Abstract
Membrane complement inhibitors (CD46, CD55 and CD59) are upregulated in some human cancers indicating that they play a role in immune evasion. We investigated complement inhibitor expression in bladder cancer and examined the hypothesis that selective pressure of an antibody response (anti-MUC1) results in the upregulated expression of complement inhibitors on tumor cells. Paired samples of tumor and normal tissue from 22 bladder cancer patients were analyzed for expression of MUC1, CD46, CD55 and CD59, and matched serum samples analyzed for anti-MUC1 IgM and IgG levels. Relationships between anti-MUC1 antibody levels and complement inhibitor expression were investigated. MUC1 mRNA was upregulated in 86% of tumor samples. CD46 was upregulated in 77%, CD55 in 55% and CD59 in 59% of tumors. Low titer anti-MUC1 IgM was detected in normal human sera, but was elevated in 41% of the bladder cancer patients. Anti-MUC1 IgG was virtually absent from normal sera, but present in 32% of the cancer patients. There was a direct relationship between anti-MUC1 antibody titer and expression level of complement inhibitors. Analysis of the correlation of each antibody with the expression of each complement inhibitor by Spearman’s rank test revealed a strong correlation between both anti-MUC1 IgM and IgG levels and increased expression of CD46 and CD55, and combined anti-MUC1 IgM/IgG levels correlated with increased expression of all 3 complement inhibitors. In conclusion, the data demonstrate upregulated complement inhibitor expression and the presence of an anti-MUC1 antibody response in bladder cancer patients and support the hypothesis of antibody-mediated immune selection.
Keywords: complement, antibody, MUC1, bladder cancer
There is strong evidence indicating that complement effector mechanisms contribute to the efficacy of some anticancer antibodies administered as therapy. Naturally elicited antibody responses to tumor-associated antigens also occur in some cancer patients. A contributing factor to the ineffectiveness of antitumor antibodies, whether they are naturally elicited or therapeutically administered, appears to be the expression of membrane bound complement inhibitory proteins that are widely expressed on both normal and cancer cells. The 3 principle human membrane-bound complement inhibitory proteins are membrane cofactor protein (MCP, CD46), decay accelerating factor (DAF, CD55) and CD59. CD46 and CD55 inhibit complement activation by inactivating or interfering with the formation of C3 convertase, an enzymatic complex formed on the activating cell surface that is central to amplification of the complement cascade. CD59 functions later in the complement pathway and inhibits formation of the membrane attack complex (MAC), a cytolytic assembly of the terminal complement proteins. Each of the 3 complement inhibitors has been reported to be upregulated in a variety of primary tumors (breast, lung, liver, kidney, prostate, cervical, gastric, colorectal, pancreatic), and on tumor cell lines.1-6 The upregulation of complement inhibitors on cancer cells indicate that they may play a role in immune evasion, but any direct evidence is lacking. In the current study we sought to provide supporting evidence by investigating whether selective pressure of an antitumor antibody response, specifically an anti-MUC1 response, is related to the upregulated expression of complement inhibitory proteins. MUC1 (mucin 1, epsialin) is an important cancer-associated antigen and is expressed by most adenocarcinomas of the breast, lung, stomach, pancreas, colon, ovary, prostate and bladder. The mucin is normally expressed on the apical surface of ductal epithelial cells, but on many types of cancer cells it loses polarity of expression, is over expressed and is underglycosylated. The loss of polarization makes MUC1 more accessible to the immune system and underglycosylation of the normally highly glycosylated mucin exposes immunodominant peptide sequences that are concealed on normal cell surfaces. These differences in MUC1 expression impart a level of tumor specificity to MUC1 epitopes on cancer cells, and low titer antibody responses to MUC1 have been reported in breast, colon, ovarian and pancreatic cancer patients.7-11 The current study investigates relationships and correlations between MUC1 expression, complement inhibitor expression and anti-MUC1 antibody response (IgM and IgG) in bladder cancer.
Material and methods
Patient samples
Paired samples of tumor and normal tissue along with matched serum from 22 bladder cancer patients were obtained from the Medical University of South Carolina Tumor Bank with appropriate IRB approval. The samples consisted of 19 transitional cell carcinomas (TCC), 2 adenocarcinomas and 1 squamous cell carcinoma (SCC). The pathology of samples was verified by a blinded observer by Hematoxylin & Eosin staining (data not shown). Normal human serum (NHS) (n = 8) was purchased from Innovative Research (Southfield, MI).
RNA extraction
Total RNA was extracted from paired normal and tumor samples using guanidine isothiocyanate and phenol-chloroform according to standard methods. Briefly, samples were homogenized in 0.75 ml RNA extraction solution (TRIzol; Invitrogen, Carlsbad, CA) using a motorized tissue homogenizer. RNA was extracted with chloroform, precipitated with isopropanol, washed with ethanol and dissolved in RNase-free water. The material was then treated with 1 U/μl DNase I at 37°C for 30 min to remove any contaminating genomic DNA, and then extracted once with phenol-chloroform-isoamyl alcohol (25:24:1 pH 4.5). Following extraction with chloroform, RNA was precipitated with 2 M sodium acetate and 95% ethanol, washed with 80% ethanol and resuspended in RNase-free water. The concentrations of RNA were measured spectrophotometrically at 260 nm.
Real-time reverse transcription-PCR
cDNA was made from 1 lg total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time RT-PCR analysis was subsequently performed using the IQ SYBR Green Supermix kit (Bio-Rad) following manufacturer’s protocols. Analyses were done using My IQ Real-Time detection system (Bio-Rad) using intron-spanning primers specific for MUC1 forward 5′-ACCATCCTATGAGCGAGTACCC-3′, reverse 5′-GCCACCATTACCTGCAGAAAC-3; CD46 forward 5′-TGCACTTCTTCCACTACAAAATCTCC-3′, reverse 5′-ATCCAAACTGTCAAGTATTCCTTCCTC-3′; CD55 forward 5′-CGTTGCCAGAGTGCAGAGAAA-3′, reverse 5′-CGTTACAGACTGTCTATATCCATAATC-3′; CD59 forward 5′-TGCGTGTCTCATTACCAAAGCTG-3′, reverse 5′-CGTTAGCTCATTTTCCCTCAAGC-3′. All reactions were done in triplicate and the β2-microglobulin gene was used as an internal control since it is considered to be a more reliable reference standard than β-actin or GAPDH.12
Anti-MUC1 antibodies determinations
Serum anti-MUC1 antibody titers (IgM and IgG) were measured by sandwich ELISA as previously described.13 The MUC1 peptide (100mer, corresponding to 5 tandem repeats of human MUC1) used to coat the ELISA plates for these assays was kindly provided by Dr. Olivera Finn (University of Pittsburg) and has been previously characterized in the detection of MUC1-specific antibodies in sera of cancer patients.8 To control for nonspecific antibody binding, the optical density values from wells not coated with MUC1 peptide were subtracted from the test wells coated with the peptide.
Immunohistochemistry
A limited number of the tissue samples utilized for the mRNA analysis were available for immunohistochemical analysis (n = 9). Frozen samples of matched normal bladder and bladder carcinoma were embedded in OCT compound and cryosectioned. For immunohistochemical analysis, the sections were stained for the presence of the complement inhibitory proteins CD55, CD46 and CD59 (antibodies purchased from BD Biosciences, Franklin Lakes, NJ) and for MUC1 (using BCP8 mAb, kindly supplied by Dr. F.C. McKenzie, Austin Research Institute, Heidelberg, Australia). Briefly, sections were incubated for 1 hr with primary antibody and washed with PBS. Binding of primary antibody was assessed using a streptavidin biotin complex system (DakoCytomation, Carpinteria, USA) and visualized by 3′3 diaminobenzidine substrate (DAB), producing a brown reaction product. The specificity of immunostaining was demonstrated by the absence of signal in sections incubated with isotype control antibodies and also by omission of primary antibody. Sections were counterstained with Carazzi’s hematoxylin and examined by light microscopy by 2 independent observers.
Statistical analysis
Wilcoxon-Mann-Whitney tests were used to compare patients with elevated anti-MUC1 antibodies (Group A) to the remaining patients (Group B) for each complement inhibitory protein. Association of anti-MUC1 antibody titers with complement inhibitor expression was evaluated using Spearman’s rank correlation. Levels of complement inhibitor expression and MUC1 expression upregulation were analyzed by calculating cancer/normal sample ratios and calculating 95% confidence intervals. For MUC1, confidence intervals were calculated on log-transformed ratios due to the variation in MUC1 expression by tumors. Exact p-values are displayed for statistical analyses with p < 0.05 considered statistically significant in the presentation of the results. All calculations were carried out using SPSS version 14.0.
Results
MUC1 expression
In a previous study, immunohistochemical analysis of biopsy specimens showed that MUC1 is overexpressed on bladder tumor cells compared to normal urothelium.14 In the current study we analyzed MUC1 mRNA expression in paired normal/cancer tissue from samples obtained from bladder cancer patients. In agreement with the earlier report on MUC1 protein expression, MUC1 mRNA was expressed in all normal and cancer samples analyzed. MUC1 mRNA expression was, however, upregulated in 86% (19/22) of the tumor samples when compared to paired normal samples (95% confidence interval 1.92-6.67). The level of mRNA upregulation ranged from 1.5- to 150-fold (Fig. 1a).
Figure 1.
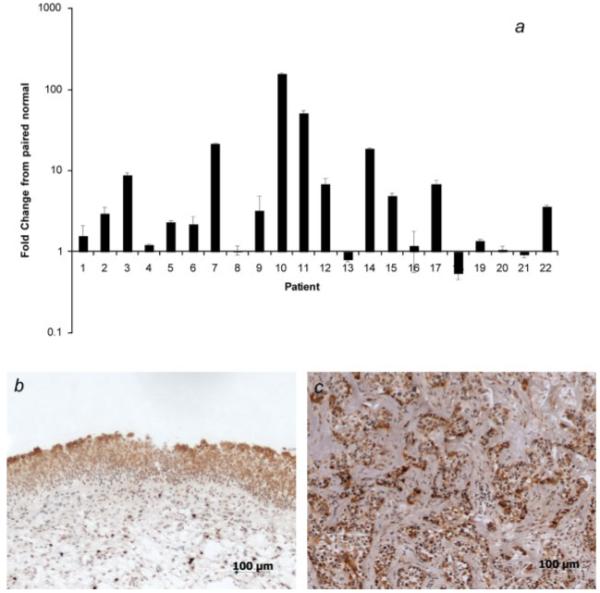
MUC1 expression in bladder carcinoma. (a) mRNA MUC1 expression was determined in paired normal/cancer samples by realtime RT-PCR. Graph shows fold change in MUC1 expression in tumor sample compared to paired normal tissue sample. Mean ± SD of 3 determinations is shown. The β2-microglobulin gene was used as an internal control. (b and c) Immunohistochemical analysis of section from normal (b) and tumor (c) sample. Sections were stained for MUC1 using anti-MUC1 mAb BCP8. The panels show that MUC1 expression is restricted to the luminal surface of normal urothelium, but is present in the luminal, intermediate and basal layers of the tumor sample. Magnification ×100. Representative images shown (n = 9).
Immunohistochemical analysis of the matched normal and tumor samples demonstrated MUC1 protein expression in all samples. Although expression was noted in both normal and cancer tissue, the distribution of MUC1 staining was markedly different between normal and cancer samples, which is consistent with previous histological studies of MUC1 positive tumor samples. In all normal bladder samples, MUC1 immunostaining was confined to the apical surface of the transitional epithelium (9/9), but polarized expression was absent in tumor samples (9/9) (Figs. 1b and 1c). We did not attempt to quantify intensity of staining since tumor expressed MUC1 is aberrantly glycosylated which can affect anti-MUC1 antibody binding.15
Expression of CD46, CD55, CD59
Previous studies have shown that one or more complement inhibitory proteins are upregulated in primary carcinomas of the breast, lung, colon, prostate, stomach, kidney, liver, ovary and pancreas as well as in some hematological malignancies.1,2,4,5,16-26 Here we demonstrate upregulation of membrane bound inhibitors of human complement in bladder cancer by analyzing mRNA expression in paired normal/cancer samples. CD46 was upregulated in 77% of tumors analyzed when compared to normal paired samples (1.5- to 11-fold, 95% confidence interval 1.59-3.72), CD55 was upregulated in 55% of tumors analyzed (1.5- to 13-fold, 95% confidence interval 1.05-3.42) and CD59 was upregulated in 59% of tumors analyzed (2-to 4-fold, 95% confidence interval 1.20-2.32) (Fig. 2). Also of note, 86% of the bladder cancer samples analyzed expressed increased levels of at least one complement inhibitor.
Figure 2.
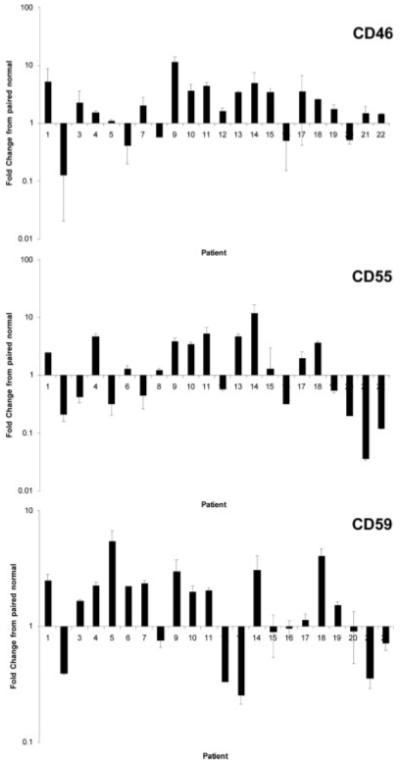
Complement inhibitor expression in normal bladder and bladder carcinomas. mRNA levels of CD46, CD55 and CD59 were determined in paired normal/cancer samples by real-time RT-PCR. Graph shows fold change in the expression of each complement inhibitor in tumor sample compared to paired normal tissue sample. Mean ± SD of 3 determinations is shown. The β2-microglobulin gene was used as an internal control.
In addition to mRNA determinations, normal and bladder cancer samples were analyzed by immunohistochemistry to characterize the in situ staining pattern of the complement inhibitors. CD55, CD46 and CD59 were present on epithelial cells, endothelial cells and stromal cells in all normal samples analyzed (9/9). Both CD46 and CD59 were expressed throughout the normal bladder epithelium (Figs. 3a and 3e). For CD55, there was more extensive expression within the stromal tissue of the normal bladder. CD55 expression in the normal transitional epithelium was similar to that seen with MUC1, with more intense staining noted at the apical surface in all analyzed samples (Fig. 3c). In tumor samples, as with the normal samples, CD55, CD46 and CD59 were expressed on epithelia, endothelia and within the stroma. The expression pattern on the tumor cells was, however, somewhat different for each complement inhibitor. CD55 was expressed by tumor cells in all samples (9/9), but interestingly, staining intensity was highest on the stromal cells surrounding the tumor cell nests (Fig. 3d). A similar expression pattern was seen with CD59, with more intensive staining on the stromal cells (Fig. 3f). The pattern of CD46 staining was different to that seen with CD55 and CD59. All tumor samples analyzed demonstrated strong positive staining for CD46, with positive staining localized to the tumor cells and absent within the intratumor stromal areas (Fig. 3b). CD46 is a transmembrane protein, whereas CD55 and CD59 are attached to the membrane via a glycosylphosphatidylinositol (GPI) anchor (see Discussion section).
Figure 3.
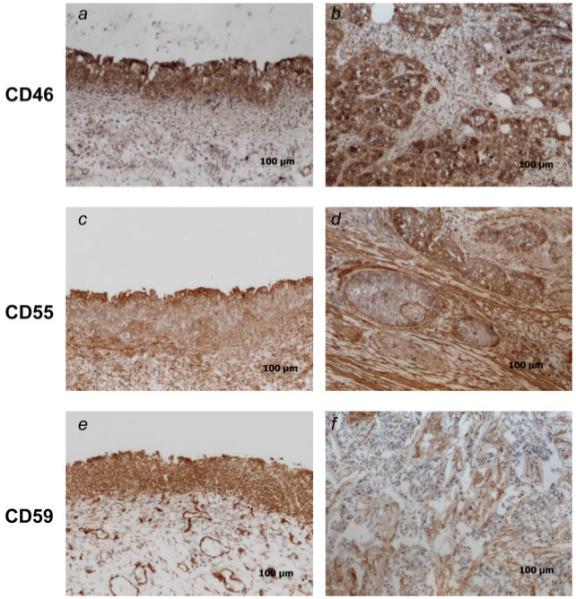
Immunohistochemical analysis of complement inhibitor expression in normal bladder and bladder carcinomas. Sections from normal (a, c, e) and tumor (b, d, f) samples were analyzed for expression of CD46, CD55 and CD59 by indirect immunohistochemistry. Magnification ×100, representative images shown (n = 9).
Anti-MUC1 antibody levels
Serum from each bladder cancer patient matched to the paired normal/tumor tissue sample was analyzed for relative levels of IgM and IgG antibody by ELISA. Anti-MUC1 IgM antibodies were present in the serum of all bladder cancer patients studied, although there was a wide variation in relative levels (OD value range 0.06-1.89) (Fig. 4a). Anti-MUC1 IgM was also present in NHS (OD value range 0.52-0.78, n = 8). Compared to normal, anti-MUC1 IgM was elevated in 9/22 of patients (more than one standard deviation higher than the average of anti-MUC1 IgM levels in NHS). Only very low levels of IgG reactive against MUC1 were detected in NHS (OD value range 0-0.23, n = 8). In 7/22 patients, anti-MUC1 IgG was significantly elevated compared to NHS (Fig. 4b). Similar antibody response profiles in patients with other types of MUC1-positive tumors have been reported previously.8,27-29
Figure 4.
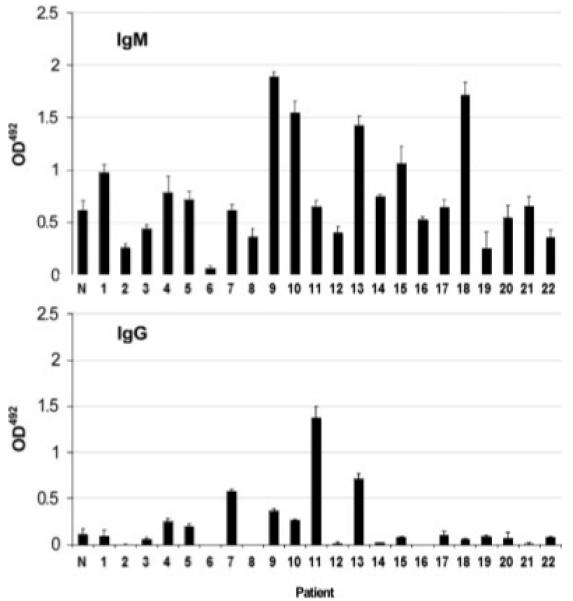
Anti-MUC1 antibody levels in serum from bladder cancer patients. Anti-MUC1 IgM and IgG in the serum of 22 bladder cancer patients was determined by ELISA. Antibody levels in serum from 8 healthy individuals were also determined and the mean value is shown in the figure, marked N. Patient numbers correspond to those in Figure 2. Mean ± SD (n = 3). All serum samples were measured undiluted and in triplicate.
Relationship between anti-MUC1 antibody levels and the expression of complement inhibitory proteins
To determine whether there was a correlation between anti-MUC1 antibody titers and upregulated complement inhibitor expression, the bladder cancer patients were divided into 2 groups for the purpose of analysis. Patients with anti-MUC1 antibody levels (either IgM or IgG) more than one standard deviation higher than the average anti-MUC1 antibody level in normal serum were placed in Group A (n = 12), with the remaining patients placed into Group B (n = 10). The average anti-MUC1 IgM and IgG antibody levels in normal serum were 0.612 ± 0.099 and 0.107 ± 0.067, respectively. Analysis of complement inhibitor mRNA levels revealed that the average expression level of each complement inhibitory protein (CD46, CD55 and CD59) was significantly higher in tumor samples from patients in Group A (i.e. patients with elevated levels of anti-MUC1 antibodies) compared to tumor samples from patients in Group B (CD46: Z = -3.36, p = 0.0003, CD55: Z = -3.29, p = 0.0004, CD59: Z = -2.63, p = 0.007) (Fig. 5). These results suggest a direct relationship between anti-MUC1 antibody titer and the expression level of CD46, CD55 and CD59 on bladder tumors. Furthermore, there was no elevation of anti-MUC1 antibody titers in any of the patients whose tumors did not have upregulated expression of any complement inhibitory protein (see above). There was no difference between the average expression level of MUC1 mRNA in patient Groups A and B (data not shown).
Figure 5.
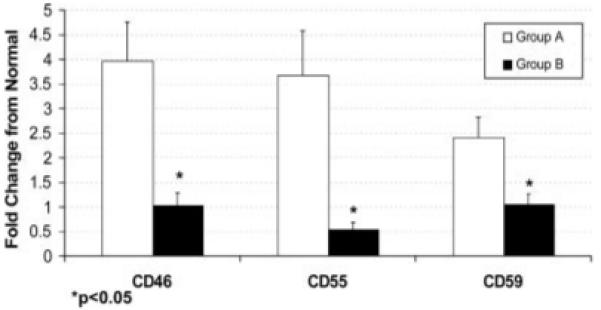
Comparison of complement inhibitor expression on tumors from patients with either high or low anti-MUC1 antibody titers. Patients were divided into 2 groups based on their anti-MUC1 antibody levels. Group A represents patients with antibody levels (either IgM or IgG) at least one standard deviation higher than the average levels from normal individuals (n = 12), and Group B represents remaining patients (n = 10). Bladder tumors from patients with high levels of anti-MUC1 antibodies (Group A) had significantly higher mRNA levels of CD46, CD55 and CD46 (p < 0.05) than tumors from patients with low titer anti-MUC1 antibodies (Group B). Mean ± SD.
Correlation analysis of anti-MUC1 antibody levels and complement inhibitor expression
To further investigate the correlative relationship between anti-MUC1 antibody titers and the expression of complement inhibitors on bladder tumors, a correlation analysis using Spearman’s rank test was carried out. The effect of IgM and of IgG on expression levels of each of CD46, CD55 and CD59 was separately evaluated. There was a very strong statistical correlation between both anti-MUC1 IgM and IgG levels and increased expression of CD46, and also a significant correlation between anti-MUC1 IgM and IgG levels and expression of CD55 (Table I). Although there was some evidence of an association between anti-MUC1 IgM and CD59, the correlation did not reach statistical significance, and there was no correlation between anti-MUC1 IgG levels and CD59 expression. An analysis was also performed by combining the values of anti-MUC1 IgM and IgG for each patient and correlating the sum with expression of each complement inhibitor. There was a strong correlation between total anti-MUC1 Ig levels and increased expression of CD46 and CD55, as when IgM and IgG were considered separately, but total Ig also correlated with increased CD59 expression, although less strongly than for CD46 and CD55 (Table I). In a separate analysis, we also found no correlation between anti-MUC1 antibody titer and cancer clinical phenotype (tumor stage) (data not shown). However, all patients had advanced disease and had undergone cystectomy for at least high grade T1 or worse disease.
TABLE I.
SPEARMAN CORRELATION ANALYSIS BETWEEN SERUM LEVELS OF ANTI-MUC1 ANTIBODIES AND EXPRESSION OF COMPLEMENT REGULATORS BY BLADDER TUMOR CELLS
| Anti-MUC1 antibody isotype | Spearman correlation value (p-value) |
||
|---|---|---|---|
| CD46 | CD55 | CD59 | |
| IgM | 0.697 | 0.576 | 0.395 |
| (0.0003)* | (0.004)* | (0.06) | |
| IgG | 0.631 | 0.466 | 0.305 |
| (0.001)* | (0.02)* | (0.166) | |
| IgM + IgG | 0.683 | 0.665 | 0.458 |
| (0.0005)* | (0.0007)* | (0.03)* | |
p-values < 0.05 were considered statistically significant.
Discussion
MUC1 is an important tumor-associated antigen and has been described as a tumor marker for bladder cancer.30 In the current study, mRNA analysis revealed that MUC1 is upregulated in a large proportion (86%) of the bladder tumors studied, a result that is in broad agreement with previous studies evaluating MUC1 protein expression.14 Anti-MUC1 IgM antibodies, and to a lesser extent IgG, have been found in the sera of patients with breast, colon, ovarian and pancreatic cancer.7-11 Studies have also shown a correlation between the presence of anti-MUC1 antibodies and better clinical outcomes in patients with pancreatic and breast cancer,31,32 although in the current study we found no correlation between antibody titer and bladder tumor stage (data not shown). However, this lack of correlation could be because all tumors were clinically advanced (all cystectomy samples), and a wider spectrum of clinical samples including low-risk patients would be required to fully investigate any relationship between anti-MUC1 antibodies and bladder tumor stage. There is evidence indicating that the expression of complement inhibitors on tumor cells can hinder the effectiveness of monoclonal antibody therapy, and the same may be true for naturally elicited antibodies. Thus, any protective effect of an elevated anti-MUC1 antibody response may be offset by the increased expression of complement inhibitors. Indeed, there were elevated levels of anti-MUC1 IgM and an IgG response in some bladder cancer patients, but there was a direct correlation between increased antibody titer and increased expression of complement inhibitors. In addition, all patients with high levels of anti-MUC1 IgG had increased tumor expression of at least one complement inhibitor. Of course it is not possible to rule out an antibody response to other unidentified tumor antigens that may provide a component of the effect seen.
This is the first report of upregulated complement inhibitor expression in bladder cancer, and the correlation of upregulated expression with antibody titer suggests a link with immune selection and an antitumor response. Each of the membrane complement inhibitors were upregulated in over 50% of the tumor samples, although anti-MUC1 IgM and IgG levels when analyzed separately correlated with increased expression of only CD46 and CD55. Total anti-MUC1 Ig levels (sum of IgM and IgG) also correlated with increased CD59 expression, but the correlation was significantly weaker than for CD46 and CD55. These latter 2 inhibitors function at the C3 level in the complement cascade and inhibit complement activation. CD59 functions late in the cascade by interfering with the formation of the terminal cytolytic MAC. The strong correlation of anti-MUC1 antibody levels with increased expression of only the inhibitors of complement activation may be indicative of a complement-mediated effector mechanism that is active against tumor cells. Thus, complement-dependent cytotoxicity (CDC) that is mediated by the MAC, although effective against many tumor cell lines in vitro (particularly when CD59 activity is reduced), does not appear to play a significant role in immune selection. On the other hand, complement-dependent enhancement of antibody-dependent cell cytotoxicity (ADCC) and/or complement-dependent cellular cytotoxicity (CDCC), which would be inhibited by CD46 and CD55, may be active antitumor mechanisms. CDCC is an effector mechanism similar to ADCC, but is dependent on ligation of complement receptors on immune effector cells.33,34 However, ADCC is mediated via FcγR’s suggesting a possible role for CDCC in IgM mediated upregulation of the complement inhibitors. Of note, previous studies in a syngeneic mouse model of lymphoma indicated that CDCC was the primary mechanism of action for IgM therapy and that ADCC was the primary mechanism for IgG therapy.35 Released complement activation products C3a and C5a, which are generated downstream of C3 activation, may also be involved via immune cell recruitment and the potentiation of an inflammatory/immune response.
As stated above, anti-MUC1 IgM antibodies, and to a lesser extent IgG, have been found previously in the sera of some cancer patients. Low levels of anti-MUC1 have also been found in healthy individuals, and in the current study we detected anti-MUC1 IgM (but not IgG) in samples of NHS. It is possible that anti-MUC1 IgM thus represents a naturally occurring antibody, and that these antibodies were upregulated as part of a tissue homeostatic response to the increased and aberrant MUC1 expression by the bladder tumor cells. Natural antibodies play homeostatic roles in the clearance of proteins from lysed cells and of altered/modified proteins, and there is also evidence that natural antibodies constitute part of an antitumor immunosurveillance mechanism. Humans can upregulate both IgM and IgG natural antibodies, and class switching may also occur as a result of a tissue homeostatic process (for reviews, see Refs. 36 and 37).
Another consideration is that complement inhibitors can modulate the induction of T-cell responses (for review, see Refs. 38 and 39), and recent studies in mice have shown that DAF can modulate T-cell differentiation and suppress immunity in a complement dependent manner.40-43 Complement independent roles for DAF and CD59 in the modulation of T cell activation have also been reported, but via the expression of the inhibitors on T cells.41,44 However, unlike antibody effector mechanisms, complement inhibitor expression and C3 deposition is not known to directly modulate T-cell effector mechanisms, and if a T-cell response was initially induced it is not clear how it would subsequently select for increased complement inhibitor expression. Nevertheless, a consequence of upregulated complement inhibitor expression may be the suppression of an ongoing T-cell response, and diminution of T-cell stimulation could lead to a decrease in T-cell expansion. It has also been shown that T-cell responses can be downregulated by the crosslinking of TCR and MCP, which induces T regulatory cells.45 Therefore, there is the possibility that decreased C3 deposition on tumor cells as a result of complement inhibitor upregulation could result in an enhanced T-cell response due to the suppression of T regulatory cells.
Immunohistochemical analysis of the expression patterns of complement inhibitors revealed marked differences among CD46, CD55 and CD59 distribution. All 3 inhibitors were expressed on both normal urothelium and tumor samples. However, CD55 and CD59 were widely expressed within the stromal compartment of the tumor, whereas CD46 expression was primarily restricted to the tumor cells. Similar findings for other types of cancer have been reported5,46 and may be related to the fact that CD46 is a trans membrane protein whereas CD55 and CD59 are GPI linked proteins. Cells can both actively cleave and directly secrete GPI-linked proteins which may account for the high levels of CD55 and CD59 in the stroma.
In conclusion, the data demonstrate the presence of an anti-MUC1 antibody response and upregulated complement inhibitor expression in bladder cancer patients and support the hypothesis of antibody-mediated immune selection. In addition to regulating direct antitumor complement effector mechanisms, it is possible that the upregulation of complement inhibitors may also regulate T-cell immunity, although we are unable to examine this hypothesis with our currently available samples. While the current study was focused on bladder cancer, similar mechanisms of immune selection may account for the upregulated expression of complement inhibitors found in other types of MUC1 positive cancer, and possibly with other tumor antigens. The data indicate that complement inhibition is an immune evasion mechanism used by tumors and strengthen the argument that the modulation of complement inhibitor activity represents a therapeutic modality that may augment passive and active antitumor antibody therapy in humans.
Acknowledgements
The authors thank Dr. Olivera Finn, University of Pittsburg, for generously providing the MUC1 peptide used in ELISA assays, and Dr. Azizul Haque, Medical University of South Carolina, for his critical reading of the manuscript. Supported by NCI grant and predoctoral fellowship.
Grant sponsor: NCI; Grant number: R21 CA 104579.
References
- 1.Rushmere NK, Knowlden JM, Gee JM, Harper ME, Robertson JF, Morgan BP, Nicholson RI. Analysis of the level of mRNA expression of the membrane regulators of complement, CD59, CD55 and CD46, in breast cancer. Int J Cancer. 2004;108:930–6. doi: 10.1002/ijc.11606. [DOI] [PubMed] [Google Scholar]
- 2.Murray KP, Mathure S, Kaul R, Khan S, Carson LF, Twiggs LB, Martens MG, Kaul A. Expression of complement regulatory proteins-CD 35, CD 46, CD 55, and CD 59-in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76:176–82. doi: 10.1006/gyno.1999.5614. [DOI] [PubMed] [Google Scholar]
- 3.Simon HG, Risse B, Jost M, Oppenheimer S, Kari C, Rodeck U. Identification of differentially expressed messenger RNAs in human melanocytes and melanoma cells. Cancer Res. 1996;56:3112–17. [PubMed] [Google Scholar]
- 4.Li L, Spendlove I, Morgan J, Durrant LG. CD55 is over-expressed in the tumour environment. Br J Cancer. 2001;84:80–6. doi: 10.1054/bjoc.2000.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement-inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay accelerating factor), and CD59 (protectin) Am J Pathol. 1996;149:129–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Seya T, Hara T, Matsumoto M, Sugita Y, Akedo H. Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein. J Exp Med. 1990;172:1673–80. doi: 10.1084/jem.172.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 8.Kotera Y, Fontenot J, Pecher G, Metzger R, Finn O. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–60. [PubMed] [Google Scholar]
- 9.Petrarca C, Casalino B, von Mensdorff-Pouilly S, Rughetti A, Rahimi H, Scambia G, Hilgers J, Frati L, Nuti M. Isolation of MUC1-primed B lymphocytes from tumour-draining lymph nodes by immunomagnetic beads. Cancer Immunol Immunother. 1999;47:272–7. doi: 10.1007/s002620050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snijdewint FG, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, Verstraeten AA, van Zanten-Przybysz I, Hummel P, Nijman HW, Kenemans P, Hilgers J. Cellular and humoral immune responses to MUC1 mucin and tandem-repeat peptides in ovarian cancer patients and controls. Cancer Immunol Immunother. 1999;48:47–55. doi: 10.1007/s002620050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura H, Hinoda Y, Nakagawa N, Makiguchi Y, Itoh F, Endo T, Imai K. Detection of circulating anti-MUC1 mucin core protein antibodies in patients with colorectal cancer. J Gastroenterol. 1998;33:354–61. doi: 10.1007/s005350050096. [DOI] [PubMed] [Google Scholar]
- 12.Mitas M, Mikhitarian K, Walters C, Baron PL, Elliott BM, Brothers TE, Robison JG, Metcalf JS, Palesch YY, Zhang Z, Gillanders WE, Cole DJ. Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel. Int J Cancer. 2001;93:162–71. doi: 10.1002/ijc.1312. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, Day R, Troetschel M, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–64. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh MD, Hohn BG, Thong W, Devine PL, Gardiner RA, Samaratunga ML, McGuckin MA. Mucin expression by transitional cell carcinomas of the bladder. Br J Urol. 1994;73:256–62. doi: 10.1111/j.1464-410x.1994.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Papadimitriou J, Burchell JM, Plunkett T, Graham R, Correa I, Miles D, Smith M. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–21. doi: 10.1023/a:1020360121451. [DOI] [PubMed] [Google Scholar]
- 16.Guc D, Canpinar H, Kucukaksu C, Kansu E. Expression of complement regulatory proteins CR1, DAF, MCP and CD59 in haematological malignancies. Eur J Haematol. 2000;64:3–9. doi: 10.1034/j.1600-0609.2000.80097.x. [DOI] [PubMed] [Google Scholar]
- 17.Hara T, Kojima A, Fukuda H, Masaoka T, Fukumori Y, Matsumoto M, Seya T. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br J Haematol. 1992;82:368–73. doi: 10.1111/j.1365-2141.1992.tb06431.x. [DOI] [PubMed] [Google Scholar]
- 18.Hofman P, Hsi BL, Manie S, Fenichel P, Thyss A, Rossi B. High expression of the antigen recognized by the monoclonal antibody GB24 on human breast carcinomas: a preventative mechanism of malignant tumor cells against complement attack? Breast Cancer Res Treat. 1994;32:213–19. doi: 10.1007/BF00665772. [DOI] [PubMed] [Google Scholar]
- 19.Kiso T, Mizuno M, Nasu J, Shimo K, Uesu T, Yamamoto K, Okada H, Fujita T, Tsuji T. Enhanced expression of decay-accelerating factor and CD59/homologous restriction factor 20 in intestinal metaplasia, gastric adenomas and intestinal-type gastric carcinomas but not in diffuse-type carcinomas. Histopathology. 2002;40:339–47. doi: 10.1046/j.1365-2559.2002.01350.x. [DOI] [PubMed] [Google Scholar]
- 20.Koretz K, Bruderlein S, Henne C, Moller P. Expression of CD59, a complement regulator protein and a second ligand of the CD2 molecule, and CD46 in normal and neoplastic colorectal epithelium. Br J Cancer. 1993;68:926–31. doi: 10.1038/bjc.1993.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loberg RD, Wojno KJ, Day LL, Pienta KJ. Analysis of membranebound complement regulatory proteins in prostate cancer. Urology. 2005;66:1321–6. doi: 10.1016/j.urology.2005.06.094. [DOI] [PubMed] [Google Scholar]
- 22.Simpson KL, Jones A, Norman S, Holmes CH. Expression of the complement regulatory proteins decay accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46) and CD59 in the normal human uterine cervix and in premalignant and malignant cervical disease. Am J Pathol. 1997;151:1455–67. [PMC free article] [PubMed] [Google Scholar]
- 23.Thorsteinsson L, O’Dowd GM, Harrington PM, Johnson PM. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS. 1998;106:869–78. doi: 10.1111/j.1699-0463.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 24.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complementregulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Shimo K, Mizuno M, Nasu J, Hiraoka S, Makidono C, Okazaki H, Yamamoto K, Okada H, Fujita T, Shiratori Y. Complement regulatory proteins in normal human esophagus and esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2004;19:643–7. doi: 10.1111/j.1440-1746.2003.03328.x. [DOI] [PubMed] [Google Scholar]
- 26.Ravindranath NM, Shuler C. Cell-surface density of complement restriction factors (CD46, CD55, and CD59): oral squamous cell carcinoma versus other solid tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:231–9. doi: 10.1016/j.tripleo.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Richards ER, Devine PL, Quin RJ, Fontenot JD, Ward BG, McGuckin MA. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol Immunother. 1998;46:245–52. doi: 10.1007/s002620050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rughetti A, Turchi V, Ghetti CA, Scambia G, Panici PB, Roncucci G, Mancuso S, Frati L, Nuti M. Human B-cell immune response to the polymorphic epithelial mucin. Cancer Res. 1993;53:2457–9. [PubMed] [Google Scholar]
- 29.von Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FG, Kok A, Van Kamp GJ, Paul MA, Van Diest PJ, Meijer S, Hilgers J. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–83. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 30.Simms MS, Hughes OD, Limb M, Price MR, Bishop MC. MUC1 mucin as a tumour marker in bladder cancer. BJU Int. 1999;84:350–2. doi: 10.1046/j.1464-410x.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 31.Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 32.von Mensdorff-Pouilly S, Gourevitch MM, Kenemans P, Verstraeten AA, Litvinov SV, van Kamp GJ, Meijer S, Vermorken J, Hilgers J. Humoral immune response to polymorphic epithelial mucin (MUC-1) in patients with benign and malignant breast tumours. Eur J Cancer. 1996;32A:1325–31. doi: 10.1016/0959-8049(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 33.Gorter A, Meri S. Immune evasion of tumor cells using membranebound complement regulatory proteins. Immunol Today. 1999;20:576–82. doi: 10.1016/s0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 34.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–41. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Imai M, Landen C, Ohta R, Cheung NK, Tomlinson S. Complementmediated mechanisms in anti-GD2 monoclonal antibody therapy of murine metastatic cancer. Cancer Res. 2005;65:10562–8. doi: 10.1158/0008-5472.CAN-05-1894. [DOI] [PubMed] [Google Scholar]
- 36.Lutz HU. Homeostatic roles of naturally occurring antibodies: an overview. J Autoimmun. 2007;29:287–94. doi: 10.1016/j.jaut.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Vollmers HP, Brandlein S. Natural antibodies and cancer. J Autoimmun. 2007;29:295–302. doi: 10.1016/j.jaut.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 39.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check—a new role for complement regulators? Trends Immunol. 2006;27:102–8. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–77. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang C, Miwa T, Shen H, Song WC. Complement-dependent enhancement of CD81 T cell immunity to lymphocytic choriomeningitis virus infection in decay-accelerating factor-deficient mice. J Immunol. 2007;179:3178–86. doi: 10.4049/jimmunol.179.5.3178. [DOI] [PubMed] [Google Scholar]
- 43.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD41 T cell activity in a complement-independent manner. J Immunol. 2005;175:7098–102. doi: 10.4049/jimmunol.175.11.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD41 cells with CD3 and CD46 induces a Tregulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 46.Ravindranath NM, Shuler C. Expression of complement restriction factors (CD46. CD55 & CD59) in head and neck squamous cell carcinomas. J Oral Pathol Med. 2006;35:560–7. doi: 10.1111/j.1600-0714.2006.00466.x. [DOI] [PubMed] [Google Scholar]


