Abstract
AIM: To investigate whether the major kavalactone kavain imposes adverse effects on the liver ultrastructure and function by affecting vascular and microvascular architecture and altering hepatocellular morphology.
METHODS: Kavain solution (10 μg/mL or 43.5 μmol/L) was perfused for 2 h in isolated rat livers. After standard fixation and tissue preparation, the samples were examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and light microscopy (LM).
RESULTS: LM, SEM, and TEM examinations indicated kavain-treated rat livers (n = 4) displayed severe vascular and endothelial damage compared to control livers (n = 4).
CONCLUSION: The data so far support the hypothesis that kavain induces adverse effects on liver; additional investigations with other kavalactones and their effects on liver are urgently needed.
Keywords: Kava, Kavalactones, Kavain, Liver, Electron microscopy
INTRODUCTION
Kava-kava, or kava, (Piper methysticum Forst.) is a South Pacific plant that has been used by indigenous people as an intoxicating traditional beverage since ancient times[1–4]. Various preparations of kava have been marketed since the 1980s, especially in Europe and North America to manage mild anxiety, tension, and restlessness. Some reports also indicate that kava preparations may have analgesic, spasmolytic, neuroprotective, and antimitotic activities[5–8]. However, a number of case reports have raised serious concerns about kava’s safety. These reports suggest that, occasionally, even normal doses (200-300 mg/d standardized to contain 70% kavalactones) of kava can cause severe liver injury[9–13]. On the basis of these reports, regulatory agencies have banned or restricted sale of kava and kava products in many countries.
The main active constituents believed to be responsible for the pharmacological (and perhaps toxicological) effects of Piper methysticum are the styryl α-pyrones (called kavapyrones or kavalactones). To date, 18 kavalactones have been identified, among these, six major kavalactones constitute approximately 95% of the lipid extract derived from the dried roots and rhizomes; these are kavain, dihydrokavain, methysticin, dihydromethysticin, yangonin, and desmethoxyyangonin. Figure 1 shows kavain’s chemical structure, its molecular formula is C14H14O3, and molecular weight is 230[14–16].
Figure 1.
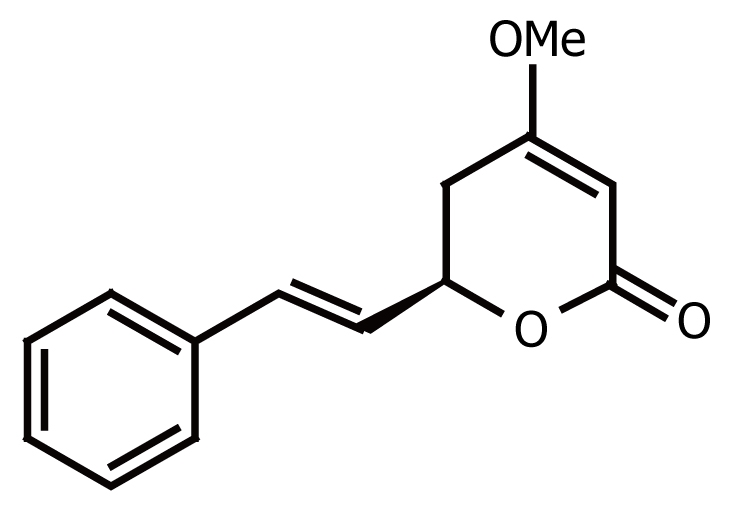
Chemical structure of kavain (KA).
The aim of this study was to investigate whether the major kavalactone, kavain, induces ultrastructural changes and function of the liver instead of biochemical perturbations. This was achieved by examining the vascular, microvascular, and hepatocellular morphological changes that might occur after perfusion of rat livers with kavain. This hypothesis was tested as there are literature evidence that kava might produce general adverse effects on the liver.
MATERIALS AND METHODS
Materials
Kavain (purity 95%) was purchased from ChromaDex, California, USA. Bovine serum albumin (BSA, Fraction V), taurocholic acid, and other chemicals were analytical grade and purchased from Sigma (Sydney, Australia).
Animals
Male Sprague-Dawley rats (Gore Hill, NSW Australia) of about 200 grams were used; prior to the experiments the animals were housed in a 12 h light-dark cycle and controlled temperature environment with free access to standard laboratory chow and water. The study was approved by the Animal Ethics Committee of The University of Sydney.
Isolated rat liver perfusions with kavain
Rat livers were perfused with 10 μg/mL (43.5 μmol/L) kavain in 100 mL perfusate (1% BSA and 0.1% glucose in Krebs-Henseleit bicarbonate buffer) for 120 min for the kavain treatment groups; livers were perfused only with 100 mL perfusate for 120 min in the control group. The surgical and perfusion techniques have been described previously[17]; briefly this involved in situ cannulation of the portal vein and inferior vena cava under barbiturate anaesthesia and harvesting of the liver for recirculating perfusion. Liver viability during the perfusion was assessed by the pH gradient between inflow and outflow perfusate, bile flow rate and the gross appearance of the liver[18]. After 120 min of perfusion, the livers were perfusion fixed with 100 mL of 1.5% glutaraldehyde in 0.12 mol/L cacodylate buffer at pH 6.9 for 7 min. Following this the livers were stored in buffer at 4°C until preparation for electron microscopic examination.
Liver tissue preparation for light and electron microscopy
Tissue samples were prepared according to standard protocols[19,20]. Briefly, glutaraldehyde perfused-fixed samples were cut into 1 mm3 blocks in 1.5% glutaraldehyde/0.12 mol/L cacodylate buffer at pH 6.9. Blocks were then submerged in 1% osmium tetroxide/0.1 mol/L cacodylate buffer for 1 h at 4°C, gradually dehydrated in ethanol and embedded in epon. 50-80 nm ultra thin sections were cut, stained first with uranyl acetate and then with lead citrate. Slides were examined in a Philips Zeiss 902 transmission electron microscope at 80 kV.
Semi-thin (1000 nm) sections of TEM embedded liver samples were cut with a glass knife for overall light microscopic observations. Sections were stained with 1% toluidine blue solution prepared in 1% sodium tetraborate buffer, mounted in DePex (Cat. No. RT 13510, Electron Microscopy Science, Hatfield PA, USA) and examined in bright field mode and recorded using a digital CCD camera.
For scanning electron microscopy (SEM), liver tissue samples were prepared according to standard protocols[19,20]. Briefly, liver tissue was glutaraldehyde-prefixed, cut to 1 mm3 samples and following glutaraldehyde fixation, tissue blocks were postfixed in 1% osmium tetroxide and dehydrated in graded ethanol solutions. The liver tissue was then dried with hexamethyldisilazane and subsequently broken in liquid nitrogen, mounted on stubs and sputter coated with a thin layer of 20 nm gold[21]. SEM-samples were examined in a Philips SEM 505 at 30 kV. Imaging and morphometric analysis was performed on randomly acquired digitized SEM images at magnifications of × 3000, × 5000 or × 20 000 as previously described[22]. For light- and electron microscope studies all experiments were repeated at least four times.
RESULTS
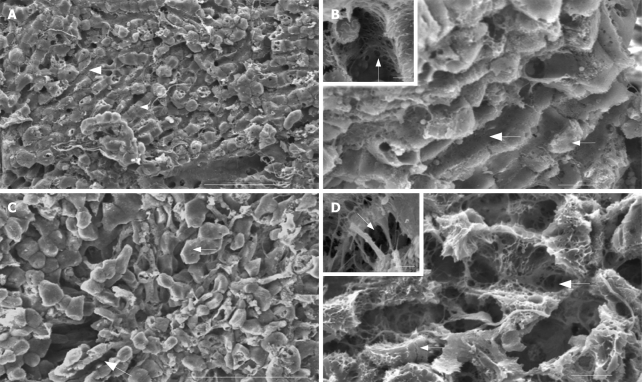
Given that there are literature indications that kava affects liver function it was logical to examine with the aid of scanning electron microscopy, the influence of kavain on the surface liver architecture and vasculature in general and on the intactness of the endothelial lining in particular. Under control (no kavain) conditions sinusoids in the liver lobule show an intact endothelial lining, consisting of endothelium with flattened processes perforated by small pores. These pores (i.e., fenestrae) measure about 150-200 nm in diameter and are arranged in groups, also called sieve plates (Figure 2, SEM images). Following kavain treatment the liver tissue displays overall loss of architecture in general; the narrowing of blood vessels, constriction of sinusoidal blood vessels and retraction of the endothelium. Only few intact pores (fenestrae) can be observed since most of the pores are lost, and gaps form in endothelium which are from the dilatation of pores, these gaps can disturb normal liver transportation.
Figure 2.
Scanning electron micrographs of control (A and B) and kavain-treated (C and D) livers. A: Overall low magnification image shows the intact histological relationship between the liver sinusoidal vascular bed (large arrow) and the neighbouring liver parenchymal cells (small arrow) of control liver tissue. Scale bar, 100 micrometer; B: Intermediate magnification of control liver tissue reveals the intact hepatic endothelial lining (large arrow) and its relationship with the neighbouring hepatocytes (small arrow). Scale bar, 40 micrometer. Inset depicts the pores or so-called fenestrae (arrow) at high magnification (bar represents 1 micrometer); C: Observation of kavain-treated liver tissue at low magnification reveals loss of the normal straight and parallel aligned sinusoidal blood vessels, instead contracted and curly microvessels can be seen (large arrow). Note that the hepatocytes appear to be swollen and have lost their relationship with the bordering endothelial lining (small arrow). Scale bar, 100 micrometer; D: When the same tissue is observed at higher magnification the endothelial damage in the form of gaps (large arrow) and the disconnection of the liver parenchymal cells (small arrow) becomes apparent. Scale bar, 40 micrometer. The inset illustrates the severely damaged endothelial lining (arrow, gaps; scale bar 1 micrometer).
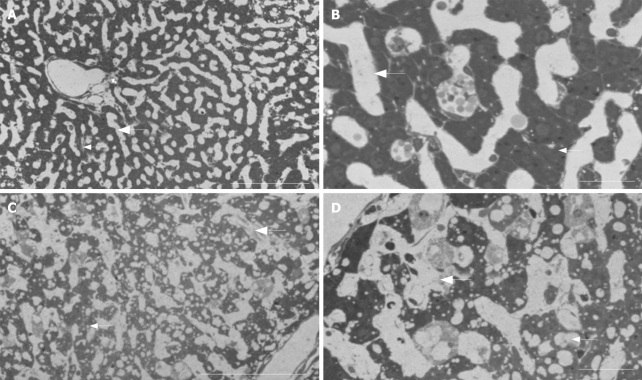
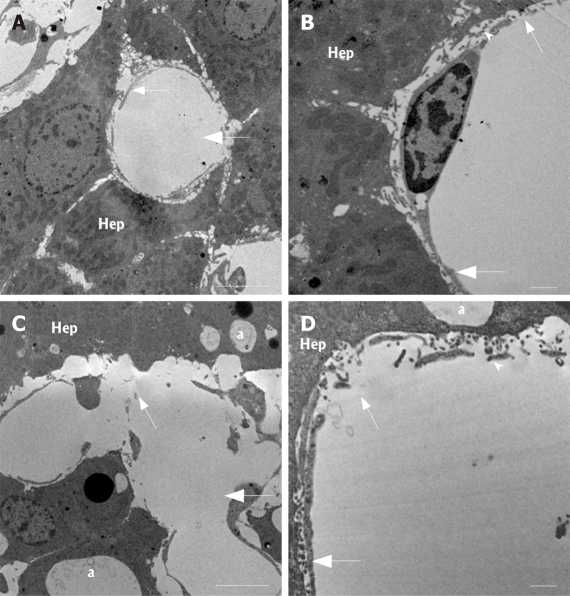
In order to investigate these changes in further detail at overall low versus high magnification light- (Figure 3, LM images) and transmission (Figure 4, TEM images) electron microscopy were applied on sectioned liver tissue, respectively. LM and TEM examination of control tissue shows an intact relationship between the sinusoidal liver vasculature and the neighbouring liver parenchymal cells; i.e., the sinusoid is patent and empty, the wall of the sinusoid is composed of a thin layer of porous (i.e., fenestrated) endothelium, which is covering the space of Disse, filled by microvilli extending from the parenchymal cell surface. These parenchymal cells contain glycogen, a few lipid vesicles, and numerous organelles in their cytoplasm. However, after 120 min of kavain treatment, the LM observations show loss of overall liver architecture; the closed, disrupted endothelium are confirmed, there are no signs of inflammatory reactions (such as platelets and white blood cells or other related cell types); the parenchymal cells of hepatocytes which are lying close to the sinusoidal vasculature are vacuolated, and the vascular bed is constricted, narrowed. The TEM results after kavain treatment confirm the SEM observations; the existence of gaps which indicate disruption of the normal liver architecture, only few pores in endothelial lining were found; and some strong vacuolized hepatocytes surround the blood vessels; finally vascular constriction can be noticed from the images, and it appears that the diameters of the blood vessels are smaller when compared to the control (no kavain) samples. In other words, after kavain treatment the normal hepatic vascular bed is lost. This may cause portal and central pressure perturbations and affect normal physiological liver function.
Figure 3.
Bright field light optical micrographs of control (A and B) and kavain-treated livers (C and D). Note, comparison with the high-magnification TEM data for comparative purposes. A: Control liver tissue at low magnification reveals normal histology: i.e., hepatocytes (small arrow) are organized in long cords following the liver sinusoids or microvascular bed (large arrow). Scale bar, 100 micrometer; B: Higher magnification of control tissue reveals the patent empty sinusoidal lumen (large arrow) and the relationship with the neighbouring hepatocytes (small arrow). Scale bar 40 micrometer; C: In contrast, kavain-treated samples reveal narrowing of the microvessels (large arrow), indicating sinusoidal blood vessel contraction (compare with A). Small arrow, hepatocytes. Scale bar, 100 micrometer; D: Intermediate magnification of kavain-treated samples reveal strong vacuolization of the liver parenchymal tissue (small arrow) and loss of normal sinusoidal vasculature (large arrow). Scale bar, 40 micrometer.
Figure 4.
Transmission electron micrographs of control (A and B) and kavain-treated livers (C and D). Note, comparisons of these high-resolution TEM data with the corresponding low-magnification light microscopy images. A: Intermediate magnification reveals the intact histological relationship between the liver sinusoidal endothelium (small arrow) and the neighbouring liver parenchymal cells (Hep) of control liver tissue. Note the lumen (large arrow) of the small liver capillaries, also called the liver sinusoids. Scale bar, 5 micrometer; B: Examination of control samples at higher magnification reveals the intact endothelial lining (large arrow) containing the typical small transportation pores (fenestrae) (small arrow). Hep, hepatocytes; arrowhead, the space of Disse (defined as the space between the endothelial processes and the hepatocytes). Scale bar, 1 micrometer; C: Kavain-treated liver tissue samples at intermediate magnification shows strong signs of vacuolization of the hepatic tissue (a), sinusoidal endothelial damage (small arrow). Large arrow, liver sinusoids; Hep, hepatocytes or liver parenchymal cells. Scale bar, 5 micrometer; D: High magnification of liver tissue of kavain-treated rats confirm the earlier SEM observations with respect to large gap formation of the endothelial lining (small arrow). Large arrow, sinusoids; arrowhead, space of Disse; asterisk, vacuolization. Scale bar, 1 micrometer.
Noteworthy is that the severe changes in sinusoidal microvasculature and overall loss of the integrity of the liver parenchymal cell architecture was accompanied with striking structural alterations of the liver macrophage population (i.e., Kupffer cells) in the sinusoids of kava-treated livers (Figure 5, high magnification TEM images). Close examination by TEM revealed that Kupffer cells appear to be swollen and the presence of large cytoplasmic vacuoles and phagocytosed material is apparent. Furthermore, the cells have lost their typical ruffled cell membrane aspect and concurrently they appear to disengage from the sinusoidal endothelial lining.
Figure 5.
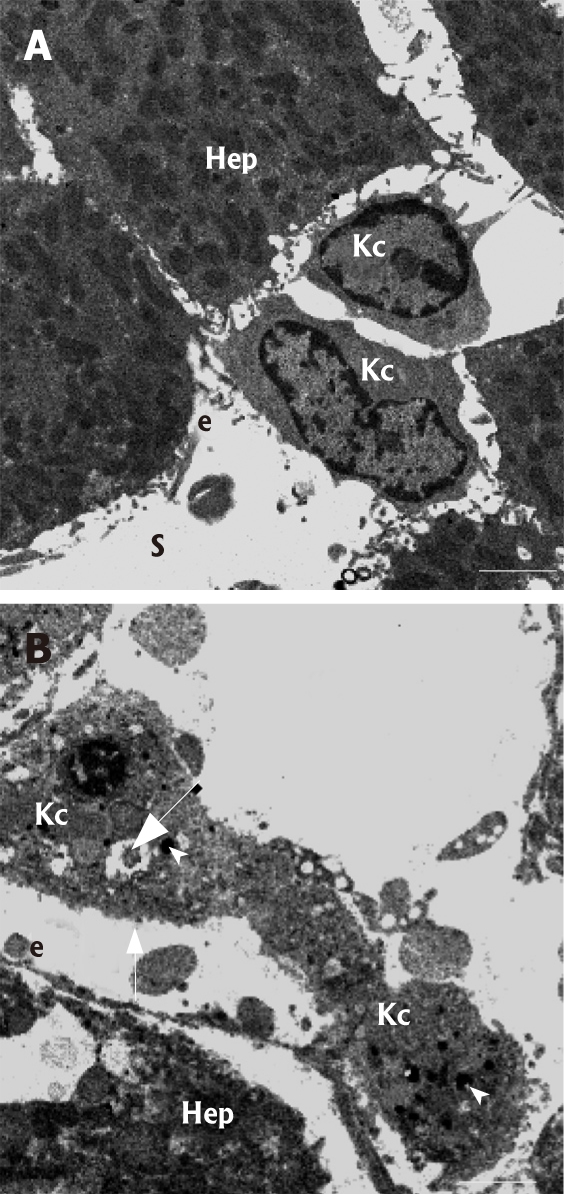
High-magnification transmission electron micro-graphs of Kupffer cells (Kc) in control (A) and kavain-treated livers (B). A: Kupffer cells are located within the sinusoid (S) and adhere to the endothelial (e) lining by means of multiple cytoplasmic extensions. Note, hepatocytes (Hep); B: In kavain-treated livers, Kupffer cells (Kc) show an entire different ultrastructure when compared to control livers (see figure 4A for the difference): i.e., the cells look swollen, contain large amounts of electron dense phagocytosed material (arrowhead), have large vacuoles (large arrow), and have lost their ruffled and microvillous cell surface characteristics (small arrow). Scale bars, 5 micrometer.
DISCUSSION
This study provides LM, TEM and SEM evidence that perfusion of rat liver with kavain (10 μg/mL or 43.5 μmol/L) for 2 h causes severe vascular and endothelial damage manifesting as vasoconstriction, gaps and loss of endothelial and subendothelial liver tissue integrity when compared to control livers. The liver parenchymal tissue looks quite normal except for the cells lying close to the Space of Disse (indicating vacuolization). No other differences were observed between the kavain treated and control livers.
This current study does not allow elucidation of the exact mechanisms by which kavain induces the severe ultrastructural changes in the liver. The severe morphological changes were mainly confined to the sinusoidal vascular bed and the parenchymal liver cells lying close to the Space of Disse. This indicates that kavain either affects the endothelial lining directly resulting in the loss of the integrity of the local sinusoidal liver architecture; or alternatively, kavain might activate the local liver-associated macrophage population (i.e., the Kupffer cells) that in turn release cytotoxic substances which cause the acute endothelial vascular- and parenchymal tissue damage. Consistent with this, it is generally accepted that the increased release of pro-inflammatory mediators and reactive oxygen species by stimulated liver macrophages causes severe and acute liver damage[23,24]. In line with this, we collected fine structural evidence showing the presence of activated liver macrophages in livers exposed to kavain. In addition, these data also support the earlier literature observations of the adverse effects of kava on liver function in general[9–13]. Hence these results open up a new avenue for future research to investigate the cellular adverse effects of kava constituents on isolated liver parenchymal-, endothelial-, and Kupffer cells in mono- or co-cultures by using cytotoxic and functional metabolic assays. Furthermore, additional in vivo experiments might address the intriguing question of whether the effects of kavain on liver tissue are reversible, and may in part explain why patients recover from kava intoxication. Mounting literature evidence is available that dysfunction of the hepatic sinusoid can be reversed by modulating the Kupffer cell population[25] or its activation status[26]. On the other hand, it has been recently shown that acute structural and pathophysiological damage of the liver sinusoidal endothelial lining can revert to normal within days[27].
The current study only examined the effects of one kavalactone, kavain on the liver. Hence it is not possible to conclude whether the other five (dihydrokavain, methysticin, dihydromethysticin, yangonin, and desmethoxyyangonin) major kavalactones have similar effects individually on the liver. In addition, since kava contains all the six kavalactones in combination, this pilot study has not examined any interactive or synergistic effects of these kavalactones with respect to adverse effects on the liver. It is also not able to be predicted whether kavain and other kavalactones have similar effects on human liver as has been observed here with rat liver.
This study also used only one (10 μg/mL or 43.5 μmol/L) concentration of kavain which was continuously exposed to the livers for 2 h. The choice of this exposure concentration and time was based on literature data. Plasma concentration of total kavalactones was about 33 μg/mL in a fatal human accident subsequent to kava ingestion with alcohol and cannabis[28]. The toxic concentration of kava extracts was 50 μg/mL in rat hepatocytes[29] while kavain toxic concentration (as judged by release of lactate dehydrogenase, LDH) was 125 μg/mL in mouse hepatocytes[30]. Based on this literature data, the kavain concentration in the current study was 10 μg/mL, and the results clearly show liver damage at this concentration. It would therefore be expected that at higher kavain concentrations adverse hepatic effects would be more severe. The exposure time of 2 h reflects literature evidence that livers remain normal during perfusions up to 3 h[18]. The structural changes observed after kavain exposure are therefore genuine as control perfusions revealed normal liver architecture. However, it is not known if the effects of kavain noted here are acute or chronic or if the effects are reversible since the livers were continuously exposed to kavain for 2 h. Since only one kavain concentration was used, it is not known if the effects observed here are concentration-dependent and what the minimum hepatotoxic concentration of kavain is.
COMMENTS
Background
Since the use of kava and kava-containing products were banned in some Western countries due to case report of liver failure, this study was aimed to examine the vascular, microvascular and hepatocellular fine morphological changes that might occur after perfusion of rat livers with major kavalactone kavain.
Research frontiers
This article demonstrates for the first time using light and electron microscopy liver vascular, microvascular architecture and hepatocellular morphologic changes after liver perfusion with kavain perfusate solution.
Innovation and breakthroughs
The isolation perfused rat liver (IPRL) method has been widely used in drug metabolism studies as it closely simulates in vivo (whole body) conditions with the advantage that experimental conditions can be precisely controlled, like perfusion time and drug concentration, since high concentrations of drugs would normally be a limitation because of their side effects in vivo. Thus IPRL combined with light and electron microscopic techniques increases the chance of determining the exact ultrastructural changes in the liver.
Applications
In this study kavain solution (10 μg/mL) was perfused for 2 h in IPRL, after standard fixation and tissue preparation, the samples were examined by scanning electron microscopy, transmission electron microscopy and light microscopy. Examinations indicated kavain-treated rat livers displayed severe vascular and endothelial damage compared to control livers. The data so far support the hypothesis that kavain induces adverse effects on liver. Further investigations with other major kavalactones and with different kavalactone concentrations to examine their effects on liver are urgently needed.
Terminology
Herb kava-kava (Piper methysticum Forst) has been used extensively in the Pacific as a drink for thousands of years. The roots of kava are used to prepare a drink for social and ceremonial occasions, similar to coffee or tea in other cultures. The root is also used for soothing the nerves, inducing relaxation and sleep. Various preparations of kava have also been marketed since 1980s in Europe and North America to manage mild anxiety and insomnia.
Peer review
The authors show that kavain induces adverse effects. The methods and histological examinations are rigid and instructive. The presentation is good. This present study is an interesting trial.
Acknowledgments
The authors acknowledge access to the facilities as well as technical and administrative assistance from staff in the NANO Major National Research Facility at the “Australian Key Centre for Microscopy and Microanalysis” of The University of Sydney.
Peer reviewer: Yasuji Arase, MD, Department of Gastroen-terology, Toranomon Hospital, 2-2-2Toranomonminato-ku, Tokyo 105-8470, Japan
S- Editor Liu Y L- Editor Mihm S E- Editor Li HY
References
- 1.Lebot V, Merlin M, Lindstrom L. Kava - The Pacific Elixir: the Definitive Guide to its Ethnobotany, History and Chemistry. 2nd ed. New Haven: Yale University Press; 1997. pp. 1–9. [Google Scholar]
- 2.Cawte J. Psychoactive substances of the South Seas: betel, kava and pituri. Aust N Z J Psychiatry. 1985;19:83–87. doi: 10.3109/00048678509158818. [DOI] [PubMed] [Google Scholar]
- 3.Norton SA. Herbal medicines in Hawaii from tradition to convention. Hawaii Med J. 1998;57:382–386. [PubMed] [Google Scholar]
- 4.Singh YN. Kava: an overview. J Ethnopharmacol. 1992;37:13–45. doi: 10.1016/0378-8741(92)90003-a. [DOI] [PubMed] [Google Scholar]
- 5.Singh YN, Singh NN. Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs. 2002;16:731–743. doi: 10.2165/00023210-200216110-00002. [DOI] [PubMed] [Google Scholar]
- 6.Connor KM, Davidson JR. A placebo-controlled study of Kava kava in generalized anxiety disorder. Int Clin Psychopharmacol. 2002;17:185–188. doi: 10.1097/00004850-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Pittler MH, Ernst E. Cochrane Database Syst Rev. (2) 2002. Kava extract for treating anxiety; p. CD003383. [DOI] [PubMed] [Google Scholar]
- 8.Wheatley D. Kava and valerian in the treatment of stress-induced insomnia. Phytother Res. 2001;15:549–551. doi: 10.1002/ptr.840. [DOI] [PubMed] [Google Scholar]
- 9.Escher M, Desmeules J, Giostra E, Mentha G. Hepatitis associated with Kava, a herbal remedy for anxiety. BMJ. 2001;322:139. doi: 10.1136/bmj.322.7279.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft M, Spahn TW, Menzel J, Senninger N, Dietl KH, Herbst H, Domschke W, Lerch MM. Fulminant liver failure after administration of the herbal antidepressant Kava-Kava. Dtsch Med Wochenschr. 2001;126:970–972. doi: 10.1055/s-2001-16966. [DOI] [PubMed] [Google Scholar]
- 11.Russmann S, Lauterburg BH, Helbling A. Kava hepatotoxicity.Ann Intern Med. 2001;135. pp. 68–69. [DOI] [PubMed] [Google Scholar]
- 12.Strahl S, Ehret V, Dahm HH, Maier KP. Necrotizing hepatitis after taking herbal remedies. Dtsch Med Wochenschr. 1998;123:1410–1414. doi: 10.1055/s-2007-1024196. [DOI] [PubMed] [Google Scholar]
- 13.Parkman CA. Another FDA warning: Kava supplements. Case Manager. 2002;13:26–28. doi: 10.1067/mcm.2002.126437. [DOI] [PubMed] [Google Scholar]
- 14.Klohs MW. Chemistry of kava. Psychopharmacol Bull. 1967;4:10. [PubMed] [Google Scholar]
- 15.Ramzan I, Tran HV. Chemistry of Kava and Kavalactones. In: Singh YN, editor. Kava from Ethnology to Pharmacology. Boca Raton: CRC Press; 2004. p. 76. [Google Scholar]
- 16.He XG, Lin LZ, Lian LZ. Electrospray high performance liquid chromatography-mass spectrometry in phytochemical analysis of kava (Piper methysticum) extract. Planta Med. 1997;63:70–74. doi: 10.1055/s-2006-957608. [DOI] [PubMed] [Google Scholar]
- 17.Hong Y, Ramzan I, McLachlan AJ. Disposition of amphotericin B in the isolated perfused rat liver. J Pharm Pharmacol. 2004;56:35–41. doi: 10.1211/0022357022502. [DOI] [PubMed] [Google Scholar]
- 18.Ross BD. Perfusion techniques in biochemistry. Oxford: Clarendon Press; 1972. p. 162. [Google Scholar]
- 19.Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- 20.Wisse E, De Zanger RB, Charels K, Van Der Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 21.Braet F, De Zanger R, Wisse E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Microsc. 1997;186:84–87. doi: 10.1046/j.1365-2818.1997.1940755.x. [DOI] [PubMed] [Google Scholar]
- 22.Braet F, De Zanger R, Sasaoki T, Baekeland M, Janssens P, Smedsrod B, Wisse E. Assessment of a method of isolation, purification, and cultivation of rat liver sinusoidal endothelial cells. Lab Invest. 1994;70:944–952. [PubMed] [Google Scholar]
- 23.Bouwens L, Wisse E. The origin of Kupffer Cells and their anatomic relationship to hepatocytes. In: Billiar TR, Curran RD Hepatocyte and Kupffer cell interactions, editors. London: CRC Press; 1992. pp. 3–21. [Google Scholar]
- 24.Tsukamoto H, Lin M. The role of Kupffer cells in liver injury. In: Wisse E, Knook DL, Balabaud C, editors. Cells of the Hepatic Sinusoid 6. Leiden: Kupffer Cell Foundation; 1997. pp. 244–250. [Google Scholar]
- 25.Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg. 2000;7:40–48. doi: 10.1007/s005340050152. [DOI] [PubMed] [Google Scholar]
- 26.Laskin DL. Sinusoidal lining cells and hepatotoxicity. Toxicol Pathol. 1996;24:112–118. doi: 10.1177/019262339602400115. [DOI] [PubMed] [Google Scholar]
- 27.Yeikilis R, Gal S, Kopeiko N, Paizi M, Pines M, Braet F, Spira G. Hydrodynamics based transfection in normal and fibrotic rats. World J Gastroenterol. 2006;12:6149–6155. doi: 10.3748/wjg.v12.i38.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarbah F, Barguil Y, Weinmann W, Mueller C, Duhet D, Cabalion P, Kardek B, Daidrup T. Death after consumption of kava beverage in combination with alcohol and cannabis. GTFCh- Symposium; April 3-5; Germany Mosbach 2003. Leiden: Kupffer Cell Foundation; 2003. pp. 101–108. [Google Scholar]
- 29.Schulze J, Raasch W, Siegers CP. Toxicity of kava pyrones, drug safety and precautions--a case study. Phytomedicine. 2003;10 Suppl 4:68–73. doi: 10.1078/1433-187x-00300. [DOI] [PubMed] [Google Scholar]
- 30.Smith DJ, Kamendulis LM, Klaunig JE. Kava induces hepatotoxicity in male B6C3F1 mice. Medical paper online, 2003-09-03, cited 2005-02-23. Available from: URL: http://www.medical-papers.com/kava+control+resin+release+dhm/ [Google Scholar]