Abstract
We have previously demonstrated that Leptin reduces extracellular amyloid β (Aβ) protein both in vitro and in vivo, and intracellular tau phosphorylation in vitro. Further, we have shown that these effects are dependent on activation of AMP-activated protein kinase (AMPK) in vitro. Herein, we investigated downstream effectors of AMPK signaling directly linked to tau phosphorylation. One such target, of relevance to Alzheimer’s disease (AD), may be GSK-3β, which has been shown to be inactivated by Leptin. We therefore dissected the role of GSK-3β in mediating Leptin’s ability to reducing tau phosphorylation in neuronal cells. Our data suggest that Leptin regulates tau phosphorylation through a pathway involving both AMPK and GSK-3β. This was based on the following: Leptin and the cell-permeable AMPK activator, 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR), reduced tau phosphorylation at AD-relevant sites similarly to the GSK-3β inhibitor, lithium chloride (LiCl). Further, this reduction of tau phosphorylation was mimicked by the downregulation of GSK-3β, achieved using siRNA technology and antagonized by the ectopic overexpression of GSK-3β. These studies provide further insight into Leptin’s mechanism of action in ameliorating AD-related pathways.
Keywords: Leptin, AICAR, GSK-3β, tau, Alzheimer’s disease
Dysregulation of glycogen synthase kinase-3β (GSK-3β), a constitutively active serine/threonine kinase, has been implicated in Alzheimer’s disease (AD) pathobiology. GSK-3β phosphorylates tau at select epitopes, thereby leading to tau hyperphosphorylation which increasesthe propensity for the formation of oligomeric tau [14]. Phosphorylation of GSK-3β at Ser9 leads to the inactivation of the enzyme which results in the reduction of tau phosphorylation [17]. Insulin inactivates GSK-3β in this manner, through phosphorylation by protein kinase B (Akt) [8]. Lithium chloride (LiCl) also inhibits GSK-3β activity [1].
Leptin, a peptide hormone produced by adipocytes, modulates metabolic pathways and energy availability [18]. This is predominantly achieved through receptors in the central nervous system, particularly in the hypothalamus [11]. However, accumulating evidence suggests that Leptin receptors are expressed in abundance in the hippocampus as well [6]. In accord, several groups have demonstrated that Leptin can regulate short- and long-term potentiation in neurons, suggesting a role in memory and learning [2, 16]. We previously demonstrated that Leptin reduces extracellular amyloid β (Aβ) protein both in vitro and in vivo [3], and intracellular tau phosphorylation in vitro [4, 5]. Further, we showed that these effects are dependent on activation of AMP-activated protein kinase (AMPK) in vitro [4, 5]. also Additionally, animal studies using a subcutaneous-fitted peristaltic pump for the delivery of Leptin, demonstrated that chronic supplementation (8 weeks) reduces total brain Aβ40 and Aβ42 in the Tg2576 mouse [3] and improves the cognitive performance of the CRND8 mouse (unpublished data).
Interestingly, Leptin has been reported to inactivate GSK-3β in mouse cortical neurons [20]. Herein, we investigated whether GSK-3β inactivation is involved in mediating Leptin’s ability to reduce tau phosphorylation.
Minimum essential medium (MEM) was purchased from ATCC (Manassas, VA). Trypsin-EDTA and penicillin solution were purchased from MP Biomedicals (Solon, Ohio). Fetal bovine serum (FBS), all-trans retinoic acid (RA) and recombinant human Leptin were purchased from Sigma-Aldrich (St. Louis, MO). 5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) was purchased from Cell Signaling Technology (Danvers, MA). LiCl was purchased from EMD Chemicals (Gibbstown, NJ).
Rabbit anti-phosphorylated GSK-3β (pSer9) and anti-GSK-3β, tau (pSer396) mAb and tau (tau46) mAb were purchased from Cell Signaling. PHF-tau mAb (clone AT8) was purchased from Pierce Biotechnology (Rockford, IL). PHF-1 mAb was a gift from Dr. Peter Davies, Albert Einstein College of Medicine (Bronx, NY). Rabbit anti-phosphorylated tau (pThr181) was purchased from Sigma-Aldrich. Rabbit anti-α-tubulin mAb was purchased from Affinity BioReagents (Golden, CO).
The human neuroblastoma cell line, SH-SY5Y, was purchased from ATCC. Cell culture was performed according to manufacturer’s specific guidelines. Cells were propagated in MEM containing 10% FBS until 80–90% confluence then detached from the flask by trypsin-EDTA and sub-cultured at a ratio of 1:5. For neuronal differentiation, 1 × 106 SY5Y cells were grown in neuronal induction medium (NIM), which consisted of MEM containing 2% FBS supplemented with 10 µM RA. Cells were incubated in NIM for 6 days, and switched to serum-free NIM prior to treatment and harvesting on day 7.
Neuronal cells were treated with Leptin (100 nM; 1600 ng/ml) for 4 hrs or AICAR (2 mM) or LiCl (10 mM) for 1 hr, and then harvested by scraping. Cell pellets were resuspended in protease and phosphatase inhibitor-supplemented 1X RIPA lysis/extraction buffer (Pierce), and then subjected to freeze/thaw cycles in a dry ice/ethanol bath. Total protein was determined with the Coomassie (Bradford) Protein Assay Kit (Pierce). Whole cell extracts (25 µg) were analyzed by immunoblot using 10% tris-glycine SDS-PAGE pre-cast gels (Lonza; Rockland, ME), and the proteins were transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were incubated overnight at 4°C with primary antibodies and then detected the following day with HRP-conjugated secondary IgG. All primary antibodies, except tau-pSer396, and total tau (1:500), and PHF-tau AT8 (1:200), and secondary antibodies were used at final dilutions of 1:1,000 and 1:10,000, respectively. HRP was developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce), and imaged using a BioRad (Hercules, CA) ChemiDoc XRS System. The membranes were stripped with Restore PLUS Western Blot Stripping Buffer (Pierce) for reprobing with other antibodies.
For knockdown of GSK-3β, differentiated SY5Y cells were transiently transfected with 50 nM SignalSilence GSK-3β siRNA (Cell Signaling) for 48 hrs using the TransIT-TKO transfection reagent (Mirus; Madison, WI), according to manufacturer’s specific instructions. Cells transfected with 50 nM SignalSilence control siRNA (fluorescein conjugate) (Cell Signaling), were used as negative controls and for measuring transfection efficiency.
For GSK-3β overexpression, differentiated SY5Y cells were transiently transfected with 2 µg of the expression vector (pCMV6-XL4) containing the full-length GSK-3β cDNA sequence (Accession No: NM_002093.2) (OriGene; Rockville, MD) for 48 hrs using the TurboFectin 8.0 transfection reagent (OriGene), according to manufacturer’s specific instructions. Cells transfected with 2 µg empty pCMV6-XL4 (OriGene) served as negative controls. Following transfection, cells were harvested and knockdown or overexpression of GSK-3β was confirmed by immunoblot.
GSK-3β levels in cellular extracts were determined using the PhosphoDetect GSK-3β (pSer9) ELISA kit (EMD Chemicals) according to manufacturer’s specific instructions. GSK-3β levels were calculated from a standard curve.
Statistical data analyses were performed with analysis of variance and Tukey-Kramer multiple comparisons test. Densitometric analyses were performed using the UN-SCAN-IT gel 6.1 software (Silk Scientific; Orem, UT). p<0.05 was considered statistically significant.
We previously utilized retinoic acid-treated SY5Y cells (RA-SY5Y) as a neuronal model to study the relationship between Leptin and tau phosphorylation [4, 5]. Treatment with Leptin, as well as AICAR, which is a cell-permeable activator of AMPK, was effective at reducing tau phosphorylation [4, 5]. Herein, we expanded these findings to explore whether the effects of Leptin and AICAR on tau phosphorylation involves modulation of GSK-3β activity.
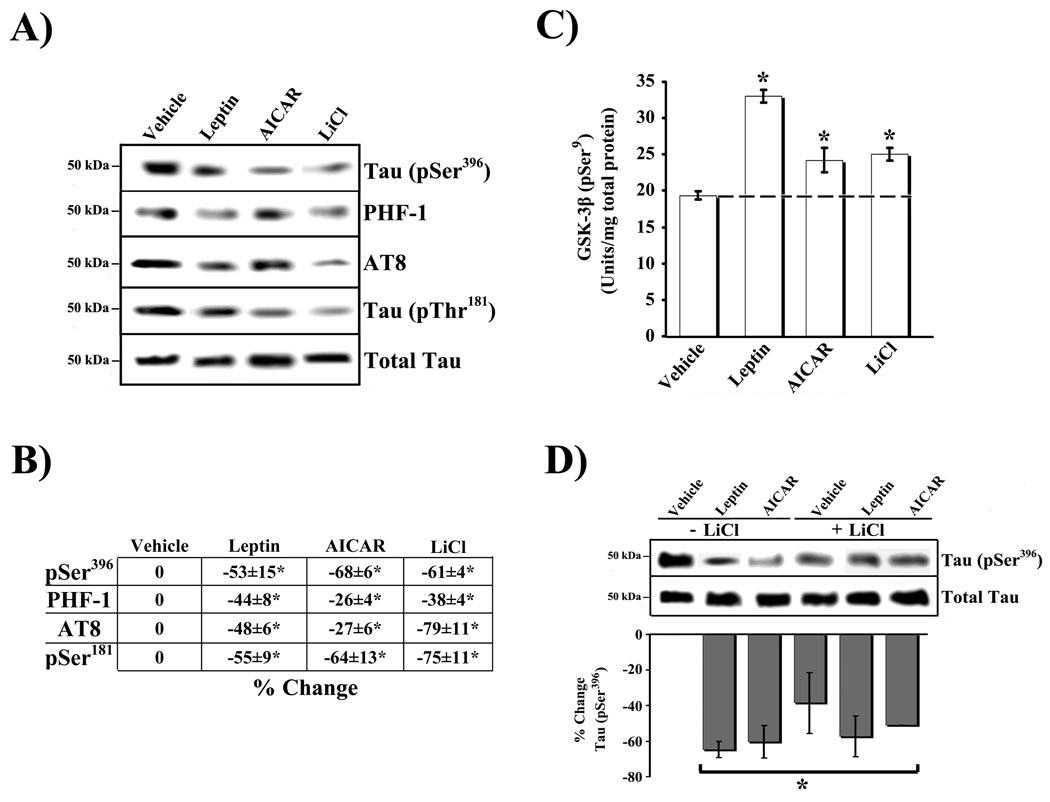
The first set of studies compared the affect of 1 h LiCl treatment (10 mM), a known GSK-3β inhibitor [7], on tau phosphorylation at AD-related sites in RA-SY5Y cells (Figure 1A) to that obtained by treatment with Leptin (100 nM; 1600 ng/ml – 4 hrs) or AICAR (2 mM – 1 hr). As expected, based on previous studies [7, 15], direct inhibition of GSK-3β with LiCl produced a significant (p<0.05) reduction in tau phosphorylation at multiple sites, [as detected by antibodies AT8 and PHF-1 and antibodies directed against Tau (pSer396) and Tau (pThr181)], compared to vehicle controls (Figure 1B). Similalry a comparable significant reduction was observed with either Leptin or AICAR.
Fig. 1.
Enzymatic regulation of tau phosphorylation in RA-SY5Y. A. RA-SY5Y were incubated with the GSK-3β inhibitor, LiCl (10 mM), for 1 hr, or non-treated (vehicle), and phosphorylation of tau at multiple sites was measured. Treatment with Leptin for 4 h (100 nM; 1600 ng/ml) or AICAR for 1 h (2 mM) served as positive control. Whole-cell lysates were prepared and analyzed by immunoblot with phosphorylated tau-specific antibodies (pSer396, PHF-1, AT8 or pSer181). Membranes were stripped and re-probed with total tau antibody for normalization. Representative blots are shown, n=3. B. Normalized bands were analyzed by densitometry and results are presented as the mean ± SD percent change, relative to vehicle. C. Lysates from A were prepared and analyzed by ELISA for GSK-3β (pSer9). Cells incubated for 1 hr with LiCl served as positive control. Results (n=3) are presented as the mean normalized GSK-3β (pSer9) concentration (Units/mg total protein) ± SD, relative to non-treated samples. D. Cells were incubated with or without LiCl, in the presence of Leptin, AICAR or no additional treatment (vehicle), and phosphorylation of tau (pSer396) was measured by immunoblot as in A. Results (n=3) are presented as in B.
*p<0.05 vs. non-treated (vehicle)
Regulation of GSK-3β is often achieved by phosphorylation at Ser9/21, which inactivates kinase activity. In contrast, the regulation of GSK-3β by phosphorylation at Tyr216/279, is less common and is associated with an augmentation of kinase activity [9]. To this end, we next determined the extent to which Leptin and AICAR affect the phosphorylation status of GSK-3β at the Ser9 inactivation site compared to LiCl (Figure 1C). A significant (p<0.05) increase in GSK-3β phosphorylation (pSer9) was observed following Leptin (second bar from left) or AICAR (second bar from right) treatment compared to vehicle. As expected, LiCl inhibited GSK-3β activity (first bar from right) under the conditions used (Ki = 2 mM) [13]. Although these findings (Figures 1A–C) validate previous studies by our group and others, in the current context they serve to document the comparable effects of these molecules in RA-SY5Y cells. Interestingly, LiCl was unable to significantly enhance the ability of either Leptin or AICAR at reducing tau phosphorylation (pSer396) when co-administered (Figure 1D). These findings suggest that Leptin, AICAR and LiCl may regulate tau phosphorylation through a common pathway.
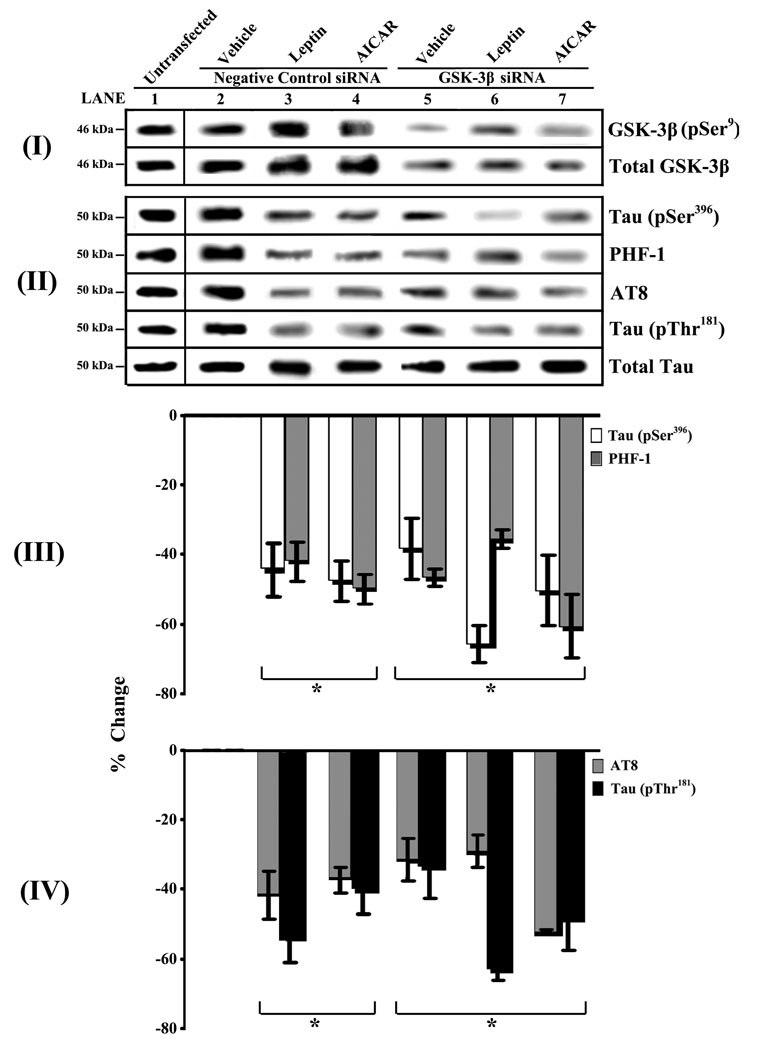
To directly confirm that Leptin and/or AICAR require GSK-3β to regulate phosphorylation of tau, we repeated the above experiments (Figures 1A and B) while ectopically modulating GSK-3β expression. To block GSK-3β expression and, ultimately, activity, we used siRNA technology (Figure 2). We also examined the effect of overexpression of GSK-3β in neuronal cells using a mammalian expression vector under the control of a strong promoter (CMV) (Figure 3).
Fig. 2.
Loss-of-function studies show that Leptin and AICAR modulate tau phosphorylation via a GSK-3β-dependent mechanism. RA-SY5Y were transiently transfected with GSK-3β-specific siRNA, or untransfected (lane 1), and later treated with Leptin for 4 h (100 nM; 1600 ng/ml – lane 6), AICAR for 1 h (2 mM – lane 7) or no treatment (vehicle – lane 5). Cells transfected with fluorescein-conjugated control siRNA with or without Leptin and AICAR treatment (lanes 2–4) were used to assess tranfection efficiency and served as negative controls. Whole-cell lysates were prepared and analyzed by immunoblot with GSK-3β-specific (panel I) or phosphorylated tau-specific antibodies (pSer396, PHF-1, AT8 or pSer181; panel II). Membranes were stripped and re-probed with total GSK-3β (panel I) or total tau (panel II) antibodies for normalization. Representative blots are shown, n=3. Normalized tau bands were analyzed by densitometry and results (panels III–IV) are presented as the mean ± SD percent change, relative to negative control samples.
*p<0.05 vs. negative control siRNA-transfected cells treated with vehicle (lane 2)
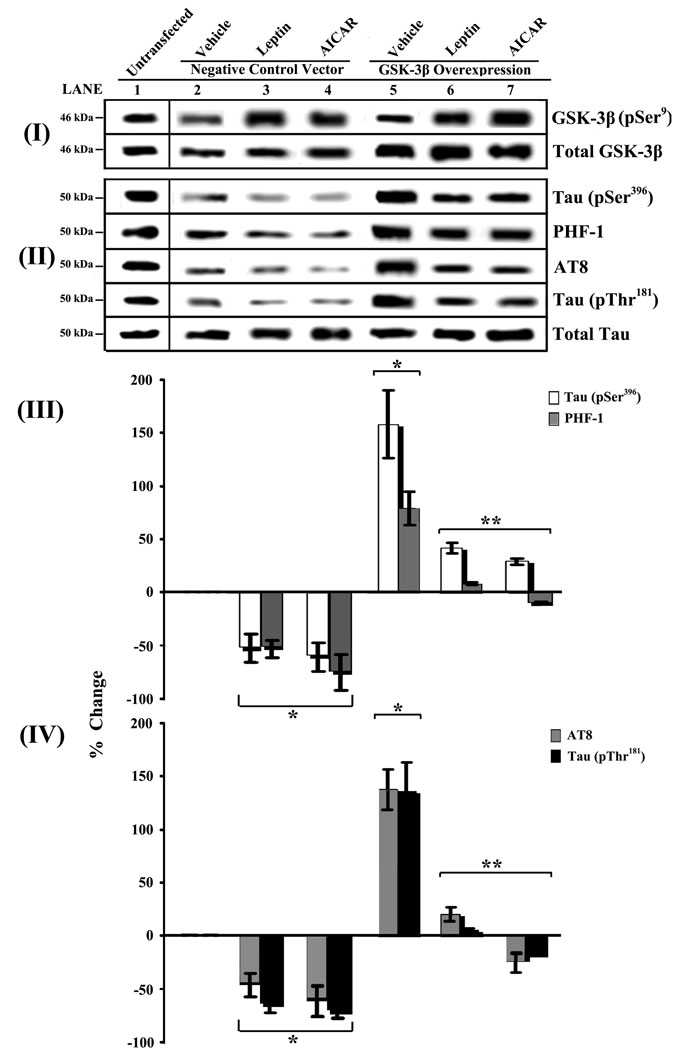
Fig. 3.
Gain-of-function studies show that Leptin and AICAR modulate tau phosphorylation via a GSK-3β-dependent mechanism. RA-SY5Y were transiently transfected with a GSK-3β full-length cDNA expression vector (lanes 5–7), or untransfected (lane 1), and later treated as described in the Legend of Fig. 2. Cells transfected with empty expression vector (lanes 2–4), with or without Leptin and AICAR treatment were used to assess transfection efficiency and served as negative controls. Whole-cell lysates were prepared, analyzed and normalized as described in the Legend of Fig. 2. Results (n=3) are presented as described in the Legend of Fig. 2
*p<0.05 vs. negative control siRNA-transfected cells treated with vehicle (lane 2)
**p<0.05 vs. GSK-3β-overexpressing cells treated with vehicle (lane 5)
We began these loss- and gain-of-function studies by transiently transfecting RA-SY5Y cells with GSK-3β-specific siRNA, followed by treatment with Leptin, AICAR or vehicle (Figure 2). GSK-3β knockdowns were compared to cells transfected with control siRNA or to cells that were not transfected. First, cells were assayed for total GSK-3β expression (active plus inactive forms) and phosphorylated GSK-3β (pSer9; inactive form) to confirm knockdown of the specific protein (panel I). Next, the effect of GSK-3β knockdown on the levels of different forms of phosphorylated tau was measured (panels II–IV). GSK-3β knockdowns (lanes 5–7) showed a significant (p<0.05) decrease in tau phosphorylation at all sites (panels II–IV) compared to untransfected and vehicle controls (lanes 1–2), but no difference compared to negative controls treated with Leptin or AICAR (lanes 3–4). In the presence of Leptin (but not AICAR) increased phosphorylation of the residual GSK-3β (panel I, lane 6) after knockdown, was associated with a further decrease in the phosphorylation of pSer396 tau (panels II and III, lane 6 white bar) and pThr181 tau (panels II and IV, lane 6 black bar), but this was not significant.
We next performed studies with RA-SY5Y cells transiently transfected to overexpress GSK-3β in order to provide further mechanistic insight (Figure 3). We first confirmed that these cells produced significantly higher levels of GSK-3β (panel I, lanes 5–7) compared to untransfected cells (lane 1) or cells transfected with empty vector (lanes 2–4). Leptin or AICAR treatment of these cells significantly increased the levels of GSK-3β phosphorylation (pSer9) (lanes 3–4, 6–7) compared to vehicle (lane 2) and untransfected controls (lane 1). Overexpression of GSK-3β was coincident with an increase of all phospho-tau sites examined (panels II–IV, lane 5). However, both Leptin and AICAR treatments significantly (p<0.05) blocked these increases (lanes 6–7) presumably by increasing GSK-3β phosphorylation and thereby leading to its inactivation. Leptin and AICAR treatment of cells transfected with empty expression vector (lanes 3–4) showed comparable levels of tau phosphorylation as untransfected cells treated similarly (Figure 1B).
In summary, the findings of the present study suggest that Leptin and its downstream signaling protein, AMPK (stimulated by AICAR) [4, 5], reduce phosphorylation of tau by inhibiting GSK-3β; thereby supporting a putative signaling cascade whereby Leptin modulates tau phosphorylation via AMPK [4, 5].
Inhibition of tau phosphorylation through inactivation of GSK-3β by Akt has been well-described [9]. AMPK has been reported to directly inactivate GSK-3β through Ser9 phosphorylation in hepatocytes [10]. A previous study in differentiated hippocampal neurons and SY5Y cells has shown that AICAR induces dephosphorylation of Akt and GSK-3β (pSer9) [12]. However, higher doses of AICAR and longer incubation periods were utilized, which may trigger negative feedback loops or be deleterious to AMPK and Akt. In addition, other signaling pathways may be involved, independent of AMPK.
Our previous findings have also shown that Leptin modulates Aβ production and release through AMPK [4, 5]. Aβ has been reported to induce neuronal tauopathy in APP-V717I×Tau-P301L biogenic mice through activation of GSK-3β [19]. Thus, at the physiological level, in addition to directly inactivating GSK-3β by kinases, the Leptin-AMPK pathway may also indirectly inactivate the enzyme through homeostatic regulation of Aβ.
None of the currently approved therapies for AD target any of the underlying etiological facets of the disease, providing only symptomatic relief, and drugs under development address at most only one aspect. In contrast, we have previously and presently identified Leptin as a modulator of both amyloidogenic and tau pathways [3,4,5]. Taken together, our studies strongly support Leptin as a potential therapeutic target.
Acknowledgments
This work was supported by the National Institute on Aging (SBIR –1R43AG029670) and the New Jersey Commission on Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aghdam SY, Barger SW. Glycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithium. Curr Alzheimer Res. 2007;4:21–31. doi: 10.2174/156720507779939832. [DOI] [PubMed] [Google Scholar]
- 2.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer's Abeta. Faseb J. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 4.Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, Ashford JW, Smith MA, Tezapsidis N. Leptin reduces Alzheimer's disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun. 2008;376:536–541. doi: 10.1016/j.bbrc.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates Tau phosphorylation and Amyloid through AMPK in Neuronal Cells. Biochem Biophys Res Commun. 2009;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–313. doi: 10.1111/j.1471-4159.2006.04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 8.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 9.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, Nishiyama K, Uchijima Y, Kurihara Y, Kurihara H, Asano T. AMP-activated Protein Kinase Activation Increases Phosphorylation of Glycogen Synthase Kinase 3{beta} and Thereby Reduces cAMP-responsive Element Transcriptional Activity and Phosphoenolpyruvate Carboxykinase C Gene Expression in the Liver. J Biol Chem. 2008;283:33902–33910. doi: 10.1074/jbc.M802537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 12.King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol. 2006;71:1637–1647. doi: 10.1016/j.bcp.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci. 2007;26:3429–3436. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovestone S, Davis DR, Webster MT, Kaech S, Brion JP, Matus A, Anderton BH. Lithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrations. Biol Psychiatry. 1999;45:995–1003. doi: 10.1016/s0006-3223(98)00183-8. [DOI] [PubMed] [Google Scholar]
- 16.Moult PR, Milojkovic B, Harvey J. Leptin reverses long-term potentiation at hippocampal CA1 synapses. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryder J, Su Y, Ni B. Akt/GSK3beta serine/threonine kinases: evidence for a signaling pathway mediated by familial Alzheimer's disease mutations. Cell Signal. 2004;16:187–200. doi: 10.1016/j.cellsig.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 19.Terwel D, Muyllaert D, Dewachter I, Borghgraef P, Croes S, Devijver H, Van Leuven F. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am J Pathol. 2008;172:786–798. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]