:1, 69.49% ± 6.11% specific lysis in CT26 TP AdVIL-12/DCs group vs 37.44% ± 4.32% specific lysis in CT26 TP DCs, aP < 0.05, Figure 6A). It is possible that a vaccination with CT26 TP AdVIL-12/DCs may enhance the anti-tumor immune response to syngeneic tumor cells.
Figure 6.
Generation of tumor-specific cytotoxic T cells ex vivo. Splenic CD3+ T cells were magnetically isolated from naïve BALB/c mice. These cells were cultured ex vivo in the presence of cytokines including IL-2 and IL-7 and with CT26 TP AdVIL-12/DCs or CT26 TP DCs at a stimulator to responder cell ratio of 1:20. The primed T cells were effector cells, CT26 or SGC-7901 tumor cells were target cells. Then these effector T cells were titrated by serial dilution (E-T mix, E:T 1:1, 5:1, 10:1, 25:1, 50:1, 100:1), and their lytic activity against CT26 or SGC-7901 were assayed by a Cytotoxicity Detection Kit. Statistical analysis used the paired Student’s t test. Data are given as mean ± SE. A aP < 0.05, CT26 TP AdVIL-12/DCs vs CT26 TP DCs or other control groups in the CT26 groups.
Together, these results suggest that mice vaccinated with CT26 TP AdVIL-12/DCs, and subjected to CT26 tumor cells, and primed T cells in vitro with AdVIL-12/DCs, were more effective in generating generated MHC class I restricted CTLs specific for CT26 tumor cells.
DISCUSSION
In our experiments we showed that murine BM-DCs could be retrovirally transduced with AdVIL-12 efficiency, and the AdVIL-12 transduced DCs could secrete high levels of IL-12. Vaccination with DCs that were engineered to express IL-12 by adenovirus-mediated gene transfer could enhance anti-tumor immunity against CT26 colon tumor in murine therapeutic and protective models. Moreover, in CTL response, vaccination with CT26 TP AdVIL-12/DCs enhanced tumor-specific CTL activity to produced high levels of IFNγ.
Successful genetic modification of murine BM-DCs with retroviral vectors was demonstrated. Previous reports illustrated the feasibility of retroviral gene modification of human DCs by tansduction of CD34+ progenitor cells with the subsequent addition of cytokine to promote differentiation and maturation[18,19]. In this study murine DCs were transduced using cocultivation with retroviral producer cells. The murine BM-DCs transduced with AdVIL-12 at different MOI or different time intervals in culture. DCs transduced with AdVIL-12 at an MOI of 100 shown limited toxicity and maximal production of IL-12, and at 48 h intervals in culture, demonstrate maximal production of IL-12. Phenotypes of DCs retrovirally transduced with IL-12 were evaluated. The experiments demonstrated expression levels of CD80, CD86, and MHC II were enhanced in AdVIL-12/DCs compared with the non-transduced DCs, without apparent surface molecules changes. These phenotypic changes could be due in part to the direct effect of IL-12 or, more likely, indirectly through IFNγ production by contaminating T cells or natural killer cells. Grohmann U et al[20] reported that IL-12 acts directly on DCs to promote nuclear localization of nuclear factor kB and primes DCs for IL-12 production. Suzuki T et al[21] also reported that the DCs transduced AdVIL-12 showed increased levels of the costimulatory molecules (CD80 and CD86), MHC II molecules, compared with the non-transduced DCs. These changes could explain, in part, why IL-12-transduced DCs are more effective in tumor treatment application.
Tumor immunity induction can be initiated by the effectors of innate immunity and can be further developed by cells of adaptive immunity, with DCs playing a central regulatory role. Several steps are involved including (a) recognition of tumor molecules by DC precursors, (b) direct and IFN-γ-mediated killing of transformed cells by NK/NK T cells activated by DCs, (c) capture and cross-presentation of released-tumor-associated Ags (TAAs) by immature DCs, (d) selection and activation of TAA-specific T cells, as well as nonspecific effectors including macrophages and eosinophils, and (e) homing of TAA-specific T cells to the tumor site and recognition elements leading to the elimination of tumor cells[22]. DCs-based vaccination had showed efficiently anti-tumor effective in many tumor models and in the clinical studies. Kono K et al[23] reported that tumor vaccination therapy with DCs pulsed with HER-2/neu-peptides may be a potential candidate for the novel treatment of gastric cancer patients. Tumor markers (CEA or CA19-9) were decreased after vaccination in 3 out of 9 patients. Two patients had more than 50% tumor regression, and two presented a mixed response. These studies revealed that such vaccines could be safely applied without significant side effects and induced antigen-specific CTL responses in vivo.
Cytokine gene therapy is becoming a promising weapon against cancer[24]. Adenovrial-based cytokine gene therapy has many advantages over other forms of cytokine delivery[25]. Adenoviral vectors allow local, high-efficiency, but transient transgene expression, generating high-level but self-limited cytokine production in treated tumors. Adenoviral vectors are transduction targets in a heterogeneously growing population of tumor cells. Recombinant IL-12 has generated interest as a potential cancer therapeutic agent due to the ability of murine IL-12 to induce tumor regression and to cure in various mouse models of cancer. Studies examining the anti-tumor mechanisms of IL-12 have implicated various cellular mediators including CD8 T cells, NK cells, and NK T cells[26,27]. Taking full advantages of characters of DCs and IL-12 respectively, constitutive production of IL-12 by AdVIL-12/DCs, coupled with their ability to take up and process tumor antigens, migrate to tumor site or lymph nodes can induce an effective immune response. In this study, a DCs-based vaccination transduced with AdVIL-12 was successfully produced using cocultivation methods.
In the experiment in vivo, significant anti-tumor effects were observed in therapeutic models or protective models when vaccination with CT26 TP AdVIL-12 transduced DCs were injected. These anti-tumor effects were not observed, however, when nontransduced DCs were injected. Complete tumor eradication was observed in 30%-50% of mice treated with a single injection of CT26 TP AdVIL-12/DCs in therapeutic models. In protective models, the mice immunized with CT26 TP AdVIL-12/DCs had significant survival rates. During the 60-d observation period, mice vaccinated with CT26 TP AdVIL-12/DCs showed 90% survival rate, compared to mice vaccinated with CT26 TP/DCs (50%). These results suggest that overexpression of IL-12 by DCs in the peripheral blood, the tumor site, or in the secondary lymphoid sites, alternatively, could be important for an anti-tumor response in addition to the phenotypic changes in AdVIL-12/DCs. Chemokine (IP-10 and Mig)[28,29] production induced by IL-12, is responsible for these effects, at least in part, through enhanced recruitment of cytolytic effector cells to develop into tumors, plus possibly antiangiogenic effects (Fas/FasL)[30].
Systemic immune responses, as demonstrated by CTL activity and IFNγ production in vivo and ex vivo, were also significantly higher and tumor specific when AdVIL-12/DCs were used. High cytolytic activity in association with a TH1-type response could possibly contribute to the profound anti-tumor effects that we observed. In contrast, responses induced by nontransduced DCs were far less potent. Tatsumi T et al[31] reported that the anti-tumor effect of DCs-based vaccination is dependent on the production of the TH1-type immunostimulatory cytokine such as IFNγ, IL-12. Therefore, the level of IFNγ production as a result of IL-12 may play an important role in the increased anti-tumor immune responses.
In summary, research data demonstrates that vaccination with recombinant adenoviruses interleukin-12 transduced dendritic cells pulsed tumor cell lysate enhance anti-tumor immunity specific to colon cancer in mice. This approach is superior to that observed by nontransduced DCs. These results suggest that an evaluation of AdVIL-12/DCs in colon cancer patients with is an important next step. A clinical trial evaluating this approach is currently in preparation.
COMMENTS
Background
Dendritic cells (DCs) play a central role in immune responses and may be useful adjuvants for tumor vaccination. Heterodimeric cytokine interleukin (IL)-12 is the key cytokine mediating the generation of a TH1-type cytokine expression pattern in both T lymphocytes and NK cells, resulting in cytotoxic cellular immune responses. IL-12 has been shown to generate powerful anti-tumor responses in many tumor models. IL-12 production by recombinant adenoviruses IL-12 (AdVIL-12) transduced DCs, coupled with their ability to take up and process tumor antigens, induce an effective anti-tumor immune response.
Research frontiers
DCs are believed to be essential for stimulating tumor-specific CTL and inducing the protective and therapeutic anti-tumor immunity against cancer cells. Attempts to produce vaccines based on DCs have been developed including immunizations by DCs pulsed with tumor lysates or apoptotic tumor cells or tumor RNA, fused with tumor cells, transduced with peptides. Now, DCs can be pulsed with synthetic peptides or proteins derived from known tumor associated antigens (TAA) such as MUC1, Her-2/neu, tyrosinase, CEA or Melan-A/MART. Vaccines based on DCs have been safely applied in clinic. Some studies have shown that IL-12 has powerful adjuvant properties and immune-modulating characteristics of IL-12 are considered to be responsible for such adjuvant effects. Therapeutic activity of IL-12 has been observed in various murine tumor models. Transduction of vaccine cells with IL-12 genes can substantially improve their immunogenicity. Trials with IL-12 gene-modified melanoma cells have also been started in humans.
Innovations and breakthroughs
Taking full advantages of characters of DCs and IL-12 respectively, a DCs-based vaccination transduced with AdVIL-12 was successfully produced using cocultivation methods. Vaccination with DC engineered to express IL-12 by adenovirus-mediated gene transfer could enhance anti-tumor immunity in murine therapeutic and protective models. Vaccination can be application for immunotherapy against colon cancer in mice successfully.
Applications
Vaccination with AdVIL-12 transduced DCs pulsed tumor cell lysate enhance anti-tumor immunity specific to colon cancer in mice. These results suggest that an evaluation of AdVIL-12/DCs in colon cancer patients with is an important next step. A clinical application of this approach is currently in preparation.
Terminology
DCs (Dendritic cells), bone marrow DCs (BM-DCs), antigen presenting cells (APCs), Interleukin-12 (IL-12), recombinant adenoviruses interleukin-12 (AdVIL-12), AdVIL-12 transduced DCs (AdVIL-12/DCs), major histocompatibility complex (MHC), cytotoxic T lymphocyte (CTL), interferon gamma (IFNγ),
Peer review
The authors examined that murine BM-DCs could be retrovirally transduced with AdVIL-12 efficiency, and the AdVIL-12 transduced DCs could be secret high levels of IL-12. Vaccination with DC engineered to express IL-12 by adenovirus-mediated gene transfer could enhance anti-tumor immunity against CT26 colon tumor in murine therapeutic and protective models. Moreover, in CTL response vaccination with CT26 TP AdVIL-12/DCs enhanced tumor-specific CTL activity, to produced high levels of IFNγ.
Peer reviewer: Lucia Malaguarnera, Associate Professor, MD, PhD, Department of Biomedical Sciences, University, Via E. De Amicis, 24 Trecastagni Catania 95039, Italy
S- Editor Liu Y L- Editor Lutze M E- Editor Li HY
References
- 1.Rougier P, Andre T, Panis Y, Colin P, Stremsdoerfer N, Laurent-Puig P. Colon cancer. Gastroenterol Clin Biol. 2006;30 Spec No 2:2S24–2S29. [PubMed] [Google Scholar]
- 2.McCormick D, Kibbe PJ, Morgan SW. Colon cancer: prevention, diagnosis, treatment. Gastroenterol Nurs. 2002;25:204–211; quiz, 211-212. [PubMed] [Google Scholar]
- 3.Colquhoun PH, Wexner SD. Surgical management of colon cancer. Curr Gastroenterol Rep. 2002;4:414–419. doi: 10.1007/s11894-002-0012-4. [DOI] [PubMed] [Google Scholar]
- 4.Saif MW. Targeted agents for adjuvant therapy of colon cancer. Clin Colorectal Cancer. 2006;6:46–51. doi: 10.3816/CCC.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 5.Okaji Y, Tsuno NH, Kitayama J, Saito S, Takahashi T, Kawai K, Yazawa K, Asakage M, Hori N, Watanabe T, et al. Vaccination with autologous endothelium inhibits angiogenesis and metastasis of colon cancer through autoimmunity. Cancer Sci. 2004;95:85–90. doi: 10.1111/j.1349-7006.2004.tb03175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Nouri-Shirazi M, Banchereau J, Fay J, Palucka K. Dendritic cell based tumor vaccines. Immunol Lett. 2000;74:5–10. doi: 10.1016/s0165-2478(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 9.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 11.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 12.Switaj T, Jalili A, Jakubowska AB, Drela N, Stoksik M, Nowis D, Basak G, Golab J, Wysocki PJ, Mackiewicz A, et al. CpG immunostimulatory oligodeoxynucleotide 1826 enhances antitumor effect of interleukin 12 gene-modified tumor vaccine in a melanoma model in mice. Clin Cancer Res. 2004;10:4165–4175. doi: 10.1158/1078-0432.CCR-04-0022. [DOI] [PubMed] [Google Scholar]
- 13.Mu J, Zou JP, Yamamoto N, Tsutsui T, Tai XG, Kobayashi M, Herrmann S, Fujiwara H, Hamaoka T. Administration of recombinant interleukin 12 prevents outgrowth of tumor cells metastasizing spontaneously to lung and lymph nodes. Cancer Res. 1995;55:4404–4408. [PubMed] [Google Scholar]
- 14.Golab J, Zagozdzon R. Antitumor effects of interleukin-12 in pre-clinical and early clinical studies (Review) Int J Mol Med. 1999;3:537–544. doi: 10.3892/ijmm.3.5.537. [DOI] [PubMed] [Google Scholar]
- 15.Saika T, Satoh T, Kusaka N, Ebara S, Mouraviev VB, Timme TL, Thompson TC. Route of administration influences the antitumor effects of bone marrow-derived dendritic cells engineered to produce interleukin-12 in a metastatic mouse prostate cancer model. Cancer Gene Ther. 2004;11:317–324. doi: 10.1038/sj.cgt.7700709. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Harada A, Wang JB, Zhang YY, Hashimoto S, Naito M, Matsushima K. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood. 1998;92:118–128. [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang YY, Ogata M, Chen P, Harada A, Hashimoto S, Matsushima K. Transforming growth factor-beta1 polarizes murine hematopoietic progenitor cells to generate Langerhans cell-like dendritic cells through a monocyte/macrophage differentiation pathway. Blood. 1999;93:1208–1220. [PubMed] [Google Scholar]
- 18.Henderson RA, Nimgaonkar MT, Watkins SC, Robbins PD, Ball ED, Finn OJ. Human dendritic cells genetically engineered to express high levels of the human epithelial tumor antigen mucin (MUC-1) Cancer Res. 1996;56:3763–3770. [PubMed] [Google Scholar]
- 19.Reeves ME, Royal RE, Lam JS, Rosenberg SA, Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996;56:5672–5677. [PubMed] [Google Scholar]
- 20.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Fukuhara T, Tanaka M, Nakamura A, Akiyama K, Sakakibara T, Koinuma D, Kikuchi T, Tazawa R, Maemondo M, et al. Vaccination of dendritic cells loaded with interleukin-12-secreting cancer cells augments in vivo antitumor immunity: characteristics of syngeneic and allogeneic antigen-presenting cell cancer hybrid cells. Clin Cancer Res. 2005;11:58–66. [PubMed] [Google Scholar]
- 22.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 23.Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394–3400. [PubMed] [Google Scholar]
- 24.Ojima T, Iwahashi M, Nakamura M, Matsuda K, Naka T, Nakamori M, Ueda K, Ishida K, Yamaue H. The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen. Int J Oncol. 2006;28:947–953. [PubMed] [Google Scholar]
- 25.Stone D, Lieber A. New serotypes of adenoviral vectors. Curr Opin Mol Ther. 2006;8:423–431. [PubMed] [Google Scholar]
- 26.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–278. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 27.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 28.Palmer K, Hitt M, Emtage PC, Gyorffy S, Gauldie J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 2001;8:282–290. doi: 10.1038/sj.gt.3301386. [DOI] [PubMed] [Google Scholar]
- 29.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 30.Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, Wiltrout TA, Nagashima K, Back TC, Wiltrout RH. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest. 2001;108:51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Miyagi T, Jinushi M, Sakamori R, Kohga K, Uemura A, Ohkawa K, et al. Injection of IL-12 gene-transduced dendritic cells into mouse liver tumor lesions activates both innate and acquired immunity. Gene Ther. 2007;14:863–871. doi: 10.1038/sj.gt.3302941. [DOI] [PubMed] [Google Scholar]