Abstract
A Somali cat was presented with recurrent anorexia, lethargy, vomiting and icterus. A macrocytic-hypochromic, regenerative haemolytic anaemia was identified and hereditary pyruvate kinase deficiency was confirmed by means of breed-specific DNA mutation analysis. The case was complicated by the presence of markedly elevated serum liver enzyme activities, hyperbilirubinaemia, coagulopathy and ultrasonographic evidence of gall bladder choleliths and extrahepatic bile duct obstruction. The choleliths consisted of 100 per cent bilirubin, likely because of chronic haemolysis and haeme degradation. In conclusion, haemosiderosis and bilirubin cholelithiasis can be a consequence of chronic haemolysis in pyruvate kinase-deficient cats, as seen in human beings with a variety of chronic haemolytic disorders.
INTRODUCTION
Erythrocytic pyruvate kinase (PK) deficiency has recently been described as a cause of hereditary haemolytic anaemia (HA) in Somali and Abyssinian cats and is caused by a splicing defect. Common clinical signs include intermittent pallor, lethargy and inappetence, while icterus is rarely present (Ford and others 1992, Giger and others 1997, Giger 2001, Kohn and others 2005, Mansfield and Clark 2005, Harvey 2006a). In human beings, chronic haemolytic disorders are major risk factors for the formation of pigment gallstones (Everson and others 1989, Aydogdu and others 2001). Altered gall bladder function is believed to contribute to the pathogenesis of bilirubin cholelith formation in human beings as not all these patients develop cholelithiasis (Everson and others 1989). The formation of bilirubin cholelithiasis and extrahepatic bile duct obstruction (EBDO) associated with chronic HA is a rare but potentially serious complication in cats (Harvey and others 2007).
CASE HISTORY
A two-and-a-half-year-old female spayed Somali cat was referred to the Department of Medicine and Clinical Biology of Small Animals at the University of Ghent, Belgium, for recurrent icterus during the previous six months. These episodes generally lasted a few days and were accompanied by lethargy, anorexia and intermittent vomiting. Previous treatment included intravenous fluids and amoxicillin clavulanate. On presentation, the cat was alert, but mucous membranes were icteric and somewhat tacky. Rectal temperature was 39·2°C, heart rate was 200 beats/minute and respiratory rate was 20 breaths/minute. Abdominal palpation revealed a large mass, suspected to be an enlarged spleen.
A complete blood count (CBC) showed a macrocytic, hypochromic regenerative anaemia [haematocrit 20·1 per cent, reference range (RR) 30 to 45 per cent; mean corpuscular volume 67 fl, RR 38 to 50 fl; mean corpuscular haemoglobin concentration 29 g/dl, RR 30 to 36 mg/dl; absolute reticulocyte count 126,000/μl, RR <40,000/μl]. There was marked hyperproteinaemia (96 g/l, RR 56 to 78 g/l) because of hyperglobulinaemia (60 g/l) with increased gamma globulins (41 g/l). Other abnormal serum biochemical test results included hyperbilirubinaemia (bilirubin 273 μmol/l, RR <7 μmol/l), elevated activities of serum aspartate aminotransferase (88 U/l, RR <46 U/l), alanine aminotransaminase (ALT; 612 U/l, RR <43 U/l), alkaline phosphatase (ALP; 332 U/l, RR <101 U/l), γ-glutamyltransferase (γ-GT; 26 U/l, RR <9 U/l) and increased fasting serum bile acid concentration (32 μmol/l, RR <10 μmol/l). However, values of all these parameters may have been affected by the high-serum bilirubin concentration. Coagulation times were moderately prolonged (prothrombin time 13 seconds, RR 8 to 9 seconds; activated partial thromboplastin time 40 seconds, RR 16 to 20 seconds). The cat tested negative for feline immunodeficiency virus antibodies (immunofluorescence antibody test) and feline leukaemia virus antigen (ELISA). Urinalysis, after a few hours of infusion therapy, revealed a specific gravity of 1·018 and 31 bilirubinuria. Microscopic examination of blood smears showed polychromasia, anisocytosis and no microorganisms (for example, Mycoplasma haemofelis). Macroscopic agglutination was not present. A direct polyvalent Coombs’ test (Nordic) was positive (titre 1:2 to 1:6, no positive controls were available and cats with immune-mediated HA have titres >1:8 in this laboratory) at 37°C. Hereditary increased erythrocytic osmotic fragility (OF), a condition with the same breed predisposition and clinical presentation, was unlikely given the fact that no in vitro haemolysis was present after an EDTA blood sample was left overnight in the refrigerator, but an actual osmotic fragility test was not performed (Kohn and others 2000).
Because of the known occurrence of hereditary PK deficiency in Somali cats, an EDTA blood sample was shipped by regular airmail for mutation-specific PK DNA testing to Philadelphia where the cat was found to be PK deficient (Giger and others 1997).
Abdominal radiographs confirmed massive splenomegaly (Fig. 1). Ultrasonographically, the spleen was homogeneously enlarged and of normal echogenicity. The dilated gall bladder contained a large amount of biliary sludge and choleliths (Fig. 2A). The common bile duct (CBD) was tortuous and enlarged, suggesting extrahepatic obstruction (Fig. 2B); however, no cause could be identified. The pancreas and kidneys appeared within normal limits. Thoracic radiographs were unremarkable.
FIG 1.
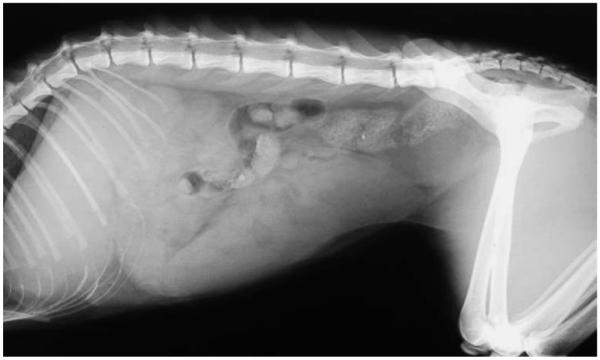
Abdominal radiograph of a pyruvate kinase-deficient Somali cat showing massive splenomegaly
FIG 2.
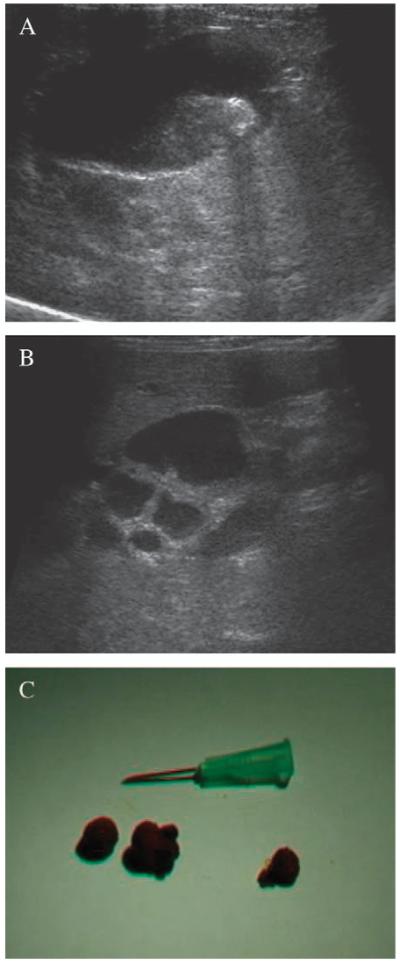
Ultrasonographic evaluation of biliary tree from a pyruvate kinase-deficient Somali cat: the enlarged gall bladder (A) contained biliary sludge and a hyperechoic focus with accompanying acoustic shadow representing a cholelith. A tortuous and dilated common bile duct, with a diameter of 6 to 7 mm diameter [normal range <5 mm (Leveillé and others 1996)], was also noted (B). Hepatic parenchyma appeared of normal echogenicity and intrahepatic bile ductules were not dilated. Three choleliths (C) of 8 to 12 mm composed exclusively of bilirubin (atomic absorption spectrophotometry) were found at necropsy
Surgical bile duct decompression, removal of choleliths, liver biopsy and splenectomy were considered. Transfusion support was deemed necessary, considering the cat’s marked anaemia and coagulopathy. However, because the cat was found to have blood type B and no type B donor was readily available, the patient was not transfused.
Initial treatment included saline infusion, 1 mg/kg vitamin K1 sc (q24h; Konakion; Roche) (coagulopathy in part caused by vitamin K deficiency because of biliary obstruction and malabsorption) and 12·5 mg/kg amoxicillin clavulanate (12 h; Synulox; Pfizer Animal Health). The following day, the cat appeared alert and the packed cell volume (PCV) was 17 per cent. Abdominal ultrasound showed resolution of CBD dilation (3 mm diameter), and the gall bladder was no longer distended, although it still contained sludge and choleliths. The cat regained appetite on the second day and continued to improve clinically. After one week, the PCV was 19 per cent and the absolute reticulocyte count 188,700/μl, indicating ongoing haemolysis. Furthermore, serum bilirubin (113 μmol/l), fasting bile acid concentrations (11 μmol/l) and liver enzyme activities (ALT 245 U/l, γ-GT 12 U/l, ALP 153 U/l) had markedly decreased. Coagulation tests were not repeated. About 1 mg/kg prednisolone (q24h; Deltacortril; Pfizer) was added orally to inhibit the macrophage system and erythrocyte destruction during haemolytic episodes, followed by tapering over weeks. Orally, 10 mg/kg ursodeoxycholic acid (q24h; Ursochol; Zambon) was also added to improve bile flow. Over the next three months, the owner reported recurrent episodes of pallor, icterus, inappetence and weakness. Further examination, laboratory testing and an exploratory celiotomy with cholecystectomy and splenectomy were offered; however, the owner elected euthanasia and permitted a limited pathological examination of abdominal organs.
There was mild hepatomegaly with a markedly distended CBD, cystic duct and gall bladder, which contained thickened, red-brown bile fluid. Three choleliths, composed of 100 per cent bilirubin, were found inside the gall bladder, cystic and CBD, respectively (Fig. 2C).The latter cholelith contributed mostly to the bile duct obstruction. Histopathology of liver and spleen showed haemosiderosis (Fig. 3A). The spleen was massively enlarged, and erythrocyte destruction and extramedullary haematopoiesis were found histopathologically (Fig. 3B). Similar findings were seen in dark brown abdominal lymph nodes. Gross and histopathological examinations of pancreas, kidneys and small intestines were unremarkable.
FIG 3.

Histopathology of liver (A) and spleen (B) from a pyruvate kinase-deficient Somali cat. Kupffer cells and hepatocytes are laden with haemosiderin (staining with Prussian blue), indicating hepatic haemosiderosis. The spleen (H&E staining) also contained excessive haemosiderin and also many megakaryocytes and erythroid precursor cells suggesting extramedullary haematopoiesis
DISCUSSION
This case report documents unusual bilirubin cholelithiasis and EBDO as serious complications of hereditary erythrocytic PK deficiency in an anaemic Somali cat. Because this case has been seen, another PK-deficient Somali cat from Britain has been reported (Harvey and others 2007), making this an important consequence in cats with this erythroenzymopathy.
The high serum liver enzyme activities and bile acid concentrations, severe hyperbilirubinaemia and bilirubin choleliths seen in the Somali cats reported here suggested that, besides haemolysis, hepatobiliary disease was also present. Indeed, PK-deficient cats usually only have mild increases in serum bilirubin values and therefore icterus is rare, likely because of the fact that their bilirubin system is accelerated and can metabolise large quantities of haeme degradation (Giger 2001). Also, in Abyssinian and Somali cats with increased OF and chronic intermittent HA, serum bilirubin concentration is only mildly elevated (Kohn and others 2000). Evidence of EBDO was found on abdominal ultrasound at initial presentation and later at necropsy (with one cholelith obstructing the cystic and another the CBD). In human beings with PK deficiency and other hereditary HAs, formation of inspissated biliary sludge, bilirubin choleliths, posthepatic biliary obstruction, secondary cholangiopathy and hepatic failure are known complications. While gallstones in human beings occur frequently, feline choleliths are uncommon and generally are incidental findings rather than causes of biliary obstruction (Center 1996, Eich and Ludwig 2002, Mayhew and others 2002). Choleliths are usually associated with altered bile composition, cholecystitis, dietary factors, biliary parasitic or bacterial infections and cholangiohepatitis, and biliary stasis is suspected to favour biliary lithogenesis (Center 1996, Eich and Ludwig 2002). There is little information on cholelith composition in cats, which can include variable amounts of cholesterol, calcium and bilirubin (Eich and Ludwig 2002). The pure bilirubin constitution of the calculi from the cat reported here is a unique finding for any cat but consistent with what is seen in human beings with chronic haemolytic disorders (Aydogdu and others 2001). Calcium bilirubinate gallstones also have been reported in mice with hereditary HAs (Trotman and others 1981). Excessive bilirubin production during haemolysis overloads the hepatocyte-conjugating capacity, increases the amount of bilirubin monoconjugates and unconjugated bilirubin in bile and results in precipitation as black gallstones (Aydogdu and others 2001). Other possible contributing factors include changes in bile pH and calcium, overproduction of organic matrix, gall bladder enlargement, bile stasis and sludge retention (Everson and others 1989, Aydogdu and others 2001). Similar pathogenesis was suspected in the present case as haemolysis, gall bladder enlargement and bile stasis were present. Possibly, the inspissated bile caused further accumulation of biliary bilirubin. In human patients with chronic haemolytic disorders and resultant cholelithiasis, cholecystectomy is performed concomitantly with splenectomy (Al-Salem 2003).
The reason for the initial resolution of ultrasonographic signs of biliary obstruction in the cat reported here may suggest that some cholelith passed, despite the fact that no choleliths were detected other than the ones in the gall bladder. Indeed, this area is more difficult to visualise ultrasonographically because of reverberation artefacts, secondary to intestinal gas (Leveillé and others 1996). No additional causes of EBDO such as neoplasia, pancreatitis or diaphragmatic hernia were identified (Cornell and others 1993, Mayhew and others 2002, Buote and others 2006). Another hypothesis for the bile duct dilation was the formation of thickened biliary sludge. During biliary stasis, bile becomes progressively thicker as water is absorbed causing inspissated biliary sludge (Partington and Biller 1996). Obstructive jaundice because of sludge in the CBD has been described in human medicine and in a cat with haemobartonellosis (Kaplan and others 1973, Riederer 2000). It should be noted that biliary sludge identified during ultrasonography is non-pathological unless there are other signs consistent with cholestasis (Center 1996).
While other acquired hepatic disorders were considered as contributing factors to the elevated serum liver enzymes and hyperbilirubinaemia, including hepatic lipidosis, lymphoma, cholangiohepatitis and feline infectious peritonitis, they were ruled out at necropsy. In human beings, chronic haemolysis is a well-known cause of haemosiderosis, leading to hepatopathies and other organ failure (Nakao and others 2001, Vichinsky and others 2005). Cholangiohepatopathies occur in human beings with PK deficiency (Hilgard and Gerken 2005) but more commonly with haemoglobinopathies (sickle cell anaemia and thalassaemia) (Olivieri and Brittenham 1997, Vichinsky and others 2005). Increased iron absorption because of haemolysis and iron overload from multiple transfusions may result in splenic and hepatic haemosiderosis and ultimately hepatic failure. While hepatic haemosiderosis generally does not cause major signs in PK-deficient dogs and cats, it was described in a PK-deficient Cairn terrier (Schaer and others 1992) with ensuing hepatic failure. This complication can be avoided by eliminating the cause of the haemolysis as shown experimentally after bone marrow transplantation in PK-deficient Basenjis (Weiden and others 1981). In this case, hepatic histological abnormalities were most likely related to a combination of biliary stasis because of the obstruction by choleliths and chronic haemolysis with subsequent haemosiderosis.
Because the owner declined further diagnostic testing before euthanasia, it remains unknown to what degree the HA, biliary obstruction and hepatopathy contributed to the intermittent recurrence of icterus, pale mucous membranes, anorexia, weakness, lethargy and demise in the cat of this report. The CBD and cystic duct obstruction found at necropsy, however, likely heightened the severity of the clinical progression.
Efforts were made to exclude specific infectious, neoplastic and toxic causes of haemolysis. Despite the fact that blood smears were screened for haemotrophic Mycoplasma species on multiple occasions, sensitivity is low and infection cannot be completely ruled out as PCR testing was not performed (Harvey 2006b). If present, haemotrophic M. haemofelis could have contributed both to the HA and to a positive Coombs’ test result. This cat had marked hyperglobulinaemia, which has previously been described in cats with PK deficiency (Giger 2001, Kohn and others 2005), but its precise cause remains to be elucidated. Immune-mediated HA is considered to occur relatively rarely in cats compared with dogs (Giger 2005, Kohn and others 2006).
CONCLUSION
In this case, the presence of CBD obstruction, choleliths and haemosiderosis complicated the diagnosis of the HA because of PK deficiency. Careful evaluation and monitoring of anaemia as well as the biliary system of PK-deficient Somali and Abyssinian cats are recommended, especially if serum bilirubin levels are high.
Acknowledgments
In part presented at the 48th BSAVA Congress, 2005, Birmingham, UK. Studies performed at the University of Pennsylvania were supported in part by the National Institutes of Health (grant no. RR02512) and the Josephine Deubler Genetic Disease Testing Laboratory.
References
- Al-Salem AH. Should cholecystectomy be performed concomitantly with splenectomy in children with sickle-cell disease? Pediatric Surgery International. 2003;19:71–74. doi: 10.1007/s00383-002-0804-5. [DOI] [PubMed] [Google Scholar]
- Aydogdu I, Sari R, Ulu R, Sevinc A. The frequency of gallbladder stones in patients with pernicious anemia. Journal of Surgical Research. 2001;101:120–123. doi: 10.1006/jsre.2001.6269. [DOI] [PubMed] [Google Scholar]
- Buote NJ, Mitchell SL, Penninck D, Freeman LM, Webster CRL. Cholecystoenterostomy for treatment of extrahepatic biliary tract obstruction in cats: 22 cases (1994-2003) Journal of the American Veterinary Medical Association. 2006;228:1376–1382. doi: 10.2460/javma.228.9.1376. [DOI] [PubMed] [Google Scholar]
- Center SA. Diagnostic procedures for evaluation of hepatic disease. In: Guilford WG, Center SA, Strombeck DR, Williams DA, Meyer DJ, editors. Strombeck’s Small Animal Gastroenterology. 3rd edn. W. B. Saunders Co; Philadelphia, PA, USA: 1996. pp. 130–188. [Google Scholar]
- Cornell KK, Jakovljevic S, Waters DJ, Prostredny J, Salisbury SK, Denicola DB. Extrahepatic biliary obstruction secondary to diaphragmatic hernia in two cats. Journal of the American Animal Hospital Association. 1993;29:502–506. [Google Scholar]
- Eich C, Ludwig LL. The surgical treatment of cholelithiasis in cats: a study of nine cases. Journal of the American Animal Hospital Association. 2002;38:290–296. doi: 10.5326/0380290. [DOI] [PubMed] [Google Scholar]
- Everson GT, Nemeth A, Kourkourian S, Zogg D, Berger Leff N, Dixon D, Githens JH, Pretorius D. Gallbladder function is altered in sickle hemoglobinopathy. Gastroenterology. 1989;96:1307–1316. doi: 10.1016/s0016-5085(89)80018-6. [DOI] [PubMed] [Google Scholar]
- Ford S, Giger U, Duesberg C, Beutler E, Wang P. Inherited erythrocyte pyruvate kinase (PK) deficiency causing hemolytic anemia in an Abyssinian cat. Journal of Veterinary Internal Medicine. 1992;6:123. [Google Scholar]
- Giger U. Hereditary erythrocyte disorders. In: August JR, editor. Consultations of Feline Med Surg. 4th edn. W. B. Saunders; Philadelphia, PA, USA: 2001. pp. 484–489. [Google Scholar]
- Giger U. Regenerative anemias caused by blood loss or hemolysis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th edn. Elsevier Saunders; St. Louis, MO, USA: 2005. pp. 1886–1907. [Google Scholar]
- Giger U, Rajpurohit Y, Wang P, Ford S, Kohn B, Niggemeier A, Patterson DF, Beutler E, Henthorn PS. Molecular basis of erythrocyte pyruvate kinase (R-PK) deficiency in cats. Blood. 1997;90:2701. [Google Scholar]
- Harvey JW. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses. Veterinary Clinical Pathology. 2006a;35:144–156. doi: 10.1111/j.1939-165x.2006.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Harvey JW. Hemotrophic mycoplasmosis (Hemobartonellosis) In: Greene CE III, editor. Infectious Diseases of the Dog and Cat. 4th edn. Elsevier Saunders; St. Louis, MO, USA: 2006b. pp. 252–260. [Google Scholar]
- Harvey AM, Holt PE, Barr FJ, Rizzo F, Tasker S. Treatment and long-term follow-up of extrahepatic biliary obstruction with bilirubin cholelithiasis in a Somali cat with pyruvate kinase deficiency. Journal of Feline Medicine Surgery. 2007;9:424–431. doi: 10.1016/j.jfms.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgard P, Gerken G. Liver cirrhosis as a consequence of iron overload caused by hereditary nonspherocytic hemolytic anemia. World Journal of Gastroenterology. 2005;11:1241–1244. doi: 10.3748/wjg.v11.i8.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Keller K, Schrodt GR. Surgical correction of obstructive jaundice in a cat suffering from hemobartonellosis. Veterinary Medicine/Small Animal Clinics. 1973;68:993–994. [PubMed] [Google Scholar]
- Kohn B, Goldschmidt MH, Hohenhaus AE, Giger U. Anemia, splenomegaly, and increased osmotic fragility of erythrocytes in Abyssinian and Somali cats. Journal of the American Veterinary Medical Association. 2000;217:1483–1497. doi: 10.2460/javma.2000.217.1483. [DOI] [PubMed] [Google Scholar]
- Kohn B, Fumi C, Seng A, Giger U. Anämie infolge erythrozytären Pyruvatkinase-Mangels und deren Verbreitung bei Somali- und Abessinierkatzen in Deutschland. Kleintierpraxis. 2005;50:305–312. [Google Scholar]
- Kohn B, Weingart C, Eckmann V, Ottenjann M, Leibold W. Primary immune-mediated hemolytic anemia in 19 cats. Journal of Veterinary Internal Medicine. 2006;20:159–166. doi: 10.1892/0891-6640(2006)20[159:pihaic]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Leveillé R, Biller DS, Shiroma JT. Sonographic evaluation of the common bile duct in cats. Journal of Veterinary Internal Medicine. 1996;10:296–299. doi: 10.1111/j.1939-1676.1996.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Mansfield CS, Clark P. Pyruvate kinase deficiency in a Somali cat in Australia. Australian Veterinary Journal. 2005;83:483–485. doi: 10.1111/j.1751-0813.2005.tb13298.x. [DOI] [PubMed] [Google Scholar]
- Mayhew PD, Holt DE, Mclear RC, Washabau RJ. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. Journal of Small Animal Practice. 2002;43:247–253. doi: 10.1111/j.1748-5827.2002.tb00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Toyozaki T, Nagakawa H, Himi T, Yamada K, Watanabe S, Masuda Y, Asai T. Cardiac dysfunction because of secondary hemochromatosis caused by congenital non-spherocytic hemolytic anemia. Japanese Circulation Journal. 2001;65:126–128. doi: 10.1253/jcj.65.126. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- Partington BP, Biller DS. Liver. In: Green RW, editor. Small Animal Ultrasound. Lippincott-Raven; Philadelphia, PA, USA: 1996. p. 105. [Google Scholar]
- Riederer J. Obstructive jaundice due to sludge in the common bile duct. Deutsche Medizinische Wochenschrift. 2000;125:11–14. doi: 10.1055/s-2007-1023877. [DOI] [PubMed] [Google Scholar]
- Schaer M, Harvey JW, Calderwood-Mays M, Giger U. Pyruvate kinase deficiency causing hemolytic anemia with secondary hemochromatosis is a Cairn terrier. Journal of the American Animal Hospital Association. 1992;28:233–239. [Google Scholar]
- Trotman BW, Bernstein SE, Balistreri WF, Wirt GD, Martin RA. Hemolysis-induced gallstones in mice: increased unconjugated bilirubin in hepatic bile predisposes to gallstone formation. Gastroenterology. 1981;81:232–236. [PubMed] [Google Scholar]
- Vichinsky E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. American Journal of Hematology. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- Weiden PL, Hackman RC, Deeg HJ, Graham TC, Thomas ED, Storb R. Long-term survival and reversal of iron overload after marrow transplantation in dogs with congenital hemolytic anemia. Blood. 1981;57:66–70. [PubMed] [Google Scholar]


