Abstract
A dual electrospray ionization source (ESI) has been modified to simultaneously produce cations and anions, one from each emitter, for performing rapid electron-transfer dissociation (ETD) ion/ion reactions on a hybrid linear ion trap–orbitrap mass spectrometer. Unlike the pulsed dual ESI sources that were used to generate ETD reagent ions, this source separates the emitters in space, rather than time, by physically switching which one is in front of the atmospheric inlet. The new arrangement allows for substantially enhanced spray stability and decreased switching times (≤30 ms), allowing for more tandem-MS spectra per unit time. Herein, we demonstrate the stability of the ETD anion population and the ability to identify several c- and z-type product ions from multiply protonated peptide cations.
The ability of electron-transfer dissociation (ETD) to produce fragmentation patterns similar to electron-capture dissociation (ECD) in the context of the ubiquitous rf quadrupole ion trap mass spectrometer has garnered the technique considerable attention.1–4 Among other attributes, ETD can allow for the identification of posttranslational modification sites and is able to dissociate large peptides and entire proteins on a chromatographic time scale.5–9 Several ion trap MS systems offer a good platform for conducting ion/ion chemistry10–14 (i.e., ETD); however, today numerous hybrid systems incorporate ion trapping devices as either mass analyzers or intermediate storage devices. In these hybrids, orbitrap (LTQ-orbitrap), time-of-flight, and Fourier transform ion cyclotron resonance (LTQ-FT-ICR) mass analyzers are frequently employed for their ability to bring higher mass resolving power and m/z measurement accuracies.
Originally, ETD was accomplished with reagent anions that were produced by a negative chemical ionization source, which had been fitted to the unoccupied end of a linear ion trap (LTQ).2 But because this modification took advantage of the unoccupied end of the stand-alone LTQ, it is not easily incorporated on hybrid instruments such as the LTQ-orbitrap or LTQ-FT-ICR. To address the problem of implementing ETD and other ion/ion reactions on hybrid mass spectrometers, McLuckey and co-workers have recently developed different strategies for introducing both cations and anions through a single atmospheric pressure (AP) inlet. Their initial strategy utilized a nanoelectrospray source for generation of peptide cations and an atmospheric pressure chemical ionization (APCI) for anion production.15,16 The anions generated via APCI were nitrobenzene and azobenzene, with azobenzene having a slightly higher ETD efficiency.
About that same time, McLuckey et al. also developed a dual AP ETD source that generates the anion reagent population via ESI.17 This scheme produces the same ETD anion as used in the initial report of ETD, but in this case, a precursor anion 9-anthracenecarboxylic acid is electrosprayed and then subjected to collision-activated dissociation (CAD). These experiments were performed utilizing multiple independent AP sources in which cation and anion populations were constantly generated and gated by downstream ion optics.
Coon and co-workers have adapted that approach for use on a single inlet LTQ-orbitrap hybrid mass spectrometer.4 Briefly, two ESI emitters, in proximity, were pulsed on and off to generate distinct populations of cations and anions for ion/ion reactions in the LTQ. An addition of 750 ms to the total scan time was necessary to pulse the high-voltage power supplies and inject the precursor anion population. Following the ETD reaction in the LTQ, product ions were m/z analyzed in either the LTQ or the orbitrap; the later produces high-resolution and high m/z measurement accuracy (~3 ppm) product ion spectra at the expense of time and sensitivity. McLuckey and co-workers have also developed a pulsed ESI source in which voltages were pulsed as quickly as 10 ms; however, this was utilized for ion/ion reactions other than ETD including charge reduction, charge inversion, and complex formation.18
Here we describe the modification of a dual ESI source, previously developed by Muddiman and co-workers,19–22 for generation of both the analyte cation population and the anion reagent population. The voltage on each of the two emitters is constant throughout a given experiment, translating to improved ESI stability (for both anions and cations) and much more rapid switching times that previous sources utilized for ETD. The fast physical switching time allows for more ETD tandem mass spectra for a given time period.
MATERIALS AND METHODS
Materials
The peptide KAAAKAAAK was synthesized at the University of Wisconsin Biotechnology Center (Madison, WI). All other peptides and chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Peptide and protein solutions were prepared by dilution of stock solutions to ~3 pmol/µL in 60:39.9:0.1 acetonitrile/water/acetic acid by volume. The reagent anion 9-anthracenecarboxylic acid was dissolved in a solution of 50:50 acetonitrile/water to a concentration of 150 µmol/µL. The solution was centrifuged for ~5 min (~9000 rcf) to prevent particulates from clogging the ESI emitter tip during infusion.
Dual Electrospray Ionization Hybrid Linear Ion Trap–Orbitrap (Dual ESI-LTQ-orbitrap)
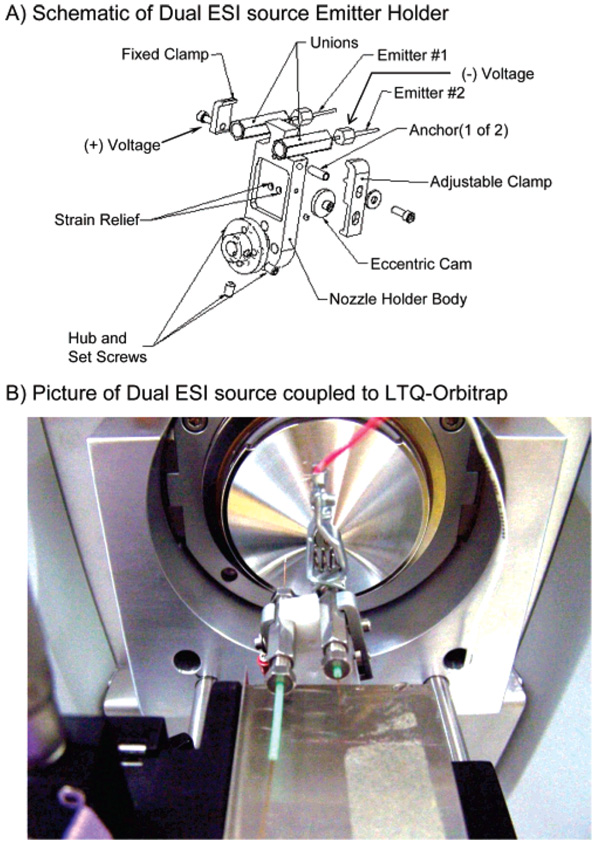
All data shown were collected on a modified LTQ-orbitrap hybrid mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). A description of the internal instrument modifications necessary for ETD (i.e., ion optics and power supplies) has been previously published, as well as the voltage changes within the instrument throughout the experiment.4 A previously developed dual ESI source,19–22 to which changes were made such that one emitter could produce cations while the other could simultaneously produce anions (Figure 1), was installed on the front end of the instrument. The necessary changes to the source included replacement of the original electrical connector that connected the two emitters to a single voltage source. This piece was replaced with two separate anchors for the fixed clamp and adjustable clamp that enabled the two emitters to be held at constant, but separate, voltages throughout the experiment. The ion trap control language was modified to trigger a TTL pulse, when desired, to induce emitter switching. The capability of this source to be modified to accommodate ion–ion chemistry was previously noted;21 however, it was not completed until recently.
Figure 1.
(A) Expanded view of the emitter holder of the dual electrospray assembly. (B) Picture of dual ESI source coupled to LTQ-orbitrap.
Samples were introduced by direct infusion using a 250-µL gastight syringe (Hamilton, Las Vegas, NV) at a flow rate of 1.5 –2.00 µL/min. The ESI emitter tips used were 360-µm o.d., 50-µm i.d. and tapered to 30 ± 1.0-µm i.d. (New Objective, Woburn, MA). The cation emitter tip was held at a potential of +2.3−2.5 kV while the anion emitter tip was held at a constant potential of −2.7 kV for all experiments.
Since both ESI emitter tips were kept at a constant voltage, throughout a given experiment, there was no need to allow the electrospray to stabilize. The maximum total time for switching between the emitters and anion injection and for these experiments was ~85 ms—significantly faster than that of other dual ESI sources.
RESULTS AND DISCUSSION
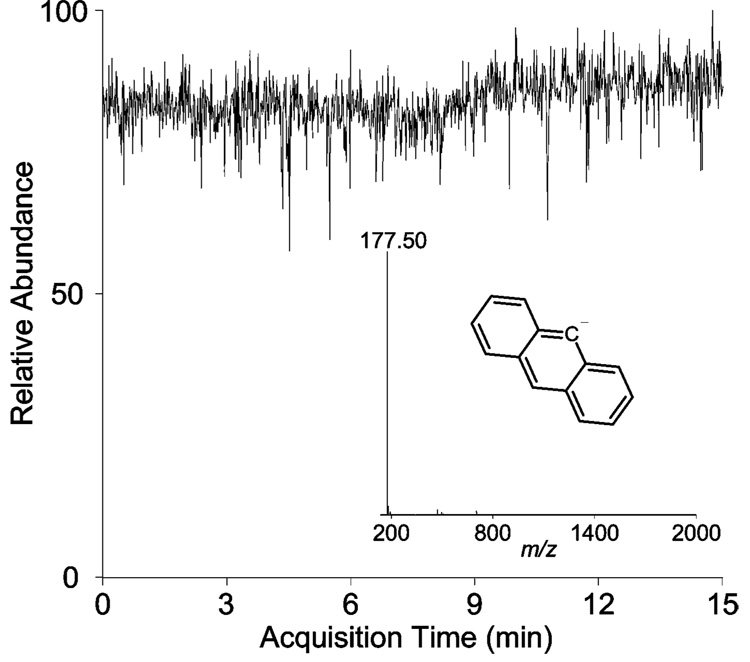
Stability of signal is one important factor to consider when implementing a new ion source. Figure 2 shows the stability of the ETD reagent at m/z value 177 (the CAD product of the anion 9-anthracenecarboxylic acid) over a 15-min period. During this collection time, the source was operating in the pulsed mode, just as in a typical ETD experiment. The inset of Figure 2 displays a single mass spectrum acquired by the LTQ and the structure of the ETD reagent. For this work, anion stability is critical as automatic gain control was not employed to meter the number of anions allowed into the instrument.
Figure 2.
Total ion chromotogram for 9-anthracenecarboxylic acid undergoing CAD to produce the ETD anion during a pulsing experiment. The inset displays a single scan mass spectrum of the ETD reagent anion and its structure.
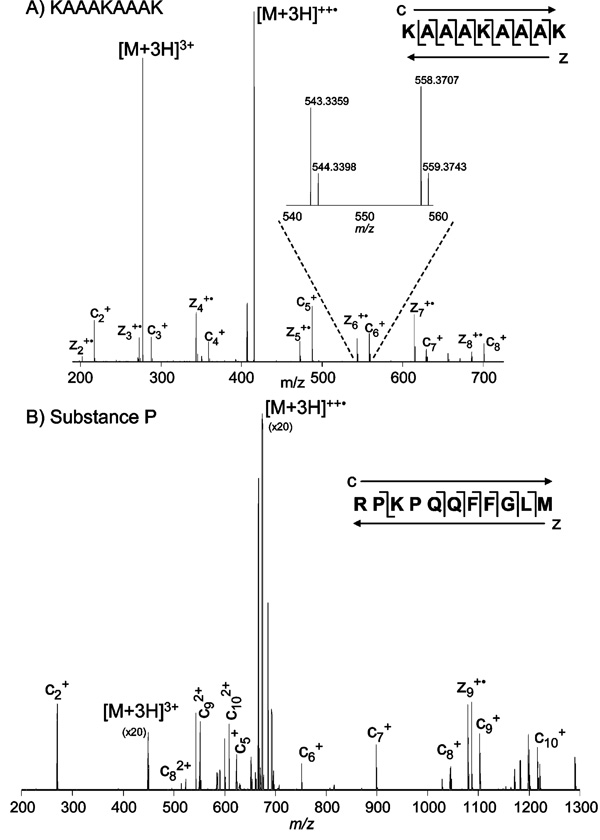
Figure 3A shows a single scan LTQ-orbitrap tandem mass spectrum produced following the reaction of a triply protonated synthetic peptide (KAAAKAAAK) and the collisionally activated 9-anthracenecarboxylic acid anion. This reaction persisted for 100 ms in the LTQ. The reaction was then quenched and the product ions transferred to the orbitrap mass analyzer for m/z analysis. Detected c- and z-type product ions are labeled on the mass spectrum and shown on the sequence. An inset showing the orbitrap’s ability to achieve isotopic resolution is also shown in Figure 3A.
Figure 3.
(A) Dual ESI LTQ-orbitrap ETD mass spectrum of the 3+ charge state of the synthetic peptide KAAAKAAK, with c- and z-type ions labeled. The inset presents an expanded view of the c6 and z6 ions to highlight the isotopic resolution achieved by use of the orbitrap mass analyzer. (B) Sequence of substance P and denotation of the observed c- and z-type product ions. Dual ESI LTQ-orbitrap ETD mass spectrum of the 3+ charge-state of substance P.
Figure 3B illustrates the sequence and labeled ETD tandem mass spectrum of the benchmark ECD peptide, substance P. This reaction proceeded for 150 ms in the LTQ before product ions were transferred to the orbitrap mass analyzer. Unlike the spectrum of KAAAKAAAK, where nearly every backbone bond cleavage was observed in both the c and z directions, the spectrum of substance P contained mostly c-type product ions. This result was similar to that achieved by Coon and co-workers utilizing their dual electrospray source.4
Coon and co-workers demonstrated the ability of their pulsed dual ESI to perform ETD, and its compatibility with automated data acquisition was also shown. In that work, however, it was realized that a limiting factor was the “relatively long times required for anion injection”— ~750 ms, due to pulsing on and off of the high voltages.4 The source presented herein does not suffer from the same time limitations because the voltages are held constant throughout an experiment, and the time needed to physically switch the emitters is quite fast (~40 ms). The 10-fold decrease in switching and injection time reported for this source equates to a 1.9–2.4-fold increase in tandem MS per unit time (respectively, LTQ and orbitrap analysis), which will aid greatly for conducting higher order experiments such as LC-MS/MS.
CONCLUSIONS
The ability to couple a rapidly spatially pulsed ESI source to a high resolving power instrument, such as the hybrid LTQ-orbitrap, and to perform ETD experiments is demonstrated. A maximum of 85 ms to inject the anion population and switch between the emitters was observed, which allows for the collection of a larger amount of data during a given time period. This increased amount of data will lead to more identifications when coupled with online liquid chromatography. Some previously developed pulsed ESI sources can switch as quickly as 10 ms,18 but this does not consider the time it takes to develop a stable electrospray. A second major benefit of the constant voltage is electrospray stability, which results in more consistent anion densities and equates to more reproducible ETD experiments. This source presents a practical solution to the problems of previously developed dual AP ETD sources, allowing the focus of experiments to shift toward finding a more efficient ETD precursor anion that can be generated via ESI. Finally, we note operation of this device is far simpler and much more convenient to implement as the distance between ESI needles need not be manually optimized.
ACKNOWLEDGMENT
D.C.M. acknowledges financial support by the National Institutes of Health (R33 CA105295), the W. M. Keck Foundation, and North Carolina State University. J.J.C. acknowledges the University of Wisconsin—Madison, Thermo Fisher, the Beckman Foundation, and the NIH (1R01GM080148) for financial support. D.M.G. and G.C.M. gratefully acknowledge support from an NIH predoctoral fellowship—the Biotechnology Training Program (NIH 5T32GM08349).
Footnotes
SUPPORTING INFORMATION AVAILABLE
A movie of the dual ESI in action carrying out an ETD experiment. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Coon JJ, Syka JEP, Schwartz JC, Shabanowitz J, Hunt DF. Int. J. Mass Spectrom. 2004;236:33–42. [Google Scholar]
- 2.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Good DM, Coon JJ. Biotechniques. 2006;40:783–789. doi: 10.2144/000112194. [DOI] [PubMed] [Google Scholar]
- 4.McAlister GC, Phanstiel D, Good DM, Berggren WT, Coon JJ. Anal. Chem. 2007;79:3525–3534. doi: 10.1021/ac070020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunawardena HP, Emory JF, McLuckey SA. Anal. Chem. 2006;78:3788–3793. doi: 10.1021/ac060164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan JM, Pitteri SJ, Chrisman PA, McLuckey SA. J. Proteome Res. 2005;4:628–632. doi: 10.1021/pr049770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Anal. Chem. 2005;77:1831–1839. doi: 10.1021/ac0483872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coon JJ, Ueberheide B, Syka JEP, Dryhurst DD, Ausio J, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaney DL, McAlister GC, Wirtala M, Schwartz JC, Syka JEP, Coon JJ. Anal. Chem. 2007;79:477–485. doi: 10.1021/ac061457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herron WJ, Goeringer DE, McLuckey SA. Anal. Chem. 1996;68:257–262. doi: 10.1021/ac950895b. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson JL, McLuckey SA. Int. J. Mass Spectrom. 1997;165:419–431. [Google Scholar]
- 12.Stephenson JL, McLuckey SA. Int. J. Mass Spectrom. Ion Processes. 1997;162:89–106. [Google Scholar]
- 13.McLuckey SA, Stephenson JL. Mass Spectrom. Rev. 1998;17:369–407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Syka JEP, Marto JA, Bai DL, Horning S, Senko MW, Schwartz JC, Ueberheide B, Garcia B, Busby S, Muratore T, Shabanowitz J, Hunt DF. J. Proteome Res. 2004;3:621–626. doi: 10.1021/pr0499794. [DOI] [PubMed] [Google Scholar]
- 15.Liang XR, Xia Y, McLuckey SA. Anal. Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y, Chrisman PA, Erickson DE, Liu J, Liang XR, Londry FA, Yang MJ, McLuckey SA. Anal. Chem. 2006;78:4146–4154. doi: 10.1021/ac0606296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang TY, Emory JF, O’Hair RAJ, McLuckey SA. Anal. Chem. 2006;78:7387–7391. doi: 10.1021/ac061409v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y, Liang XR, McLuckey SA. J. Am. Soc. Mass Spectrom. 2005;16:1750–1756. doi: 10.1016/j.jasms.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Hannis JC, Muddiman DC. J. Am. Soc. Mass Spectrom. 2000;11:876–883. doi: 10.1016/S1044-0305(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 20.Flora JW, Hannis JC, Muddiman DC. Anal. Chem. 2001;73:1247–1251. doi: 10.1021/ac0011282. [DOI] [PubMed] [Google Scholar]
- 21.Nepomuceno AI, Muddiman DC, Bergen HR, Craighead JR, Burke MJ, Caskey PE, Allan JA. Anal. Chem. 2003;75:3411–3418. doi: 10.1021/ac0342471. [DOI] [PubMed] [Google Scholar]
- 22.Williams K, Hawkridge AM, Muddiman DC. J. Am. Soc. Mass Spectrom. 2007;18:1–7. doi: 10.1016/j.jasms.2006.08.014. [DOI] [PubMed] [Google Scholar]