Abstract
Glutamate facilitates calcium influx via NMDAR, and excess calcium influx increases excitotoxicity – a pathological characteristic of neurological diseases. Both 17β-estradiol (E2) and lithium influence NMDAR expression/signaling and excitotoxicity. This led us to hypothesize that combined E2 and lithium will alter NMDAR expression and excitotoxicity. We tested this hypothesis using primary cell cultures from the cortex and hippocampus of C57BL/6J fetal mice pretreated with E2, lithium chloride (LiCl) and combined E2/LiCl for 12, 24 or 48 h. We examined cultures for brain cell type and changes in cell type caused by experimental procedures using glia and neuron gene specific primers. These cultures expressed increased glial fibrillary acidic protein (GFAP) mRNA with low neurofilament-heavy chain (NF-H) mRNA expression. Subsequent analysis of cortical cell cultures indicated that combined E2/LiCl decreased NR1 mRNA expression after a 12 and 48 h treatment period. Combined E2/LiCl also reduced NR1 mRNA expression in hippocampal cultures but only after a 48 h treatment period. LiCl-treated hippocampal cultures also reduced NR1 mRNA expression after a 24 and 48 h treatment. We next examined the response of 48 h pretreated cultures to a toxic level of glutamate. Excitotoxicity was measured using fluorescein diacetate/propidium iodide (FDA/PI) cell viability assay. Results from FDA/PI assay revealed that LiCl pretreatment increased viability for cortical cultures while E2 and combined E2/LiCl reduced viability. All pretreatments for hippocampal cultures failed to increase viability. Our results showed combined E2/LiCl reduced NR1 mRNA and prevented protection against glutamate excitotoxicity in glial primary cultures.
Keywords: estradiol, lithium chloride, NMDA receptor, glia, excitotoxicity
1. Introduction
Estrogen-mediated neurobiological processes are involved in a myriad of signal transduction pathways. These pathways result in enhancing synaptic plasticity (Leranth et al., 2002), reducing apoptotic activity in cortical neurons (Honda et al., 2001), regulating neurotrophic factors (Solum and Handa, 2002), and facilitating transcription factor activation – e.g. cAMP response element binding protein (CREB) (McEwen, 2001). Estrogen is considered an activating agent in N-methyl-d-aspartate receptor (NMDAR) signaling pathways (Jelks et al., 2007), and NMDAR forms a heteromeric glutamate receptor composed of the subunits NR1, NR2A-D and NR3 (Stephenson, 2001). Expression of NR1, NMDAR critical subunit, is increased in estrogen-induced primary hippocampal neuronal cell cultures (Jelks et al., 2007). Similar cultures show increased levels of intracellular calcium, mediated by the activation of L-type calcium channels, which in turn promote signaling pathways that activate/phosphorylate CREB. This activation/phosphorylation promotes anti-apoptotic events (Wu et al., 2005). Estrogen also facilitates a transient activation of glycogen synthase kinase-3beta (GSK-3β). Estrogen receptor-α, when bound by its ligand estrogen, forms a complex with GSK-3β that facilitates regulation of beta-catenin transcriptional properties (Cardona-Gomez et al., 2004). Estrogen receptor-α response element binding relies on GSK-3β phosphorylation and this phosphorylation is inhibited in the presence of lithium (Medunjanin et al., 2005). Contrary to this synergistic relation of estrogen receptor-α and GSK-3β, other studies show estrogen facilitating an inhibition of GSK-3β. This inhibition is dependent on kinase phosphorylation mediated by estrogen receptor signaling (Goodenough et al., 2005). Interestingly, GSK-3β activity influences NMDAR signaling and NMDAR subunit expression. Electrophysiological studies using mouse hippocampal slices report that GSK-3β activity is increased during long-term depression. This increase reduces during NMDAR-mediated long-term potentiation (Peineau et al., 2007). Selective GSK-3β inhibitors also reduce NMDAR synaptic currents and receptor internalization in cortical cell cultures. Cultures expressing a knockdown of GSK-3β exhibit similar effects (Chen et al., 2007).
Over a decade has passed since Klein and Melton (1996) demonstrated lithium specifically inhibiting GSK-3β. This inhibition is associated with reducing apoptotic activity (Hongisto et al., 2003), increasing neurotrophic factors (Angelucci et al., 2003), and facilitating CREB DNA binding (Grimes and Jope, 2001; Ozaki and Chuang, 1997). Though the mechanism remains elusive, lithium indirectly inhibits GSK-3β by activating protein kinases that phosphorylate GSK-3β at serine 9 and/or by inactivating phosphatases that remove this phosphate group (Jope, 2003). Direct inhibition involves lithium acting as a competitive inhibitor of magnesium; magnesium being a cofactor for ATP in GSK-3β activation (Ryves and Harwood, 2001). Aside from lithium's inhibitory action on GSK-3β, there is an indirect effect on NMDAR signaling pathway. This involves reducing phosphorylation/activation of Src tyrosine kinases, potentially affecting any NMDAR-regulated excitotoxicity. Phosphorylation of NR2 subunits of NMDAR by Src tyrosine kinases is essential for the receptor's signal transduction activity (see Hashimoto et al., 2003). Using lithium-treated rat cerebral cortex neurons, Hashimoto et al. (2003) demonstrated that phosphorylated levels of Src tyrosine kinase is decreased in a time-dependent fashion. Lithium, however, does not seem to influence NMDAR subunit protein expression (Nonaka et al., 1998).
NR1 expression increases in estrogen-treated primary hippocampal neuronal cultures (Jelks et al., 2007), and similar cultures also show increase intracellular calcium levels (Wu et al., 2005). But lithium reduces NMDAR-mediated glutamate excitotoxicity by reducing calcium influx (Chuang et al., 2002). While NMDAR is classically regarded as a neuronal receptor, these receptors are also found on cortical astrocytes (Conti et al., 1996). Astrocytes process the neurotransmitter glutamate to reduce excitotoxic insults to neurons; however, astrocytes themselves are not as susceptible to excitotoxicity as are neurons (see Matute et al., 2002). In astrocyte cultures of the rat olfactory bulb, estrogen receptor agonists reduce secretion of specific markers responsible for inflammatory responses (e.g. interleukin-1, tumor necrosis factor-α, and matrix metallopeptidase 9) (Lewis et al., 2008). As well, astrocytes produce and secrete estrogen, influencing communication among neurons. When cultures of rat cortical neurons are exposed to media from cultured astrocytes, neuronal synaptic formation and impulse transmission increase, due to the presence of estrogen in the astrocytic media (Hu et al., 2007).
NMDAR-mediated glutamate excitotoxicity is a pathological element in many neurodegenerative diseases and analysis of how combined estrogen and lithium influence NMDAR regulation and its mediated pathways are wanting. Since estrogen and lithium are involved in regulating NMDAR activation/function we hypothesized that combined estrogen (specifically 17β-estradiol; E2) and lithium chloride (LiCl) (E2/LiCl) will alter mRNA expression of NMDAR subunit NR1 in primary cultures harvested from mouse cortex and hippocampus. The premise of this hypothesis is that estrogen receptor-α facilitates NMDAR subunit expression and studies have shown that estrogen receptor-α response element binding is attenuated in the presence of lithium (Medunjanin et al., 2005). Our results indicate that combined E2/LiCl treatment reduce NR1 mRNA expression of primary cultures expressing high levels of glial-specific mRNA. We chose this course of study since glia express NR1 (Conti et al., 1996), and NR1 is the critical subunit of a functional NMDAR. These results led us to further hypothesize that amongst these primary glial cultures combined E2/LiCl will alter protective features (cell viability) against glutamate insult. We tested this hypothesis using a cell viability assay with 48 h pre-treated primary cultures exposed to high levels of glutamate. High glutamate concentration was necessary since glia are extremely tolerant to excitotoxicity (see Matute et al., 2002). Our results indicated that while LiCl reduced NR1 mRNA in E2-treated cultures, E2 compromised cell viability in LiCl treated cultures. In many instances post-menopausal women undergoing hormone therapy are also prescribed lithium (for depression) and the interaction of these two agents are poorly understood. Ours is the first study to note the influence of combined E2/LiCl on expression of receptors involved in learning, memory and mediating excitotoxic effects.
2. Results
2.1. Brain cell typing of primary cultures indicated predominance for glia
Figures 1A-D depicts densitometric data (gene/HPRT) obtained after RT-PCR and gel electrophoresis (gels represented above the graph). We chose to analyze brain cell type using gene specific primers for neurons and glia. This allowed us to monitor brain cell type and to monitor how our experimental parameters affected neuronal and glial gene expression. We used gene specific primers for glial fibrillary acidic protein (GFAP) and neurofilament heavy chain (NF-H), markers readily used for identifying glial and neuronal populations, respectively. ANOVA of densitometric data indicated a significant difference in gene expression (p < .001). These primary cell cultures expressed high levels of GFAP mRNA (figures 1A & C) but with consistently low levels of NF-H mRNA (figures 1B & D).
Figures 1 A-D.
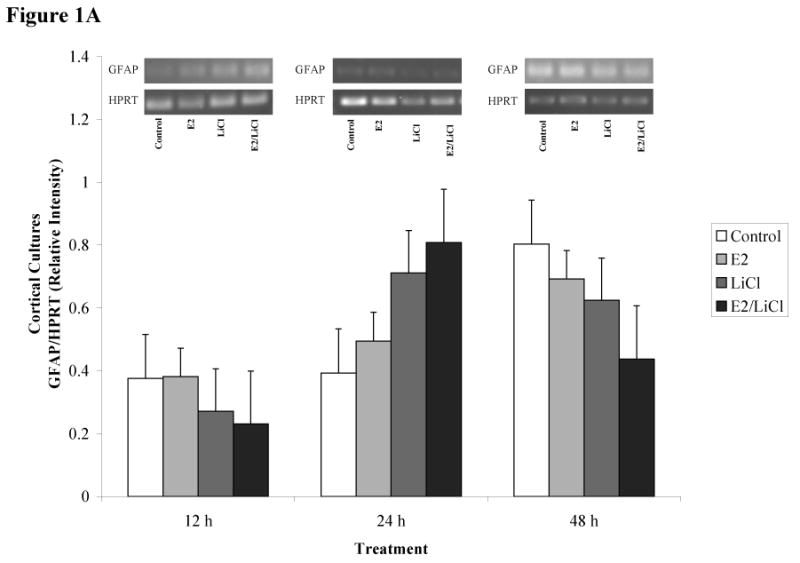
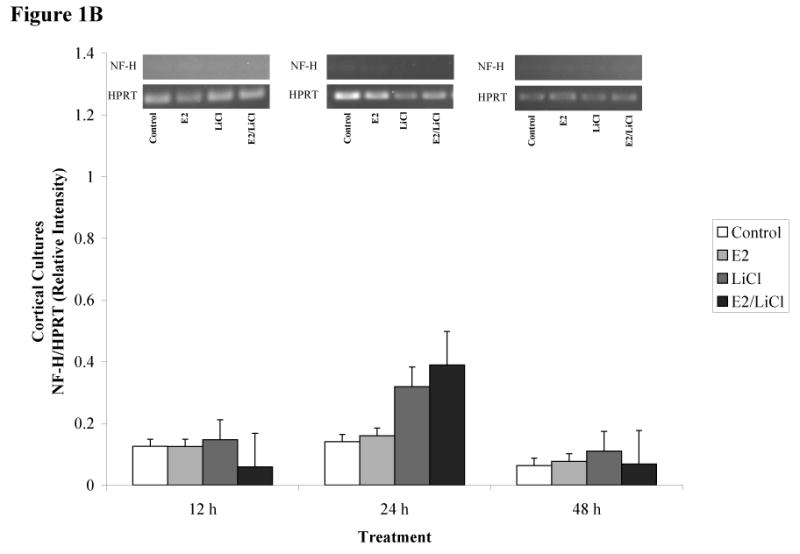
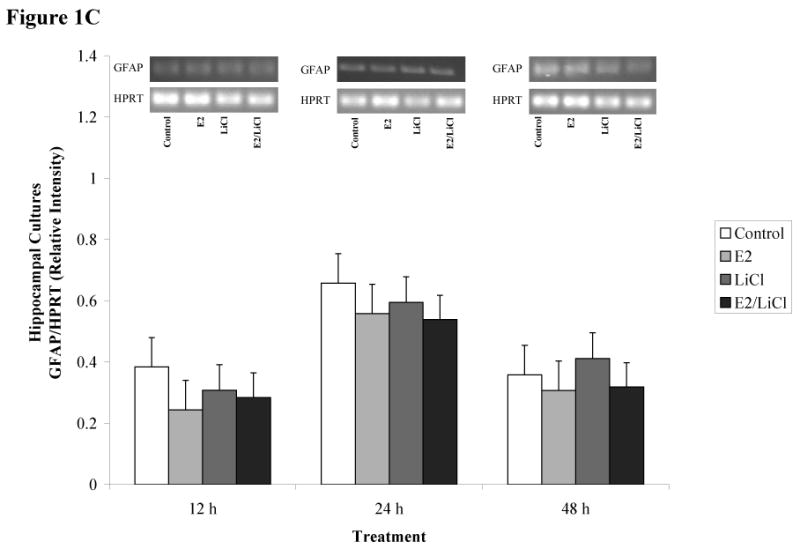
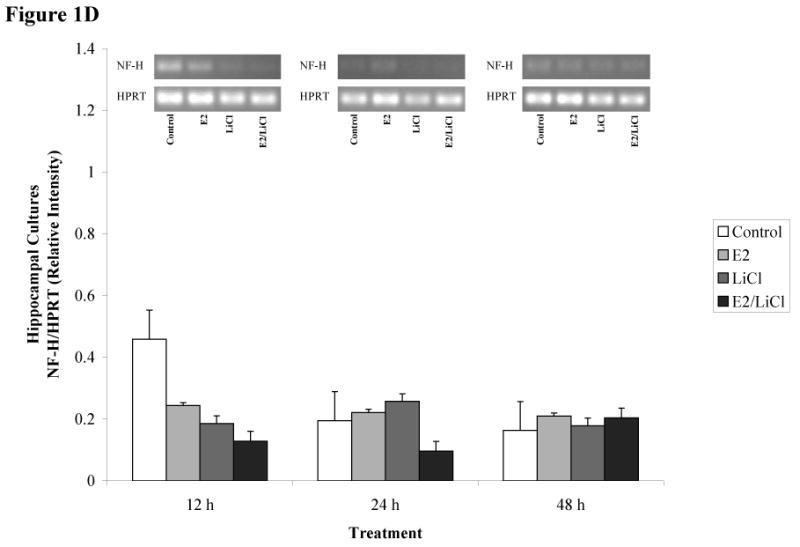
ImageJ densitometric data obtained from RT-PCR and electrophoresis. Analysis was performed via electrophoresis of RT-PCR samples using a 1.5% agarose gel, gel images captured, and analyzed using ImageJ. Low visibilities of bands are due to low mRNA expression. Total RNA from and reverse transcribed E18.5 cortical (A & B) and hippocampal (C & D) primary cell cultures were isolated from Control (no treatment), 0.04μM E2, 10mM LiCl, and combined E2/LiCl treated for 12, 24, and 48 h. cDNA was then amplified via PCR using gene specific primers for GFAP (A & C) or NF-H (B & D). Measurements were based on relative pixel intensity (integrated intensity of GFAP/HPRT or NF-H/HPRT) and expressed in arbitrary units. Gels are represented for each gene and treatment period (represented above the graph).
Our experimental parameters altered genes expression for cortical but not hippocampal cultures. GFAP mRNA expression gradually increased for cortical cultures during the 12, 24, and 48 h period (figure 1A), while hippocampal cultures maintained consistency in GFAP expression (figure 1B). NF-H, however, was more abundant in hippocampal cultures (figure 1D) compared with cortical cultures (figure 1B) and this was consistent throughout treatment periods. Treatment did not affect GFAP or NF-H mRNA expression within each respective culture type (cortical or hippocampal). Overall, densitometric data demonstrated that both culture types expressed high levels of GFAP mRNA compared with NF-H indicating these cultures were predominantly glial.
2.2. Combined E2/LiCl treatment reduced NR1 mRNA expression
We next examined the effect of our experimental design on NR1 mRNA expression. NR1 is a critical subunit of the NMDAR and no have studies investigated how combined E2/LiCl will affect NR1 expression in glial cultures. Additionally, in analyzing NR1 gene expression we can postulate transcriptional activity caused by our treatment parameters. Interpretation of ANOVA on real time RT-PCR data showed significant differences (p < .001) in NR1 mRNA expression related to treatment. Both treated culture types (cortical and hippocampal) slightly differed in overall NR1 mRNA expression (figures 2A & B), but this difference was not significant (p > .057). No significant changes were noted across treatment periods (p > .2) – e.g. in a time-dependent fashion; although, NR1 expression significantly changed among treated culture types at specific time periods (p < .001).
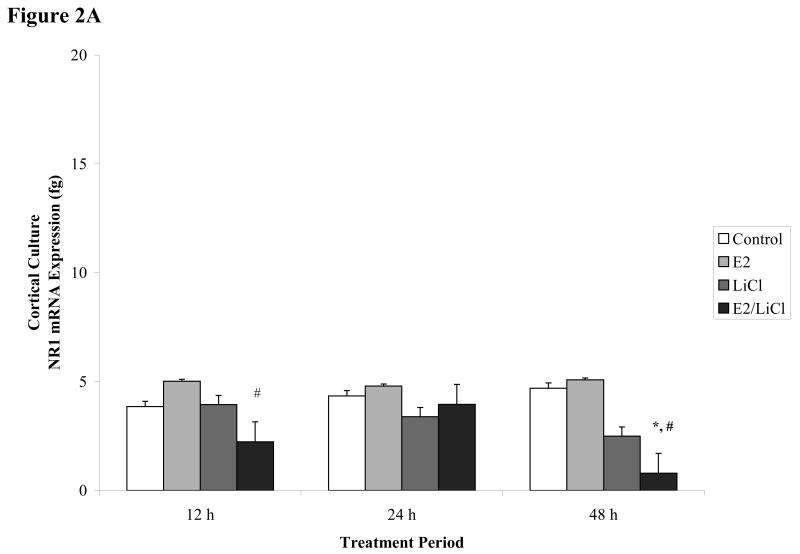
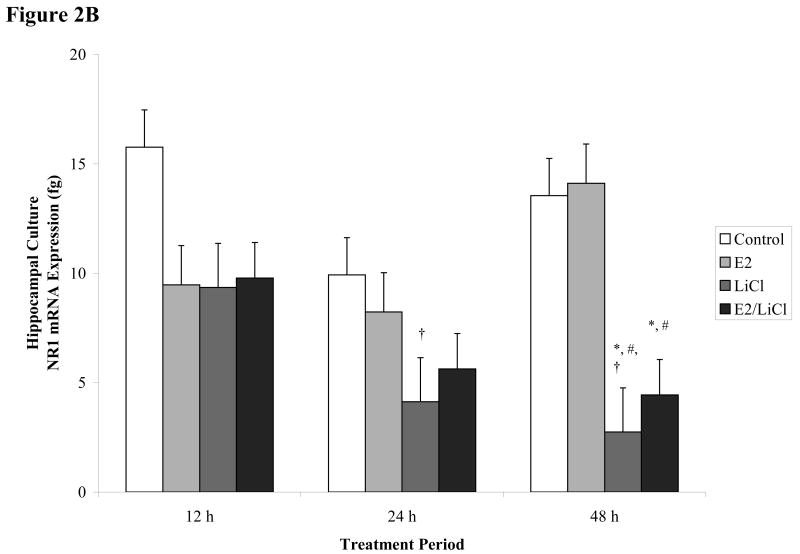
Figures 2 A-B.
Real time RT-PCR of NR1 mRNA expression. E18.5 cortical (A) and hippocampal (B) primary cell cultures treated for 12, 24, and 48 h with 0.04μM E2, 10mM LiCl, combined E2/LiCl, or Control (no treatment). After temporal treatment total RNA was isolated and reverse transcribed. The figure denotes NR1 mRNA expression in femtograms (y-axis) and treatment duration (x-axis). *, p-value < .05 compared with control; #, p-value < .05 compared with E2; †, p-value < .001 compared with 12 h treatment.
No significant effects were detected for Control or E2 treatment on NR1 mRNA expression across treatment periods and culture type (figure 2A & B). LiCl influenced NR1 expression but this alteration was dependent on culture type. In cortical cultures (figure 2A), LiCl seemed to reduce NR1 gradually and specifically after a 48 h treatment period, but according to Fisher's least significant difference (LSD) this decrease proved to be non-significant (p > .1). For hippocampal cultures, Fisher's LSD indicated that LiCl significantly decreased (p < 0.05) NR1 mRNA expression after a 24 and 48 h treatment period when compared with the 12 h treatment period (figure 2B). LiCl also significantly reduced (p < 0.05) NR1 expression in hippocampal cultures after a 48 h treatment period when compared with Control and E2 treatments (figure 2B). Although all treatments appeared to reduce hippocampal culture NR1 gene expression after 12 h when compared with Control (figure 2B), Fisher's LSD indicated this change was non-significant (p > .05).
Combined E2/LiCl treatment significantly decreased (p < .05) NR1 mRNA expression in both cortical and hippocampal cultures (figure 2A & B). Fisher's LSD showed that combined E2/LiCl treatment significantly reduced cortical NR1 mRNA expression after 12 h when compared with E2 treated cultures (figure 2A). This significant decrease reoccurred after cortical cultures were treated with combined E2/LiCl for 48 h. The 48 h decrease was noted when combined E2/LiCl was compared with E2 and Control (figure 2A). Cortical cultures treated after 24 h did not alter NR1 mRNA expression. In hippocampal cultures, combined E2/LiCl treatment significantly reduced NR1 expression. Combined E2/LiCl treatment when compared with Control and E2 significantly reduced (p < 0.05) NR1 expression after a 48 h treatment period (figure 2B). No significant differences were noted for combined E2/LiCl treated hippocampal cultures after a 12 and 24 h treatment, though.
These results demonstrated that combined E2/LiCl treatment decreased NR1 gene expression for both culture types, specifically after a 48 h treatment period. Cortical cultures showed significantly decreased NR1 gene expression after 12 and 48 h treatment periods and hippocampal cultures showed significantly decreased expression only after a 48 h treatment period. Additionally, NR1 gene expression in LiCl-treated hippocampal cultures decreased after a 24 and 48 h when compared with 12 h treatment period. E2-treated cultures did not affect NR1 mRNA expression across all experimental parameters.
2.3. E2 and combined E2/LiCl failed to reduce excitotoxicity
Glia are extremely tolerant to excitotoxicity (see Matute et al., 2002) and since our primary cultures express high glial mRNA (see figures 1A-D) a high dosage of glutamate (100μM) was necessary. In our experiment, primary cell cultures were pretreated for 48 h, subjected to excitotoxicity with glutamate, and assessed for cell viability using fluorescein diacetate/propidium iodide assay (FDA/PI). Figure 3A were captured images of primary cultures after experimental procedures. Live cells were labeled green (FDA) and dead cells were labeled red (PI). These primary cultures maintained a tolerance for glutamate toxicity without treatment (Control) when compared with Initial Viability (e.g. assayed prior to any experimental procedure). Additionally, Control cortical cultures compared with Initial Viability showed an increase in FDA labeling (figure 3A). Increased Control FDA labeling did not occur for hippocampal cultures, though (figure 3A). Pretreatment with E2 and combined E2/LiCl decreased viability for both cortical or hippocampal cultures, as indicated by the intense PI labeling; however, LiCl-treated cortical cultures showed increased cell viability which did not occur for hippocampal (figure 3A). These images were then processed using ImageJ cell counter (Rasband, 1997-2007) and data generated was statistically analyzed.
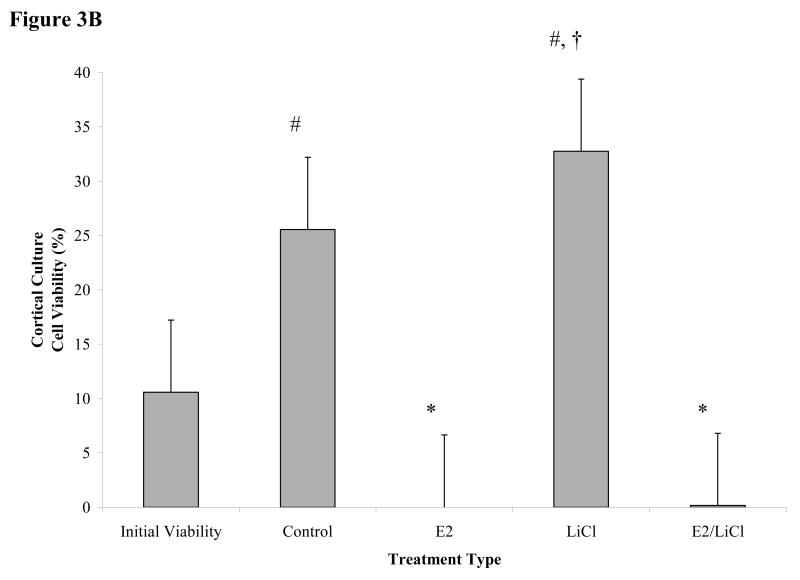
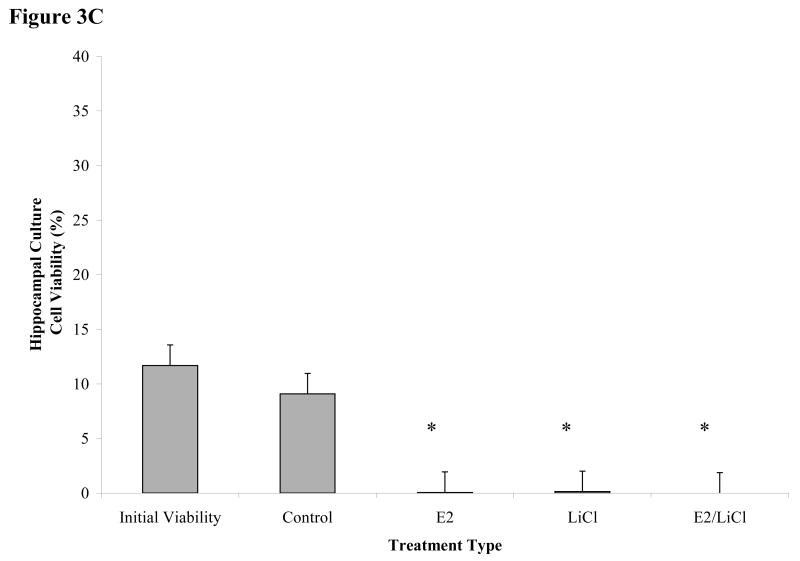
Figures 3 A-C.
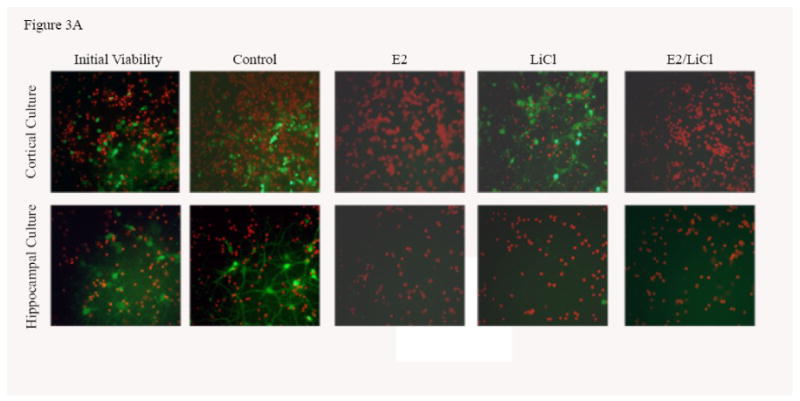
Mean viability for glutamate excitotoxicity from 48 h treated cortical and hippocampal cell cultures. Excitotoxicity was induced by incubating primary cultures with 100μM glutamic acid for 1 h at 37°C after pre-treating for 48 h with with 0.04μM E2, 10mM LiCl, combined E2/LiCl, or Control (no treatment). A pre-treatment measurement (Initial viability) was assayed to note any changes caused by the treatment and glutamate excitotoxicity. Images (A) were captured (green labeling, FDA (live cells) and red labeling, PI (dead cells)) and viability (FDA/(FDA+PI)) was assessed using ImageJ Cell Counter for both cortical (B) and hippocampal (C) cultures. *, significantly reduced cell viability compare with Initial Viability and Control (p-value < .05); #, increased cell viability when compared with Initial Viability (p-value < .05); †, increased cell viability when compared with other treatments (p-value < .05).
Viability was not reduced for Control cortical and hippocampal cultures when compared with Initial Viability after glutamate excitotoxicity (figure 3B & C); specifically in Control cortical cultures where viability significantly increased (p < .05) (figure 3B). Figure 3B indicated cortical cultures pretreated with E2 and combined E2/LiCl significantly failed to rescue cells from glutamate excitotoxicity when compared with Control and Initial Viability. LiCl-treated cortical cultures, however, exhibited increased cell viability when compared with other treatment types and Initial Viability (figure 3B). Figure 3C depicts that all pretreated hippocampal cultures failed to rescue cells from glutamate excitotoxicity when compared with Control and Initial Viability. Hippocampal cultures also did not display increased cell viability due only to glutamate (e.g. Control) as did cortical cultures.
3. Discussion
Several cell signaling transduction pathways are affected by E2 and LiCl, but few studies concentrate on any neurobiological effects the two combined may present. Additionally, investigations are wanting on how combined E2 and LiCl affect glia. In the mammalian brain glia are ten times more numerous than neurons and such would be expected to play a role in the progression of neurodegenerative diseases (see Van Eldik et al., 2007). In this study, densitometric results showed high levels of GFAP mRNA expression among cortical and hippocampal cultures (see figures 1A & C) compared with NF-H (see figures 1B & D), suggesting a predominance of glia. We chose to use GFAP and NF-H since these are standard markers in identifying brain cell type (glia or neuron, respectively). Cultures used in this study were harvested at a late embryonic age (E18.5) when most neurons have differentiated. As expected, neurons harvested at E18.5 would have a greater propensity for cell death during mechanical trituration, hence the low NF-H expression (see figures 1B & D). B27/Neurobasal medium is primarily used for culturing cortical and hippocampal neurons and this medium generates a 90% neuronal population compared with a 10% glial population as described by immunocytochemistry (Brewer, 1995). This percentage coincides with our FDA/PI assay of Initial Viability (e.g. viability assayed prior to any experimental procedures; see figures 3B & C) for both cortical and hippocampal cultures since this media maintained a neuronal population while suppressing glia proliferation. This allowed us to maintain cultures that sustained neurons (see figures 1B & D), suppressed glia proliferation, but still with a predominance of glial cells (see figures 1A & C). Densitometric data (figures 1A-D) indicated that cortical cultures increased and were higher in GFAP mRNA expression across treatment periods (see figure 1A) compared with hippocampal cultures (see figure 1C). Glia are constantly proliferating and this gradual increase and higher GFAP expression in cortical cultures could be explained since cortical cultures were plated at a higher density than hippocampal cultures (see Experimental Procedures). Hippocampal cultures expressed higher levels of NF-H mRNA (see figure 1D) compared with cortical cultures (see figure 1B), but still, with higher GFAP levels than NF-H (see figure 1C & D). Rocha et al. (1994) indicated that GFAP protein expression increased in the caudate nucleus and hippocampus of 4 week lithium-treated rats (Rocha and Rodnight, 1994) and though we noted GFAP mRNA increased in cortical cultures (specifically at 24 and 48 h; see figure 1A) this was not apparent in hippocampal cultures. This may be due to our treatment being an acute (up to 48 h) rather than a chronic one (4 weeks).
Glia, like neurons, are also known to express functional NMDAR and non-NMDAR (Conti et al., 1996; Lalo et al., 2006). Glia NMDAR expression increases susceptibility to excitotoxicity and facilitates glutamate transport (see Matute et al., 2002). Glial susceptibility to excitotoxicity, however, is less severe than neuronal. This is of specific interest since NMDAR subunit expression and excitotoxicity of glia are rarely studied. NR1 is a critical subunit of the NMDAR and without it there is no functional receptor. In studying glial NR1 gene expression within our experimental parameters we can postulate transcriptional activities affected (e.g. estrogen receptor-α/GSK-3β-mediated pathways or CREB). Among both culture types, hippocampal cultures expressed higher levels of NR1 mRNA (see figure 2B) compared with cortical cultures; this difference is worth discussing even with p-value = .058 (see figure 2A). Both cultures expressed high levels of GFAP but hippocampal cultures also expressed higher levels of NF-H (see figure 1D) compared with cortical cultures (see figure 1B). The higher levels of NR1 mRNA expression in hippocampal cultures is that glia express functional NMDAR at a lower level than neurons (see Conti et al., 1999). Additionally, autoradiography using NMDAR-specific ligands depict higher density of NMDAR in hippocampal regions than cortical (Mugnaini et al., 1996). Our study showed no effect of E2 treatment on NR1 mRNA expression for both culture types (see figures 2A & B). This contradicts Jelks et al. (2007) findings where E2 treatment increased NR1 in neuronal cultures (Jelks et al., 2007), but our cultures did express higher levels of GFAP mRNA (see figures 1A & C). Few studies investigate how E2 (or lithium) influence glia but we suggest our glia cultures reached a saturation point since glia also produce estrogen – as noted in astrocytic culture media (Hu et al., 2007). E2-estrogen receptor complex have a Kd of 150pM (Sasson and Notides, 1983), therefore, our doses of .04μM E2 may have been negligible in altering NR1 mRNA expression. NR1 mRNA expression did not change in LiCl-treated cortical cultures (see figures 2A) across treatment periods which coincides with Hashimoto et al. (2002) who showed that lithium does not influence NR1 protein expression in neuronal cultures (Hashimoto et al., 2002). LiCl, however, did reduce NR1 mRNA expression in hippocampal cultures after a 24 and 48 h treatment period compared with 12 h LiCl-treatment (see figures 2B). Lithium diffuses into a cell via sodium channel but also through glutamate receptors (Kabakov et al., 1998). We clearly show that hippocampal cultures express higher levels of NR1 mRNA (see figure 2B) than cortical cultures (see figure 2A) and this may be indicative of functionally expressed NMDAR. Hippocampal NR1 mRNA down-regulation by LiCl may be caused by the influx rate of LiCl. This rate will change NR1 expression since estrogen receptor-α is involved in facilitating NMDAR subunit expression (see Watanabe et al., 1999) that is mediated by GSK-3β (see Medunjanin et al., 2005); and lithium is known as a selective inhibitor of GSK-3β (Jope, 2003).
The purpose of this study was to note the combined affect of E2 and LiCl on NR1 mRNA expression and excitotoxicity. After a 12 h treatment, NR1 expression for cortical cultures was not altered with individual treatment of E2 or LiCl; however, when LiCl was combined with E2, NR1 expression decreased after 12 h (see figure 2A). Hippocampal cultures did not display this reduction at 12 h, though (see figure 2B). Studies show that in rat cerebral cortex hormone and dopamine-stimulated adenyl cyclase activity is hindered by lithium (Newman and Belmaker, 1987). Adenyl cyclase produces cAMP, a main constituent in CREB signaling pathway. CREB is highly involved in facilitating NMDAR subunit expression (Qiang and Ticku, 2005) and reduced NR1 at 12 h may be caused by LiCl inhibiting cortical adenyl cyclase activity triggered by E2. Combined E2/LiCl did not alter NR1 expression in cortical and hippocampal cultures after 24 h treatment (see figure 2A & B). Therefore, the 12 h decrease in cortical NR1 mRNA may be caused by the fast action of extracellular LiCl concentration inhibiting adenyl cyclase activity (Campbell and Siddle, 1978) as opposed to intracellular concentration since reduced cortical NR1 mRNA reoccurred at 48 h treatment period for both culture types. This 48 h decrease in NR1 was significant compared with Control and E2 treated cortical and hippocampal cultures (see figures 2A & B). Reduced NR1 at 48 h with combined E2/LiCl occurred for both cultures indicating a similar pathway involved, possibly through the intracellular estrogen receptor-α/GSK-3β-mediated pathway (Medunjanin et al., 2005). NR1 down-regulation could be explained since estrogen receptor-α, when bound by its ligand E2, facilitates NMDAR subunit gene expression by recognizing palindromic DNA sequences (Watanabe et al., 1999); thus, E2 has a direct genomic role in NMDAR subunit expression. Additionally, response element binding of estrogen receptor-α relies on GSK-3β phosphorylation and this phosphorylation is inhibited by lithium (Medunjanin et al., 2005) since lithium is a selective inhibitor of GSK-3β (Jope, 2003). We chose such high concentrations for E2 and LiCl based on the literature, since 10mM LiCl inhibits ∼70% GSK-3β protein activity (Ryves and Harwood, 2001), and a 0.04μM concentration of E2 significantly increases bcl-2 expression – an anti-apoptotic protein – via calcium influx in cell cultures (Wu et al., 2005). Additionally, Medunjanin et al. (2001) used MELN cells treated for 48 h with 30mM LiCl which decreased estrogen receptor-α response element binding and this decrease was due to GSK-3β inhibition (Medunjanin et al., 2005). The aforementioned references provide insight into our results since LiCl significantly decreased NR1 mRNA expression in E2-treated cortical (see figure 2A) and hippocampal cultures (see figure 2B) after 48 h treatment period. Since NR1 mRNA in E2-treated cells did not change, we deduce this reduction is due to LiCl (potentially via inhibition of GSK-3β activity). Whether or not LiCl influences estrogen receptor-α/GSK-3β-mediated pathway within our experimental still remains to be elucidated. We are currently working on experiments to test these effects. Studies on NR1 protein level should be conducted under our experimental and treatment conditions. Present results are represented in mRNA expression and post-transcription and/or post-translational influences may render differences at the protein level when compared. Both estrogen and lithium alter the phosphorylation state of NMDAR subunits reducing NMDAR-mediated excitotoxicity (Dominguez et al., 2007; Hashimoto et al., 2002; Ma et al., 2004). It will be interesting to note the phosphorylated state of NMDARs within our experimental parameters; however, within the scope of this project, we provide substantial evidence on how combined E2 and LiCl (as opposed to individual treatment) alter excitotoxicity and NR1 gene expression.
NMDAR-mediated excitotoxicity characterizes many neurological diseases (see Lipton, 2005). The E2 concentration we chose for our experimental procedures (0.04μM) also provides neuroprotection for hippocampal cultures (Chen et al., 2006), and as mentioned, increases bcl-2 expression (Wu et al., 2005). E2 at 0.04μM should provide neuroprotection against excitotoxicity, however, glia may respond differently to this treatment. After pre-treating primary cultures for 48 h with E2 and/or LiCl we exposed these glial cultures to a toxic level of glutamate for 1 h. Cortical cultures displayed higher viability with LiCl treatment compared with hippocampal cultures (see figures 3B & C). NR1 (see figure 2B) and NF-H mRNA expression (see figure 1D) were higher in hippocampal cultures than cortical cultures (see figure 1B & 2A). These observations could explain the extremely low viability in treated hippocampal cultures (see figure 3C) since excitotoxicity is mediated via NMDAR signaling. The literature indicates that E2 and lithium affect NMDAR, but with opposing actions. On the one hand, estrogen facilitates NMDAR-regulated calcium influx (Wu et al., 2005), but studies show estrogen's neuroprotective qualities against glutamate insult are through calcium-independent pathways (Perrella and Bhavnani, 2005). On the other hand, lithium reduces calcium influx and NMDAR-mediated glutamate excitotoxicity (Chuang et al., 2002; Hashimoto et al., 2003). Normally, inducing excitotoxicity reduces viability among neuronal cultures, but glia are more tolerant to this insult (see Matute et al., 2002). Our cortical cultures exhibited high glia-associated GFAP mRNA expression (figures 1A). Although hippocampal cultures showed this as well (see figures 1C), hippocampal cultures also expressed higher levels of neuron-associated NF-H mRNA expression compared with cortical cultures (see figures 1B & D). Results from FDA/PI assay (figures 3A-C) strengthen densitometric data since cortical and hippocampal cultures tolerated excitotoxicity as observed with Control, which was not significantly affected by such a high dosage of glutamate (see figures 3A-C). Cortical cultures expressing higher levels GFAP mRNA (see figure 1A) also explain increased cell viability in Control and LiCl-treated cortical cultures (see figure 3B). Treated hippocampal cultures (figure 3C) did not display this protective quality since hippocampal cultures also expressed higher levels of NF-H mRNA (see figure 1D).
Previous literature specifies that both estrogen and lithium act as neuroprotective agents and may be used as a palliative treatment for neurodegeneration (Chuang et al., 2002; Goodenough et al., 2003; Rowe and Chuang, 2004). Our study is the first to show that combined E2 and LiCl affect NR1 mRNA expression and excitotoxicity in cortical and hippocampal primary mixed brain cell cultures. Studies of this caliber are wanting since little is known about how E2 and LiCl influence molecular processes of the brain. Analysis of NMDAR subunit mRNA and protein expression using post-mortem Alzheimer's disease (AD) brains show a decrease in NR1 and NR2B in the hippocampal formation with advancement of the disease (Mishizen-Eberz et al., 2004). In a study using ovariectomized rats, estrogen-replacement did not affect NMDAR subunit mRNA expression; although, duration of ovariectomy did affect this expression (Adams et al., 2001). In recent years, post-menopausal women are diagnosed with AD more often than men and this statistic is related to estrogen deficiency (Garcia-Segura et al., 2001). Estrogen replacement therapy (ERT) proves to be beneficial to post-menopausal women in reducing the risk of developing AD (see Garcia-Segura et al., 2001). Studies demonstrate that the dominant form of estrogen, estradiol, plays an important role in neuroprotection and enhancement of learning and memory (Henderson et al., 1994). ERT is a subject of debate due to the risk of developing breast cancer. This is marked by an increased incidence of lobular carcinoma in post-menopausal women under ERT (Newcomer et al., 2003) and women suffering from depression during peri- and post-menopause are often prescribed lithium (Burt and Rasgon, 2004; Kukopulos et al., 1985). Estrogen and lithium also affects the dopamine pathway, a pathway implicated in many clinical disorders and neurodegenerative diseases (Morissette et al., 2008; Silverstone, 1985). An increase in dopaminergic pathways is a characteristic of bipolar disorder (manic-depression) and lithium is readily prescribed as a prophylactic for this disorder (Silverstone, 1985). Studies show that combined E2/LiCl alter serotonin and dopamine metabolites. Ovariectomized rats treated with E2 show no change in frontal cortices levels of serotonin, dopamine or dopamine metabolites. When E2 is combined with LiCl, though, dopamine is greatly decreased but show higher levels of serotonin and dopamine metabolites Morissette (Morissette and Di Paolo, 1996). Additionally, NMDA agonist increase dopamine release modulated by hormones (Cabrera et al., 2002). It will be worth studying the involvement of combined E2 and LiCl in the dopamine pathway and how these two agents affect NMDAR expression and function. An understanding of underlying molecular mechanism in regards to how E2 and LiCl interact is lacking. The present study introduces and advances perspectives for additional studies directed towards developing palliative therapies for neurodegeneration and postmenopausal-related cognitive decline.
4. Experimental Procedures
4.1. Primary mixed brain cell cultures
C57BL/6J mice were bred at Florida International University. Animals were kept in an environment of 22°C temperature, 60% humidity, within polycarbonate transparent cages (26.7 cm × 20.6 cm × 14 cm) on a 12 h day-night cycle with free access to water and food. All experiments were approved by the Institutional Animal Care and Use Committee. Primary cultures were harvested from E18.5 C57BL/6J mice. Pregnant mice (n = 5) were euthanized by cervical dislocation, E18.5 fetal mice were then removed via caesarian section, decapitated, brains removed, and placed in ice-cold Earle's balanced salt solution (EBSS; Hyclone, Utah). The cortex and hippocampus from each hemisphere were isolated under a dissecting microscope and washed with fresh ice-cold EBSS. Tissue was mechanically triturated, passed through a 70μm pore filter, and cells were counted using a hemocytometer. Disassociated cells were randomly plated (6 × 106 for cortical cultures and 3 × 106 for hippocampal cultures) on poly-d-lysine (100μg/mL; Sigma-Aldrich, Missouri) coated 12-well plates containing B27/Neurobasal medium (Invitrogen, California) with 0.5mM L-glutamine and 4μg/mL gentamicin. Additional 12-well plates, with poly-d-lysine (100μg/mL) coated coverslips were set aside for viability assessment. Cells were maintained in a humidified, 5%CO2 atmosphere incubator with a set temperature of 37°C. After 4 days in culture, the media was removed entirely and replaced with fresh media; half of the media was changed every 4 days, thereafter, until E2 and/or LiCl treatment.
4.2. Chemicals and Treatments
After twelve days in culture, the media was removed entirely and replaced with media treated with 10mM LiCl (Sigma-Aldrich, Missouri), 0.04μM E2 (Sigma-Aldrich, Missouri), or combined E2 and LiCl. Duration of treatment for each group was 12, 24, and 48 h. Additionally, cell viability was measured using 48 h pretreated cells exposed to 100μM glutamatic acid (Sigma-Aldrich, Missouri) for 1 h at 37°C.
4.3. Experimental Techniques
4.3.1. RNA Isolation and cDNA Synthesis
Total RNA was extracted from cultured cells after treatment with TRIzol reagent (GIBCO, California), according to manufacturer's protocol. Briefly, cultures were rinsed with ice-cold DPBS, TRIzol Reagent (600μL-1mL) was added directly into each well, and cultures were homogenized. Homogenized cells were then transferred to a fresh tube, and incubated at room temperature. Chloroform was added and centrifuge at 10, 000 rpm at 4°C. Total RNA (aqueous phase) was transferred to a sterile tube and allowed to precipitate with isopropanol and glycogen overnight at -20°C. RNA was pelleted via centrifugation at 10, 000 rpm at 4°C, supernatant was decanted and RNA pellet was washed twice with 75 % ethanol. Pellets were then air dried and resuspended with DEPC-treated water. DNA contaminants were removed with the RQ1 DNase kit (Promega, Wisconsin). Samples were stored at -80°C until further processed.
Reverse transcription (RT) was performed using SUPERSCRIPT™ II RNase H-free reverse transcriptase (Invitrogen, California), according to the manufacturer's protocol. Briefly, 500ng of total RNA was reverse transcribed with 0.05μg/μl of Oligo (dT)12-18 at 65°C for 5 minutes. cDNA was synthesized with 5mM MgCl2, 10mM DTT, and 2.5 units Superscript II and incubated at 42°C for 50 minutes; reaction was terminated at 70°C for 15 minutes. RNase H (Invitrogen, California) was added once the first strand was synthesized to remove any remaining RNA. RNase H was incubated at 37°C for 20 minutes.
4.3.2. Cell-Typing Cultures using RT-PCR and Gel Electrophoresis
cDNA was amplified via real time polymerase chain reaction (PCR) using NF-H (forward primer 5′-AGCCCAAGGACTCTACAGCA-3′; reverse primer, 5′-CTTTGGCTTTTCCGTCTCTG-3′) and GFAP (forward primer 5′-AGAAAACCGCATCACCATTC-3′; reverse primer, 5′-TCACATCACCACGTCCTTGT -3′) and GoTaq® (Promega, Wisconsin). Cycling parameters for an Eppendorf Mastercycler Gradient were set for: denaturing at 95°C for 30 seconds, annealing temperature of 57°C or 64°C for 30 seconds, and extension at 72°C for 30 seconds, for a total of 30 cycles, followed by a final extension at 72°C for 10 minutes. After amplification, samples were subjected to gel electrophoresis using a 1.5% agarose gel stained with ethidium bromide and photographed using Digital Imaging Filter/Ethidium Bromide (Fotodyne, Wisconsin) under UV light. Resulting band intensities were based on two replicates and analyzed using ImageJ software band analyzer (Rasband, 1997-2007). Measurements are represented in arbitrary units and obtained by dividing the pixel integrated density (integrated density = area × mean pixel value) from each band of NF-H or GFAP by the absolute intensity of the standard – HPRT.
4.3.3. Quantitative Real Time RT-PCR
cDNA was amplified via real time PCR using SYBR Green PCR master mix (ABgene, New York), 200-300nM of forward and reverse primers using iCycler (BioRad, California) with IQ iCycler software (version 3.1). Cycling parameters were set at: 95°C 15s, 57°C 30s, and extension at 72°C for 30s, for a total of 40 cycles, followed by a final extension at 72°C for 10 minutes. The specific primer pairs were: NMDAR subunit NR1: forward primer, 5′-ACTCCCAACGACCACTTCAC-3; reverse primer, 5′-GTAGACGCGCATCATCTCAA-3′; HPRT: forward primer, 5′-GGAGCGGTAGCACCTCCT-3′; reverse primer, 5′-AATCCAGCAGGTCAGCAAAG-3′. All samples were compared with a standard curve comprised of pDNA of HPRT and NR1 with pDNA generated using TOPO TA Cloning® Kit (Invitrogen, California). Readings were normalized by dividing mRNA starting quantity (SQ) of NR1 by the house-keeping gene HPRT mRNA SQ; output is in femtograms (fg) of mRNA.
4.3.4. Cell Viability – Fluorescein Diacetate/Propidium Iodide Assay segment
Cortical and hippocampal cells were cultured on 18mm coverslips cultures and pretreated for 48 h with E2, LiCl, and combined E2 and LiCl (as described previously). Excitotoxicity was induced for 1 h with 100μM glutamate. Cell viability of cultures was determined after glutamate excitotoxicity through the use of a fluorescein diacetate-propidium iodide labeling technique (Jones and Senft, 1985). Briefly, cells were quickly rinsed with ice-cold Dulbecco's Phosphate Buffer Saline (DPBS). Approximately 150μL cocktail of 2μg/mL of fluorescein diacetate and 0.6μg/mL propidium iodide was added directly to the coverslip with adhered cells and incubated at room temperature. Each 18mm coverslip was placed against a grid and images were captured from five subdivisions on the grid (upper right, upper left, lower right, lower left, and center). Images were captured from 3 replicates per subdivision – a total of 15 images per coverslip – with a Leica inverted epifluorescent microscope with a QImaging Micropublisher 3.3 cooled RTV camera and processed using ImageJ Cell Counter. For a comparison prior to any experimental procedure we assayed a separate culture designated as Initial Viability.
4.3.5. Statistical Analysis
Data is presented as the mean ± S.E.M and statistical significance was determined by ANOVA followed by Fisher's LSD post-hoc testing. Significant differences have a p-value < .05. All results were obtained from two separate experimental procedures – except for glutamate excitotoxicity which was based on triplicates from one experiment (see section 4.3.4 in Experimental Procedures). Each level for all factors was randomly determined.
Acknowledgments
Funding has been provided in part by intramural funding through the Biomedical Research Initiative and NIH/National Institute of Child Health and Human Development through the Extramural Associates Research Development Award Program. We will also like to acknowledge the laboratories of Dr. R. J. Herrera, Dr. F. Noriega and Dr. L. Kim.
Abbreviations used
- AD
Alzheimer's disease
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- cDNA
complementary deoxyribonucleic acid
- CREB
Cyclic adenosine monophosphate (cAMP) response element binding protein
- E2
17β-estradiol
- FDA/PI
fluorescein diacetate/propidium iodide
- GFAP
glial fibrillary acidic protein
- GSK-3β
glycogen synthase kinase-3beta
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- Fisher's LSD
Fisher's least significant difference
- LiCl
lithium chloride
- mRNA
messenger ribonucleic acid
- NF-H
neurofilament-heavy chain
- NMDAR
N-methyl-d-aspartate receptor
- NR1
N-methyl-d-aspartate receptor subunit 1
- RT-PCR
reverse transcription polymerase chain reaction
Footnotes
Clarification of Terms: 1. Cellular and Molecular Biology of Nervous Systems
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Oung T, Morrison JH, Gore AC. Length of Postovariectomy Interval and Age, but Not Estrogen Replacement, Regulate N-Methyl-d-Aspartate Receptor mRNA Levels in the Hippocampus of Female Rats. Experimental Neurology. 2001;170:345–356. doi: 10.1006/exnr.2001.7716. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Aloe L, Jiménez-Vasquez P, Mathé AA. Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depression. The International Journal of Neuropsychopharmacology. 2003;6:225–231. doi: 10.1017/S1461145703003468. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. Journal of Neuroscience Research. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Burt VK, Rasgon N. Special considerations in treating bipolar disorder in women. Bipolar Disorders. 2004;6:2–13. doi: 10.1046/j.1399-5618.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- Cabrera RJ, Bregonzio C, Laconi M, Mampel A. Allopregnanolone Increase in Striatal N-Methyl-D-aspartic Acid Evoked [3H]Dopamine Release Is Estrogen and Progesterone Dependent. Cellular and Molecular Neurobiology. 2002;22:445–454. doi: 10.1023/a:1021015705597. [DOI] [PubMed] [Google Scholar]
- Campbell AK, Siddle K. Effect of replacement of extracellular sodium ions and of D-600 on the activation by adrenalin of adenylate cyclase in intact pigeon erythrocytes. Molecular and Cellular Endocrinology. 1978;11:79–89. doi: 10.1016/0303-7207(78)90034-5. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Molecular and Cellular Neuroscience. 2004;25:363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, Yan Z. Glycogen Synthase Kinase 3 Regulates N-Methyl-D-aspartate Receptor Channel Trafficking and Function in Cortical Neurons. Molecular Pharmacology. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Brinton RD. Dose and Temporal Pattern of Estrogen Exposure Determines Neuroprotective Outcome in Hippocampal Neurons: Therapeutic Implications. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Chen RW, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disorders. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Melone M. Expression of NR1 and NR2A/B subunits of the NMDA receptor in cortical astrocytes. Glia. 1996;17:254–258. doi: 10.1002/(SICI)1098-1136(199607)17:3<254::AID-GLIA7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and Glial Localization of NR1 and NR2A/B Subunits of the NMDA Receptor in the Human Cerebral Cortex. Cerebral Cortex. 1999;9:110–120. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Liu R, Baudry M. 17β-Estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-d-aspartate receptor phosphorylation in cortical synaptoneurosomes. Jounal of Neurochemistry. 2007;101:232–240. doi: 10.1111/j.1471-4159.2006.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in Neurobiology. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Schafer M, Behl C. Estrogen-induced cell signalling in a cellular model of Alzheimer's disease. The Journal of Steroid Biochemistry and Molecular Biology. 2003;84:301–305. doi: 10.1016/s0960-0760(03)00043-8. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Schleusner D, Pietrzik C, Skutella T, Behl C. Glycogen synthase kinase 3β links neuroprotection by 17β-estradiol to key Alzheimer processes. Neuroscience. 2005;132:581–589. doi: 10.1016/j.neuroscience.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3β and facilitated by lithium. Journal of Neurochemistry. 2001;78:1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. Journal of Neurochemistry. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Fujimaki K, Jeong MR, Christ L, Chuang DM. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-d-aspartate receptor-mediated excitotoxicity. FEBS Letters. 2003;538:145–148. doi: 10.1016/s0014-5793(03)00167-4. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer's disease cases and nondemented control subjects. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. Journal of Neuroscience Research. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- Hongisto V, Smeds N, Brecht S, Herdegen T, Courtney MJ, Coffey ET. Lithium Blocks the c-Jun Stress Response and Protects Neurons via Its Action on Glycogen Synthase Kinase 3. Molecular and Cellular Biology. 2003;23:6027–6036. doi: 10.1128/MCB.23.17.6027-6036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Cai WQ, Wu XG, Yang Z. Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience. 2007;144:1229–1240. doi: 10.1016/j.neuroscience.2006.09.056. [DOI] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol Targets Synaptic Proteins to Induce Glutamatergic Synapse Formation in Cultured Hippocampal Neurons: Critical Role of Estrogen Receptor-α. Journal of Neuroscience. 2007;27:6903–6913. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends in Pharmacological Sciences. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Kabakov AY, Karkanias NB, Lenox RH, Papke RL. Synapse-specific accumulation of lithium in intracellular microdomains: A model for uncoupling coincidence detection in the brain. Synapse. 1998;28:271–279. doi: 10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kukopulos A, Minnai G, Muller-Oerlinghausen B. The influence of mania and depression on the pharmacokinetics of lithium: A longitudinal single-case study. Journal of Affective Disorders. 1985;8:159–166. doi: 10.1016/0165-0327(85)90039-4. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA Receptors Mediate Neuron-to-Glia Signaling in Mouse Cortical Astrocytes. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. The Journal of Comparative Neurology. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. Journal of Neuroimmunology. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. The Molecular Basis of Memantine Action in Alzheimer's Disease and Other Neurologic Disorders: Low-affinity, Uncompetitive Antagonism. Current Alzheimer Research. 2005;2:155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang GY, Liu Y, Yan JZ, Hao ZB. Lithium suppressed Tyr-402 phosphorylation of proline-rich tyrosine kinase (Pyk2) and interactions of Pyk2 and PSD-95 with NR2A in rat hippocampus following cerebral ischemia. Neuroscience Research. 2004;49:357–362. doi: 10.1016/j.neures.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Ibarretxe G, Sánchez-Gómez MV. Excitotoxicity in glial cells. European Journal of Pharmacology. 2002;447:239–246. doi: 10.1016/s0014-2999(02)01847-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Genome and Hormones: Gender Differences in Physiology: Invited Review: Estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of Applied Physiology. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Medunjanin S, Hermani A, De Servi B, Grisouard J, Rincke G, Mayer D. Glycogen Synthase Kinase-3 Interacts with and Phosphorylates Estrogen Receptor α and Is Involved in the Regulation of Receptor Activity. Journal of Biological Chemistry. 2005;280:33006–33014. doi: 10.1074/jbc.M506758200. [DOI] [PubMed] [Google Scholar]
- Mishizen-Eberz AJ, Rissman RA, Carter TL, Ikonomovic MD, Wolfe BB, Armstrong DM. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer's disease pathology. Neurobiology of Disease. 2004;15:80–92. doi: 10.1016/j.nbd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Acute effect of 17[beta]-estradiol and lithium on ovariectomized rat brain biogenic amines metabolism. Journal of Psychiatric Research. 1996;30:95. doi: 10.1016/0022-3956(95)00037-2. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D'Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. The Journal of Steroid Biochemistry and Molecular Biology. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, van Amsterdam F, Ratti E, Trist D, Bowery N. Regionally different N-methyl-D-aspartate receptors distinguished by ligand binding and quantitative autoradiography of [3H]-CGP 39653 in rat brain. British Journal of Pharmacology. 1996;119:819–828. doi: 10.1111/j.1476-5381.1996.tb15746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer LM, Newcomb PA, Potter JD, Yasui Y, Trentham-Dietz A, Storer BE, Longnecker MP, Baron JA, Daling JR. Postmenopausal hormone therapy and risk of breast cancer by histologic type United States. Cancer Causes and Control. 2003;14:225–233. doi: 10.1023/a:1023634907723. [DOI] [PubMed] [Google Scholar]
- Newman ME, Belmaker RH. Effects of lithium in vitro and ex vivo on components of the adenylate cyclase system in membranes from the cerebral cortex of the rat. Neuropharmacology. 1987;26:211–217. doi: 10.1016/0028-3908(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-d-aspartate receptor-mediated calcium influx. PNAS. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Chuang DM. Lithium Increases Transcription Factor Binding to AP-1 and Cyclic AMP-Responsive Element in Cultured Neurons and Rat Brain. Journal of Neurochemistry. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JTR, Bortolotto ZA, Wang YT, Collingridge GL. LTP Inhibits LTD in the Hippocampus via Regulation of GSK3β. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Perrella J, Bhavnani B. Protection of cortical cells by equine estrogens against glutamate-induced excitotoxicity is mediated through a calcium independent mechanism. BMC Neuroscience. 2005;6:34–51. doi: 10.1186/1471-2202-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Ticku MK. Role of AP-1 in ethanol-induced N-methyl-d-aspartate receptor 2B subunit gene up-regulation in mouse cortical neurons. Journal of Neurochemistry. 2005;95:1332–1341. doi: 10.1111/j.1471-4159.2005.03464.x. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda: 19972007. [Google Scholar]
- Rocha E, Rodnight R. Chronic Administration of Lithium Chloride Increases Immunodetectable Glial Fibrillary Acidic Protein in the Rat Hippocampus. Journal of Neurochemistry. 1994;63:1582–1584. doi: 10.1046/j.1471-4159.1994.63041582.x. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Chuang D. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Reviews in Molecular Medicines. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ. Lithium Inhibits Glycogen Synthase Kinase-3 by Competition for Magnesium. Biochemical and Biophysical Research Communications. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- Sasson S, Notides AC. Estriol and Estrone Interaction with the Estrogen Receptor: Temperature-induced modulation of the cooperative binding of [3H] estriol and [3H] estrone to the estrogen receptor. The Journal of Biological Chemistry. 1983;258:8113–8117. [PubMed] [Google Scholar]
- Silverstone T. Dopamine in manic depressive illness : A pharmacological synthesis. Journal of Affective Disorders. 1985;8:225–231. doi: 10.1016/0165-0327(85)90020-5. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen Regulates the Development of Brain-Derived Neurotrophic Factor mRNA and Protein in the Rat Hippocampus. Journal of Neuroscience. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson FA. Subunit Characterization of NMDA Receptors. Current Drug Targets. 2001;2:233–239. doi: 10.2174/1389450013348461. [DOI] [PubMed] [Google Scholar]
- Van Eldik LJ, Thompson WL, Ranaivo HR, Behanna HA, Martin Watterson D. Glia Proinflammatory Cytokine Upregulation as a Therapeutic Target for Neurodegenerative Diseases: Function-Based and Target-Based Discovery Approaches. International Review of Neurobiology. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Inoue S, Hiroi H, Orimo A, Muramatsu M. NMDA receptor type 2D gene as target for estrogen receptor in the brain. Molecular Brain Research. 1999;63:375–379. doi: 10.1016/s0169-328x(98)00304-0. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17β-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]