Summary
The neurotrophin receptor p75 is induced by various injuries to the nervous system, but its role after injury has remained unclear. Here, we report that p75 is required for the death of oligodendrocytes following spinal cord injury, and its action is mediated mainly by proNGF. Oligodendrocytes undergoing apoptosis expressed p75, and the absence of p75 resulted in a decrease in the number of apoptotic oligodendrocytes and increased survival of oligodendrocytes. ProNGF is likely responsible for activating p75 in vivo, since the proNGF from the injured spinal cord induced apoptosis among p75+/+, but not among p75-/-, oligodendrocytes in culture, and its action was blocked by proNGF-specific antibody. Together, these data suggest that the role of proNGF is to eliminate damaged cells by activating the apoptotic machinery of p75 after injury.
Introduction
It has been recently discovered that unprocessed NGF precursor, proNGF, binds p75 preferentially over TrkA, and this selective binding of proNGF to p75 leads to apoptotic death of cells that express both TrkA and p75 (Lee et al., 2001). Mature NGF, on the other hand, binds and activates both receptors, which results in promotion of cell survival due to the TrkA-mediated survival signal overriding p75-mediated apoptotic signal (Yoon et al., 1998; Friedman, 2000; Harrington et al., 2002). Before the discovery of an independent function for proNGF, p75 was thought to activate its apoptotic program when TrkA was not expressed in a cell. With the finding that proNGF can activate p75 regardless of the presence of TrkA, the ratio of proNGF to mature NGF now emerges as a critical regulatory factor for the maintenance of the balance between survival and death (Chao and Bothwell, 2002).
In vivo, p75 is often induced by an injury or a disease state among neurons, oligodendrocytes, or Schwann cells. p75 expression was induced in dying neurons following seizure (Roux et al., 1999), ischemia (Park et al., 2000), excitotoxic agents (Oh et al., 2000), axotomy (Giehl et al., 2001), and in cortical neurons following experimental allergic encephalomyelitis (Calza et al., 1997; Nataf et al., 1998). In the white matter plaques of multiple sclerosis patients, p75 induction was also observed among oligodendrocytes and their precursors (Chang et al., 2000; Dowling et al., 1999). In neonatal Schwann cells, p75 has been shown to play a death-inducing role following axotomy (Syroid et al., 2000). In the CNS, however, the consequence of this induced p75 expression has been unclear. Induction of p75 could be one of the first steps that initiate the apoptotic cascade after injury, or it may signify regenerative responses undertaken by the injured system, perhaps in cooperation with resident Trks.
It is well established in the literature that neurotrophins are induced or their level is greatly increased by pathological conditions that are known to cause induction of p75 (Bengzon et al., 1992; Donovan et al., 1995; Heumann et al., 1987; Widenfalk et al., 2001). The recent discovery of the functionality of proNGF leads to the prediction that the presence or absence of pro neurotrophins under injury conditions will determine whether activation of p75 induction will lead to a proapoptotic or anti-apoptotic response. For instance, in Alzheimer's patients, the level of proNGF is increased (Fahnestock et al., 2001), suggesting a possible role for p75 in Alzheimer's disease. Induction of p75 among cortical neurons in Alzheimer's patients has been reported (Mufson and Kordower, 1992).
In this report, we investigated whether expression of p75 and proNGF is responsible for injury-mediated apoptosis of oligodendrocytes in vivo, using spinal cord injury as an injury model. As one of the secondary events triggered by the initial spinal cord injury, oligodendrocytes undergo apoptosis (Crowe et al., 1997), and the presence of apoptotic oligodendrocytes was shown to correlate closely with lesion extension along fiber tracts undergoing Wallerian degeneration after spinal cord injury (Shuman et al., 1997). The death of oligodendrocytes might contribute to chronic demyelination, resulting in spinal cord dysfunction (Beattie et al., 2000; Blight, 1993; Crowe et al., 1997; Li et al., 1996; Liu et al., 1997; Shuman et al., 1997; Warden et al., 2001). Although the temporal and spatial patterns of oligodendrocyte death have been well characterized in a number of studies (reviewed in Beattie et al., 2000; Warden et al., 2001), the mechanisms by which oligodendrocytes die during Wallerian degeneration is unknown.
Here, we report that p75 and proNGF are both induced following spinal cord injury. p75 expression was specifically induced among oligodendrocytes, and the majority of p75+ cells was positive for cleaved caspase 3, suggesting that they were undergoing apoptosis. Corollary to these data, the number of cleaved caspase 3+ oligodendrocytes was reduced after spinal cord injury in the absence of p75. Likewise, the absence of p75 permitted survival of oligodendrocytes under conditions that would otherwise lead to apoptosis both in vivo and in vitro. In addition, we demonstrate that proNGF present in the extracts from the injured spinal cord is active in inducing apoptosis among oligodendrocytes, while its action can be blocked by a proNGF-specific, but not proBDNF-specific, antibody. Together, these results support the hypothesis that a consequence of p75 expression after injury is, in part, to eliminate damaged cells in the CNS, and its activation is mediated predominantly by proNGF.
Results
Temporal and Spatial Correlation between Apoptotic Profile and Actual Loss of Oligodendrocytes after Spinal Cord Injury
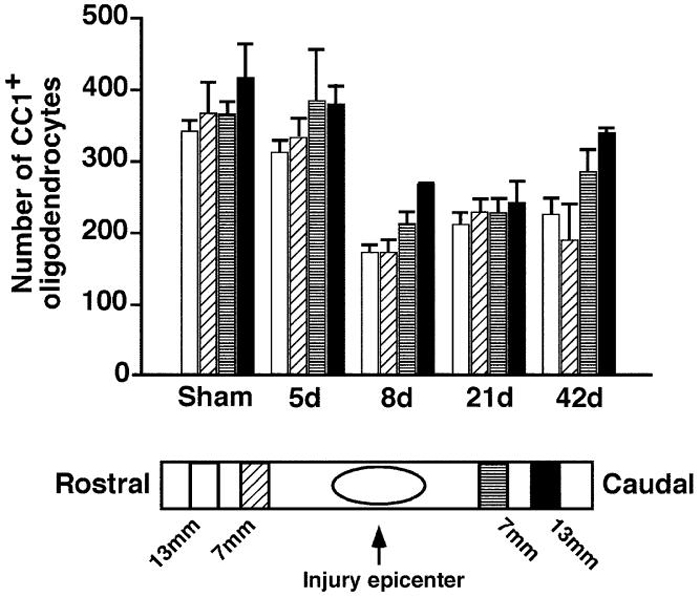
Although the presence of apoptotic oligodendrocytes has been documented after spinal cord injury (Crowe et al., 1997), it has not been determined whether this results in significant cell loss, which could potentially contribute to eventual demyelination. To address this question, we performed thoracic spinal cord contusion lesions on rats and assessed the changes in the number of oligodendrocytes in regions rostral and caudal to the lesion center at 5, 8, 21, and 42 days after the injury. The oligodendrocytes were identified by CC1/APC immunoreactivity (Bhat et al., 1996; Crowe et al., 1997; Rosenberg et al., 1999). At the fifth day postinjury, there was a small reduction in the number of oligodendrocytes in the dorsal columns, but by the eighth day postinjury, the extent of reduction reached 30%-50% throughout the four sampled regions (Figure 1). The greatest reduction, which was approximately 50%, was found in the dorsal columns rostral to the injury center. Previously, the number of apoptotic cells was found to be highest in this region (Crowe et al., 1997), indicating a spatial correlation between the number of apoptotic cells and the loss of oligodendrocytes. Temporally, the greatest loss in oligodendrocytes was found at the eighth day postinjury, which also coincides with the time when the number of apoptotic cells reached its peak (Crowe et al., 1997). This temporal and spatial correlation between the extent of apoptosis and actual cell loss indicates that apoptosis in oligodendrocytes after spinal cord injury leads to the eventual loss of oligodendrocytes.
Figure 1. Spinal Cord Injury Leads to a Loss of Oligodendrocytes Contusion injury in rats leads to a loss of oligodendrocytes.
Estimated total number of oligodendrocytes within the confines of the rat dorsal columns at 7 and 13 mm rostral and 7 and 13 mm caudal to a contusion lesion at spinal level T10 (25 mm, NYU device). Counts were made using a stereology program (see Experimental Procedures). An approximate 50% reduction in numbers was seen after 8 and 21 days with significant reductions continuing to 42 days (p < 0.05). Similar results were obtained when measuring the density (number per mm3).
Spinal Cord Injury-Specific Induction of p75 among Oligodendrocytes
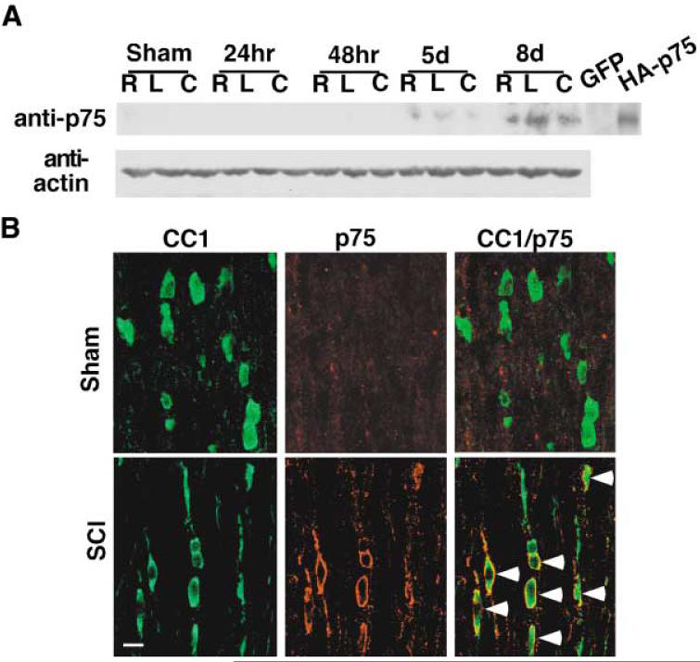
It has been shown that the presence of p75 is required for the NGF-dependent death of oligodendrocytes in culture (Harrington et al., 2002). In addition, p75 expression has been observed in the white matter plaques of multiple sclerosis patients (Chang et al., 2000; Dowling et al., 1999) and near the lesion epicenter following spinal cord injury (Brandoli et al., 2001; Casha et al., 2001; Reynolds et al., 1991). We asked whether spinal cord injury induces p75 expression among oligodendrocytes and whether its expression correlates with the observed pattern of cell loss in the dorsal columns after spinal cord injury. Contusion injuries were performed on rats, and the presence of p75 was determined at various time points after spinal cord injury by performing Western analyses using 5 mm spinal cord blocks taken from the lesion centers, as well as from regions rostral and caudal to the central samples. p75 expression was not detected in sham-operated animals, but first observed at 5 days postinjury, continuing to 8 days postinjury (Figure 2A). This temporal expression pattern of p75 correlates closely to the actual loss of oligodendrocytes (compare with Figure 1). It should be noted that there was very little p75 protein detected in sham-operated animals, although sensory neuron projections into the spinal cord are known to contain p75 in their terminals (Richardson and Riopelle, 1984). This may be due to a low level of p75 present in these fibers. Taniuchi et al. (1988) have reported that 125I-NGF binding in the spinal cord was less than 5% of the level found in the periphery. Our Western data agree with this observation and also with a report that demonstrated low levels of p75 in the adult spinal cord, except among motor neurons during development or after axotomy (Ernfors et al., 1989).
Figure 2. Spinal Cord Injury-Specific Induction of p75 among Oligodendrocytes.
(A) Injury-specific induction of p75 in rats after contusion injury. A 5 mm spinal cord block was taken from rostral (R), caudal (C), as well as from the injury epicenter (L) at the indicated times after initial injury and processed for anti-p75 Western. The data are from a representative Western blot, and the same pattern was observed from five independent sets of samples. The lysates from 293 cells transfected with vector (GFP) or rat-p75 cDNA (HA-p75) were used as a negative or positive control, respectively. The same blot was reprobed for actin as a negative control.
(B) Oligodendrocytes express p75 after spinal cord injury. Colocalization of CC1 and p75 in the dorsal column region 13 mm rostral to the injury epicenter and 8 days after the lesion. The arrows point to CC1+/p75+ cells. Scale bar, 12.5 μm.
Since p75 expression correlates with oligodendrocyte loss temporally, we tested whether oligodendrocytes express p75 upon spinal cord injury by performing immunohistochemistry. Similarly to the Western data, p75+ cells were mainly found at 8 days postinjury in the dorsal funiculus, although we also observed some positive cells at 48 hr postinjury (data not shown). These p75+ cells were positive for CC1/APC, indicating that oligodendrocytes in the dorsal funiculus expressed p75 (Figure 2B). p75 expression was not observed in sham-operated animals, as was also the case with Western analyses (Figure 2A). Together, these results indicate that p75 expression is induced among oligodendrocytes in a manner specific to spinal cord injury, and its temporalexpression profile correlates with actual loss of oligodendrocytes.
Majority of p75+ Oligodendrocytes Are Apoptotic
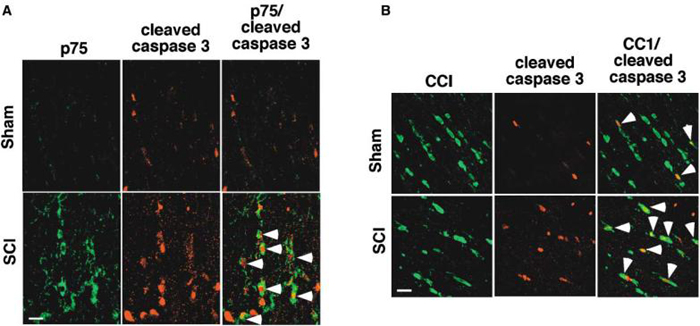
The temporal course of p75 expression correlated with the loss of oligodendrocytes and the apoptotic profile previously reported after spinal cord injury (Crowe et al., 1997). We therefore tested whether p75+ cells were undergoing apoptosis by measuring caspase 3 activation. For this, the sections taken from 8 days postinjury were processed for double immunohistochemistry for p75 and active caspase 3. In injured animals, the majority of p75+ cells were also positively stained with active, cleaved caspase 3 antibody (Figure 3A). In sham-operated animals, the number of cells positive for active caspase 3 was much smaller than in injured animals, and no cells double-positive for p75 and active caspase 3 were observed.
Figure 3. p75+ Oligodendrocytes Are Positive for Active Caspase 3.
(A) Colocalization of p75+ and active caspase 3 in the rostral region at 8 days after spinal cord injury. The arrows point to cells that are positive for both.
(B) Colocalization of CC1+ and active caspase 3 in the rostral region at 8 days after spinal cord injury. The arrows point to cells that are positive for both. Scale bar, 12.5 μm.
A spinal cord injury-mediated increase in caspase 3 activity in oligodendrocytes has been reported, but only up to 2 days postinjury (Springer et al., 1999). Since the greatest loss of oligodendrocytes is observed from 5 days postinjury, we asked whether caspase 3 was still activated among oligodendrocytes at 8 days postinjury. If so, this would suggest that the induction of apoptosis among oligodendrocytes is continuous, rather than initiated at a given point and then ceasing. As shown in Figure 3B, the majority of oligodendrocytes in the dorsal funiculus was positive for active caspase 3 stain, even at 8 days postinjury. The number of oligodendrocytes positive for active caspase 3 was much reduced in sham-operated animals. Together with the data for p75 and active caspase 3, these results suggest that p75+ oligodendrocytes undergo apoptosis continuously after spinal cord injury.
p75 Is Required for the Apoptosis of Spinal Cord Oligodendrocytes in Culture
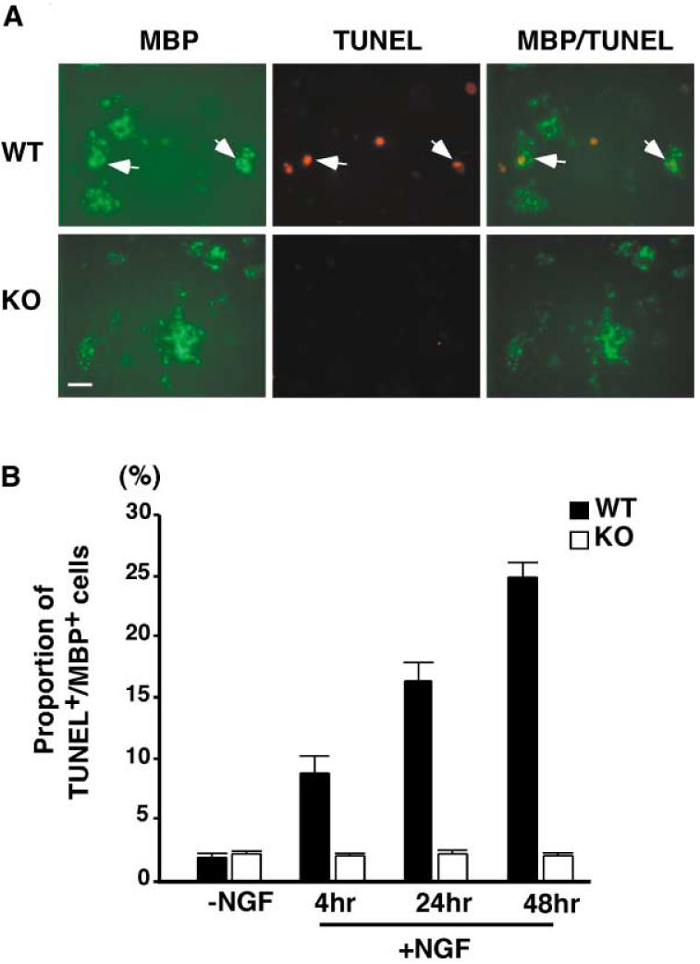
We next tested whether ligand-dependent activation of p75 is necessary for apoptosis of spinal cord oligodendrocytes. As the first step, we investigated whether the absence of p75 would render spinal cord oligodendrocytes resistant to NGF-dependent apoptosis by culturing the spinal oligodendrocytes from p75+/+ and p75-/- mice. In culture, spinal cord oligodendrocytes taken from postnatal day (P)12-14 pups expressed p75, as did their cortical counterparts (data not shown; Harrington et al., 2002). When NGF purified from the submaxillary gland (Harlan Bioproducts for Science) was added to p75+/+ oligodendrocytes at 100 ng/ml for 4-48hr, the proportion of TUNEL+ and MBP+ cells increased to 24% from a basal level of 2% (Figure 4B). Among p75-/- oligodendrocytes, however, for the entire period of NGF treatment, the proportion of TUNEL+ and MBP+ cells remained the same as for those left untreated. A representative picture is shown in Figure 4A. These data therefore indicate that p75 is required for NGF-dependent apoptosis in spinal cord oligodendrocytes in culture.
Figure 4. Mouse Oligodendrocytes Fail to Die in Culture in the Absence of p75.
(A) A representative picture of MBP+ cells counted for data in Figure 4B. After 6 days in culture, cells were treated with purified mature NGF at 100 ng/ml. At the amounts of time indicated in Figure 4B, cells were fixed, stained for TUNEL, then subsequently for MBP. The arrows point to TUNEL+ cells in the field. Scale bar, 20 μm.
(B) Quantification of TUNEL+ MBP+ cells following purified NGF treatment at 100 ng/ml. The quantification data are from six independent experiments, with 80-120 cells counted in each experiment, for a total of 480-720 cells counted.
p75 Plays a Critical Role for Apoptosis of Oligodendrocytes after Spinal Cord Injury
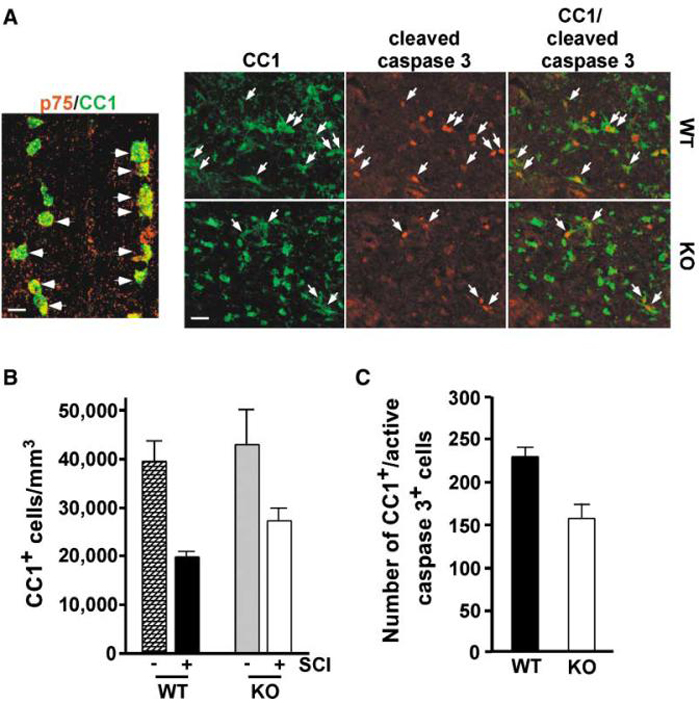
As the second step, we investigated whether p75 is required for apoptosis of oligodendrocytes in vivo by injuring the spinal cord in p75+/+ and p75-/- mice. After spinal cord injury, mouse oligodendrocytes express p75 in the dorsal column, as their rat counterparts do (Figure 5A, left). For spinal cord injury in mice, dorsal hemisection was chosen as our method instead of contusion, since hemisection also induces axon degeneration and subsequent oligodendrocyte death without the potential complications associated with a temporally expanding contusion injury in the small mouse spinal cord. Two different approaches were taken to assess the role of p75 following spinal cord hemisection. One was to measure the extent of oligodendrocyte survival (Figure 5B), and the other was to measure the extent of oligodendrocyte apoptosis (Figure 5C) in the absence of p75. The extent of oligodendrocyte survival was first determined by estimating the density of the CC1+ cells per mm3, using sections taken from 1.8 mm and 1.2 mm rostral and 1.2 mm and 1.8 mm caudal to the injury epicenter on the eighth day following spinal cord injury (Figure 5B). Following hemisection, the density of oligodendrocytes in the dorsal columns dropped 51% from 39,600 to 19,500 per mm3 in p75+/+ mice, while it dropped 36% from 42,600 to 27,100 per mm3 in p75-/- mice. This result indicates that the lack of p75 resulted in a 15% increase in oligodendrocyte survival.
Figure 5. Increased Survival and Decreased Apoptosis of Oligodendrocytes after Spinal Cord Injury in p75-/- Mice.
(A) Left: induction of p75 among oligodendrocytes in mice after hemisection. Scale bar, 12.5 μm. Right: representative pictures of cells stained for cleaved caspase 3 and CC1 at 5 days postinjury. The arrows point to cells that are stained for both antibodies. Note the decrease in the number of CC1+/cleaved caspase 3+ cells in p75-/- mice. Scale bar, 20 μm.
(B) The loss of oligodendrocytes is attenuated in the dorsal funiculus of p75-/- mice after dorsal hemisection (p < 0.05). The number is presented as the density of oligodendrocytes per mm3. Counts were made using a stereology program.
(C) Reduction in the number of oligodendrocytes expressing active caspase 3 in p75-/- mice after dorsal hemisection (p < 0.01). The number represents average cell counts in the dorsal columns per coronal section of the spinal cord.
As the CC1+ cell counts include mainly the healthy surviving cells, it is likely that the density estimation does not fully represent the extent of protection in the absence of p75. For this reason, we examined the number of apoptotic oligodendrocytes in p75+/+ and p75-/- mice at 5 days postinjury. As a marker for apoptosis, we stained sections with cleaved caspase 3 in addition to CC1. A representative picture is shown in Figure 5A (right). The average number of CC1+/cleaved caspase 3+ cells in the dorsal columns was 229 per section in p75+/+ mice, while it was 155 in p75-/- mice, representing a 32% reduction in the number of apoptotic oligodendrocytes in the absence of p75 (Figure 5C). The extent of protection assessed by cleaved caspase 3 is higher than the estimation of surviving oligodendrocytes, since the staining of cleaved caspase 3 allows detection of oligodendrocytes that are at an early stage in the apoptotic process. Together, these data indicate that p75 is one of the critical components for inducing apoptosis of oligodendrocytes following spinal cord injury.
Spinal Cord Injury-Mediated Production of ProNGF Is Responsible for the Death of Oligodendrocytes
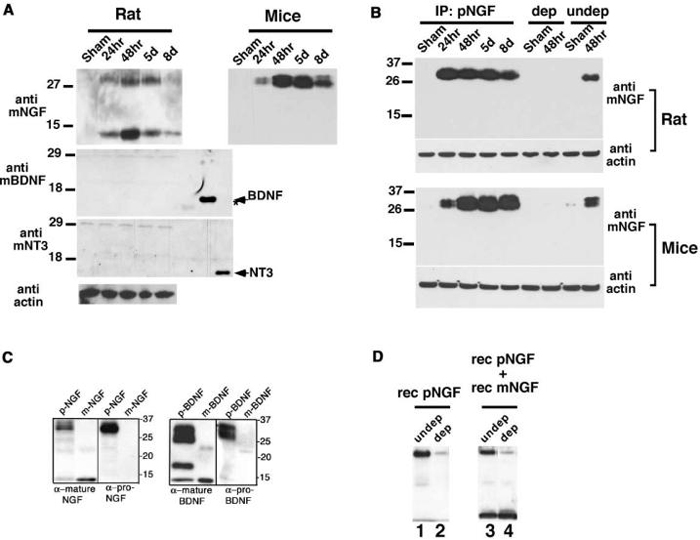
What is activating p75 to elicit apoptotic programs in vivo? It has been known that NGF expression is strongly induced among astrocytes, activated microglia, and meningeal cells in the spinal cord by spinal cord injury in the rat (Krenz and Weaver, 2000; Widenfalk et al., 2001). Since neurotrophins are known to be present in pro forms in many brain tissues (Chao and Bothwell, 2002) and in the case of proNGF its expression is increased in Alzheimer's patients (Fahnestock et al., 2001), we decided to examine the profiles of neurotrophin expression in our spinal cord injury paradigm. BDNF and NT3 were present in pro forms as reported (Lee et al., 2001), based on the predicted size, but no significant increase was detected in the amount of pro or mature forms of BDNF or NT3 produced after spinal cord injury (Figure 6A). The lack of induction of BDNF was further confirmed using proBDNF-specific antibody, which produced similar results to those shown in Figure 6A (data not shown). In contrast, NGF expression was strongly induced in a manner specific to spinal cord injury based on Western analyses, using an anti-mature-NGF antibody (Figure 6A). The size of induced proteins that are detected with anti-mature-NGF antibody (14 kDa, 24-32 kDa) suggests that both mature and proNGF are induced in rats, but the extent of induction of proNGF is greater than that observed with mature NGF (Figure 6A). Interestingly, only proNGF and not mature NGF was induced following spinal cord injury in mice (Figure 6A). This may be due to species differences, or it may represent the difference in the type of injury inflicted, that is, contusion lesion in rats versus hemisection in mice.
Figure 6. Spinal Cord Injury-Specific Induction of ProNGF.
(A) High-molecular weight NGF is predominantly induced after spinal cord injury. 30 μg of extracts were analyzed at each time point in Western analyses for NGF (Chemicon), BDNF (Promega), and NT3 (Promega). The extracts were prepared from rostral 5 mm (rats) or 3 mm (mice) blocks from the lesion center, but the pattern of NGF expression did not vary whether the sample was taken caudally or from the lesion center. The arrow indicates the position of recombinant BDNF and NT3, which were used at 250 ng as a control for antibody specificity. The star points to purified NGF being weakly recognized by anti-BDNF antibody.
(B) The high-molecular weight NGF is proNGF. 350 μg of lysates was immunoprecipitated with proNGF antibody, and probed with anti-mature-NGF antibody (Chemicon). The bands at 26-32 KDa disappear almost completely following depletion with proNGF antibody, indicating that these bands are proNGF (compare lanes dep and undep). Although not shown, mature NGF is present in undepleted rat samples in longer exposure.
(C) Specificity of proNGF and proBDNF antibodies. Western blot of 50 ng of mature NGF (Harlan Bioproducts for Science), 50 ng of mature BDNF (Promega), 50 μg of lysate from 293 cells stably expressing cleavage resistant proNGF, and 250 ng of supernatant from 293 cells infected with cleavage-resistant adenoviral proBDNF. A set of pro and mature NGF was probed with anti-pro/mature NGF antibody (Cedarlane) and proNGF antibody, and a set of pro and mature BDNF was probed with anti-mature-BDNF (Santa Cruz) and anti-proBDNF antibody.
(D) ProNGF antibody depletes proNGF, but not mature NGF. Recombinant proNGF (lanes 1 and 2) or recombinant pro and mature NGF (lanes 3 and 4) were subjected to immunodepletion using proNGF antibody. Note that proNGF antibody depletes only proNGF, but not mature NGF (compare lanes 3 and 4).
To confirm that the high-molecular weight bands are indeed proNGF, we utilized proNGF-specific antibody. ProNGF antibody was generated using a 51 amino acid sequence present in the pro domain of NGF (See Experimental Procedures for details). The specificity of this proNGF antibody was tested both in biochemical and biological assays. In Western analyses and in immunodepletion assays, the proNGF antibody detects only recombinant, cleavage-resistant proNGF, and not recombinant mature NGF (Figures 6C and 6D). In a functional assay using oligodendrocytes, the antibody blocks the apoptotic action of recombinant proNGF, but not that of recombinant mature NGF (Figure 7D). These results confirm that the proNGF antibody specifically detects and binds proNGF, but not mature NGF. When the rat lysates containing both proNGF and mature NGF were subjected to immunoprecipitation using this proNGF-specific antibody, only high-molecular weight bands of 26-32 kDa were detected and not the band of 14 kDa (Figure 6B, top). Similar data were obtained with mouse lysates as well (Figure 6B, bottom). In addition, when both the rat and mouse lysates were subjected to immunodepletion using the proNGF antibody, these high-molecular weight bands were no longer detected, while the undepleted samples demonstrated immunoreactive species of 26-32 kDa in Western analyses (Figure 6B). These data therefore confirm that the high-molecular weight bands of 26-32 kDa are proNGF.
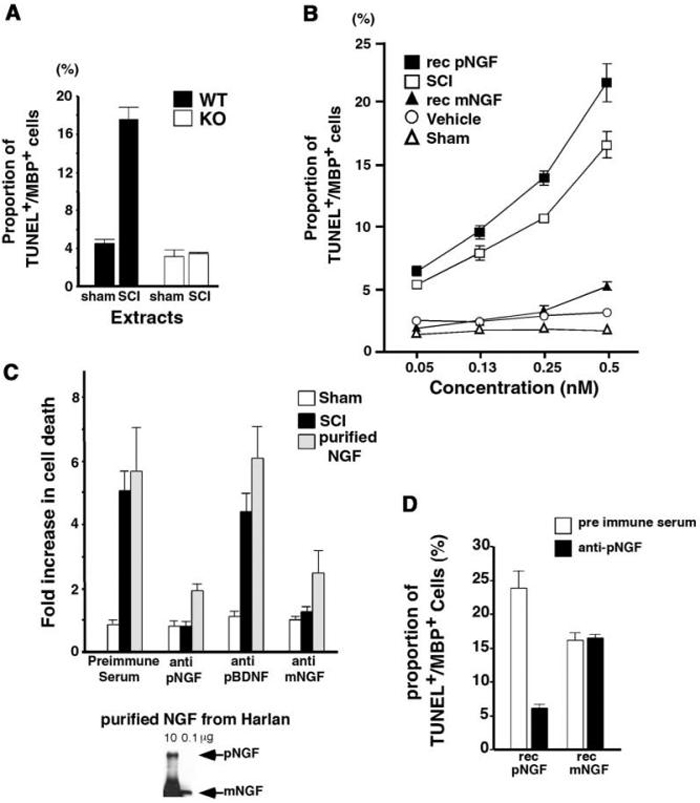
Figure 7. ProNGF Present in Injured Mouse Spinal Cord Extracts Is Active in p75-Mediated Apoptosis of Oligodendrocytes in Culture.
(A) p75 is required for apoptosis mediated by the injured spinal cord extracts. Extracts from sham-operated or injured mouse spinal cord extracts were added to either p75+/+ orp75-/- mouse oligodendrocytes. After 24 hr, cells were processed for TUNEL/MBP stain as described. For quantification of apoptotic cells, 150-200 cells were counted in each of two to four independent experiments to yield the total cell count of 300-800.
(B) ProNGF in the injured spinal cord extracts behaves similarly to recombinant, cleavage-resistant proNGF in its affinity to induce apoptosis of oligodendrocytes. The concentration of proNGF in the extracts was estimated when added to 1 ml media. After 24 hr, cells were processed for TUNEL/MBP staining as described. For quantification of apoptotic cells, 250-350 cells were counted in each of three independent experiments to yield the total cell count of 750-1050. Abbreviations: rec pNGF, recombinant, cleavage-resistant proNGF; SCI, spinal cord injury extracts; rec mNGF, recombinant mature NGF.
(C) ProNGF present in the spinal cord injury extracts is responsible for apoptosis of oligodendrocytes. Top: the data are represented in terms of the fold increase in the proportion of TUNEL+ MBP+ cells compared to that with the samples treated with individual antibody. The death mediated by the spinal cord injury extract is significantly attenuated with Chem-icon anti-NGF and proNGF antibodies, but not by preimmune serum or proBDNF antibody. The cells treated with purified NGF (Harlan Bioproducts for Science) were used as a positive control. For quantification of apoptotic cells, 250-350 cells were counted in each of three independent experiments to yield the total cell count of 750-1050. Bottom: purified NGF from Harlan contains proNGF.
(D) Functional specificity of proNGF antibody. Rat oligodendrocytes were treated with 50 ng/ml of purified, recombinant mature NGF or 0.5 ng/ml of purified, recombinant, cleavage-resistant proNGF in the presence of either preimmune serum or anti-proNGF antibody. After 24 hr, cells were processed for TUNEL/MBP staining as described. For quantification of apoptotic cells, 200-300 cells were counted in each of three independent experiments to yield the total cell count of 600-900. Note that both proNGF and mature NGF can induce apoptosis, but proNGF is at least 50-fold more active than mature NGF.
We next investigated whether proNGF present in the spinal cord extracts activates p75. Since only proNGF and not mature NGF was induced in mice after spinal cord injury, we used mouse extracts obtained after spinal cord injury as a source of proNGF in functional assays using p75+/+ and p75-/- mouse oligodendrocytes. When the spinal cord injury extracts were added at 0.14 μl (350 ng total protein added in 1 ml media) to p75+/+ mouse oligodendrocytes for 24 hr, the proportion of TUNEL+ and MBP+ cells reached 17%, while it remained at 4% with extracts from sham-operated mice. With p75-/- mouse oligodendrocytes, the proportion of TUNEL+ and MBP+ cells remained below 4% (Figure 7A). This 17% value is very close to that obtained on p75+/+ mouse oligodendrocytes using 100 ng/ml (4 nM) of NGF purified from submaxillary gland (Figure 4B). Based on estimation using purified mature NGF as a control in Western analysis, 0.14 μl of mouse extracts contains approximately 10-20 ng of proNGF (0.15-0.30 nM) in proNGF concentration. This represents approximately a 13- to 26-fold increase in the action of proNGF on p75 compared to mature NGF, which is comparable to that reported for smooth muscle cells (Lee et al., 2001).
We next compared the activity of proNGF in the spinal cord extracts to that of recombinant proNGF and recombinant mature NGF in oligodendrocytes. Generation and purification of recombinant, cleavage-resistant proNGF and comparably purified mature NGF were previously described (Lee et al., 2001). Within an estimated concentration range from 0.05 nM to 0.5 nM, proNGF in the spinal cord extracts induced apoptosis comparably to recombinant proNGF and more effectively than that observed with recombinant mature NGF (Figure 7B). These results therefore suggest that the apoptotic factor present in the injured spinal cord acts via p75, and its dose response suggests that the activity reflects a proneurotrophin.
In order to confirm that the activity was due to proNGF, we preincubated the injured spinal cord extracts with preimmune serum, anti-mature-NGF antibody, proNGF-specific antibody, or proBDNF-specific antibody before they were added to oligodendrocytes. The specificity of proNGF antibody was discussed earlier. ProBDNF-specific antibody was generated using peptide sequences present in the pro domain of BDNF in a manner similar to proNGF antibody (see Experimental Procedures for detail). In Western analyses, proBDNF antibody detects only proBDNF and not mature BDNF (Figure 6C). As a negative control, parallel cultures were treated with extracts from sham-operated mice, while cells treated with purified NGF from the submaxillary gland (Harlan Bioproducts for Science) were used as a positive control for the antibody action. The extent of apoptosis was assessed after 24 hr and is expressed in terms of the fold increase observed in TUNEL+ and MBP+ cells over that observed with samples treated with the individual antibody alone (Figure 7C). The extent of apoptosis in cells treated with spinal cord injury extracts was significantly attenuated with proNGF-specific antibody as well as anti-mature-NGF antibody. Preimmune serum or proBDNF-specific antibody did not have any effect.
ProNGF-specific antibody was also effective in attenuating the extent of apoptosis of cells treated with purified NGF. NGF purified from the submaxillary gland has been reported to contain proNGF, which we confirmed (Reinshagen et al., 2000; Figure 7C, bottom). The extent of apoptosis mediated by submaxillary gland derived NGF typically ranges from 5- to 7-fold above control in oligodendrocytes (Figure 4; Harrington et al., 2002). In the presence of proNGF antibody, the extent of apoptosis was reduced to 2-fold above control (Figure 7C). This result suggests that proNGF present in submaxillary gland preparations is largely responsible for its apoptotic action, although it comprises only 2%-6% of total NGF. To directly compare the effects of mature NGF and proNGF, recombinant preparations were utilized (Lee et al., 2001) where the mature NGF preparations contained less than 2% proNGF. Utilizing these reagents, mature NGF can induce apoptosis of oligodendrocytes but less effectively than proNGF: 0.5 ng/ml of recombinant proNGF yielded 23% apoptosis, whereas 50 ng/ml recombinant mature NGF yielded 17% apoptosis (Figure 7D). These results, together with the observed inability of proNGF antisera to reduce the apoptosis induced by mature NGF (Figure 7D), suggest that although both proNGF and mature NGF are each capable of inducing apoptosis, proNGF is more active than mature NGF in inducing oligodendrocyte cell death. Together, these results indicate that proNGF present in the spinal cord injury extracts was largely responsible for inducing apoptosis in oligodendrocytes in culture. In addition, these data strongly suggest that proNGF is most likely a factor responsible for inducing apoptosis in vivo after spinal cord injury.
Discussion
In this report, we present data that support a physiological role of proNGF in p75-mediated apoptosis after spinal cord injury. The temporal induction of p75 correlates with the loss of oligodendrocytes following spinal cord injury. Consistent with these observations, in p75-/- mice after spinal cord injury, there is both a reduction in the number of cleaved caspase 3+ oligodendrocytes and an increase in the number of surviving oligodendrocytes, indicating a predominantly proapoptotic role for p75 activation following injury. We further demonstrated that this apoptotic action of p75 is likely to be mediated by proNGF in vivo by showing that proNGF in extracts from the injured spinal cord induces apoptosis of oligodendrocytes in culture at an affinity comparable to that exhibited by purified, recombinant, cleavage-resistant proNGF. Together, these data provide strong evidence for the apoptotic role of p75 and proNGF after injury to the spinal cord.
Proapoptotic Role of p75 following Injury In Vivo
Injury-mediated induction of p75 in the CNS has been well documented in the literature (Calza et al., 1997; Koliatsos et al., 1991; Nataf et al., 1998; Park et al., 2000; Reynolds et al., 1991; Roux et al., 1999). The role of p75 under these circumstances, however, had not yet been clearly determined in vivo. The present report provides direct evidence that p75 plays a proapoptotic role in oligodendrocyte cell death after spinal cord injury. Oligodendrocytes in the spinal cord express significant levels of p75 in response to spinal cord injury and, in p75-/- mice, their apoptosis is attenuated while their survival is enhanced.
The proapoptotic role of p75 in oligodendrocytes in vivo was further supported by our spinal cord oligodendrocyte culture data. Like their cortical counterparts, spinal cord oligodendrocytes express p75 in culture, and NGF binding to p75 leads to apoptosis in the absence of TrkA. Spinal cord oligodendrocytes do not express TrkA either in culture or in vivo (data not shown). Although it is not clear whether p75 is expressed in cortical oligodendrocytes in vivo, it has been argued that culture conditions represent a stress situation that models in vivo injury. In support of this contention, p75 was shown to activate an injury-specific JNK3 in cortical oligodendrocyte cultures (Harrington et al., 2002). Our current finding, that injury induces p75 among oligodendrocytes in the spinal cord and that these cells also express p75 in culture and die upon binding NGF, provides in vivo evidence in support of this contention.
Although the absence of p75 was fully protective of spinal cord oligodendrocytes in culture, its effect in vivo was not complete. These observations suggest that p75 is but one contributing factor in vivo where other factors and molecules are also involved. Likely candidates include cytokines, such as TNFα, and excitatory amino acids (Beattie et al., 2000; McDonald et al., 1998). The analyses of TNF receptor knockout mice suggest that TNFα plays a protective role by activating NF-κB pathways after spinal cord injury (Kim et al., 2001). This result differs from previous reports where TNFα induced apoptosis of oligodendrocytes in culture (Hisahara et al., 1997; Ladiwala et al., 1998; Louis et al., 1993). When TNFα was applied to dorsal columns, it failed to induce apoptosis among oligodendrocytes (Schnell et al., 1999). When applied together with sublethal doses of kainic acid, however, TNFα induced rapid, massive cell death in the spinal cord gray matter (Hermann et al., 2001). These results suggest that induction of secondary injury is likely to involve interactions among multiple pathways. In this report, we identify the p75 neurotrophin receptor as a key player in the downstream apoptotic cascade that could lead to functional loss following spinal cord injury.
In culture, p75-/- mouse oligodendrocytes were resistant to the cell killing effect of the injured spinal cord extracts. This seems surprising, since spinal cord injury induces expression of an array of potential apoptotic agents that act on receptors other than p75, as was discussed previously. A possible explanation may be that our culture conditions provide compensatory mechanisms that result in overall protection against such insults. One potential protective reagent is insulin present in our serum-free media. Expression of the IGF family of factors has been reported to be induced among reactive astrocytes after spinal cord injury (Hammarberg et al., 1998; Yao et al., 1995a). Administration of these factors at the time of demyelinating injuries was known to exert a protective and regenerative effect (Pulford et al., 1999; Sharma et al., 1997; Yao et al., 1995b).
Selective Upregulation of NGF and ProNGF upon Injury
In brain extracts, NGF and BDNF were reported present predominantly as pro forms (Fahnestock et al., 2001; Lee et al., 2001). In the spinal cord, NT3 also exists mainly as 30 kDa proNT3 (Figure 6). Of the three neurotrophins, only NGF was induced after spinal cord injury. When comparing NGF and proNGF induced in rats after spinal cord injury, the level of proNGF is at least equivalent to or higher than the level of mature NGF. The significance of this preferential induction of proNGF as compared to mature NGF is not clear with regard to oligodendrocyte survival, as significant levels of TrkA are not expressed either before or after spinal cord injury (data not shown). In the absence of TrkA, either form of NGF should be capable of activating p75 to induce apoptosis, albeit at a different affinity (Figure 7D). Perhaps a different population of cells express proNGF or mature NGF, or the secretion mechanism or the site of release for the two forms may differ (Farhadi et al., 2000). These different factors are likely to affect the accessibility of mature NGF/proNGF to p75+ oligodendrocytes.
The NGF level has been shown to increase both in meningeal layer (Widenfalk et al., 2001) and also among astrocytes and activated microglia (Krenz and Weaver, 2000) after spinal cord injury. Activated microglia are often found juxtaposed to apoptotic oligodendrocytes during Wallerian degeneration (Shuman et al., 1997), suggesting that NGF produced by microglia may activate p75 expressed among adjacent oligodendrocytes. Although it is not yet known which cell types express proNGF after spinal cord injury, a scenario analogous to what was reported in the developing retina where NGF secreted by microglia promoted apoptotic actions of p75 (Frade and Barde, 1998) may apply as well.
In hippocampal cultures, the release of BDNF and NT3 required stimuli such as depolarization (Farhadi et al., 2000). NGF, on the other hand, was constitutively released both in a 32 kDa precursor form as well as a 14 kDa mature form when expressed in hippocampal neurons (Mowla et al., 1999). Following injury, a 32 kDa precursor was the predominant form of NGF present in the spinal cord, at least after hemisection in mice. Since a 32 kDa precursor can be released from the cell, at least in culture, this result suggests that proteolytic processing that cleaves proNGF to mature NGF may be a critical step in determining the extent of oligodendrocyte death. In culture, proNGF is significantly more potent than mature NGF in inducing apoptosis of oligodendrocytes, suggesting that inhibition of the protease activity responsible for the conversion of proNGF to mature NGF can provide a potential therapeutic means following spinal cord injury. Lee et al. (2001) have identified matrix metalloproteinase (MMP) 3 and plasmin as a protease that can cleave proNGF and proBDNF in vitro. MMP 2 and 9 expression increases following spinal cord injury (de Castro et al., 2000), however, proNGF was not cleaved by MMP 2 or 9 in vitro (Lee et al., 2001). It is plausible that as yet unidentified MMPs or other proteases regulate the proteolytic processing of proNGF once it is released from the cell.
In conclusion, we report that proNGF is induced by spinal cord injury, and it is likely to play a role in inducing apoptosis in vivo by activating p75. Activation of p75 contributes to the demise of oligodendrocytes. The p75 neurotrophin receptor is therefore identified as a key player in the downstream apoptotic cascade in secondary degeneration following spinal cord injury and other CNS injuries.
Experimental Procedures
Animals Used in the Study
Mice: two groups of p75+/+ and p75-/- mice were used for the study. For the injury study, a congenic C57/BL6 line that carries a mutation in exon 3 of the p75 gene (Lee et al., 1992) was purchased from the Jackson Laboratory (Bar Harbor, ME). For the culture study, the p75-/- and p75+/+ mice were obtained from heterozygote mating as littermates. Their genotype was determined by PCR analyses of tail DNA according to Bentley and Lee (Bentley and Lee, 2000).
Rats: adult female Long-Evans hooded rats were obtained from Simonsen Labs (Los Angeles, CA).
Spinal Cord Injuries
Mice were anesthetized with isoflurane and the spinal cord was exposed at T10. A Beaver blade was used to produce a dorsal hemisection of the cord, including the dorsal columns and the dorsal part of the lateral funiculus. Contusion injuries in rats were made using the NYU device (Gruner, 1992). Under pentobarbital anesthesia, the spinal cord was exposed at T10 and a 10g weight was dropped from 25 mm onto the dural surface as previously described (Basso et al., 1996). All procedures were approved by the Institutional Laboratory Animal Care and Use Committee and followed the NIH Guidelines for the proper use and care of laboratory animals.
Perfusion
Under deep anesthesia (80 mg/kg ketamine, Fort Dodge Animal Health, Fort Dodge, IA; 10 mg/kg xylazine, Vedco, Inc., St. Joseph, MO), rats and mice were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde fixative. The spinal cords were removed, and three adjacent blocks were cut from the cords, each being either 3 mm long for the mouse cords or 5 mm long for the rat cords. In each case, one block was centered on the lesion, and the others were rostral and caudal to that block. The blocks were sectioned at 20 μm thickness on a cryostat.
Immunohistochemistry
Sections were incubated in blocking solution containing 10% goat serum, 10% horse serum, 1% BSA, and 0.3% Triton X-100 in 0.1M PB for 2 hr at room temperature. For double-staining for p75 and oligodendrocyte cell bodies, the sections were incubated simultaneously with an anti-p75 antibody, 9651 (Huber and Chao, 1995), and CC1 antibody (Bhat et al., 1996; Crowe et al., 1997) in 5% goat serum, 5% horse serum, and 0.1% BSA in 0.1M PB at room temperature overnight. 9651 recognizes the extracellular domain of p75. CC1 antibody recognizes the APC gene product, which is expressed in rat oligodendrocyte somata and proximal processes (Bhat et al., 1996). Although CC1 antibody can detect GFAP+ astrocytes, less than 0.5% of cells were positive for both CC1 and GFAP in our rat and mouse spinal cord tissue. Sections were then incubated with biotinylated anti-rabbit antibody (Vectorlabs) for p75 stain and an anti-mouse antibody conjugated to Alexa 488 (Molecular Probes) for CC1. P75 staining was visualized using Extravidin Cy3 (Sigma). For double-staining for p75 and active caspase 3, 192 anti-mouse anti-p75 antibody was used simultaneously with active caspase 3 anti-rabbit antibody (Cell Signaling, Beverly, MA). For 192 immuno-staining, a biotinylated anti-mouse secondary and an anti-mouse antibody conjugated to Alexa 488 (Molecular Probes) was used, and for active caspase 3, an anti-rabbit secondary conjugated to Cy3 (Jackson ImmunoResearch, West Grove, PA) was used. For double-staining for CC1 and active caspase 3, a biotinylated anti-mouse secondary and an anti-mouse antibody conjugated to Alexa 488 (Molecular Probes) was used for CC1. Active caspase 3 was detected with anti-rabbit secondary conjugated to Cy3 (Jackson ImmunoResearch, West Grove, PA). The sections were mounted with Vectashield containing DAPI to label the nuclei (Vector Labs). For confocal microscopy, BioRad MRC 1024 attached to a Nikon Optiphot-2 was used.
For cell counts shown in Figures 1 and 5A, CC1 staining of rat and mouse tissues were done in the same way as the fluorescence staining, except that the positive staining was visualized using DAB and the Vectastain ABC kit (Vector Labs).
Cell Counts
All the counts were done blind to mouse genotype or lesion condition. Cell counts were made on rat and mouse coronal sections at a series of rostral and caudal locations relative to the contusion or hemisection lesions. Counts and reference volumes were estimated using procedures specified in the Stereologer ™ program (Systems Planning and Associates, Inc., Alexandria, VA). For rat contusion injuries, three sections were randomly sampled from 1 mm blocks taken from 13 and 7 mm rostral and 7 and 13 mm caudal to the lesion epicenter. A pilot study was run to determine the optimal disector size and spacing to allow for counts of at least 100 cells per block. CC1 positive cells were only counted when the cell body and proximal processes were darkly labeled and were within the inclusive zone of each disector frame. Results are reported as total number of oligodendrocytes and as density (number per mm3). Data were gathered from rats with 25 mm spinal cord injury surviving for 5 or 8 days (n = 4/time point) or 3 or 6 weeks (n = 3/time point), and control uninjured rats (n = 3). Mouse CC1+ cells were counted in a similar fashion at 8 days postinjury, except that the distance from the lesion center sampled was 1.2 mm and 1.8 mm rostral (R1 and R2) and 1.2 mm and 1.8 mm caudal (C1 and C2). The number of mice analyzed was n = 6 for p75+/+ and n = 5 for p75-/-. For quantification of CC1+/active caspase 3+ cells in Figure 5B, p75+/+ (n = 5) and p75-/- (n = 5) mice were analyzed at 5 days postinjury, using rostral 4 mm blocks.
Primary Oligodendrocyte Cultures
The p75 knockout and the wild-type mice were obtained from heterozygote mating as littermates. For spinal cord oligodendrocytes (Figure 4), mouse pups at postnatal days 12-14 and for cortical oligodendrocytes (Figure 7), mouse pups at postnatal days 15-16 were used. Cell suspension obtained from the triturated tissues was loaded onto a 36% Percoll gradient, and oligodendrocytes were isolated following centrifugation at 10,000 g (Fuss et al., 2000; Lubetzki et al., 1991). Isolated oligodendrocytes were resuspended in 10% FBS in DMEM and plated onto poly-D-Lysine coated 4-well slide dishes at 0.1 × 106 per well. The following day, the medium was changed to a differentiation medium with no serum, as previously described (Yoon et al., 1998). The culture was kept for 4 days before NGF was added at 100 ng/ml for the indicated amount of time. Rat oligodendrocytes were cultured as described (Harrington et al., 2002).
Quantification of Apoptotic Oligodendrocytes in Culture
For quantification of apoptotic mouse oligodendrocytes, cells were fixed at indicated times after NGF treatment and incubated with anti-myelin basic protein (MBP) antibody (Boehringer-Mannheim). Cells were then stained for TUNEL and processed for visualization of MBP stain using an anti-mouse secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR).
Western Analyses
The spinal cords were homogenized in a lysis buffer containing 1% Nonidet P-40, 20 mM Tris (pH 8.0), 137 mM NaCl, 0.5 mM EDTA, 10% glycerol, 10 mM Na2P2O7, 10 mM NaF, 1 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM vanadate, and 1 mM phenylmethylfulfonyl fluoride. Induction of p75 by spinal cord injury was detected on Western analyses using an anti-rabbit, anti-p75 antibody from Co-vance (Berkeley, CA). For detection of proNGF and mature NGF, anti-mouse anti-NGF from Chemicon International (Temecula, CA) was used, but the same data were obtained with anti-rabbit anti-NGF from Cedarlane (Hornby, Ontario). The samples for neurotrophin Western analyses were prepared in Laemli buffer that was supplemented with 20 mM DTT and 100 mM iodoacetamide to prevent any potential dimeric interaction between mature NGFs. BDNF and NT3 antibodies were from Promega (Madison, WI).
Immunodepletion
The lysates were subjected to two rounds of immunoprecipitation using proNGF antiserum. The supernatants resulting from immuno-precipitation were analyzed in Western analyses with NGF antibody (Chemicon International, Temecular, CA) to assess the extent of depletion. The lysates taken before immunodepletion was used as undepleted controls in Figure 6B.
Generation of Recombinant ProNGF and Mature NGF
The cDNA of murine NGF was amplified by RT-PCR and sequenced in both directions for any errors. To improve translation initiation, 11 bases from the mouse untranslated region of murine NT-3, including the Kozak consensus site, was exchanged for the murine NGF sequence. PCR-mutagenesis was performed to add six histidine (His) residues at the C terminus, and residues RR (bp 1008-1013) near the C terminus were mutated to AA to impair cleavage of the His tag. To generate proNGF with impaired furin cleavage (proNGF), the KR (bp 651-657) was mutated AA. After bidirectional sequencing, the constructs were cloned into pcDNA, and stable 293 transfectants expressing pcDNA, pcDNA-proNGF, and pcDNA-mature-NGF were isolated following G418 treatment. For purification, cells were cultured for 18 hr in serum-free media, and the resulting media were collected after removing cells by centrifugation. His-tagged mature or cleavage resistant proNGF was purified using Ni-bead chromatography (Xpress System Protein purification, Invitrogen) as per the manufacturer's instructions using imidazole (350 mM) for elution. Medium from cells stably transfected with pcDNA vector alone was harvested and purified in parallel. The concentration of proNGF or mature NGF was estimated by silver stain, using known concentrations of mature NGF (Harlan Bioproducts for science) in parallel.
Generation of ProNGF- and ProBDNF-Specific Antibodies
GST fusion proteins encoding amino acids 23-81 (asp23 to arg81) of human proNGF or amino acids 25-90 (asn25 to asp90) of human proBDNF were generated in bacteria and purified by chromatography with glutathione-sepharose. Rabbits (using GST-proNGF) or chickens (using GST-proBDNF) were immunized to generate antisera. Specific antisera were purified by first incubating whole serum with GST to adsorb GST-specific immunoreactivity and then by adsorption to and elution from a glutathione column to which GST-proBDNF or GST-proNGF had been irreversibly coupled.
Antibody Blocking Experiments Using Injured Spinal Cord Extracts
The extracts from injured spinal cord were added at 0.14 μl in volume, which was estimated to give a final proNGF concentration of 14 ng/ml based on Western analyses. Extracts from sham-operated spinal cord were used at the same volume. For the dose curves in Figure 7B, rat oligodendrocytes were treated with column-purified recombinant proNGF, column-purified recombinant mature NGF, injured spinal cord extracts from mice, sham extracts, or vehicle at the indicated concentrations. For the vehicle control for the recombinant NGFs, the elution buffer containing 350 mM imidazole was used. The final concentration of imidazole therefore ranged from 250 μM to 5 mM. Following a 24 hr incubation period, samples were processed for TUNEL and MBP staining as described. For antibody blocking experiments, either the injured or sham extracts (0.14 μl) were preincubated with mature NGF (5 μl; Chemicon), proNGF (5 μl), proBDNF (10 μl) antibodies, or pre-immune serum (10 μl) for 2 hrat 4°C. The extract and antibody mix was then added to oligodendrocytes for 24 hr before they were processed for TUNEL and MBP stain.
Statistical Methods
A two-way ANOVA (site × time) was used for the cell counts in the rat and a Student's t test in the mice to evaluate the number of surviving and apoptotic oligodendrocytes.
Acknowledgments
We thank Drs. Pilar Perez, Bruce Carter, John Oberdick, Tsonwin Hai, and Moses Chao for discussion. We especially thank Dr. Juan C. Arevalo for the help with antibody production and Dr. Paul Worley for an insightful suggestion. Dr. Stan Baldwin helped establish stereological methods. John Komon, Amy Tovar, and Tina van Meter provided excellent technical assistance for the in vivo studies. This project was supported by grants to S.O.Y. from Whitehall Foundation, ACS, and NIH (RO1: NS39472); to J.C.B. and M.S.B. from NIH (RO1: NS 38079) and the Spinal Cord Research Foundation (SCRF 1734); to F.M.L. from NIH (R01 AG09873) and the Veterans Administration; and to B.L.H. from NIH (RO1: NS30687).
References
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J. Neuro-trauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Soderstrom S, Kokaia Z, Kokaia M, Ernfors P, Persson H, Ebendal T, Lindvall O. Widespread increase of nerve growth factor protein in the rat forebrain after kindling-induced seizures. Brain Res. 1992;587:338–342. doi: 10.1016/0006-8993(92)91016-8. [DOI] [PubMed] [Google Scholar]
- Bentley CA, Lee KF. p75 is important for axon growth and schwann cell migration during development. J. Neurosci. 2000;20:7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Blight AR. Remyelination, revascularization, and recovery of function in experimental spinal cord injury. Adv. Neurol. 1993;59:91–104. [PubMed] [Google Scholar]
- Brandoli C, Shi B, Pflug B, Andrews P, Wrathall JR, Mocchetti I. Dexamethasone reduces the expression of p75 neurotrophin receptor and apoptosis in contused spinal cord. Brain Res. Mol. Brain Res. 2001;87:61–70. doi: 10.1016/s0169-328x(00)00284-9. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Pozza M, Micera A, Aloe L. Time-course changes of nerve growth factor, corticotropin-releasing hormone, and nitric oxide synthase isoforms and their possible role in the development of inflammatory response in experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. USA. 1997;94:3368–3373. doi: 10.1073/pnas.94.7.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuro-science. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins. To cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- de Castro RC, Jr., Burns CL, McAdoo DJ, Romanic AM. Metalloproteinase increases in the injured rat spinal cord. Neuroreport. 2000;11:3551–3554. doi: 10.1097/00001756-200011090-00029. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am. J. Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- Dowling P, Ming X, Raval S, Husar W, Casaccia-Bonnefil P, Chao M, Cook S, Blumberg B. Up-regulated p75NTR neurotrophin receptor on glial cells in MS plaques. Neurology. 1999;53:1676–1682. doi: 10.1212/wnl.53.8.1676. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Henschen A, Olson L, Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989;2:1605–1613. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Mol. Cell. Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Farhadi HF, Mowla SJ, Petrecca K, Morris SJ, Seidah NG, Murphy RA. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J. Neurosci. 2000;20:4059–4068. doi: 10.1523/JNEUROSCI.20-11-04059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J. Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss B, Mallon B, Phan T, Ohlemeyer C, Kirchhoff F, Nishiyama A, Macklin WB. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev. Biol. 2000;218:259–274. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Rohrig S, Bonatz H, Gutjahr M, Leiner B, Bartke I, Yan Q, Reichardt LF, Backus C, Welcher AA, et al. Endogenous brain-derived neurotrophic factor and neurotrophin-3 antagonistically regulate survival of axotomized corticospinal neurons in vivo. J. Neurosci. 2001;21:3492–3502. doi: 10.1523/JNEUROSCI.21-10-03492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126-128. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Risling M, Hokfelt T, Cullheim S, Piehl F. Expression of insulin-like growth factors and corresponding binding proteins (IGFBP 1-6) in rat spinal cord and peripheral nerve after axonal injuries. J. Comp. Neurol. 1998;400:57–72. [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun n-terminal kinase-mediated apoptosis. J. Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol. Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Shoji S, Okano H, Miura M. ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J. Neurochem. 1997;69:10–20. doi: 10.1046/j.1471-4159.1997.69010010.x. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev. Biol. 1995;167:227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Kim GM, Xu J, Song SK, Yan P, Ku G, Xu XM, Hsu CY. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J. Neurosci. 2001;21:6617–6625. doi: 10.1523/JNEUROSCI.21-17-06617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Crawford TO, Price DL. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 1991;549:297–304. doi: 10.1016/0006-8993(91)90471-7. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J. Neurochem. 2000;74:730–739. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- Ladiwala U, Lachance C, Simoneau SJ, Bhakar A, Barker PA, Antel JP. p75 neurotrophin receptor expression on adult human oligodendrocytes: signaling without cell death in response to NGF. J. Neurosci. 1998;18:1297–1304. doi: 10.1523/JNEUROSCI.18-04-01297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J. Neuropathol. Exp. Neurol. 1996;55:280–289. doi: 10.1097/00005072-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Goujet-Zalc C, Gansmuller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J. Neurochem. 1991;56:671–680. doi: 10.1111/j.1471-4159.1991.tb08202.x. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat. Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J. Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Kordower JH. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1992;89:569–573. doi: 10.1073/pnas.89.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf S, Naveilhan P, Sindji L, Darcy F, Brachet P, Montero-Menei CN. Low affinity NGF receptor expression in the central nervous system during experimental allergic encephalomyelitis. J. Neurosci. Res. 1998;52:83–92. doi: 10.1002/(SICI)1097-4547(19980401)52:1<83::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Oh JD, Chartisathian K, Chase TN, Butcher LL. Overexpression of neurotrophin receptor p75 contributes to the excitotoxin-induced cholinergic neuronal death in rat basal forebrain. Brain Res. 2000;853:174–185. doi: 10.1016/s0006-8993(99)02054-5. [DOI] [PubMed] [Google Scholar]
- Park JA, Lee JY, Sato TA, Koh JY. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the Rat. J. Neurosci. 2000;20:9096–9103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford BE, Whalen LR, Ishii DN. Peripherally administered insulin-like growth factor-I preserves hindlimb reflex and spinal cord noradrenergic circuitry following a central nervous system lesion in rats. Exp. Neurol. 1999;159:114–123. doi: 10.1006/exnr.1999.7143. [DOI] [PubMed] [Google Scholar]
- Reinshagen M, Geerling I, Eysselein VE, Adler G, Huff KR, Moore GP, Lakshmanan J. Commercial recombinant human beta-nerve growth factor and adult rat dorsal root ganglia contain an identical molecular species of nerve growth factor pro-hormone. J. Neurochem. 2000;74:2127–2133. doi: 10.1046/j.1471-4159.2000.0742127.x. [DOI] [PubMed] [Google Scholar]
- Reynolds ME, Brunello N, Mocchetti I, Wrathall JR. Localization of nerve growth factor receptor mRNA in contused rat spinal cord by in situ hybridization. Brain Res. 1991;559:149–153. doi: 10.1016/0006-8993(91)90298-a. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Riopelle RJ. Uptake of nerve growth factor along peripheral and spinal axons of primary sensory neurons. J. Neurosci. 1984;4:1683–1689. doi: 10.1523/JNEUROSCI.04-07-01683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J. Neurosci. 1999;19:464–475. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J. Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J. Neuropathol. Exp. Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Nyberg F, Gordh T, Alm P, Westman J. Topical application of insulin like growth factor-1 reduces edema and upregulation of neuronal nitric oxide synthase following trauma to the rat spinal cord. Acta Neurochir. Suppl. 1997;70:130–133. doi: 10.1007/978-3-7091-6837-0_40. [DOI] [PubMed] [Google Scholar]
- Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J. Neurosci. Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat. Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J. Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schweitzer JB, Johnson EM., Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J. Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden P, Bamber NI, Li H, Esposito A, Ahmad KA, Hsu CY, Xu XM. Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats. Exp. Neurol. 2001;168:213–224. doi: 10.1006/exnr.2000.7622. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao DL, West NR, Bondy CA, Brenner M, Hudson LD, Zhou J, Collins GH, Webster HD. Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration. J. Neurosci. Res. 1995a;40:647–659. doi: 10.1002/jnr.490400510. [DOI] [PubMed] [Google Scholar]
- Yao DL, Liu X, Hudson LD, Webster HD. Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 1995b;92:6190–6194. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter BD, Chao MV. Competitive signaling between TrkA and p75 determines cell survival. J. Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]