Abstract
Complement-inhibitory proteins expressed on cancer cells can provide protection from antitumor antibodies and may potentially modulate the induction of an immune response to tumor-associated antigens. In the current study, we investigated the consequences of complement inhibitor down-regulation on the effector and inductive phases of an immune response. Stable small interfering RNA-mediated down-regulation of the complement inhibitor Crry on MB49 murine bladder cancer cells increased their susceptibility to monoclonal antibody and complement in vitro. In a syngeneic model of metastatic cancer, the down-regulation of Crry on i.v.-injected MB49 cells was associated with a significant decrease in tumor burden and an increase in the survival of challenged mice. However, monoclonal antibody therapy had no additional benefit. There was an antitumor IgG response, but the response was not effected by Crry down-regulation on inoculated tumor cells. Down-regulation of Crry on MB49 cells resulted in an enhanced antitumor T-cell response in challenged mice (measured by lymphocyte IFN-γ; secretion), and CD8+ T cell depletion of mice prior to injection of MB49 cells completely abrogated the effect of Crry down-regulation on tumor burden and survival. Deficiency of C3 also abrogated the effect of Crry down-regulation on the survival of MB49-challenged mice, indicating a complement-dependent mechanism. These data indicate that complement inhibitors expressed on a tumor cell can suppress a T cell response and that enhancing complement activation on a tumor cell surface can promote protective T cell immunity.
Introduction
Normal cells and cancer cells are protected from complement attack by membrane-bound complement inhibitors. An important role for complement inhibitors in tumor immune evasion is indicated by the fact that their expression is up-regulated in various types of cancer (1-10). Human membrane inhibitors of complement are decay-accelerating factor (DAF; CD55), membrane cofactor protein (MCP; CD46), and CD59. DAF and MCP inhibit complement activation early in the pathway at the C3 level, whereas CD59 inhibits the formation of the terminal cytolytic membrane attack complex (C5b-9). Mice express an additional and widely distributed C3 inhibitor termed Crry.
As an immune effector mechanism, complement activation can lead to tumor cell opsonization with C3, the release of bioactive and proinflammatory complement activation products (C3a and C5a), and direct tumor cell lysis. C3 deposited on tumor cells can engage C3 receptors on immune cells and induce complement-dependent cell-mediated cytotoxicity and enhance antibody-dependent cell-mediated cell cytotoxicity. Thus, regulating the activity of complement inhibitors on target cells has the potential to enhance complement effector mechanisms, and there is strong evidence indicating that interfering with complement inhibitors expressed on tumor cells will enhance antitumor antibody therapy (reviewed in refs. 11-13). A number of approaches have been used to down-regulate or block the activity of complement inhibitors and increase complement activation on tumor cells, including the use of phospholipase C, various cytokines, neutralizing antibodies, bispecific antibodies, and small interfering RNAs (siRNA; refs. 14-22).
Almost all studies involving the regulation of complement inhibitor expression on tumor cells have been done in the context of enhancing immune effector function, and more specifically, enhancing antibody effector function. However, complement can also modulate the induction of an adaptive immune response. A link between complement and acquired humoral immunity has been known for some time, and it is now clear that complement plays an important role in modulating T cell responses (for reviews, see refs. 23-25). Recent studies have shown that DAF suppresses T-cell immunity against autoantigens and alloantigens and virus via its regulatory effect on complement activation, although the studies differed with respect to whether DAF expression on both T cells and antigen-presenting cells (APC) or T cells alone modulated T-cell proliferation (26-29). Other studies have shown that complement regulators can regulate T-cell responses independent of complement (25, 30). There is no data available on the role of complement inhibitors and complement in T cell immunity to tumors, although an earlier study showed that mice vaccinated with irradiated tumor cells pretreated with anti-Crry antibody were protected from subsequent challenge, and that survival correlated with increased IgM titers and high splenocyte cytotoxicity in two of three samples isolated from surviving mice (22).
The initial aim of the current study was to determine if the down-regulation of an inhibitor of complement activation (Crry) expressed on tumor cells would enhance the outcome of antibody-mediated immunotherapy. For these studies, monoclonal antibody (mAb) therapy was directed against the human cancer-associated antigen MUC1, and we used MUC1 transgenic mice inoculated with the syngeneic bladder cancer cell line MB49 stably expressing MUC1. We report that the down-regulation of Crry on inoculated tumor cells significantly enhanced the survival of mice receiving anti-MUC1 mAb therapy, but that the down-regulation of Crry in the absence of mAb therapy resulted in a similar survival benefit. We therefore investigated the role of tumor-expressed Crry and complement on humoral and cellular antitumor immunity in terms of both an immune response to MB49 cells and to the tumor-associated antigen MUC1. The use of MUC1 transgenic mice, which express the transgene in a pattern and level consistent with that seen in humans (31), allowed the study of immunogenic responses to a clinically relevant antigen within the context of autoimmunity and tolerance. We report that the down-regulation of a C3 inhibitor (Crry) on MB49 had no effect on antibody response, but conferred protective immunity against MB49 tumors that was dependent on complement and a cytotoxic T cell response.
Materials and Methods
Cell lines
The mouse bladder cancer cell line MB49 was provided by Dr. Timothy Ratliff (Department of Urology, University of Iowa, Iowa City, Iowa) and was grown at 37°C in 5%CO2 in RPMI 1640 with 10% heat-inactivated fetal bovine serum, 100 units/mL of penicillin, and 100 μg/mL of streptomycin.
siRNAs
A total of eight anti-mouse Crry siRNA were designed by and purchased from Qiagen as part of their “4-for-silencing” product line. The anti-Crry siRNA most effective at down-regulating Crry expression on MB49 cells in vitro (5-CCAGAGAAGACUUUCAUUAdTT-3′) was used in all subsequent experiments and for the preparation of stable transfectants (below).
Vectors and stable cell lines
Vectors expressing human MUC1 (phCMV1-MUC1) and/or anti-Crry siRNAs were transfected into MB49 cells. phCMV1-MUC1 was kindly provided by Dr. Sandra Gendler (Mayo Clinic, Scottsdale, AZ). The vector encoding the anti-Crry siRNAs was constructed using the psilencer-2.1-U6 hygro siRNA expression vector kit from Ambion following the manufacturer’s protocol. Each of the vectors were subsequently transfected into MB49 cells using LipofectAMINE reagent according to the manufacturer’s protocol (Invitrogen). Following selection, stable populations of cells expressing low levels of Crry (MB49/Crrylow) and a cell line expressing human MUC1 and low levels of Crry (MB49/MUC1+/Crrylow) were isolated by flow cytometry. Control cell populations for in vitro and in vivo experiments were prepared by transfecting MB49 cells with empty pHCMV1 vector and/or with scrambled anti-siRNA sequence (MB49/Crrynormal and MB49/MUC1+/Crrynormal cells). Stable populations of desired cells were selected by fluorescence-activated cell sorting as previously described (32).
Antibodies
BCP8 (33), an anti-MUC1 IgG2b antibody was kindly provided by Dr. I.F. McKenzie (Austin Research Institute, Heidelberg, Australia). Anti-mouse CD8 antibody (53.6.72) was obtained from Bio-Express Cell Culture Services. Anti-mouse Crry mAb 5D5 was provided by Dr. V.M. Holers (University of Colorado Health Science Center, Denver, CO), anti-mouse DAF mAb Riko-3 by Dr. H. Okada (Nagoya City University School of Medicine, Aichi, Japan), and the anti-mouse CD59 mAb 3B3 by Dr. B.P. Morgan (Cardiff University, Cardiff, United Kingdom). FITC-conjugated anti-mouse C3 was purchased from ICN Biomedicals, Inc., and all other FITC-conjugated antibodies for flow cytometry were purchased from Sigma.
Mice
MUC1 transgenic mice (MUC1Tg) were purchased from the Mayo Clinic or raised from an in-house colony at the Medical University of South Carolina (Charleston, SC). Wild-type C57BL/6 mice were obtained from the National Cancer Institute. C3-deficient mice were purchased from Jackson Laboratories. Male mice were used for experiments, but females were included in some groups of MUC1Tg mice (there was no difference in measurable outcomes between males and females). Mice were housed in a clean room and food and water was sterilized. All animal procedures conformed to the rules and regulations provided by the Institutional Animal Care and Use Committee.
In vitro assays
Analysis of membrane complement inhibitor expression was performed by flow cytometry as described (32). For in vitro analysis of C3 deposition on MB49 cells transfected with human MUC1 and/or anti-Crry siRNA, 5 × 105 cells were resuspended in 50 μL of PBS with or without BCP8at 20 μg/mL and incubated for 30 min at 4°C. After washing, cells were resuspended in 50 μL of 30% mouse serum diluted in gelatin veronal-buffered saline (Sigma) and incubated for 30 min at 37°C. Cells were then washed with gelatin veronal-buffered saline containing 10 mmol/L of EDTA, incubated with FITC-conjugated goat anti-mouse C3 (30 min/4°C), and washed twice. Finally, cells were suspended in PBS containing propidium iodide (10 μg/mL) and analyzed by flow cytometry. Crry expression was analyzed in metastatic lung nodules and analyzed on isolated tumor cells by flow cytometry as previously described (34). Complement-mediated cytotoxicity was determined by propidium iodide incorporation using flow cytometry as previously described (35).
Metastatic bladder cancer model and anti-MUC1 antibody treatment
Mice were inoculated with tumor cells (5 × 105) suspended in 0.1 mL of PBS by tail vein injection. Some groups were given i.v. injections of 100 μg of BCP8 on days 1 and 3 following tumor cell injection. For survival studies, mice were followed until the time of death, signs of suffering or until weight loss was determined to be >15% of their initial body weight. Mice were examined postmortem for the presence of metastatic lesions in the lungs. In alternative studies, all mice were sacrificed at day 17 following tumor cell injection and necropsies were done to examine the number of lung metastases, lung weight and antitumor antibody, and T-cell immune responses. In addition, lung sections were cut for H&E staining.
Analysis of antitumor antibody responses
The serum of treated mice was analyzed for the presence of anti-MUC1 and anti-MB49 IgM and IgG antibodies using a flow cytometry-based method. Serum was collected from mice prior to tumor cell injection (day 0) and at the time of sacrifice (day 17). Tumor cells were incubated with diluted (1:3) serum samples. To detect MUC1-specific antibody responses, MB49/MUC1+ target cells were used with vector-transfected MB49 as control, and to detect MB49-specifc responses, MB49/vector cells were used. After washing, cells were resuspended in 50 μL of anti-mouse IgM or IgG FITC-labeled antibody (final concentration, 1:100). Cells were then washed, resuspended in a PBS/isoelectric point solution and analyzed in a flow cytometer.
Analysis of antitumor T cell responses
Mice were sacrificed at day 17 following tumor cell injection and their spleens removed. Splenocytes were isolated and resuspended in complete medium (RPMI 1640 with 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin) at a concentration of 1 × 106/mL. Tumor cells used as targets for the assays were irradiated (30 Gy), washed thrice with PBS, and resuspended in complete medium at a concentration of 1 × 106/mL. Subsequently, splenocytes and tumor cells were incubated at a ratio of 1:1 (104 cells) in 96-well plates at 37°C. Forty-eight hours later, supernatants were collected and analyzed for the presence of IFN-γ via ELISA according to the manufacturer’s protocol (BD Biosciences). As a confirmatory procedure, ELISPOT assays for IFN-γ were also carried out using T cells purified from MUC1Tg mice. CD3+ cells were isolated by cell sorting using a BD Biosciences FACSaria system in which isolated splenocytes were labeled with a PE-conjugated anti-CD3 antibody and subsequently selected by cell sorting. The purity of the isolated CD3+ cells was consistently >98%. T cells and tumor cells were incubated in the wells of ELISPOT plates at an effector/target (E:T) ratio of 20:1. Forty-eight hours later, cells and supernatants were removed, and the ELISPOT membranes stained for IFN-γ-secreting cells.
In vivo depletion of CD8+ cells
To achieve depletion of CD8+ cells in vivo, mice were injected on 3 consecutive days and every 3rd day thereafter with 200 μg of anti-mouse CD8 antibody (53.7.62). Tumor cells were injected on day 6 after the first antibody injection and mice were followed until the time of death or until weight loss of >15% of their original body weight was observed. Depletion was verified by anti-CD8 flow cytometric analysis of isolated splenocytes and circulating lymphocytes.
Histology
Lungs were harvested at the time of death and fixed in 10% formalin. Fixed tissues were processed, embedded in paraffin, sectioned at 5 μm, and stained with H&E. Lung sections were analyzed for the presence of metastatic lesions. Paraffin-embedded sections of lung tissue were also stained for C3d (Dako Cytomation). Primary antibody binding was visualized by streptavidin biotin complex detection system and visualized by 3′3-diaminobenzidine substrate (Sigma). The specificity of immunostaining was shown by the absence of signal in sections incubated with isotype control antibody and by omission of primary antibody. Sections were counterstained with Carazzi hematoxylin and examined by light microscopy.
Statistical analysis
Student’s t tests were used to determine statistical differences. The log-rank test was applied to determine differences in survival curves. Significance was accepted at the P < 0.05 level.
Results
In vitro characterization of cell lines
Initial studies using flow cytometry determined that the MB49 mouse bladder cancer cell line did not express DAF (CD55), MCP (CD46), or CD59, but expressed high levels of Crry (data not shown). A total of eight siRNA’s were tested for their efficacy at down-regulating the surface expression of Crry on MB49 cells. Of the eight siRNA’s tested, one construct (see Materials and Methods) down-regulated the expression of Crry by >90% and was used for the preparation of the stable transfectants described below. An initial aim of the current investigation was to determine the effect of complement inhibitor down-regulation on the outcome of mAb therapy in the context of autoimmunity and tolerance, and four MB49 cell lines were prepared for study in syngeneic wild-type and MUC1 transgenic mice: (a) wild-type control-transfected (MB49/Crrynormal); (b) MUC1-transfected (MB49/MUC1+/Crrynormal); (c) wild-type anti-Crry siRNA-transfected (MB49/Crrylow); (d) MUC1-transfected, anti-Crry siRNA-transfected (MB49/MUC1+/Crrylow; refer to Fig. 1A).
Figure 1.
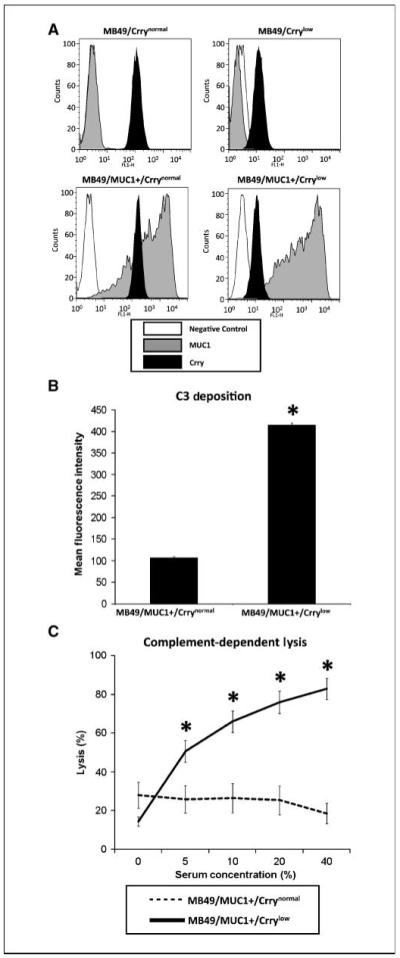
In vitro analysis of MB49 cell lines. A, stable cell lines expressing human MUC1 and/or anti-Crry siRNA. MB49 cells were transfected with a vector expressing human MUC1 and/or a vector expressing an anti-Crry siRNA. Stable populations were selected by multiple rounds of fluorescence-activated cell sorting. Representative analysis of sorted populations by anti-MUC1 and anti-Crry flow cytometry. B, effect of siRNA-mediated down-regulation of Crry on the susceptibility of MUC1-positive MB49 cells to C3 deposition. MB49/MUC1+ cells were sensitized with anti-MUC1 mAb BCP8 and incubated in 30% MUC1Tg mouse serum (30 min/37°C). Cells were washed and C3 deposition analyzed by anti-C3 flow cytometry. Cells expressing anti-Crry siRNA had significantly higher amounts of deposited C3 (*, P < 0.00001). C, effect of siRNA-mediated down-regulation of Crry on susceptibility of MUC1-positive MB49 cells to complement-dependent lysis. MB49/MUC1+ cells were sensitized with anti-MUC1 mAb BCP8 and incubated in various concentrations of rat serum (1 h/37°C). Lysis was determined by flow cytometric analysis of propidium iodide incorporation. Crrylow cells were significantly more susceptible to lysis than Crrynormal cells (P < 0.001 for 10-40% serum; n = 6 for all experiments).
The effect of Crry down-regulation on the complement-regulatory activity of MB49 cells was investigated using the stable MUC1 transfectants and anti-MUC1 mAb BCP8to activate complement. Following incubation of cells with BCP8and mouse serum, there was a significantly increased amount of C3 deposited on MB49/MUC1+/Crrylow compared with MB49/MUC1+/Crrynormal (∼75% increase, P < 0.001; Fig. 1B). In addition, MB49/MUC1+/Crrylow were significantly more sensitive to rat complement-mediated lysis than MB49/MUC1+/Crrynormal in the presence of BCP8 (Fig. 1C). Thus, Crry down-regulation increases the susceptibility of MB49 cells to complement. Mouse Crry is active against rat complement and rat serum was used in lysis assays because mouse serum is poorly lytic in vitro. C3 deposition and lysis of MB49/Crrynormal and MB49/Crrylow in vitro was not determined due to the unavailability of a complement-activating antibody. The down-regulation of Crry on MB49 cells had no significant effect on in vitro activation of the alternative pathway of complement; in a separate experiment, when the above C3 deposition assay was performed using either C4-deficient (no classical pathway) of fB-deficient (no alternative pathway) serum, C3 deposition levels on Crrylow cells obtained with C4-deficient serum were <10% of levels obtained with fB-deficient serum (data not shown).
Effect of Crry down-regulation on mAb therapy
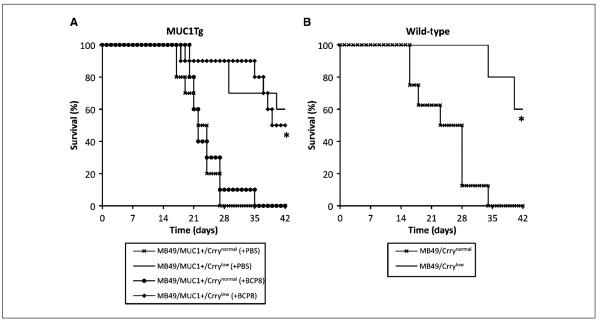
To determine the effect of Crry down-regulation on the outcome of anti-MUC1 mAb therapy in a metastatic setting, MUC1Tg mice were inoculated i.v. with either MB49/MUC1+/Crrynormal or MB49/MUC1+/Crrylow and treated with mAb BCP8(100 μg, 1 and 3 days after tumor challenge). Anti-MUC1 mAb therapy had no effect on the survival of MUC1Tg mice inoculated with Crrynormal cells, with mice receiving either PBS or BCP8 therapy having similar mean survival times (22 ± 3.7 and 23 ± 4.6 days, respectively; Fig. 2A). In contrast, BCP8 therapy significantly increased the survival of MUC1Tg mice that were inoculated with Crrylow cells, with these mice surviving an average of 38 ± 7.3 days and with 50% long-term survival (60 days; P < 0.001). Surprisingly, however, there was no difference in survival between BCP8and PBS-treated MUC1Tg mice that were inoculated with Crrylow cells (Fig. 2A). These data indicate that the down-regulated expression of Crry on tumor cells was responsible for the prolonged survival of mice inoculated with MB49 independent of mAb therapy. We therefore inoculated wild-type mice with MB49/Crrynormal and MB49/Crrylow to determine the effect of Crry down-regulation independent of MUC1 antigen expression and mAb therapy. Similar to the MUC1Tg mice, wild-type mice inoculated with Crrylow cells survived significantly longer than wild-type mice inoculated with Crrynormal cells (P < 0.01; Fig. 2B); the mean survival time of wild-type mice inoculated with Crrynormal was 23.8 ± 6.6 days with no long-term survival, where as 60% of wild-type mice inoculated with Crrylow survived long-term with the remaining mice having a mean survival of time of 37 ± 4.2 days. We did not complete studies involving antibody therapy of wild-type mice inoculated with MUC1-transfected tumor cells because a protective anti-MUC1 immune response is elicited in wild-type mice (31, 36).
Figure 2.
Effect of Crry down-regulation and antibody therapy on survival of mice challenged with tumor cells. Groups of MUC1Tg mice (A) or wild-type mice (B) were inoculated i.v. with Crrynormal or Crrylow MB49 cells as indicated. In the MUC1Tg model, groups inoculated with Crrynormal and Crrylow also received anti-MUC1 mAb BCP8 therapy (100 μg antibody on days 1 and 3 after challenge). A, MUC1Tg mice challenged with Crrylow cells survived significantly longer than MUC1Tg mice inoculated with Crryhigh cells (*, P < 0.001), independent of whether they received anti-MUC1 mAb therapy. B, wild-type mice challenged with Crrylow cells survived significantly longer than wild-type mice challenged with Crrynormal cells (*, P < 0.01; n = 5-10 per group).
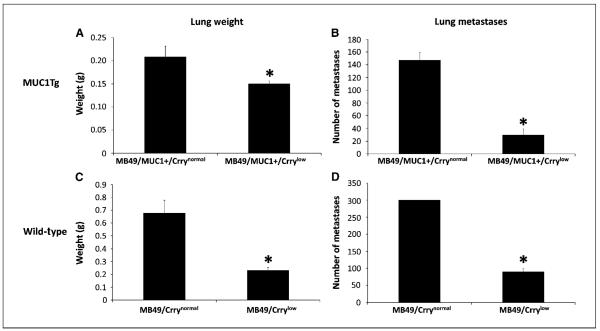
Following i.v. injection of MB49 cells, mice developed lung metastases, and the effect of Crry down-regulation on metastatatic tumor burden was investigated. MUC1Tg mice were challenged with either MB49/MUC1+/Crrynormal or MB49/MUC1+/Crrylow, and wild-type mice challenged with either MB49/Crrynormal or MB49/Crrylow. Seventeen days after challenge, lungs from MUC1Tg and wild-type mice injected with Crrylow cells had significantly fewer metastases and a significantly lower mass than lungs from mice inoculated with Crrynormal cells (Fig. 3). Macroscopically, lungs from mice (both MUC1Tg and wild-type) challenged with Crrynormal cells had a high number of large metastatic surface tumors with little normal lung tissue visible. Microscopically, these lungs had large multifocal tumors located throughout the lung parenchymal tissue with areas of vascular infiltration also noted (Fig. 4). In contrast, macroscopic examination of lungs from mice challenged with Crrylow revealed few, if any, visible tumor deposits (Fig. 4). Tumors were also examined for C3 deposition. Analysis of lung nodules by anti-C3d immunohistochemistry showed C3 deposition on tumors in mice inoculated with either MB49/Crrylow or MB49/Crrynormal cells. It was not possible to discern any significant difference in staining intensity. Of note, however, there were very few metastatic nodules found in any of the mice inoculated with MB49/Crrylow, and flow cytometric analysis of isolated tumor cells showed that the cells had up-regulated their expression of Crry (data not shown).
Figure 3.
Down-regulation of Crry on MB49 cells reduces their metastatic potential. MUC1Tg or wild-type mice were inoculated with either Crrynormal or Crrylow cells. At day 17, lungs were harvested and metastasis analyzed by liver mass and number of metastatic nodules. Lungs from both MUC1Tg mice (A and B) and wild-type mice (C and D) injected with Crrylow cells weighed significantly less and had significantly fewer metastatic nodules than lungs from mice inoculated with Crrynormal cells. Note that the number of metastatic nodules in lungs of mice inoculated with MB49/Crrynormal cells was >300 (D), but because of the high number of nodules, a more accurate determination was not possible (liver mass, P < 0.05 for MUC1Tg and P < 0.05 for wild-type; number of metastatic nodules, P < 0.001 for MUC1Tg and P < 0.001 for wild-type). Columns, mean; bars, SE (n = 7).
Figure 4.
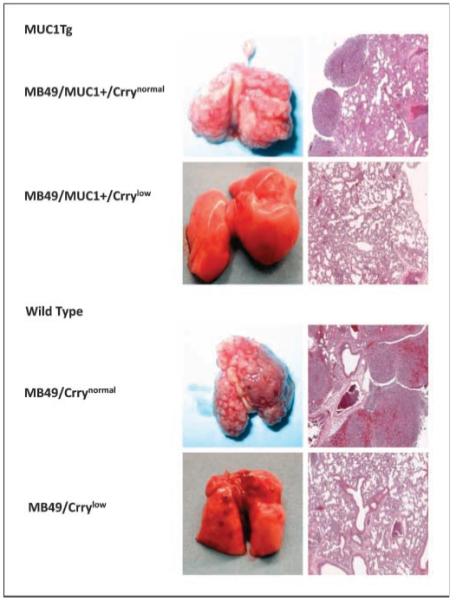
Examination of lungs from mice challenged with Crrynormal and Crrylow MB49 cells. MUC1Tg and wild-type mice were inoculated with either Crrynormal or Crrylow cells and lungs harvested at day 17. Macroscopic appearance of lungs and H&E-stained lung sections. Mice injected with Crrylow cells had a marked reduction in tumor burden compared with mice injected with Crryhigh cells.
Effect of Crry down-regulation on antitumor and anti-MUC1 antibody response
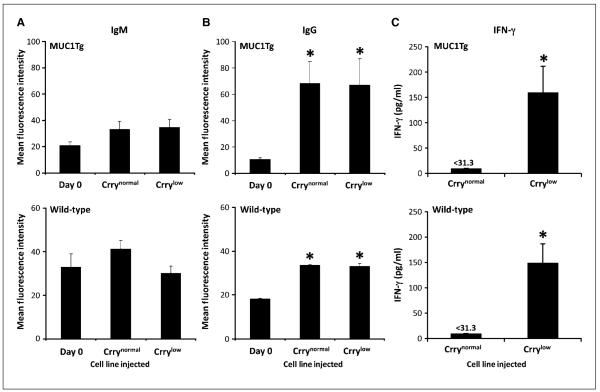
Complement plays an important role in modulating humoral immunity, and C3 opsonization of an antigen can influence the induction and enhancement of an antibody response (23, 24). We therefore investigated whether the decreased tumor burden and increased survival that was associated with Crry down-regulation on MB49 might be due to the induction/enhancement of an antitumor antibody response resulting from the expected enhancement of C3 deposition on the tumor cells/MUC1. As in previous experiments, MUC1Tg mice were challenged with either MB49/MUC1+/Crrynormal or MB49/MUC1+/Crrylow, and wild-type mice with MB49/Crrynormal or MB49/Crrylow. Serum was prepared from blood collected before tumor challenge (day 0) and at day 17, and analyzed for relative levels of anti-MUC1 antibodies and anti-MB49 antibodies in MUC1Tg and wild-type mice, respectively. As has been reported previously (31), MUC1-reactive IgM was present in the serum of naïve MUC1Tg mice. However, there was no significant increase in the relative levels of anti-MUC1 IgM in MUC1Tg mice challenged with either Crrynormal or Crrylow MB49/MUC1+ cells (Fig. 5A). Similarly, there were MB49-reactive IgMs in serum from naïve wild-type mice, and there was no increase in relative IgM titers following challenge with either Crrynormal or Crrylow MB49 cells (Fig. 5A). Analysis of IgG, on the other hand, revealed a significant increase in both anti-MUC1 and anti-MB49 titers in challenged mice. However, for both MUC1Tg and wild-type mice, there was no difference between relative IgG titers in mice challenged with either Crrynormal or Crrylow cells (Fig. 5B). Thus, although an IgG response to MB49 as well as MUC1 was elicited in wild-type and MUC1Tg mice, respectively, it was not affected by decreased Crry expression and, by inference, increased C3 deposition on tumor cells.
Figure 5.
Analysis of humoral and cellular immune response following challenge with MB49 cells. Serum from MUC1Tg mice (challenged with MB49/MUC1+ cells) was analyzed for relative levels of anti-MUC1 IgM (A, top) and IgG (B, top), and serum from wild-type mice (challenged with MB49 cells) was analyzed for relative levels of anti-MB49 IgM (A, bottom) and IgG (B, bottom). Analysis was performed by flow cytometric assay using MB49/MUC1+ or MB49 cells as targets. Seventeen days after tumor challenge, there were no increases in anti-MUC1 or MB49 IgM titers (A). Relative IgG titers, however, were significantly higher in mice challenged with both Crrynormal and Crrylow cells (B;*, P < 0.05). Columns, mean; bars, SE (n = 7). C, down-regulation of Crry on inoculated MB49 cells leads to the induction of an antitumor T-cell response. MUC1Tg mice were inoculated with MB49/MUC1+/Crrynormal or MB49/MUC1+/Crrylow, and wild-type mice inoculated with MB49/Crrynormal or MB49/Crrylow. Splenocytes were isolated 17 days after inoculation and stimulated in vitro with MB49/MUC1+ cells (MUC1Tg splenocytes) or MB49 cells (wild-type splenocytes). IFN-γ levels in culture supernatant were determined after 48 h by ELISA. Columns, mean; bars, SE (n = 7); assays were performed in triplicate (*, P < 0.05).
Effect of Crry down-regulation on antitumor T-cell response
Complement is known to play a role in the induction and effector phases of a T cell response and recent studies have shown that the complement regulator DAF, which like Crry functions at the C3 activation stage, can suppress T cell immunity in a complement-dependent manner (27, 28). These previous studies differed with respect to whether DAF expression on both T cells and APCs or T cells alone modulated T cell proliferation. We investigated whether the down-regulation of Crry on a tumor cell influenced the induction of an antitumor T cell response.
MUC1Tg and wild-type mice were inoculated with either Crrynormal or Crrylow tumor cells, and on day 17, the mice were sacrificed and their splenocytes isolated. Following in vitro stimulation, the amount of IFN-γ released by the splenocytes was quantified. Splenocytes from MUC1Tg and wild-type mice challenged with Crrylow tumor cells secreted significantly higher amounts of IFN-γ compared with splenocytes isolated from mice challenged with Crrynormal tumor cells (P < 0.01; Fig. 5C). Similar relative levels of IFN-γ secretion were measured when splenocytes were incubated with MUC1-negative MB49, indicating that the response was not MUC1-specific. These results provide evidence that expression of Crry on tumor cells suppresses antitumor T cell immunity in vivo and that the down-regulation of Crry leads to the induction of an antitumor T cell response in this model of metastatic cancer. T-cell response in MUC1Tg mice was also assayed by IFN-γ ELISPOT assay using purified T cells, and a similar result was obtained. The average number of IFN-γ-secreting T cells per well was significantly higher in mice inoculated with MB49/MUC1+/Crrylow cells compared with T cells from mice injected with MB49/MUC1+/Crrynormal cells (data not shown). Note that Crrynormal cells used for inoculation were control-transfected with scrambled anti-Crry siRNA to rule out the nonspecific effect of plasmid transfection on immune response. There was no significant difference in IFN-γ release from splenocytes isolated from MUC1Tg or wild-type mice in assays using either Crrylow or Crrynormal cells as target cells (data not shown).
Survival of mice challenged with Crrylow tumor cells was dependent on CD8+ T cells
An antitumor IgG response was elicited in MB49 challenged mice, and although this response was independent of Crry expression, the Crrylow tumor cells may be more susceptible to IgG-mediated destruction due to their enhanced susceptibility to complement. Therefore, to determine if the antitumor T-cell response, specifically a cytotoxic T-cell response, occurring in mice inoculated with Crrylow cells was responsible for the protection of challenged mice, we investigated the effect of CD8+ T-cell depletion on the survival of mice inoculated with Crrylow tumor cells.
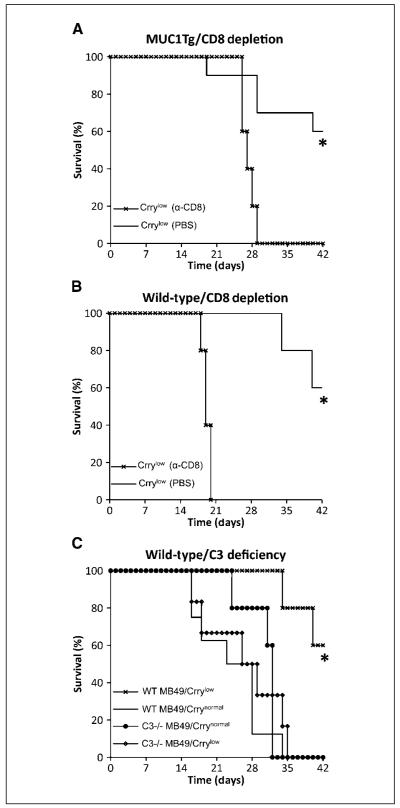
MUC1Tg and wild-type mice were depleted of CD8+ T-cells by treatment with anti-CD8 mAb as previously described (37-39), and depletion from the circulation and spleen verified by flow cytometric analysis (see Materials and Methods; data not shown). Normal and CD8+ T-cell-depleted MUC1Tg and wild-type mice were challenged with MB49/MUC1+/Crrylow and MB49/Crrylow, respectively. Challenged CD8+ T-cell-depleted MUC1Tg mice had a significantly shorter survival (27.2 ± 1.3 days with no long-term survival) than normal MUC1Tg mice (37 ± 8.3 days with 60% long-term survival; P < 0.01; Fig. 6A). Similar results were obtained with wild-type mice (CD8+ T-cell-depleted, 19.2 ± 0.83 days with no long-term survival versus normal, 37 ± 4.2 days with 60% long-term survival; P < 0.01; Fig. 6B). For both MUC1Tg and wild-type mice, there was no difference between the survival of CD8+ T-cell-depleted mice inoculated with Crrylow cells and undepleted mice inoculated with Crrynormal cells, indicating a primary role for CD8+ T-cells in the protective antitumor response resulting from Crry down-regulation.
Figure 6.
Effect of CD8+ T cell depletion and C3 deficiency on survival of challenged mice. Untreated and CD8+ T cell-depleted MUC1Tg mice (A) and wild-type mice (B) were challenged with MB49/MUC1+/Crrylow and MB49/Crrylow, respectively. Depletion of CD8+ T cells led to a significant decrease in the survival of challenged MUC1Tg and wild-type mice (*, P < 0.01), indicating that increased survival of mice associated with Crry down-regulation on inoculated MB49 cells is dependent on CD8+ T cells (n = 5-10 per group). C, increased survival of mice associated with Crry down-regulation on inoculated MB49 cells is dependent on complement. C3-deficient and wild-type mice were challenged with either MB49/Crrynormal or MB49/Crrylow cells. The survival of C3-deficient mice challenged with Crrylow cells was significantly shorter than that of wild-type mice injected with Crrylow cells (*, P < 0.01, n = 5-6 per group). Data from one of two experiments, each with similar results.
Survival of mice challenged with Crrylow tumor cells was dependent on complement
Previous studies have indicated that complement inhibitors can influence T-cell responses via both complement-dependent and complement-independent mechanisms. In addition, a previous study in a rat model of cancer showed that expression of Crry on tumor cells inhibited natural killer cell-mediated cytotoxicity by an apparently complement-independent mechanism (although Crry did not have a similar effect in a mouse system; ref. 40). The above in vitro data show that complement deposition on MB49 cells is significantly enhanced if Crry is down-regulated, and we therefore investigated whether antitumor T-cell immunity induced as a result of Crry down-regulation on MB49 cells is complement-dependent.
C3-deficient (C3-/-) mice were inoculated with either MB49/Crrynormal or MB49/Crrylow and their survival compared with inoculated wild-type mice. There was no significant difference in the survival of C3-/- mice inoculated with either Crrynormal or Crrylow cells, and the survival of the C3-/- mice challenged with Crrylow cells was significantly shorter than that of wild-type mice injected with the same cell line (26.3 ± 7.9 days with no long-term survival versus 37.2 ± 4.2 days with 60% long-term survival; P = 0.009; Fig. 6C). Thus, C3 deficiency abrogated the effect of Crry down-regulation on the survival of MB49 challenged mice. These data indicate that the induction of an antitumor T-cell response resulting from the down-regulation of Crry is largely complement-dependent and indicate that enhancing complement activation by a tumor cell can elicit protective T-cell immunity. On a technical note, we were unable to obtain any C57BL/6 MUC1Tg/C3-/- offspring following the crossing of C57BL/6 MUC1Tg mice with C57BL/6 C3-/- mice.
Discussion
Interfering with the function of complement inhibitors increases the susceptibility of tumor cells to antibody and complement-mediated immune mechanisms, both in vitro and in vivo (reviewed in refs. 11-13, 17, 41), and complement inhibitor expression is up-regulated in many types of cancer (1-10). These findings suggest a role for complement inhibitors in tumor immune evasion and resistance to humoral immunity. In particular, complement inhibitors expressed on tumor cells are considered to hinder the effectiveness of mAb therapy. In this study, we show that complement inhibitor expression on a tumor cell can also modulate protective T cell immunity.
The initial aim of this study was to investigate the role of membrane-expressed complement inhibitors in the resistance of tumor cells to mAb therapy, and we developed a syngeneic model based on anti-MUC1 mAb therapy in MUC1Tg mice to study therapy against a relevant human tumor antigen in the context of autoimmunity and tolerance. However, in our model, down-regulation of the complement inhibitor Crry on MUC1+ MB49 cells prior to their inoculation was protective for mice whether or not they received anti-MUC1 mAb therapy. A potential explanation for this finding is that Crry down-regulation on MB49 cells resulted in the induction/enhancement of an effective antitumor antibody response due to increased complement activation and C3 deposition on MB49/Crrylow cells relative to MB49/Crrynormal cells. In this regard, it is known that complement activation products and complement opsonization of antigen can significantly enhance the antibody response (23, 24). However, although there was an IgG response to both MUC1 and MB49 cells, it was unaffected by the down-regulation of Crry on the tumor cells. Thus, although anti-MUC1 and anti-MB49 IgM was present in naïve mouse serum, and the down-regulation of Crry on MB49 cells would, in principle, result in increased C3 deposition on the tumor cells, the level of Crry expression did not influence antibody response. Another possible reason for the protective effect of Crry down-regulation is an enhanced susceptibility of the tumor cells to antibody effector function, i.e., C3 opsonization and complement-dependent lysis. However, the down-regulation of Crry on MB49 cells resulted in an enhanced antitumor T cell response, and CD8+ T cell depletion completely abrogated the increased survival seen in mice challenged with Crrylow versus Crrynormal cells. These data indicate that a cytotoxic T cell response induced as a result of Crry down-regulation on the tumor cells was primarily responsible for providing protective immunity.
Previous studies have shown that complement inhibitors expressed on APCs and/or T cells can modulate T cell immunity, and both complement-dependent and complement-independent mechanisms have been described (for review, see refs. 25, 30). Studies on T cell immunity in DAF (CD55)-deficient mice have shown that DAF can regulate T cell immunity against model antigens, autoantigens and alloantigens, and during a natural immune response to viral infection (26-29). These previous studies also showed that the effect of DAF in modulating T cell immunity was dependent on an active complement system, although there were discrepancies on the relative role of APC-specific versus T cell-specific expression of DAF. The current data relate to the expression of a complement inhibitor on a tumor cell, and because the survival benefit of MB49 Crry down-regulation was abrogated by C3 deficiency, the data indicate that Crry modulates T-cell immunity via a largely complement-dependent mechanism.
Although there may be some differences depending on the immune model, recent investigations into the mechanism of complement-dependent T cell differentiation indicate an important role for C5aR signaling (26, 27). Furthermore, data indicate that locally generated C5a produced following APC and T-cell interaction can play an important role in T cell differentiation (via APC IL-12 production; ref. 26). In these previous studies, T cell responses were studied in the context of DAF expression on APCs and T cells. In the current study, if C5a is playing an important role in the development of antitumor T cell immunity via its interaction with C5aR on APCs and/or T cells, it is presumably doing so as a result of increased complement activation at the tumor cell surface. In the case of APCs or T cells, the absence or down-regulation of a complement regulator is thought to permit complement activation. The mechanism of complement activation is not clear, although the alternative pathway, which can activate spontaneously on surfaces lacking complement inhibitors as well as function as an amplification loop, has been implicated in the T cell response (27). In the current tumor model, we have shown the presence of anti-MUC1 and anti-MB49 IgM in naïve mice, and these antibodies may be responsible for activating complement on the tumor cells. Anticancer antibodies are frequently found in patients with cancer, and in particular, anti-MUC1 IgM is found in patients with MUC1-positive cancer (42-46).
An anti-MUC1/MB49 IgG response was also induced in our model, but is unlikely to have contributed to the induction of the complement-dependent T cell response that was measured 17 days after tumor cell inoculation. Nevertheless, IgG can enhance T-cell priming by providing antigen access to exogenous and cross-presentation processing pathways via activating FcgRs on APCs (47, 48), and it is possible that antitumor antibodies, either induced or passively administered for therapy, may positively modulate T cell immunity. In view of the current data, it is also possible that antitumor IgG may enhance T cell immunity via its complement-activating properties, and strategies currently under investigation to enhance complement-dependent antitumor effector function of antibodies (11-13) may also promote a T-cell immune response.
Increased complement opsonization of tumor cells as a result of Crry down-regulation could also potentially promote T cell activation via the engagement of C3 receptors on APCs. The engagement of APC C3 receptors by complement-opsonized cells and antigens can modulate T cell responses by influencing APC cytokine expression profiles and by enhancing the uptake of complement opsonized material for antigen presentation (reviewed in refs. 25, 30). The complement regulators MCP (CD46) and CD59 have also been shown to influence T-cell responses. The cross-linking of TCR (CD3) and MCP, by either antibody or C3b ligand, can induce a T-regulatory cell phenotype. In addition, the cross-linking of Crry on murine T cells can promote T-cell activation and a Th2 response (25). Accordingly, increased C3 deposition on tumor cells resulting from Crry down-regulation could potentially lead to the induction of T-regulatory cells and suppression of a T-cell response, although such a model is not consistent with the current data. Deficiency of CD59, an inhibitor of the terminal cytolytic membrane attack complex, has also been shown to enhance T-cell activity in mice, although independent of complement activation (30, 49).
In summary, we have shown that complement inhibitor expression on a tumor cell can modulate protective T cell immunity via a largely complement-dependent mechanism. This finding may have significant therapeutic implications, and strategies under investigation to enhance antitumor mAb effector function by increasing complement activation on tumor cells may also promote an antitumor T cell response.
Acknowledgments
Grant support: NIH grants R21 CA 104579 and CA1132694.
We thank Dr. Sandra Gendler for providing the MUC1 expression vector and for her assistance in obtaining MUC1Tg mice; Drs. Azizul Haque, Mohamed Salem, and Michael Nishimura for their critical discussions and comments concerning the work and manuscript; and Dr. Yuxiang Huang for helping with flow cytometry and C3 deposition assays.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Li L, Spendlove I, Morgan J, Durrant LG. CD55 is over-expressed in the tumour environment. Br J Cancer. 2001;84:80–6. doi: 10.1054/bjoc.2000.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hara T, Kojima A, Fukuda H, et al. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br J Haematol. 1992;82:368–73. doi: 10.1111/j.1365-2141.1992.tb06431.x. [DOI] [PubMed] [Google Scholar]
- 3.Kiso T, Mizuno M, Nasu J, et al. Enhanced expression of decay-accelerating factor and CD59/homologous restriction factor 20 in intestinal metaplasia, gastric adenomas and intestinal-type gastric carcinomas but not in diffuse-type carcinomas. Histopathology. 2002;40:339–47. doi: 10.1046/j.1365-2559.2002.01350.x. [DOI] [PubMed] [Google Scholar]
- 4.Loberg RD, Wojno KJ, Day LL, Pienta KJ. Analysis of membrane-bound complement regulatory proteins in prostate cancer. Urology. 2005;66:1321–6. doi: 10.1016/j.urology.2005.06.094. [DOI] [PubMed] [Google Scholar]
- 5.Murray KP, Mathure S, Kaul R, et al. Expression of complement regulatory proteins—CD 35, CD 46, CD 55, and CD 59—in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76:176–82. doi: 10.1006/gyno.1999.5614. [DOI] [PubMed] [Google Scholar]
- 6.Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement-inhibitory proteins CD46 (membrane co-factor protein), CD55 (decay accelerating factor), and CD59 (protectin) Am J Pathol. 1996;149:129–42. [PMC free article] [PubMed] [Google Scholar]
- 7.Rushmere NK, Knowlden JM, Gee JM, et al. Analysis of the level of mRNA expression of the membrane regulators of complement, CD59, CD55 and CD46, in breast cancer. Int J Cancer. 2004;108:930–6. doi: 10.1002/ijc.11606. [DOI] [PubMed] [Google Scholar]
- 8.Thorsteinsson L, O’Dowd GM, Harrington PM, Johnson PM. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS. 1998;106:869–78. doi: 10.1111/j.1699-0463.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 9.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Ravindranath NM, Shuler C. Expression of complement restriction factors (CD46, CD55 & CD59) in head and neck squamous cell carcinomas. J Oral Pathol Med. 2006;35:560–7. doi: 10.1111/j.1600-0714.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 11.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–23. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 12.Gelderman KA, Tomlinson S, Ross GD, Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–64. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999;20:576–82. doi: 10.1016/s0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 14.Ajona D, Hsu YF, Corrales L, Montuenga LM, Pio R. Down-regulation of human complement factor H sensitizes non-small cell lung cancer cells to complement attack and reduces in vivo tumor growth. J Immunol. 2007;178:5991–8. doi: 10.4049/jimmunol.178.9.5991. [DOI] [PubMed] [Google Scholar]
- 15.Blok VT, Gelderman KA, Tijsma OH, Daha MR, Gorter A. Cytokines affect resistance of human renal tumour cells to complement-mediated injury. Scand J Immunol. 2003;57:591–9. doi: 10.1046/j.1365-3083.2003.01265.x. [DOI] [PubMed] [Google Scholar]
- 16.Brasoveanu LI, Altomonte M, Fonsatti E, et al. Levels of cell membrane CD59 regulate the extent of complement-mediated lysis of human melanoma cells. Lab Invest. 1996;74:33–42. [PubMed] [Google Scholar]
- 17.Macor P, Tedesco F. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007;111:6–13. doi: 10.1016/j.imlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt CA, Schwaeble W, Wittig BM, Meyer zum Buschenfelde KH, Dippold WG. Expression and regulation by interferon-γ of the membrane-bound complement regulators CD46 (MCP), CD55 (DAF) and CD59 in gastrointestinal tumours. Eur J Cancer. 1999;35:117–24. doi: 10.1016/s0959-8049(98)00290-1. [DOI] [PubMed] [Google Scholar]
- 19.Gelderman KA, Kuppen PJ, Bruin W, Fleuren GJ, Gorter A. Enhancement of the complement activating capacity of 17-1A mAb to overcome the effect of membrane-bound complement regulatory proteins on colorectal carcinoma. Eur J Immunol. 2002;32:128–35. doi: 10.1002/1521-4141(200201)32:1<128::AID-IMMU128>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Gelderman KA, Kuppen PJ, Okada N, Fleuren GJ, Gorter A. Tumor-specific inhibition of membrane-bound complement regulatory protein Crry with bispecific monoclonal antibodies prevents tumor out-growth in a rat colorectal cancer lung metastases model. Cancer Res. 2004;64:4366–72. doi: 10.1158/0008-5472.CAN-03-2131. [DOI] [PubMed] [Google Scholar]
- 21.Imai M, Ohta R, Okada N, Tomlinson S. Inhibition of a complement regulator in vivo enhances antibody therapy in a model of mammary adenocarcinoma. Int J Cancer. 2004;110:875–81. doi: 10.1002/ijc.20178. [DOI] [PubMed] [Google Scholar]
- 22.Ohta R, Kondor N, Dohi N, et al. Mouse complement receptor-related gene y/p65-neutralized tumor vaccine induces antitumor activity in vivo. J Immunol. 2004;173:205–13. doi: 10.4049/jimmunol.173.1.205. [DOI] [PubMed] [Google Scholar]
- 23.Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004;41:141–6. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 25.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 26.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-γ-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heeger PS, Lalli PN, Lin F, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Miwa T, Hilliard B, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–77. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang C, Miwa T, Shen H, Song WC. Complement-dependent enhancement of CD8+ T cell immunity to lymphocytic choriomeningitis virus infection in decay-accelerating factor-deficient mice. J Immunol. 2007;179:3178–86. doi: 10.4049/jimmunol.179.5.3178. [DOI] [PubMed] [Google Scholar]
- 30.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check-a new role for complement regulators? Trends Immunol. 2006;27:102–8. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 32.Yu J, Abagyan RA, Dong S, Gilbert A, Nussenzweig V, Tomlinson S. Mapping the active site of CD59. J Exp Med. 1997;185:745–53. doi: 10.1084/jem.185.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rye PD, Price MR. Tumor biology; International workshop on monoclonal antibodies against MUC-1; Basel: Karger. 1998. [Google Scholar]
- 34.Imai M, Hwang HY, Norris JS, Tomlinson S. The effect of dexamethasone on human mucin 1 expression and antibody-dependent complement sensitivity in a prostate cancer cell line in vitro and in vivo. Immunology. 2004;111:291–7. doi: 10.1111/j.0019-2805.2004.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Smith CA, Song H, Morgan BP, Abagyan R, Tomlinson S. Insights into the human CD59 complement binding interface toward engineering new therapeutics. J Biol Chem. 2005;280:34073–9. doi: 10.1074/jbc.M504922200. [DOI] [PubMed] [Google Scholar]
- 36.Hong F, Yan J, Baran JT, et al. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin RJ, Cobbold SP, Clark MR, Waldmann H. Tolerance to rat monoclonal antibodies. Implications for serotherapy. J Exp Med. 1986;163:1539–52. doi: 10.1084/jem.163.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamin RJ, Waldmann H. Induction of tolerance by monoclonal antibody therapy. Nature. 1986;320:449–51. doi: 10.1038/320449a0. [DOI] [PubMed] [Google Scholar]
- 39.Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986;323:164–6. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- 40.Caragine TA, Imai M, Frey AB, Tomlinson S. Expression of rat complement control protein Crry on tumor cells inhibits rat natural killer cell-mediated cytotoxicity. Blood. 2002;100:3304–10. doi: 10.1182/blood.V100.9.3304. [DOI] [PubMed] [Google Scholar]
- 41.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27:375–87. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 42.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–60. [PubMed] [Google Scholar]
- 43.Nakamura H, Hinoda Y, Nakagawa N, et al. Detection of circulating anti-MUC1 mucin core protein antibodies in patients with colorectal cancer. J Gastroenterol. 1998;33:354–61. doi: 10.1007/s005350050096. [DOI] [PubMed] [Google Scholar]
- 44.Petrarca C, Casalino B, von Mensdorff-Pouilly S, et al. Isolation of MUC1-primed B lymphocytes from tumour-draining lymph nodes by immunomagnetic beads. Cancer Immunol Immunother. 1999;47:272–7. doi: 10.1007/s002620050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snijdewint FG, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, et al. Cellular and humoral immune responses to MUC1 mucin and tandem-repeat peptides in ovarian cancer patients and controls. Cancer Immunol Immunother. 1999;48:47–55. doi: 10.1007/s002620050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 47.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–34. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 48.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 49.Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD4+ T cell activity in a complement-independent manner. J Immunol. 2005;175:7098–102. doi: 10.4049/jimmunol.175.11.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]