Abstract
Purpose
The humanized anti-CD74 monoclonal antibody, milatuzumab, is in clinical evaluation for the therapy of multiple myeloma (MM). The ability of milatuzumab to increase the efficacy of bortezomib, doxorubicin, and dexamethasone was examined in three human CD74+ MM cell lines, CAG, KMS11, KMS12-PE, and one CD74− MM cell line, OPM-2.
Experimental Design
Activity of milatuzumab as a monotherapy and combined with the drugs was evaluated by studying in vitro cytotoxicity, signaling and apoptotic pathways, and in vivo therapeutic activity in SCID mouse models of MM.
Results
Given as a monotherapy, crosslinked milatuzumab, but not milatuzumab alone, yielded significant anti-proliferative effects in CD74+ cells in vitro. The combination of crosslinked milatuzumab with bortezomib, doxorubicin, or dexamethasone caused more growth inhibition than either crosslinked milatuzumab or drug alone, producing significant reductions in the IC50 of the drugs when combined. Efficacy of combined treatments was accompanied by increased levels of apoptosis measured by increases of activated caspase-3 and hypodiploid DNA. Both milatuzumab and bortezomib affect the NF-κB pathway in CAG MM cells, although in opposite direction, with milatuzumab inhibiting and bortezomib stimulating phosphorylated NF-κB. In CAG and KMS11 SCID-mouse xenograft models of disseminated MM, milatuzumab more than doubled median survival time, compared to up to a 33% increase in median survival with bortezomib, but no significant benefit with doxorubicin. Moreover, combining milatuzumab and bortezomib increased survival significantly compared to either treatment alone.
Conclusions
The therapeutic efficacies of bortezomib, doxorubicin, and dexamethasone are enhanced in MM cell lines when given in combination with milatuzumab, suggesting testing these combinations clinically.
Keywords: CD74, milatuzumab, monoclonal antibody, multiple myeloma, therapy
INTRODUCTION
Multiple myeloma (MM) is the second most prevalent blood cancer after non-Hodgkin lymphoma (NHL) (1, 2). It represents approximately 1% of all cancers and 2% of all cancer deaths. Approximately 50,000 Americans currently have myeloma, and the American Cancer Society estimates 19,920 new cases of myeloma and 10,690 deaths in 2008. Currently available therapies for myeloma include chemotherapy and stem cell transplantation, as well as new emerging therapies and combination therapy regimens that are being tested in clinical trials (2, 3). High-dose melphalan followed by autologous stem cell transplantation is currently the standard of care because of its high response rate and relatively low morbidity and mortality. Because stem cell transplantation is neither curative nor applicable to all patients and improved complete response rates have been seen in recent clinical trials, the National Comprehensive Cancer Network (NCCN) issued treatment guidelines recently that include recommendations for the use of newer treatment options for myeloma patients. Lenalidomide and bortezomib regimens involving combination therapy with doxorubicin and/or dexamethasone are now recommended in initial therapy protocols for transplant candidates and for treatment of relapsed/refractory disease. Combination of melphalan and prednisone with thalidomide or bortezomib are recommended as initial therapy for non-transplant candidates. Thus, there has been considerable progress in the field of myeloma therapy. Although cures have not been documented, molecular complete responses have been achieved with some of the new therapies. However, relapses still occur after molecular complete response, usually after a longer period of event-free survival.
Despite the substantial progress, the unmet need for improved therapy of multiple myeloma has led to the evaluation of cell surface targets expressed by the myeloma cells. Although antigens such as CD19 and CD20 are important targets for NHL, they are expressed in only a minority of myeloma cases. In contrast, the cell surface protein CD74 is expressed at fairly high levels in nearly 90% of MM clinical specimens, but not on most normal human cells (4). In addition, the humanized anti-CD74 monoclonal antibody, milatuzumab (hLL1), has in vitro growth inhibitory effects on MM cell lines and therapeutic effects in MM models (5, 6). Based on these preclinical studies, milatuzumab is currently in clinical evaluation for therapy of multiple myeloma. Here we provide in vitro and in vivo data corroborating the therapeutic efficacy of milatuzumab in human MM model systems and expand our observations to show that this mAb is more effective than key therapeutic agents for MM treatment, including bortezomib, doxorubicin, and dexamethasone, when each agent is given alone. In addition, the efficacies of the chemotherapeutics are enhanced when given in combination with milatuzumab.
METHODS
Cells
The cell lines were obtained as follows: KMS11 and KMS12-PE from Dr. T. Otsuki (Kawasaki Medical School, Okayama, Japan), CAG from Dr. Joshua Epstein (University of Arkansas, Fayetteville, AR), and OPM-2 from Dr. Kenji Oritani (Osaka University, Osaka, Japan). The cells were grown as suspension cultures in DMEM (Life Technologies, Gaithersburg, MD), supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and L-glutamine (2 mM).
Antibodies and drugs
Antibodies LL1 (7) (the parental murine mAb formerly called EPB-1), milatuzumab (6) (also called hLL1 or IMMU-115; the humanized IgG1 version), and hMN-14 (8) (labetuzumab, anti-CD66e, anti-carcinoembryonic antigen or -CEACAM5 IgG1, used here as a humanized isotype control), were provided by Immunomedics, Inc. (Morris Plains, NJ). Bortezomib was purchased from Millennium Pharmaceuticals (Cambridge, MA). Doxorubicin and dexamethasone were purchased from Florida Infusion (Palm Harbor, FL). The murine monoclonal antibody 2B8 (anti-CD20) was purified from hybridoma supernatant of cells obtained from the American Type Culture Collection. B-B4 (anti-CD138) was purchased from BD Biosciences.
Immunophenotyping
Determination of antigen expression levels on MM cells was performed by indirect immunofluorescence assays using FITC-goat anti–mouse IgG, purchased from Invitrogen Corporation (Carlsbad, CA), as described previously (9). All flow cytometry experiments were performed and analyzed using a FACSCalibur (Becton Dickinson, San Jose, CA).
In-vitro 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
The cytotoxicity assay was based on the method of Mosmann (10). Briefly, cell lines were plated at 1–2×104 cells/well (100 μl) in 96-well plates, to which antibodies and/or drugs were added (100 μl). After incubation for 4 days at 37°C in a humidified CO2 (5%) incubator, 25 μl of 5.0 mg/ml MTT were added, and the cells were incubated for an additional 4 h at 37°C. Plates were then centrifuged and supernatants were removed. Pellets were dissolved using 100 μl DMSO/well and optical density was measured at 570 nm on a microplate reader (Molecular Devices, Sunnyvale, CA). Because unlabeled milatuzumab was reported previously to require crosslinking for cytotoxic activity (6), goat anti-human IgG (GAH) was added to some of the wells. Milatuzumab was used at a final concentration of 5 μg/ml and GAH was used at 20 μg/ml. Percent growth inhibition and IC50 values were determined using 4 replicates.
DNA fragmentation
Flow cytometric analysis of cellular DNA was performed following propidium iodide (PI) staining (11, 12). Cells were placed in 24-well plates (1.5 to 3 × 105 cells per well) and treated with drugs (at concentrations indicated for each experiment) or mAbs (5 μg/mL) in the presence or absence of a second antibody (20 μg/mL). Percent apoptotic cells (hypodiploid cells) was determined following a 48-h incubation.
Cleaved caspase-3
Cells were incubated in the presence or absence of the drugs (at concentrations indicated for each experiment) and/or mAbs for 48 h. Changes in the intracellular levels of cleaved caspase-3 were measured using FITC–conjugated rabbit anti-activated caspase-3 (BD Bioscience, San Jose CA) as per the manufacturer’s directions. Analyses were performed on the FACSCalibur.
Western blots
Cells were cultured in the presence or absence of the mAbs and/or drugs for the indicated times, pelleted, washed three times in PBS, then lysed in ice cold RIPA buffer containing 100 μg/ml PMSF and 1 μg/ml aprotinin on ice for 30 min. Bortezomib was used at 1 ng/ml. Milatuzumab was used at a final concentration of 5 μg/ml and GAH was used at 20 μg/ml. The lysates were centrifuged at 1200 × g for 15 min at 4°C. Supernatants were removed and separated on 10–20% SDS-PAGE (Tris-HCl, Ready Gel; BioRad, Hercules, CA) followed by transfer to Immobilon-P membrane (Millipore, Bedford, MA). Membranes were blocked with 5% nonfat dry milk-0.05% Tween-20 (Sigma-Aldrich Corp. St. Louis, MO) in PBS for 1 h and then incubated overnight in the presence of antibody at 4°C. Primary antibodies were from Cell Signaling Technology (Beverly, MA) and were used at 1:1000 dilution, except Bid from Trevigen (Gaithersburg, MD) also used at 1:1000, and Mcl-1 from BD Pharmingen and Bcl-2 from Santa Cruz Biotechnology (Santa Cruz, CA) used at 1:500. The blots were incubated in secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences, Piscataway, NJ) for 1 h, followed by chemiluminescent detection (ECL, Amersham).
In-vivo studies in SCID mice bearing disseminated MM tumors
For studies on the therapeutic effect of the mAbs and drugs in CAG-bearing SCID mice (female, 6 to 8 weeks old, Charles River Laboratories, Frederick, MD), the mice were immunosuppressed by pretreatment with fludarabine and cyclophosphamide 3 days before intravenous injection of 5–10 × 106 tumor cells, as previously described (6). Pretreatment with fludarabine and cyclophosphamide was not used for implantation of KMS11 or KMS12-PE. Dosing is described in figure legends. Mice were examined daily for signs of distress or hind leg paralysis, and weighed weekly. Paralysis of the hind legs or a weight loss of >20% was used as the survival endpoint. Animals were euthanized at these endpoints. Experiments used groups of 8–10 mice. Animal studies were performed under protocols approved by the Institutional Animal Care and Use Committee.
Statistical considerations
For in-vitro studies, means and standard deviations were calculated. Differences between treatment groups were compared using the Students t-test. In the in-vivo studies, overall survival was evaluated graphically by the methods of Kaplan and Meier. Pairwise comparisons between specific groups were made using log-rank statistics. Significance for all tests was declared at P values less than 0.05.
RESULTS
In vitro cytotoxicity of milatuzumab on multiple myeloma cell lines
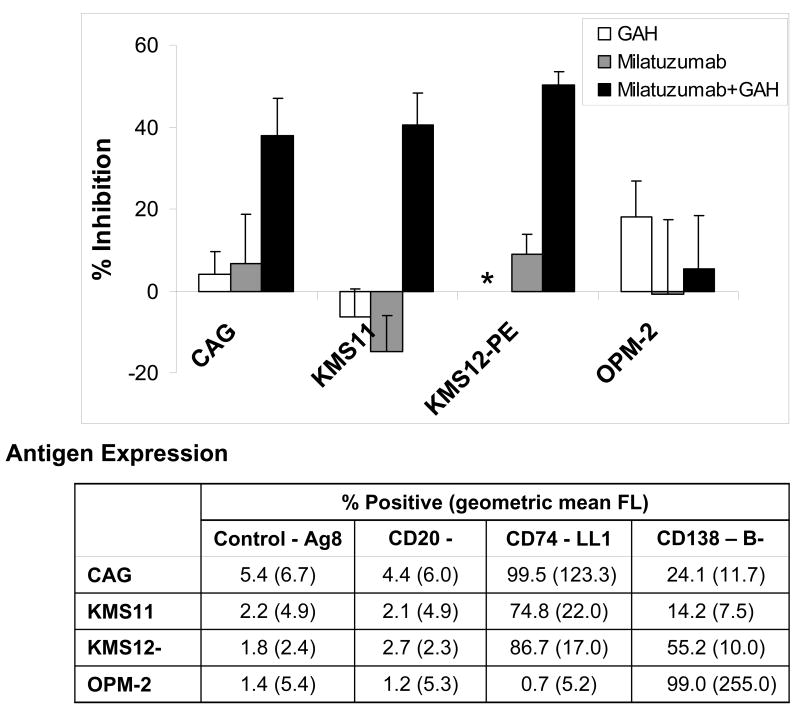
The cytotoxicity of milatuzumab was investigated on cell lines derived from MM patients, alone or crosslinked with GAH. CAG, KMS11, and KMS12-PE are positive for CD138 (syndecan-1) and CD74, and CD20-negative. OPM-2 is CD138-positive, but does not express cell surface CD74 or CD20. Antigen expression of these cell lines is tabulated in the table in figure 1. As shown in Figure 1, the CD74+ cell lines are sensitive to killing by crosslinked milatuzumab but not milatuzumab in the absence of crosslinking, or the crosslinking antibody (GAH) alone. For example, in the representative data shown, milatuzumab+GAH yielded 38.0±8.9% inhibition (P=0.0025) in CAG cells compared to 5.5±12.8% inhibition in OPM-2 cells (P=0.54) relative to untreated cells. The absence of cytotoxicity of crosslinked milatuzumab in OPM-2, which is CD74−, demonstrates specificity. Thus, crosslinked milatuzumab specifically and effectively kills CD74+ multiple myeloma cells in vitro.
Figure 1. Effects of milatuzumab and GAH second antibody on proliferation of cell lines.
Anti-proliferative effects of milatuzumab were determined by MTT assays. Cells were cultured with 5 μg/ml milatuzumab with or without 20 μg/ml second antibody for crosslinking to mimic the role of effector cells or crosslinking molecules present in vivo. Percent inhibition is calculated relative to untreated control cells. Error bars represent standard deviations of 4 replicates. *, not determined.
In-vitro cytotoxicity of bortezomib, dexamethasone, and doxorubicin with and without milatuzumab
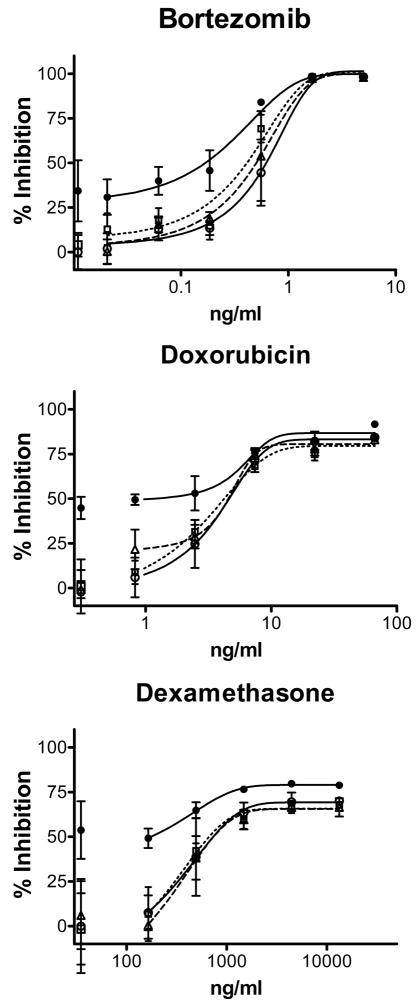
MTT assays were performed to examine the effects of the drugs, alone and combined with milatuzumab or milatuzumab+2nd antibody (GAH). Representative titrations are shown in Figure 2 for CAG (data not shown for KMS11, KMS12-PE, and OPM-2). Results from these titrations were used to calculate IC50 values of the drugs alone and in the presence of milatuzumab+GAH, shown in Table 1. Crosslinked milatuzumab, but not milatuzumab alone, yielded significant reductions in the IC50 values of bortezomib and doxorubicin in CAG, KMS11, and KMS12PE. For example, in CAG, crosslinked milatuzumab led to decreases of 6.7- 3.4-, and 13.8-fold in the IC50 values of doxorubicin, bortezomib, and dexamethasone, respectively. Crosslinked milatuzumab did not affect the IC50 values in the CD74-negative cell line, OPM-2. KMS11 and KMS12-PE were relatively insensitive to dexamethasone. The IC50 was not reached using dexamethasone concentrations up to 40 μg/ml. Dexamethasone was not tested on OPM-2.
Figure 2. In vitro cytotoxicity of bortezomib, dexamethasone, and doxorubicin with and without milatuzumab in CAG.
Cells were cultured with the drugs, milatuzumab (5 μg/ml), and GAH second antibody (20 μg/ml) as shown, then assayed using MTT. Percent inhibition of absorbance at 570 nm, relative to media only, was measured. ○, solid line, drug only; △, dashed line, drug + milatuzumab; □, dotted line, drug + GAH; ●, solid line, drug + milatuzumab + GAH. Error bars represent standard deviations of 4 replicates.
TABLE 1. Combining Milatuzumab with Drugs in MM cell lines.
IC50 values were determined using MTT cytotoxicity assays. Cells were incubated with the indicated drugs in the absence (−) or presence (+) of milatuzumab and GAH for four days.
| IC50 (ng/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|
| CAG | KMS11 | KMS12-PE | OPM-2 | |||||
| − | + | − | + | − | + | − | + | |
| Doxorubicin | 7.4±2.1 | 1.1±0.7 | 16.7±3.6 | 0.13±0.10 | 62.1±2.6 | 5.8±4.5 | 28.1±4.1 | 27.9±4.0 |
| 6.7-fold, P= 0.0063 | 128-fold, P=0.0027 | 10.7-fold, P=0.0205 | unchanged, P=0.6360 | |||||
|
| ||||||||
| Bortezomib | 0.40±0.08 | 0.12±0.06 | 0.45±0.04 | 0.18±0.02 | 1.36±0.04 | 0.50±0.14 | 0.53±0.04 | 0.52±0.02 |
| 3.4-fold, P= 0.0015 | 2.5-fold, P=0.0002 | 2.7-fold, P=0.0006 | unchanged, P=0.6953 | |||||
|
| ||||||||
| Dexamethasone | 20,038±550 | 145±86 | >40,000 | § | >40,000 | 76±99 | ND | ND |
| 13.8-fold, P= 0.0059 | ||||||||
IC50 value were not calculated because the combination of milatuzumab plus GAH yielded ≥50% inhibition of proliferation relative to untreated cells in the absence of drug.
Thus, the combination of crosslinked milatuzumab (milatuzumab+GAH) with bortezomib, doxorubicin, or dexamethasone causes more growth inhibition than either crosslinked milatuzumab or drug alone. This can be clearly observed by examining single sub- maximal points on the drug titration curves. For example, growth inhibition of CAG cells was 44.6±18.6% following incubation with 0.55 ng/ml bortezomib and 34.4±17.1% with milatuzumab+GAH, whereas incubation with these agents in combination increases the inhibition to 84.0±1.4% (P<0.025 vs. either 0.55 ng/ml bortezomib or milatuzumab+GAH).
Induction of apoptosis and signaling
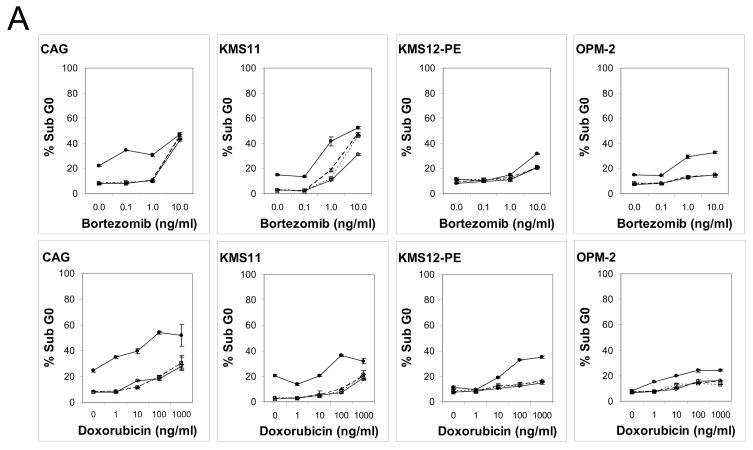
Induction of apoptosis was evaluated by determining percentage of hypodiploid DNA and induction of activated caspase-3 on the panel MM cell lines. Cells were cultured with increasing doses of bortezomib or doxorubicin in the presence or absence of milatuzumab or milatuzumab+GAH for 48 h, followed by DNA staining with propidium iodide. Cells were analyzed by flow cytometry, and positive fluorescence below the G0/G1 region (hypodiploid) represents DNA fragmentation and is a measure of apoptosis. The percentage of hypodiploid DNA induced by these treatments is shown in Figure 3A. Baseline values of sub-G0 DNA in untreated cells ranged from 2.7–8.3%. Bortezomib and doxorubicin used as single agents increased hypodiploid DNA a further 20–35% in CAG and KMS11, when used at concentrations up to 10 ng/ml and 1000 ng/ml, respectively. KMS12-PE and OPM-2 were less sensitive to induction of hypodiploid DNA by these drugs, with increases by the drugs alone remaining within 12% of baseline. In all cell lines tested, milatuzumab that was not crosslinked with second antibody did not affect the percent of hypodiploid DNA induced by the drug alone, percentages were generally within 1–5% of the drug only values. Similar results were obtained in the controls when second antibody was given in the absence of milatuzumab. However, milatuzumab + GAH led to statistically significant increased levels of hypodiploid DNA (P<0.05). For example in CAG, levels of sub G0 DNA were 8.4±0.5%, 8.0±0.3%, and 9.0±0.5% following treatment with 0.1 ng/ml bortezomib alone, 0.1 ng/ml bortezomib + milatuzumab, and 0.1 ng/ml bortezomib + GAH, respectively (no significant differences; P values >0.05). However, 0.1 ng/ml of bortezomib + milatuzumab + GAH increased sub G0 DNA to 34.5±0.3% (P vs. drug alone <0.00001).
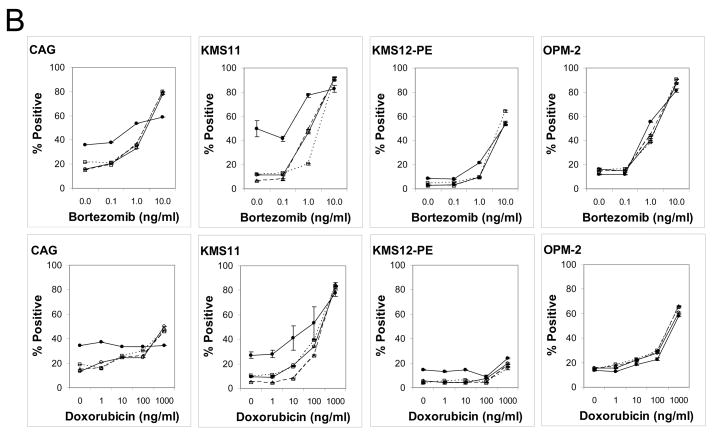
Figure 3. Apoptotic effect of mAbs on MM cell lines.
Induction of apoptosis was evaluated by flow cytometry determination of (A) hypodiploid DNA and (B) activated caspase-3. Cells were incubated with drugs alone, milatuzumab alone, or combinations of drug + milatuzumab with and without a second antibody for cross-linking for 48 h. For determination of hypodiploid DNA, staining was done with propidium iodide. Changes in the intracellular levels of cleaved caspase-3 were measured using FITC–conjugated rabbit anti-activated caspase-3. Analyses were performed on the FACSCalibur. ○, solid line, drug only; △, dashed line, drug + milatuzumab; □, dotted line, drug + GAH; ●, solid line, drug + milatuzumab + GAH. Error bars represent standard deviations of 3 replicates, and at some points are hidden by the symbol.
Induction of activated caspase-3 was similarly analyzed by flow cytometry following incubation with increasing doses of bortezomib and doxorubicin. Induction of activated caspase-3 was observed (Figure 3B). KMS12-PE was the least sensitive cell line in this test. Crosslinked milatuzumab yielded statistically significant increases in the induction of activated caspase-3 in CAG and KMS11. For example, in KMS11 levels of activated caspase-3 were 11.4±0.6%, 8.0±0.7%, and 13.1.0±0.3% following treatment with 0.1 ng/ml bortezomib alone, 0.1 ng/ml bortezomib + milatuzumab, and 0.1 ng/ml bortezomib + GAH, respectively. However, 0.1 ng/ml of bortezomib + milatuzumab + GAH yielded a value of 41.6±2.1% (P vs. drug alone=0.0009). Increases were not seen in OPM-2 and KMS12-PE, presumably due to the lack of CD74 in OPM-2 and the low caspase-3 induction by the drugs in KMS12-PE. These data show that doxorubicin and bortezomib induce apoptosis, as measured here by induction of hypodiploid DNA and activated caspase-3. Moreover, crosslinked milatuzumab amplifies this effect, corroborating the benefit of using these agents in combination.
We have begun to examine the intracellular interactions of antibody and drug by investigating signaling and apoptotic pathways in CAG cells using Western blot analyses. No alterations in Bid, Bax, Bcl-2 nor Mcl-1 were observed following exposure of CAG cells to crosslinked milatuzumab (data not shown). Thus, signaling through the extrinsic, but not the mitochondrial, apoptotic pathways is suggested.
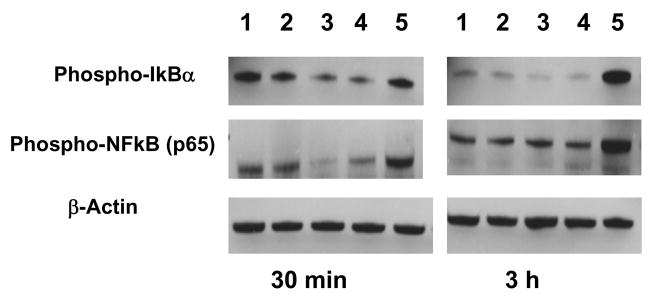
CD74 activation by its ligand MIF signals through NF-κB in chronic lymphocytic leukemia (CLL) cells (13). Moreover, stimulation of CLL cells with C-16, an agonistic anti-CD74 mAb, leads to NF-κB activation, and increased survival in CLL cells. Here we find decreases in phosphorylated IκBα and phosphorylated NF-κB 30 min after incubation of CAG cells with crosslinked milatuzumab, with recovery of phosphorylated NF-κB to control levels at 3 h (Figure 4). In contrast, bortezomib increased phosphorylated IκBα and phosphorylated NF-κB at 3 h, a result in contrast to the expected decreases in these values. The combination of crosslinked milatuzumab plus bortezomib yielded an intermediate level of phosphorylated NF-κB at 30 min. These data show that both milatuzumab binding to CD74 and bortezomib treatment induce intracellular alterations of the NF-κB signal pathway in CAG myeloma cells, although in opposite directions. Future studies examining total IKBα levels (e.g., by electrophoretic mobility shift assays) are necessary to clarify the observed effects on the NF-κB pathway under the various treatment conditions.
Figure 4. Western blot analyses of lysates of CAG cell treated with milatuzumab and bortezomib.
CAG cells were cultured in the presence or absence of the indicated reagents for 30 min or 3 h, pelleted, then washed three times in PBS, and lysed in ice cold RIPA buffer containing 100 μg/ml PMSF and 1 μg/ml aprotinin. Milatuzumab was used at 5 μg/ml, GAH at 20 μg/ml, and bortezomib at 1 ng/ml. Equivalent amounts of protein were analyzed by Western blots and actin levels were compared as a control for protein loading. Lanes are as follows: 1, untreated control cells; 2, milatuzumab; 3, milatuzumab + GAH; 4, milatuzumab + GAH + bortezomib; and 5, bortezomib.
In-vivo therapeutic activity of milatuzumab as a monotherapy and combined with drugs
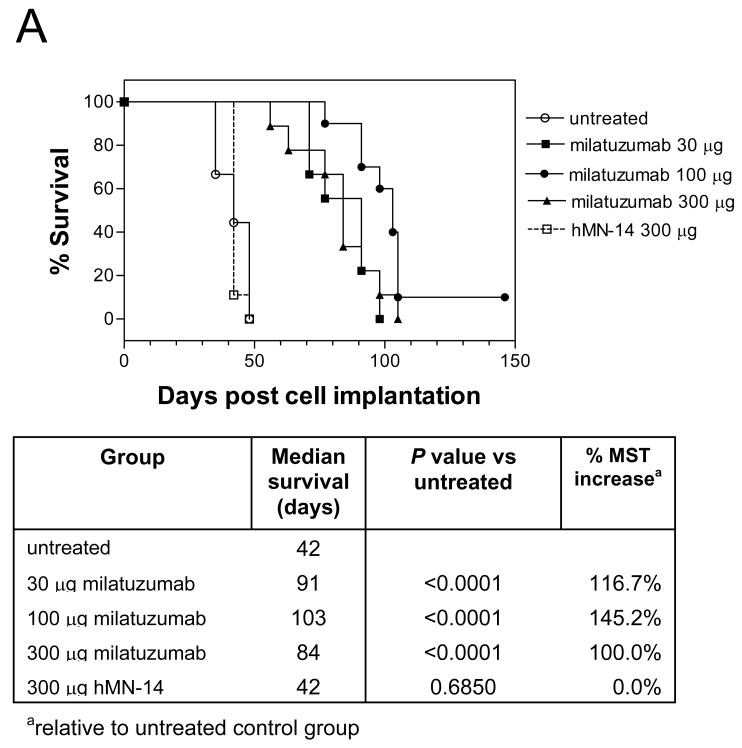
A dose-response therapy study in mice bearing the CAG cell line is shown in Figure 5A. Milatuzumab was given at 30, 100, and 300 μg/injection, twice weekly for 4 weeks, starting 1 day after injection of CAG cells. Animals were monitored for survival and hind leg paralysis, and body weights were measured weekly. Results of this study demonstrated that treatment with milatuzumab yielded a significant survival benefit in this model. Median survival times in both untreated CAG-bearing mice and mice given an unreactive isotype control mAb were 42 days. The median survival in the milatuzumab-treated groups was at least doubled, ranging between 84 and 103 days (P<0.0001 vs. untreated). A dose-response was not observed in this dose range.
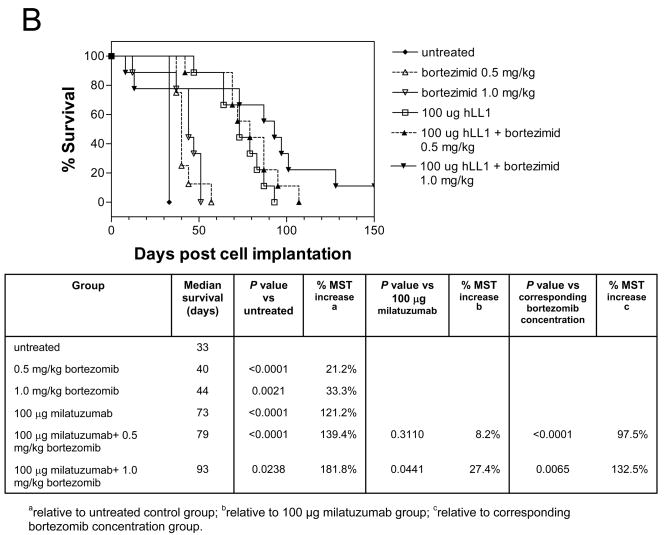
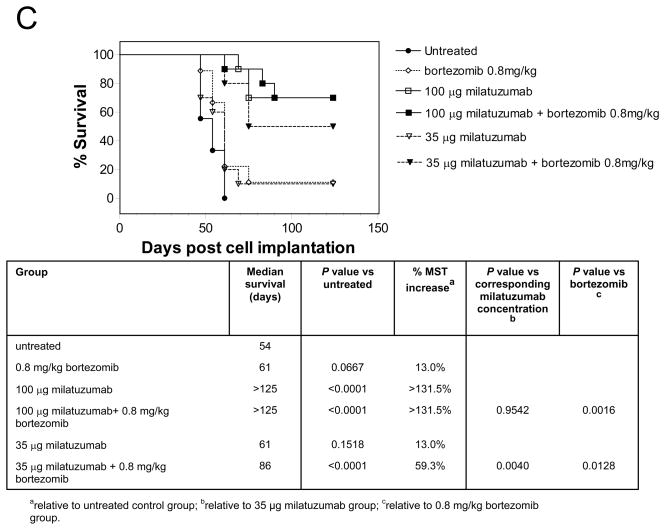
Figure 5. Therapeutic efficacy of bortezomib plus milatuzumab in MM-bearing SCID mice.
A, SCID mice were immunosuppressed by pretreatment with fludarabine phosphate and cyclophosphamide 3 d before injection of CAG cells. Treatments were given twice weekly for 4 wk, starting 1 d after injection of the CAG cells. SCID mice bearing CAG- (B) or KMS11- (C) were treated with bortezomib, milatuzumab, and mixtures of milatuzumab + bortezomib. Treatments for experiments shown in panels B and C were given as 2 i.p. doses/wk for 3 wk, initiated on day 5 after injection of tumor cells.
The effects of combining doxorubicin or bortezomib with milatuzumab were evaluated in SCID mice bearing CAG, KMS11, and KMS12-PE cells. Bortezomib dosing was selected from reports of MTD values in SCID mice (14). Treatments were given as 2 i.p. doses/week for 3 weeks, initiated on day 5 after injection of tumor cells. Figure 5B shows the tumor growth curves for CAG-bearing SCID mice treated with bortezomib and milatuzumab. Given as a single agent, 1.0 mg/kg bortezomib caused an apparent treatment-related death of 1/10 mice 12 days after administration of the drug, and given in combination with 100 μg milatuzumab, 2/10 mice died of treatment-related death (days 8 and 13). The 0.5 mg/kg dose was well tolerated with no loss of body weight. Median survival in untreated control mice in this study was 33 days. Bortezomib alone increased median survival times (MST) to 40 and 44 days, representing MST increases of 21.2% (P<0.0001) and 33.3% (P=0.0021) at 0.5 and 1.0 mg/kg, respectively. Treatment with milatuzumab (100 μg/mouse) increased the MST to 73 days. When bortezomib and milatuzumab treatments were combined, MSTs were increased further to 79 days and 93 days for 0.5 and 1.0 mg/kg bortezomib, respectively (P=0.0441 and P=0.0065 for the 100 μg milatuzumab+1.0 mg/kg bortezomib combination vs. milatuzumab alone and 1.0 mg/kg bortezomib alone, respectively).
The tumor growth curves for KMS11-bearing SCID mice treated with bortezomib and milatuzumab are shown in Figure 5C. Based on the results of the CAG study, bortezomib was given at 0.8 mg/kg/dose. Milatuzumab was administered at two dose levels, 35 and 100 μg/dose. The 0.8 mg/kg dose was well tolerated with no loss of body weight. Median survival in untreated KMS11 control mice in this study was 54 days. Bortezomib alone increased MST to 61 days (P=0.0667 vs. untreated), representing a MST increase of 13.0%. Treatment with 35 μg milatuzumab alone also increased MST to 61 days (P=0.1518 vs. untreated). MST was increased further to 86 days for the combination of 35 μg milatuzumab and 0.8 mg/kg bortezomib, representing a survival increase of 59.3% relative to untreated mice (P<0.0001). The MST increase due to combination of 35 μg milatuzumab and 0.8 mg/kg bortezomib was statistically significant compared to untreated and both single agent treatment groups (P=0.0040 and P=0.0128 for the 35 μg milatuzumab+0.8 mg/kg bortezomib combination vs. milatuzumab alone and bortezomib alone, respectively). There were five long-term survivors (> 4 months) in the 35 μg milatuzumab+0.8 mg/kg bortezomib treatment group, compared to one or none in the control and single agent groups at theses doses. Increasing the milatuzumab dose to 100 μg/dose increased its effect. Median survival was not reached at 4 months; there were seven long-term survivors each with 100 μg milatuzumab, and 100 μg milatuzumab+0.8 mg/kg bortezomib.
KMS12-PE is a slower growing tumor than CAG or KMS11. Median survival in untreated KMS12-PE-bearing SCID mice is 110 days (survival curve not shown). At 6 months post tumor injection, 2/9 mice were still alive in the untreated group, compared to 3/9 given 0.8 mg/kg bortezomib (124 days median survival), 3/9 given 100 μg milatuzumab (110 days median survival), and 6/9 given the combined treatment (median survival time not reached at 180 days). Thus, the combined treatment yielded a minimum increase in median survival of 64%. Statistical significance was not reached in this study, presumably due to the slow progression of tumor in this model. P values were 0.0962, 0.2126, and 0.1603 for milatuzumab + bortezomib vs. untreated, bortezomib alone, and milatuzumab alone, respectively.
Thus, when given as a monotherapy, milatuzumab is more effective than bortezomib in the CAG and KMS11 MM models. Moreover, combining milatuzumab and bortezomib increases survival compared to either treatment alone in the CAG, KMS11, and KMS12-PE models. Importantly, these studies confirm the therapeutic efficacy of milatuzumab previously reported in MC/CAR-bearing SCID mice (6) in additional MM cell lines.
The efficacy of milatuzumab also was compared to that of doxorubicin and a mixture of doxorubicin and milatuzumab in CAG-bearing SCID mice. Doxorubicin was administered at its MTD in this model, 35 μg, and at one-fourth the MTD, 8.65 μg. Treatments were given as a single i.p. dose 5 days after injection of tumor cells. Treatment with a single dose of milatuzumab (341 μg/mouse) increased the MST from 33 to 69 days, with no significant difference from the milatuzumab plus doxorubicin group, or the 100 μg milatuzumab/mouse twice-weekly for three weeks dose schedule, shown in Figure 5B. Given alone, doxorubicin yielded little or no effect on survival compared with untreated animals (data not shown).
DISCUSSION
CD74 (invariant chain, Ii) is a type-II transmembrane glycoprotein that associates with the major histocompatibilty (MHC) class II α and β chains and directs the transport of the αβμIi complexes to endosomes and lysosomes (15–17). Because the chaperone function of CD74 was thought to be its primary function, it was not anticipated that an anti-CD74 mAb would possess growth–inhibitory activity. However, it is now understood that CD74 is the cellular receptor for the proinflammatory cytokine, macrophage migration-inhibitory factor (MIF) (18), and that the binding of MIF to cell surface CD74 initiates a signaling cascade resulting in proliferation and survival (19). Shachar and co-workers have shown that following MIF binding to the CD74 extracellular domain, the cytosolic region of CD74 is cleaved by a two-step process (20). The cytosolic fragment then translocates to the cell nucleus, resulting in activation of NF-κB (21), and induction of a survival cascade in which interleukin-8 (IL-8) levels are elevated, as well as levels of the anti-apoptotic protein, Bcl-2 (13). In addition, Helicobacter pylori was shown to bind CD74 on gastric epithelial cells and stimulate NF-κB and IL-8 production, thus corroborating the involvement of these factors in the signal cascade initiated by CD74 activation (22, 23). Milatuzumab apparently acts as an antagonistic antibody, blocking this signal cascade by binding to cell-surface CD74. Using B-CLL patient specimens, Binsky et al. demonstrated that milatuzumab specifically blocked MIF-induced upregulation of Bcl-2 mRNA levels, inhibited IL-8 transcription, and increased the percentage of apoptotic cells (13).
The humanized anti-CD74 monoclonal antibody, milatuzumab, causes specific growth inhibition and induction of apoptosis in B-cell lines in the presence of a second crosslinking antibody. In addition, significant survival extensions were observed in NHL- and MM-bearing SCID mice treated with naked milatuzumab without the need for an exogenous crosslinking agent (6). Thus, crosslinking agents are necessary for milatuzumab activity in in-vitro studies but are not required for milatuzumab activity in vivo. Crosslinking with secondary antibodies is believed to function by generating stronger and/or more sustained signals than simple ligation of surface antigens with primary mAbs to the antigens alone (12). A requirement for extensive crosslinking of surface molecules has been noted by several groups studying lymphoma cell growth inhibition, cell cycle arrest, and/or apoptosis in vitro. Specifically, hyper-crosslinking with secondary antibodies has been used for in vitro evaluations of anti-CD20, -CD19, -CD22, -MHC class II, -sIgM and -APO-1 mAbs (12, 24–27). However, we and others have observed that crosslinking by GAH is not needed for in vivo activity. Presumably the function of the secondary antibodies in vitro is fulfilled in vivo by other molecules present in the cellular environment. These may include crosslinking with Fc-receptor-expressing cells (e.g., macrophages, monocytes, or dendritic cells), or other molecules involved in the signaling pathway of the target antigen that cause further aggregation after preliminary stimulation by the primary mAb.
Based on the preclinical evidence of activity, milatuzumab is currently in clinical evaluation for therapy of MM, NHL, and CLL. In this paper, we demonstrated the ability of milatuzumab to increase the efficacy of bortezomib, doxorubicin, and dexamethasone in MM cell lines. In vitro, crosslinked milatuzumab yielded significant cytotoxicity on CD74-positive MM cell lines when given as a single agent and caused significant reductions in the IC50 values of the anti-MM drugs. In vivo, milatuzumab given as a monotherapy was more effective than bortezomib or doxorubicin, while the combination of milatuzumab and bortezomib was more effective than either agent alone.
Bortezomib is a proteasome-inhibitor, whose mechanism of action is partly mediated through NF-κB (reviewed in (28, 29)). NF-κB is constitutively active in MM as well as other tumors and, by influencing transcription of multiple gene targets, supports proliferation and suppresses apoptosis. However, differential responses of NF-κB to bortezomib have been noted in MM as well as other hematologic and non-hematologic cancers and may contribute to heterogeneous responses to bortezomib therapy. Failure of bortezomib to decrease NF-κB activation or to actually cause increased NF-κB activation have been recently reported in MM (30), mantle cell leukemia (31), and endometrial (32), hepatocellular (33), and other carcinomas, and therefore may be more prevalent than previously thought. For example, in a recent study of primary tumor cells from MM patients, Markovina et al. (30) found that all patients’ cells constitutively expressed NF-κB activity, and that bortezomib effectively blocked proteasome activity in all. However, in 10/14 cases, bortezomib failed to inhibit NF-κB activity, and in 8 of these cases bortezomib augmented NF-κB activity. Thus, the combination of bortezomib with an agent such as milatuzumab, which can independently down-regulate NF-κB, may be especially relevant.
Bortezomib may cause cell death through additional paths not dependent on NF-κB. Thus, various scenarios can be envisioned to explain the enhancement of cytotoxicity by the combination of milatuzumab and bortezomib. If the tumor has constitutive NF-κB that is resistant to bortezomib, then bortezomib toxicity will only be due to apoptotic effects that are independent of NF-κB activation (32, 33). Down-regulation of NF-κB by milatuzumab will add to the toxicity by introducing a second mechanism of killing. Alternatively, if the tumor is one in which bortezomib does overcome NF-κB activation, then the combination of milatuzumab and bortezomib would be expected to yield greater decreases in NF-κB activity, again leading to greater cell killing. Since myeloma cell lines differ in baseline signaling characteristics (34), CD74 expression, and activation of tBid after signaling through caspase-8, as well as NF-κB response to bortezomib, a more detailed evaluation of concentration and time effects on a panel of cell lines will be necessary to fully characterize CD74 and bortezomib interactions at the subcellular level.
Although our in vitro studies demonstrated the sensitivity of CD74+ MM cell lines to doxorubicin, no significant effect was observed when doxorubicin was used as a single agent in CAG-bearing SCID mice. Milatuzumab monotherapy was markedly more effective than doxorubicin, and the doxorubicin + milatuzumab treatment protocol administered did not significantly improve the efficacy over that of milatuzumab alone. However, it should be noted that other protocols of doxorubicin administration, such as fractionated or multiple dosing, have not yet been evaluated in this model, and may yield some efficacy in vivo. Moreover, in light of results of a Phase III study in relapsed or refractory multiple myeloma, which demonstrated that the combination of pegylated liposomal doxorubicin plus bortezomib is superior to bortezomib monotherapy (35), combinations of milatuzumab with bortezomib plus doxorubicin will be tested.
In conclusion, CD74’s function as a survival receptor, combined with the expression of CD74 on malignant B cells and limited expression on normal tissues, implicate CD74 as a potential therapeutic target. In vivo, milatuzumab alone or in combination with the drugs evaluated in this report is effective in MM therapy. Moreover, the therapeutic efficacies of bortezomib, doxorubicin, and dexamethasone are enhanced when given in combination with milatuzumab. Although gaps remain in our understanding of the mechanisms of cytotoxicity of milatuzumab, it seems likely that interference with the survival functions of CD74 is involved. Future studies will focus on this issue as well as gaining a greater understanding of milatuzumab and drug interactions at the subcellular level, in order to aid in the rational design of combined modality regimens.
Acknowledgments
We thank Indira Joshi for excellent technical assistance.
Grant support: This work was supported in part by USPHS grants P01-CA103985 and R01-CA109474 from the National Cancer Institute, and grants from the Thomas and Agnes Carvel Foundation and the Walter and Louise Sutcliffe Foundation.
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE.
CD74’s function as a survival receptor, combined with the expression of CD74 on malignant B cells and limited expression on normal tissues, implicate CD74 as a potential therapeutic target. Based on our previous preclinical studies, the anti-CD74 humanized monoclonal antibody, milatuzumab, is currently in clinical evaluation for therapy of multiple myeloma. In this paper, we demonstrate the ability of milatuzumab to increase the efficacy of bortezomib, doxorubicin, and dexamethasone in multiple myeloma cell lines and animal models. The results presented here now suggest testing these combinations clinically, with the goal of improving the outcome for patients with multiple myeloma.
References
- 1.Cancer Facts and Figures. Atlanta: American Cancer Society, Inc.; 2008. [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlogie B, Shaughnessy J, Tricot G, et al. Treatment of multiple myeloma. Blood. 2004;103:20–32. doi: 10.1182/blood-2003-04-1045. [DOI] [PubMed] [Google Scholar]
- 4.Burton JD, Ely S, Reddy PK, et al. CD74 is expressed by multiple myeloma cells and is a promising target for therapy. Clin Cancer Res. 2004;10:6606–11. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- 5.Stein R, Mattes MJ, Cardillo TM, et al. CD74: A new candidate target for the immunotherapy of hematological malignancies. Clin Cancer Res. 2007;13:5556s–63s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 6.Stein R, Qu Z, Cardillo TM, et al. Anti-proliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood. 2004;104:3705–11. doi: 10.1182/blood-2004-03-0890. [DOI] [PubMed] [Google Scholar]
- 7.Pawlak-Byczkowska EJ, Hansen HJ, Dion AS, Goldenberg DM. Two new monoclonal antibodies, EPB-1 and EPB-2, reactive with human lymphoma. Cancer Res. 1989;49:4568–77. [PubMed] [Google Scholar]
- 8.Sharkey RM, Juweid M, Shevitz J, et al. Evaluation of a complementarity-determining region-grafted (humanized) anti-carcinoembryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res. 1995;55:5935s–45s. [PubMed] [Google Scholar]
- 9.Stein R, Chen S, Grossman W, Goldenberg DM. Human lung carcinoma monoclonal antibody specific for the Thomsen-Friedenreich antigen. Cancer Res. 1989;49:32–7. [PubMed] [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 12.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation with monoclonal antibodies. Blood. 1998;91:1644–52. [PubMed] [Google Scholar]
- 13.Binsky I, Haran M, Starlets D, et al. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104:13408–13. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 15.Claesson L, Larhammer D, Rask L, Peterson PA. cDNA clone for the human invariant gamma chain of class II histocompatibility antigens and its implications for the protein structure. Proc Natl Acad Sci USA. 1983;80:7395–9. doi: 10.1073/pnas.80.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roche PA, Cresswell P. Intracellular transport of class II MHC molecules directed by invarient chain. Nature (London) 1990;345:615–8. [Google Scholar]
- 17.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci U S A. 1993;90:8581–5. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starlets D, Gore Y, Binsky I, et al. Cell surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–16. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 20.Matza D, Lantner F, Bogoch Y, Flaishon L, Hershkoviz R, Shachar I. Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc Natl Acad Sci U S A. 2002;99:3018–23. doi: 10.1073/pnas.052703299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell. 2005;16:5061–9. doi: 10.1091/mbc.E05-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beswick EJ, Bland DA, Suarez G, Barrera CA, Fan X, Reyes VE. Helicobacter pylori binds to CD74 on gastric epithelial cells and stimulates interleukin-8 production. Infect Immun. 2005;73:2736–43. doi: 10.1128/IAI.73.5.2736-2743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beswick EJ, Pinchuk IV, Minch K, et al. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect Immun. 2006;74:1148–55. doi: 10.1128/IAI.74.2.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaouchi N, Vazquez A, Galanaud P, Leprince C. B cell antigen receptor-mediated apoptosis. Importance of accessory molecules CD19 and CD22, and of surface IgM cross-linking. J Immunol. 1995;154:3096–104. [PubMed] [Google Scholar]
- 25.Dhein J, Daniel P, Trauth BC, Oehm A, Moller P, Krammer PH. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol. 1992;149:3166–73. [PubMed] [Google Scholar]
- 26.Marches R, Racila E, Tucker TF, et al. Tumour dormancy and cell signaling-III: Role of hypercrosslinking of IgM and CD40 on the induction of cell cycle arrest and apoptosis in B lymphoma cells. Therapeutic Immunol. 1995;2:125–36. [PubMed] [Google Scholar]
- 27.Newell MK, VanderWall J, Beard KS, Freed JH. Ligation of major histocompatibility complex class II molecules mediates apoptotic cell death in resting B lymphocytes. Proc Natl Acad Sci U S A. 1993;90:10459–63. doi: 10.1073/pnas.90.22.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armand J-P, Burnett AK, Drach J, Harousseau J-L, Lowenberg BL, San Miguel J. The emerging role of targeted therapy for hematologic malignancies: update on bortezomib and tipifarnib. Oncologist. 2007;12:281–90. doi: 10.1634/theoncologist.12-3-281. [DOI] [PubMed] [Google Scholar]
- 29.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Markovina S, Callander NS, O’Connor SL, et al. Bortezomib-resistant nuclear factor-KB activity in multiple myeloma cells. Mol Cancer Res. 2008;6:1357–64. doi: 10.1158/1541-7786.MCR-08-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Molecular Cancer. 2008;7 doi: 10.1186/476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolcet X, Llobet D, Encinas M, et al. Proteasome inhibitors induce death but activate NF-kB on endometrial carcinoma cell lines and primary culture explants. J Biol Chem. 2006;281:22118–30. doi: 10.1074/jbc.M601350200. [DOI] [PubMed] [Google Scholar]
- 33.Chen K-F, Yeh P-Y, Yeh K-h, Lu Y-S, Huang S-Y, Cheng A-L. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008;68:6698–707. doi: 10.1158/0008-5472.CAN-08-0257. [DOI] [PubMed] [Google Scholar]
- 34.Sanda T, Iida S, Ogura H, et al. Growth inhibition of multiple myeloma cells by a novel IkB kinase inhibitor. Clin Cancer Res. 2005;11:1974–82. doi: 10.1158/1078-0432.CCR-04-1936. [DOI] [PubMed] [Google Scholar]
- 35.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]