Abstract
Measures of explicit rule-based category learning are commonly used in neuropsychological evaluation of individuals with Parkinson’s disease (PD) and the pattern of PD performance on these measures tends to be highly varied. We review the neuropsychological literature to clarify the manner in which PD affects the component processes of rule-based category learning and work to identify and resolve discrepancies within this literature. In particular, we address the manner in which PD and its common treatments affect the processes of rule generation, maintenance, shifting and selection. We then integrate the neuropsychological research with relevant neuroimaging and computational modeling evidence to clarify the neurobiological impact of PD on each process. Current evidence indicates that neurochemical changes associated with PD primarily disrupt rule shifting, and may disturb feedback-mediated learning processes that guide rule selection. Although surgical and pharmacological therapies remediate this deficit, it appears that the same treatments may contribute to impaired rule generation, maintenance and selection processes. These data emphasize the importance of distinguishing between the impact of PD and its common treatments when considering the neuropsychological profile of the disease.
Keywords: PARKINSON’S DISEASE, STRIATUM, CATEGORY LEARNING, DOPAMINE, EXECUTIVE FUNCTION
As a fundamental aspect of human cognition, categorization enables appropriate responses to a variety of familiar and novel stimuli. The same categorization processes that govern decisions of dire importance, such as whether or not the street sign ahead signals a road hazard, also govern more mundane behaviors such as filing papers or sorting laundry. The ubiquitous nature of categorization has led to extensive examination of the cognitive and, more recently, neurobiological processes underlying its performance.
Much work in the area of categorization has focused on the processes underlying the acquisition of new categories and it is generally accepted that multiple learning systems operate in parallel to support such learning (Ashby, Alfonso-Reese, Turken & Waldron, 1998; Ashby & Valentin, 2005; Erickson & Kruschke, 1998; Maddox & Ashby 2004; Reber & Squire, 1994; Smith & Grossman, 2008; Smith, Patalano, & Jonides, 1998; but see Nosofsky & Johansen, 2000 for discussion of a unitary system of categorization). At least one of these systems supports implicit category learning, which develops gradually with little intention, and at least one other system supports explicit category learning, which requires effortful hypothesis testing and memorization processes (Ashby et al., 1998; Maddox & Ashby, 2004; Poldrack et al., 2001; Poldrack & Packard, 2003; Smith & Grossman, 2008; Smith et al., 1998).
In this review, we focus specifically on the neurobiological aspects of explicit, rule-based category learning as a detailed examination of both implicit and explicit systems is outside the scope of the present article (for details on the neural mechanisms of implicit category learning, see Ashby & Ennis, 2006; Shohamy, Myers & Kalanithi, 2008). Explicit rule-based category structures are learned using logical reasoning and hypothesis testing processes that demand working memory (WM) resources. Typically, the optimal classification rule is easily verbalized, although rules vary in complexity (Bruner, Goodnow & Austin, 1956). Often, categorization depends upon a single dimension that must be appropriately mapped onto each stimulus. One of the most commonly used measures of explicit rule-based category learning is the Wisconsin Card Sorting Task (WCST; Berg, 1948; Heaton, 1981), in which stimuli vary along several dimensions and participants must sort according to one while ignoring the others. Additionally, some tasks rely upon multi-dimensional rules that require the consideration of several stimulus dimensions.
Much of the current knowledge regarding the cognitive neuroscience of explicit rule-based category learning stems from research with individuals with damage to specific brain areas. In particular, considerable work has focused on patients with fronto-striatal dysfunction due to Parkinson’s disease (PD). PD is associated with degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNPC), leading to a substantial drop in DA within the dorsal striatum (putamen and caudate) (Agid, Javoy-Agid, & Ruberg, 1987; Albin, Young & Penney, 1989; Robertson & Robertson, 1988). This results in excessive inhibition of thalamic projections to cortical regions, namely premotor and prefrontal structures (de Long, 1990; Scatton, Worms, Lloyd, & Bartholini, 1982). As PD progresses, DA neurons in the ventral tegmental area (VTA) are also lost, resulting in dysfunction of the ventral striatum and its cortical projections, including orbitofrontal cortex (OFC) and anterior cingulate (ACC) (Farley, Price & Hornykiewicz, 1977; Kish, Shannak & Hornykiewicz, 1988; Uhl, Hedreen & Price, 1985). Additionally, significant degeneration of the locus ceruleus results in dysfunction of the noradrenergic system, including the ceruleo-cortical projections to PFC (see Rommelfanger & Weinshenker, 2007 for a review).
The neurochemical changes associated with PD often lead to dysfunction of a number of cognitive processes (Brown & Marsden, 1990; Salmon, Lineweaver, & Heindel, 1998). This includes impairment in WM, selective attention and cognitive flexibility, each of which are fundamental to explicit, rule-based category learning. Patients with PD commonly exhibit rule-based category learning impairment but the nature of this impairment is highly variable. In the present review, we examine the particular conditions in which rule-based category learning deficits emerge in PD and consider how these deficits are explained by the neurobiological dysfunction associated with the disease. While dysfunction in a number of brain regions and neurotransmitter systems might contribute to rule-based category learning deficits in PD, in the present review we primarily focus on the potential role of the caudate nucleus, nucleus accumbens, prefrontal cortex, and dopaminergic systems as these brain regions and neurotransmitter have been highly implicated in explicit rule-based category learning.
A better understanding of the mechanisms that underlie rule-based category learning impairments in PD holds several benefits. Although it is the case that deficits on other cognitive tasks predict future cognitive decline in PD, measures of rule based category learning, such as performance on the WCST, are highly predictive as well (Dujardin, Degreef, Rogelet, Defebvre & Destee 1999; Jacobs et al., 1995; Janvin, Aarsland, & Larsen, 2005; Levy et al., 2002; Maddox, Filoteo & Zeithamova, in press; Mahieux et al., 1998; Piccirilli, D’Alessandro, Finali, Piccinin, & Agostini, 1989; Woods & Troster, 2003). Thus from a clinical perspective, increased understanding of PD-related deficits may enhance knowledge regarding the predictors and causes of dementia. Such knowledge may also stimulate the development of additional neuropsychological tools to predict the onset of dementia.
Further, integration of findings across multiple paradigms enriches our understanding of the neurobiological mechanisms that support category learning and helps to clarify the sources of rule-based category learning impairment in PD. The evidence we review here indicates that particular components of explicit rule-based category learning are differently affected by PD. Variability in PD performance appears to reflect deficits attributable to the disease itself and its commonly used treatments. Accumulating evidence indicates that pharmacological therapies ameliorate certain cognitive deficits while creating other forms of cognitive impairment (Cools, 2006; Cools, Barker, Sahakian, & Robbins, 2001; 2003; Cools, Altamirano & D’Esposito, 2006; Cools, Lewis, Clark, Barker & Robbins, 2007; Gotham, Brown & Marsden, 1988; Owen, Roberts, Hodges, Summers, Polkey & Robbins, 1993). Increased knowledge regarding currently available PD treatments and their impact on cognitive function informs clinical decision making and improves understanding of the potential cognitive impact of emerging therapeutic interventions (e.g. stem cell therapy, novel drug therapies, gene therapy).
Rule-Based Category Learning
Several computational models of category learning have addressed the explicit, rule-based system, and each tends to invoke similar components, namely WM and hypothesis testing processes (Ashby et al., 1998; Ashby & Valentin, 2005; Erickson & Krushke, 1998; 2002). One of the most successful neurobiological models of rule-based category learning is the COmpetition between Verbal and Implicit Systems (COVIS) model proposed by Ashby and colleagues (Ashby et al., 1998; Ashby & Valentin, 2005; Maddox & Ashby, 2004)1. FF According to the COVIS model, rule-based category learning depends upon hypothesis testing processes that place heavy demand on WM, including the selection, maintenance, and shifting of classification rules. Here, we elaborate on the COVIS model to better address patterns of variation in the performance of PD patients on these particular aspects of hypothesis testing. In addition, we extend the model to address the process of rule generation and further articulate the process of rule selection, each of which are often overlooked in empirical and theoretical examinations of category learning.
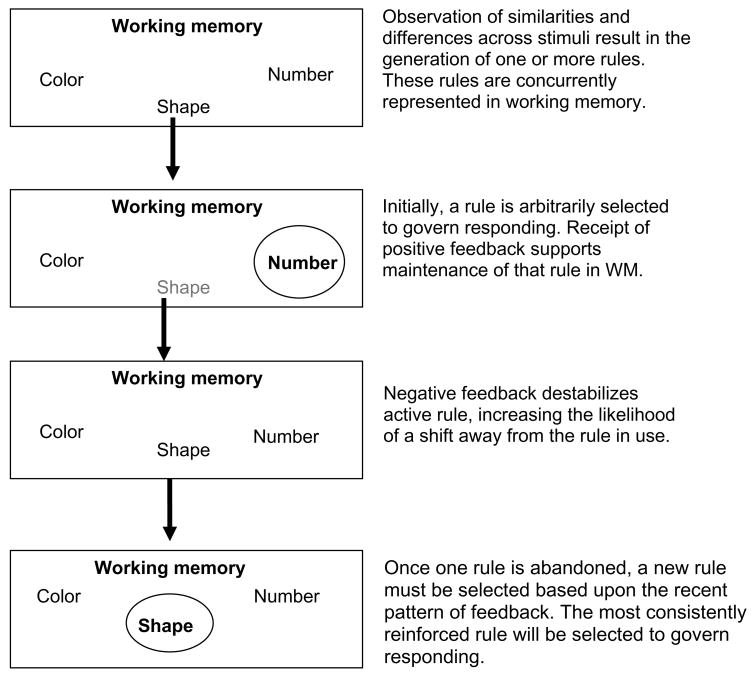
Although each hypothesis testing component represents a distinct phase within the category learning process, the components are interdependent. Figure 1 summarizes the manner in which these processes interact to support rule-based category learning. On the WCST, for example, participants must sort a deck of cards according to one of three dimensions (color, shape or number), but they are not told which dimension is relevant and must learn this information through feedback. Before beginning to sort, most participants will readily generate several possible sorting rules (e.g. sort according to color). The process of rule generation, which refers to the initial activation of one or more rule representations in WM, demands inductive reasoning and spontaneous cognitive flexibility, but places little demand on WM. Although several rules may be maintained in WM, one must select a particular rule to guide classification. Initially, rule selection is likely to be fairly random but as the task progresses, the pattern of recent feedback will increase or decrease activation of a particular rule representation in WM. The most strongly activated rule is selected to guide responding. If the selected rule remains associated with a consistent history of positive reinforcement, that rule should be maintained in WM, protected from interference by irrelevant information/rules. If the selected rule is not associated with a recent history of positive reinforcement, then one should shift away from that rule. Once the incorrect rule is abandoned, one must either guess until a new rule can be generated or select a new rule based upon its relative activation in WM.
Figure 1.
Summary of the component processes supporting rule based category learning, specifically rule generation, maintenance, shifting and selection.
In the sections that follow, we examine the impact of PD on the component processes of rule-based category learning, namely rule generation, maintenance, shifting and selection. In doing so, we have worked to identify and, where possible, resolve discrepancies within the neuropsychological literature to clarify the manner in which PD affects each component process. Often, variability in performance may be attributable to whether patients are tested while on or off their usual medication and we have worked to specify the potential role of PD-related medication on patients’ performance. For each component process of hypothesis testing, we integrate relevant neuropsychological evidence from patients with PD with neuroimaging and computational modeling evidence to clarify the neural architecture of rule-based category learning and the neurobiological impact of PD. The proposed neural architecture of rule based category learning is illustrated in Figure 2.
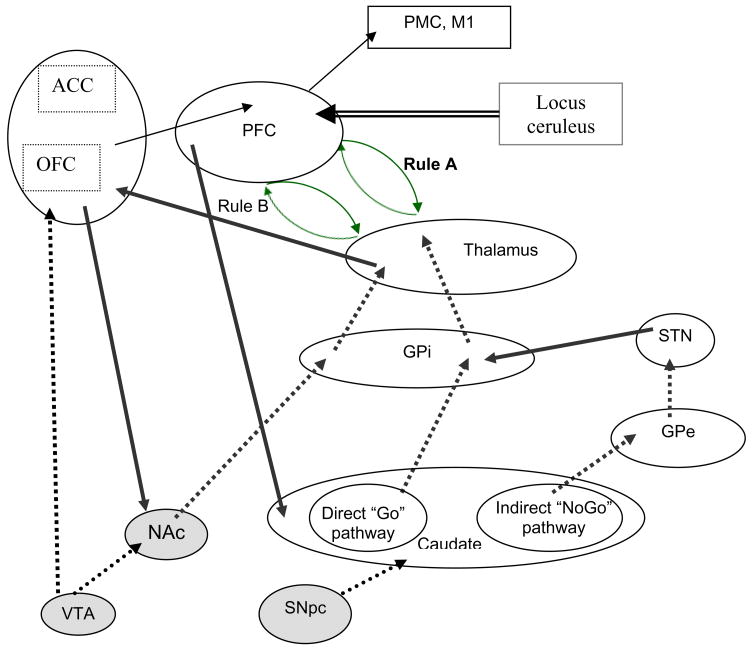
Figure 2.
Neural architecture of explicit, rule-based category learning. Solid lines indicate excitatory input; dashed lines indicate inhibitory input. Generation of rules depends upon dopamine (DA) activity within the ventral cortico-striatal circuit, including nucleus accumbens (NAc), orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC). Active maintenance of the rule in use depends upon recurrent excitation of the fronto-thalamic loop that represents that rule. Positive feedback supports rule maintenance through a phasic DA bursts to caudate, which increases excitation of the “Go” pathway and inhibition of the “NoGo” pathway and enables disinhibition of the relevant fronto-thalamic loop. Negative feedback triggers a phasic dip in DA availability in caudate, resulting in enhanced inhibitory output from GPi. This destabilizes the relevant rule representation in WM and increases the likelihood of a shift. Release of norepinephrine from the locus ceruleus may also govern shifting activity. Selection of the new rule depends upon the relative pattern of activation within ACC, which biases the relative patterns of activation of rule representations in prefrontal cortex (PFC). Unexpected negative or positive feedback triggers a phasic dip or burst, respectively, in DA release from the ventral tegmental area. These phasic changes alter activation in NA and ACC and these error-driven changes in ACC activation regulate activation of relevant rule representations in PFC.
1BRule Generation
Examinations of rule generation in PD, which are summarized in Table 1, typically employ one of two techniques. The first requires participants to state the rule they are testing as they perform feedback-mediated category learning. On these tasks, patients with PD tend to verbalize fewer hypotheses, regardless of whether the task involves unidimensional (e.g. respond according to shape; Channon, 1997) or multi-dimensional rules (e.g. respond based upon the shape, color and number of stimuli showing; Price, 2006). Unfortunately, this measure of rule generation is confounded by the operation of rule shifting and selection processes. Further, it is not clear whether deficits reflect an impairment to rule generation or feedback-mediated learning, which some have argued is disrupted in PD (Charbonneau, Riopelle & Beninger, 1996; Frank, 2005; Frank, Seeberger, & O’Reilly, 2004; Shohamy et al., 2005; Vriezen & Moscovitch, 1990).
Table 1.
| Source | Task | Patient sample | Result |
|---|---|---|---|
| Beatty & Monson, 1990 | California Card Sort Task (Delis et al., 1989) | 16 PD; Stages 1–4; M = 2.6 | PD= NC (d =.57) |
| Channon, 1997 | Unidimensional classification learning | 20 PD | PD < NC (d =.41) |
| Dimitrov, Grafman, Soares & Clark, 1999 | California Card Sort Task | 8 PD; Stages 1–4; M=3.0 | PD = NC (d =.93) |
| Farina et al., 1994 | Picture sorting task (Incisa della Rocchetta, 1986) | 22 PD Stages 1–2 | PD < NC |
| Hanes, Andrewes & Pantelis, 1995 | Verbal solutions task (Reitan, 1972) | 25 PD Stages 1–4; M = 2.76 | PD < NC (d =.41) |
| Price, 2006 | Multi-dimension classification learning | 16 PD; Stages 1–3; M = 2.2 | PD < NC (d = 1.0) |
| Swainson et al., 2006 | Five Dimension Task (Swainson & Robbins, 2001) | 11 mild PDOFF 27 mild PDON 14 severe PDON |
mild PDOFF = NC; mild PDON < NC severe PDON = NC |
Summary of studies examining rule generation in PD
Note. Stages refers to Hoehn & Yahr (1967) ratings of PD severity; M = mean level of PD severity; d = Cohen’s d measure of effect size
To more specifically target rule generation, the second type of measure presents participants with a set of stimuli that vary along multiple dimensions and participants must generate as many sorting rules as possible without receiving any form of feedback. Studies using this type of measure have produced evidence of normal (Beatty & Monson, 1990; Dimitrov, Grafman, Soares & Clark, 1999) and impaired rule generation performance (Farina, 1994; Swainson et al., 2006) in PD. One recent study suggests this discrepancy may reflect variations in disease severity and patients’ medication status (Swainson et al., 2006). Specifically, medication worsened rule generation performance in patients with early PD but improved performance among patients with moderate or severe PD.
Detrimental effects of medication in PD have been observed with other tasks and it has been argued that medication may “overdose” particular neural processes (Cools, 2006; Cools et al., 2003; 2007; Gotham et al., 1988; Swainson et al., 2000). More specifically, the “overdose hypothesis” posits that the medication required to treat motor impairment, associated with degradation of the dorsal striatum, results in excessive DA in ventral striatum, which is relatively unaffected in early PD (Kish et al., 1988; Farley et al., 1977). Consistent with this “overdose hypothesis”, medication in early PD has been shown to disrupt activity within the ventral striatum (Cools et al., 2007). As PD advances, DA neurons in the ventral striatum degenerate and the ventral cortico-striatal circuits become increasingly compromised (Kish et al., 1988; Farley et al., 1977). Among moderately affected PD patients, medication appears to sufficiently overcome this deficit and improve performance on tasks mediated by the ventral circuit (Cools et al., 2001; 2006; Swainson et al., 2006). However, once the ventral striatum is heavily compromised, as in severe PD, medication may be insufficient to support normal task performance, resulting in impaired performance both on and off medication.
The overdose hypothesis posits that for processes mediated by ventral cortico-striatal circuitry, the effect of medication in PD interacts with disease severity according to an “inverted U” function. Specifically, such processes are impaired in medicated, early PD patients, due to excessive DA, and medicated, late PD patients, due to insufficient DA; medicated patients with moderate PD perform normally (Cools et al., 2001; 2006; Swainson et al., 2006). This “inverted U” pattern has been observed with rule generation (Swainson et al., 2006) and may explain the variation in rule generation performance across studies. Those studies demonstrating normal rule generation involved medicated patients of moderate severity (Beatty & Monson, 1990; Dimitrov et al., 1999) whereas those studies reporting impairment involved medicated patients of mild severity (Farina, 1994; Price, 2006).
The possibility that rule generation depends upon ventral cortico-striatal circuitry is supported by converging neuroimaging and neuropsychological evidence from a variety of problem solving tasks indicating involvement of the OFC and ACC in hypothesis generation (Burgess, 2000, Dimitrov et al., 1999; Elliot & Dolan, 1998; Goel & Dolan, 2000; Goel & Grafman, 2000; Miller & Tippett, 1996; Reverberi, D’Agostini, & Skrap, 2002; Reverberi, Lavaroni, Gigli, Skrap & Shallice, 2005; Vartanian & Goel, 2005). Anti-Parkinsonian medications may “overdose” the ventral striatum, thereby creating dysfunction in its cortical targets, namely OFC and ACC. Taken together, the general pattern of evidence suggests that PD patients experience difficulty generating rules, a problem that may stem from PD-related treatments rather than the disease itself.
2BRule Maintenance
Once a rule is generated, it must be maintained in WM to guide responding. Maintaining rule representations within WM demands selective attention, which consists of two general processes: a) active maintenance of relevant information and b) inhibition of irrelevant information. In this context, active maintenance refers to processes which sustain activation of a distinct pattern of neural activity associated with relevant information. This pattern of activation persists unless destabilized by a signal to update WM. On the other hand, inhibition in this context refers to those processes that are responsible for actively reducing the likelihood that irrelevant items will achieve a representation in WM and potentially disrupt the maintenance of relevant information. Together, active maintenance of relevant information and inhibition of irrelevant information support rule maintenance.. Generally, PD has little impact on WM maintenance (Lewis, Cools et al., 2003; Lewis, Dove et al., 2003; Lewis, Salbosz, Robbins, Barker & Owen, 2005; Owen, Bekinska et al., 1993; Owen, Roberts et al., 1993), whereas inhibitory processes are less stable (Dujardin et al., 1999; Filoteo & Maddox, 1999; Maddox, Filoteo, Delis, & Salmon, 1996; McDowell & Harris, 1997; Sharpe, 1990). Thus, rule maintenance deficits in PD may emerge under conditions of high interference by irrelevant information.
In category learning tasks, rule maintenance is typically measured by the frequency of set-loss errors, which occur when one abandons a rule that has been consistently reinforced. Table 2 summarizes findings from examinations of rule maintenance in PD. Most commonly, rule maintenance has been examined using the WCST and a large scale analysis of set loss errors on the WCST, involving over 180 PD patients, revealed normal patterns of rule maintenance (Paolo, Troester, Axelrod, & Koller, 1995). A number of studies involving the WCST and other category learning tasks have reported similar findings in PD, although a few have reported impairment (see Table 2). In contrast to generally preserved rule maintenance on the WCST, PD patients exhibit deficits on the similarly structured Odd-Man-Out task (OMO; Flowers & Robertson, 1985; Richards, Cote & Stern, 1993). On the OMO, participants must indicate which of three stimuli is different from the others using a simple categorization rule (e.g. letter or shape) and this rule changes several times over the course of the task. PD patients show normal rule maintenance for the first few rules but set loss errors increase following several rule switches. Previously reinforced rules create considerable proactive interference and increased inhibition is necessary to prevent these rules from disrupting maintenance of the currently relevant rule. This may be especially problematic for PD patients, who tend to be more susceptible to proactive interference (Helkala, Laulumaa, Soininen, & Riekkinen, 1989; Rouleau, Imbault, Laframboise & Bedard, 2001), a deficit that likely stems from prefrontal dysfunction associated with PD (Feredoes, Tononi & Postle, 2006; Turner, Cipolotti, Yousry & Shallice, 2007).
Table 2.
Summary of studies examining rule maintenance in PD
| Source | Task | Patient sample | Result |
|---|---|---|---|
| Beatty & Monson, 1990 | WCST (Heaton, 1993) | 27 PD Stages 1–4 M=3.3 | PD = NC (d =.07) |
| Beatty, Staton, Weir & Monson & Whitaker, 1989 | WCST (Heaton, 1993) | 25 PD Stages 2–3 | PD < NC (d=.86) |
| Channon, Jones & Stephenson, 1993 | Unidimensional classification learning task (single rule; no switching) | 12 PD Stages 2–3 | PD = NC (d =.13) |
| Cooper, Sagar, Jordan, Harvey, Sullivan, 1992 | WCST (Heaton, 1993) | 61 PD; newly diagnosed; unmedicated |
PD = NC (d =.06) |
| Filoteo, Maddox, Ing, Zizak & Sang, 2005 | Unidimensional classification learning task (single rule; no switching) | 19 PD Stages 1–3 | PD < NC |
| Filoteo, Maddox, Ing, Zizak & Sang, 2005 | WCST (Heaton, 1993) | 19 PD Stages 1–3 | PD =NC |
| Flowers & Robertson 1985 | Odd-Man-Out task | 49 PD | PD < NC |
| Gauntlett-Gilbert, Roberts & Brown, 1999 | ID/ED shift task; (Owen, et al., 1993 | 10 PD Stages 1–3 M = 2.26 | PD < NC |
| Green et al., 2002 | WCST (Heaton, 1993) | 61 PD; Stage 3–5 | PD < NC (d = 1.5) |
| Monchi et al., 2004 | WCST (Heaton, 1993) | 8 PD Stages 1–2 off meds |
PD < NC |
| Paolo, Troester, Axelrod & Kaller, 1995 | WCST (Heaton, 1993) | 181 PD Stages 1–4 | PD = NC (d =.12) |
| Price, 2006 | Multidimensional classification learning task | 16 PD Stage 1–2 | PD = NC |
| Price, 2006 | WCST (Hart et al., 1998) | 16 PD Stages 1–2 | PD = NC |
| Richards, Cote, Stern, 1993 | Odd-Man-Out task (Flowers & Robertson, 1985) | 45 PD Stage M = 2.6 | PD < NC |
| Tomer, Fisher, Giladi & Ahron-Peretz, 2002 | ID/ED task of CANTAB (Robbins et al., 1998) | 24 PD; Stage M = 1.6 | PD = NC |
Note. Stages refers to Hoehn & Yahr (1967) ratings of PD severity; M = mean level of PD severity; d = Cohen’s d measure of effect size
If set loss errors increase following multiple rule switches, it is possible that rule maintenance deficits may be underestimated by the WCST. Due to a number of issues, PD patients tend to achieve fewer WCST categories than controls, and therefore experience fewer rule switches. Patients who would have difficulty inhibiting previously successful rules may also fail to achieve many previous rules and therefore experience little proactive interference. It would be worthwhile to systematically examine whether set loss errors increase as a function of previously reinforced rules. On tasks like the WCST, where the rule frequently changes, set loss errors should increase as patients achieve more categories. In contrast, if the rule remains the same throughout the task, set loss errors should be fairly low. Several studies that examined category learning using a single-rule task found no evidence of increased set loss errors among PD patients (Channon, Jones & Stephenson, 1993; Filoteo, Maddox, Ing, Zizak & Song, 2005; Price, 2006), suggesting that rule maintenance is more challenging under conditions of high proactive interference.
Additionally, it appears that high levels of concurrent interference within a task create problems for PD patients. On single-rule tasks, set loss errors among PD patients are more common when irrelevant dimensions vary randomly (Filoteo, Maddox, Ing & Song, 2007), and this occurs even when participants are explicitly told which rule to use (Filoteo & Maddox, 1999; Maddox et al., 1996). In contrast, set loss errors are not as prevalent in PD patients when there is no irrelevant dimension variability, despite an increase in active maintenance demands within WM (Filoteo et al., 2007). These data suggest that PD has relatively little impact on rule maintenance unless conditions require a high level of selective attention.
Computational models of WM typically depict active maintenance as a function of recurrent excitation among frontal neurons (Braver & Cohen, 1999; 2000; Frank, Loughry & O’Reilly, 2001; Moody, Wise, & Pellegrino, 1998; O’Reilly & Frank, 2006; O’Reilly & Munakata, 2000) or recurrent connections between PFC and thalamus (Ashby et al., 1998; 2005; Dominey, Arbib & Joseph, 1995; Goldman-Rakic & Friedman, 1991; Houk & Wise, 1995; f, 2000; Taylor & Taylor, 2000; Zipser, 1991). If WM maintenance depends upon low levels of tonic DA stimulation in PFC, then among PD patients, available DA should be sufficient to support the maintenance of WM. Alternatively, the FROntal- Striatal-Thalamic model of WM (FROST; Ashby et al., 2005), proposes that WM maintenance depends upon excitatory projections from PFC to associated neurons within the head of the caudate. When WM load is high or complex, the caudate becomes necessary to inhibit the GP and support prolonged maintenance. Striatal dysfunction, such as in PD, limits the caudate’s ability to inhibit the GP from disrupting active thalamo-cortical loops. Thus, the FROST model predicts that PD impairs active maintenance only when the WM load is especially high.
Whereas active maintenance is generally preserved in PD, patients may have greater difficulty inhibiting irrelevant information from accessing WM. This type of inhibition is commonly attributed to some type of WM gating mechanism (Ashby et al., 2005; Braver & Cohen, 2000; Cohen, Braver & O’Reilly, 1996; Frank et al., 2001; Moody et al., 1998; O’Reilly, Braver & Cohen, 1999; O’Reilly & Munakata, 2000; Zipser, 1991). Rougier et al. (2005) recently demonstrated the necessity of a gating mechanism in rule-based category learning through a simulation of WCST performance. When WM was modeled to include a gating mechanism, relevant features were rapidly encoded, resulting in activation of the correct rule. Simpler models without a gating mechanism tended to activate new rule representations with each stimulus presentation and failed to settle on a particular rule.
A number of WM models emphasize the importance of some type of gating mechanism, which is thought to rely upon phasic changes in DA. Brief, phasic changes in DA are known to follow reinforcement prediction errors, which reflect differences between an expected and actual outcome (Bayer & Glimcher, 2005; Delgado, Locke, Stenger & Fiez, 2003; Frank, Woroch & Curran, 2005; Holroyd & Coles, 2002; Shultz, Dayan & Montague, 1997; Schultz, 2007). In addition, DA appears to enhance the contrast between the relevant and irrelevant dimensions of a stimulus by enhancing the firing rate of currently active cells and suppressing less active cells (Cohen, Braver, & Brown, 2002; Cohen & Servan-Schreiber, 1992; Foote & Morrison, 1987; Hernandez-Lopez et al., 1997; Nicola, Surmeier & Malenka, 2000; Rolls, Thorpe, Boytim, Szabo, & Perrett, 1984). Thus, gating supports selective attention via active suppression of the representation(s) associated with the irrelevant dimension(s) and maintenance of the representation of the relevant dimension(s).
Several WM models posit that gating is accomplished by DA input to PFC (Braver & Cohen, 1999; O’Reilly et al., 1999). Consistent with this, profound DA depletion within PFC leads to high distractibility and great difficulty maintaining mental set (Collins, Wilkinson, Everitt, Robbins & Roberts, 2000; Crofts et al., 2001). The impact of DA on WM performance appears to be largely dose-dependent, such that especially high or low levels of DA increase one’s susceptibility to distraction (Kimberg, D’Esposito & Farah, 2007; Williams & Goldman-Rakic, 1995). In early PD, prefrontal DA availability is relatively normal (Brück et al., 2006; Sawamoto et al., 2008) and medication may yield excessive DA activation in PFC. Among healthy adults, WM under conditions of high distraction is impaired by D2 agonists that target receptors abundant in PFC and striatum, presumably because the excessive DA disrupts the gating mechanism, allowing both relevant and irrelevant information to access WM (Frank & O’Reilly, 2006). Pharmacological treatments for PD, which increase the tonic availability of DA within PFC and caudate, may similarly reduce the discriminatory power of phasic changes in DA (Frank et al., 2004). Excessive prefrontal DA may disrupt the WM gating mechanism by lowering the threshold for updating the contents of WM, resulting in rule maintenance deficits, especially under conditions of high interference.
Alternatively, difficulty filtering irrelevant information may reflect disturbances to a DA-mediated gating mechanism within the striatum (Frank et al., 2001; Hazy, Frank & O’Reilly, 2007; O’Reilly & Frank, 2006). According to the Prefrontal, Basal ganglia, Working Memory (PBWM) model, each viable cognitive response is separately maintained in the PFC as a distinct stripe, or “isolated group of interconnected neurons” (Hazy et al., 2007, p. 4). Each PFC stripe is interconnected with striatal neurons that govern WM updating based upon recent feedback. Receipt of unexpected positive feedback triggers a phasic burst of DA within the caudate. This excites the direct, or “Go” pathway of the basal ganglia and inhibits the indirect, or “NoGo”, pathway, thereby preventing the GPi from inhibiting relevant fronto-thalamic activity and increasing the likelihood that relevant information accesses WM. Irrelevant stimulus dimensions are not updated despite receiving intermittent reinforcement. This is because the frequent non-reinforcement of irrelevant dimensions results in inhibition of the direct, “Go” pathway and excitation of the indirect, or “NoGo” pathway, which indirectly allows GPi to inhibit the thalamus and decreases the likelihood that the irrelevant dimension(s) will access WM. In this way, striatal DA selectively gates only the most relevant representations, namely those consistently associated with positive reinforcement. Consistent with this, McNab & Klingberg (2008) demonstrated that high levels of within-task distraction were associated with increased GPi activation, presumably reflecting the gating mechanisms of the “Go” and “NoGo” pathways. Moreover, the extent of GPi activation was associated with WM capacity across participants, suggesting that this gating mechanism within the basal ganglia has important implications for selective attention processes in WM.
In PD, significant DA depletion results in too little inhibition of the “NoGo” pathway and excessive inhibition of the thalamus by the GPi (Frank, 2005; Frank & O’Reilly, 2006). Animal research indicates that DA depletion in the caudate leads to decreased updating and enhanced resistance to distraction (Crofts et al., 2001; Collins et al., 2000). Thus, it is unlikely that the increased susceptibility to interference among PD patients stems from striatal DA depletion. Instead, patients’ increased distractibility could reflect elevated DA levels due to levodopa or DA agonist medication, which increase tonic DA stimulation within the caudate. Although a phasic increase in DA should selectively enhance only the most active synapses, excessive tonic DA stimulation, due to medication, may enhance all active synapses, thereby reducing the discriminatory power of phasic changes in DA and lowering the threshold for updating WM. Notably, prolonged levodopa ingestion may create a secondary problem by sensitizing striatal DA receptors, resulting in excessive response to DA stimulation (Calon et al., 2002; Deogaonkar, Piallat & Subramanian; Filion, Tremblay & Bedard, 1991; Obeso, et al 2000; Papa, Desimone, Fiorani, & Oldfield, 1999). Once this occurs, medications that target DA receptors will inappropriately increase excitation along the “Go” pathway and decrease inhibition along the “NoGo” pathway. Consonant with this hypothesis, prolonged use of levodopa is associated with inappropriate motor activity, specifically uncontrollable, choreatic movements known as dyskinesias. The mechanism that causes drug-induced dyskinesias may also be reduce the ability to inhibit inappropriate cognitive responses. Future research is needed to clarify the possible relationship between WM impairment in PD and specific forms of PD treatment.
To summarize, neuropsychological evidence suggests that PD patients exhibit increased susceptibility to irrelevant stimulus dimensions during category learning, which disrupts rule maintenance under conditions of high concurrent or proactive interference. It appears this deficit may stem from excessive tonic DA stimulation in PFC and/or caudate. Additional work is necessary to clarify how pharmacological therapies impact rule maintenance performance in PD.
Rule Shifting
In contrast to rule maintenance, which occurs when one persists with a successful rule, rule shifting occurs when one ceases with an unsuccessful rule. Discussions of executive function in PD commonly assert that patients exhibit rule shifting deficits (e.g. Brown & Marsden, 1990; Lees & Smith, 1983; Salmon et al., 1998; Taylor, Saint-Cyr & Lang,1986). However, as shown in Table 3, this generalization fails to capture the variability across studies.
Table 3.
Summary of studies examining rule shifting in PD
| Source | Task | Patient sample | Result |
|---|---|---|---|
| Beatty & Monson, 1990 | WCST (Heaton et al., 1993) | 27 PD Stages 1–4; M=2.6 | PD = NC (d=.34) |
| Beatty, 1989 | WCST (Heaton et al., 1993) | 43 PD; Stages 1–4 | PD = NC (d =.56) |
| Beatty, Staton, Weir & Monson, 1989 | WCST (Heaton et al., 1993) | 25 PD; Stages 2–3 | PD = NC (d =.43) |
| Bowen, Kamienny, Burns & Yahr, 1975 | WCST (Berg, 1948) | 18 PDOFF 53 PDON |
PDOFF = NC PDON = NC |
| Canavan et al., 1989 | WCST (Nelson, 1976) | 19 PD; M = 1.5 | PD < NC |
| Channon, Jones, & Stephenson, 1993 | Unidimensional classification learning | 12 PD Stages 2–3, M = 2.75 | PD < NC (d =.50) |
| Cools, van den Bercken, Horstink, van Spaendonck, & Berger, 1984 | Block sorting task | 18 PD Stages 1–4; M = 2.3 | PD < NC |
| Cools, Barker, Sahakian & Robbins, 2001 | ID/ED task (Downes et al., 1989 | 14 PDON; M = 1.75 15 PDOFF; M = 1.80 |
PDOFF = PDON < NC |
| Cooper, Sagar, Jordan, Harvey, & Sullivan, 1991 | WCST (Milner, 1963) | 60 PD; newly diagnosed; unmedicated |
PD = NC (d =.43) |
| Cooper, Sagar, Jordan, Harvey, Sullivan, 1992 | WCST(Heaton et al., 1981) | 82 newly diagnosed PD; randomly assigned on or off meds |
PD = NC in each case |
| Dalrymple-Alford, Kaldters, Jones, & Watson, 1994 | WCST (Heaton et al., 1993) | 8 PD | PD = NC (d = 0) |
| Downes et al., 1989 | ED/ID task | 16 PDOFF M = 1.1 6 PDON M = 2.4 |
PDOFF < NC PDON < NC |
| Fimm, Barti, Zimmerman & Wallesch, 1994. | modified ID/ED task | 19 PDON Stages 2–3 M=2; 8 PDOFF Stages 1–3; M= 2. |
PDON = NC; PDOFF < NC |
| Gauntlett-Gilbert, Roberts & Brown, 1999 | modified ID/ED task | 10 PD Stages 1–3; M=2.2 | PD < NC |
| Gotham, Brown & Marsden, 1988 | WCST (Nelson, 1976) | 16 PD Compared ON and OFF | PDON < NC (d =.90) PDOFF < NC (d = 1.1) |
| Green, 2002 | WCST (Heaton et al., 1993) | 61 PDOFF; Stage 3–4 | PDOFF < NC |
| Kulisevsky et al., 1996. | WCST (Heaton et al., 1993) | 20 PD Compared ON and OFF | PDON = NC PDOFF = NC |
| Lange, Robbins, Marsden, Owen & Paul, 1992 | ID/ED task (Downes et al., 1989) | 10 PD Stages 3–5, M = 3.5; Compared ON and OFF |
PDOFF = PDON < NC |
| Lees & Smith, 1983 | WCST (Neslon, 1976) | 12 PD | PD = NC |
| Lewis et al., 2005 | Modified ID/ED task | 20 PD; M = 1.9 Compared ON and OFF |
PDOFF = PDON < NC |
| Monchi et al., 2004 | WCST | 8 PDOFF | PDOFF < NC |
| Owen et al., 1993 | ID/ED task (Downes et al., 1989) | 26 PDOFF (stages 1–3) 23 PDON (stages 1–4) |
PDOFF < NC (d= 2.4) PDON = NC |
| Paolo, Troester, Axelrod & Kaller, 1995 | WCST (Heaton et al., 1993) | 181 PD Stage 1–4 | PD < NC (d= 1.03) |
| Price, 2006 | WCST (Hart et al., 1998 | 17 PD Stages 1–4, M = 2.3 | PD = NC |
| Slabosz et al., 2006 | Modified ID/ED task | 20 PD Stage M = 1.9 Compared ON and OFF |
PDOFF = PDON < NC |
| Starkstein et al., 1989 | WCST (Heaton et al., 1993) | 48 mild PD; 20 moderate PD 26 severe PD |
severe < moderate < mild PD; mild PD = NC |
| Tomer, Aharon-Peretz, & Tsitrinbaum, 2007 | ID/ED task of CANTAB (Robbins et al., 1998) | 35 PD | PD < NC (d = 1.08) |
| van Spaendonck, Berger, Horstink, Brom, & Cools, 1995 | WCST and 2 variants (follwing Nelson, 1976) | 45 PD | PD = NC |
| Witt et al., 2006 | WCST (version not given) | 20 PD Stages 1–3 | PD = NC |
| Zakzanis & Freedman | WCST (Heaton et al. et al., 1993) | Meta-analysis (N= 11) | mean(d) =.46 Range(d) = −.66 − +.92 |
Note. Stages refers to Hoehn & Yahr (1967) ratings of PD severity; M = mean level of PD severity; d = Cohen’s d measure of effect size
Neuropsychological examinations of rule shifting have typically relied upon the WCST and the Intra-Extra Dimensional Set Shift task (ID/ED task; Downes et al., 1989). These tasks require a participant to categorize simple stimuli according to some rule that is not specified. Following a certain number of correct sorts, the experimenter changes the rule without notice. [The exception to this is the modified WCST (Nelson 1976), in which participants are told that a switch has occurred.] Since the previously relevant rule no longer receives reinforcement, it should be abandoned. However, participants may occasionally perseverate with that rule and a high frequency of preservative responses indicates rule shifting impairment.
As detailed in Table 3, patients with PD often exhibit increased perseverative responses during category learning. One meta-analysis of rule shifting on the WCST found an increase in perseverative responding among PD patients of moderate effect size, although considerable variability existed across studies (Zakzanis & Freedman, 1999, N = 11, mean(d) =.46, range(d) = −.66 − +.92). This variability may reflect differences in the patient samples across studies. In particular, increased disease severity may be associated with increased shifting impairment (Starkstein et al.,1989; but see Gotham et al., 1988). Medication, however, appears to have little effect as it neither improves (Gotham et al., 1988) nor worsens shifting performance on the WCST (Bowen, Kamienny, Burns & Yahr, 1975; Cooper et al., 1991; 1992; Kulisevsky et al., 1996).
Though variation across patient samples may explain some of the inconsistencies across studies, shifting deficits also appear to vary depending upon the WCST version in use. In the original 128 card version of the WCST (Berg, 1948; Heaton, 1981; Milner, 1963), a sort could be correct along several dimensions. In contrast, simplified versions (Hart, Kwentus, Wade & Taylor, 1988; Nelson, 1976) include only those cards that can only be matched along a single dimension. Although healthy older adults perform comparably on the two versions (Greve & Smith, 1991), it appears that PD patients have greater difficulty with the simplified version. Studies using the simpler version often indicate rule shifting deficits among PD patients, whereas the majority of studies using the original WCST report normal shifting performance (see Table 3). On the simplified versions of the WCST, rule shifting deficits emerge in patients of various stages of severity; on the original WCST, deficits primarily emerge among patients with more severe PD.
It can be difficult to interpret a group’s performance on the WCST, however, because the task measures myriad processes. For this reason, researchers also rely on the ID/ED task (Downes et al., 1989). On this task, participants view two stimuli and must determine which is correct based upon trial-by-trial feedback. The task proceeds through a series of stages, each of which is designed to measure a particular type of shift. On the final, extra-dimensional shift (EDS), stage participants must begin to respond according to the previously irrelevant dimension and ignore the previously relevant dimension. Rule shifting is determined either through the number of errors made on this phase, the number of trials required before the participant reaches criterion, or the percentage of participants who reach criterion. Each of these methods indicate rule shifting impairment in PD (Downes et al., 1989; Gauntlett-Gilbert, Roberts & Brown, 1999; Lange et al., 1992; Slabosz et al, 2006; Tomer, Aharon-Peretz, & Tsitrinbaum, 2007).
The generally poor rule shifting performance of patients on the ED/ID task mirrors the poor performance of patients on the simplified versions of WCST and contrasts with the generally preserved performance on the traditional WCST (see Table 3). In contrast to the traditional WCST, positive feedback on the simpler WCST (Nelson, 1976; Hart et al., 1988) and the ID/ED (Downes et al., 1989) task reinforces only the appropriate dimension. By insuring that irrelevant dimensions do not receive any reinforcement, the modification was designed to simplify the WCST. Unfortunately, this change may create particular difficulty for patients with PD. Slabosz et al. (2006) demonstrated that PD patients experience shifting impairment, but only when the newly relevant dimension had previously been completely irrelevant, as with the simplified WCST and ID/ED tasks. When the newly relevant dimension had previously received some reinforcement, as it does on the traditional WCST, patients shifted normally.
As discussed in the previous section, medicated PD patients are more susceptible to distraction by irrelevant dimensions that randomly vary with the relevant dimension. Although this may disrupt rule maintenance, it also enables patients to more readily shift toward the dimension once it becomes relevant. Consequently, medicated patients shift normally when the newly relevant dimension had previously received intermittent reinforcement (Kulisevsky et al., 1996; Slabosz et al., 2006). In contrast, when a newly relevant dimension has no history of reinforcement, or a history of complete non-reinforcement, patients have difficulty identifying the newly relevant rule (Channon et al., 1993; Price, 2006; van Spaendonck, Berger, Horstink, Brom & Cools, 1995). This possibility is discussed further in the Rule Selection section. These findings suggest that medicated PD patients may not necessarily have problems shifting away from an inappropriate rule. Instead they may have difficulty selecting the newly appropriate rule, especially if the new rule has no prior history of reinforcement.
By contrast, rule shifting is generally impaired among PD patients off medication, which suggests a role for striatal DA in rule shifting. This possibility is illustrated in the PBWM model Frank et al., 2001; O’Reilly & Frank, 2006), outlined in the previous section. According to the model, the occurrence of unexpected negative feedback triggers a phasic dip of DA within the caudate that decreases excitation of the “GO” pathway and inhibition of the “NoGo” pathway. The net effect is inhibition and destabilization of the stripe of prefrontal cortex that is associated with the rule in use. Negative feedback decreases the likelihood that the rule will be updated into WM the next time it is presented, and increases the likelihood of a rule shift (Frank et al., 2001; Hazy et al., 2007; O’Reilly & Frank, 2006). Among PD patients off medication, the model further predicts that depletion of striatal DA reduces the efficacy of phasic changes in DA following reinforcement prediction errors (Frank, 2005; Frank & O’Reilly, 2006). In the context of category learning, this means PD patients off medication should exhibit reduced distractibility and increased perseverative behavior. However, DA depletion within the dorsal striatum of non-human animals has no impact on set shifting, although animals did exhibit reduced distractibility (Crofts et al., 2001; Collins et al., 2000). These data call into question the assertion that rule shifting processes are mediated by DA activity within the caudate.
Instead, shifting may depend upon DA activity throughout the dorsal fronto-striatal circuit. Though rule shifting is unaffected by selective DA depletion in PFC (Crofts et al., 2001; Roberts et al., 1994) or caudate (Crofts et al.; Collins et al., 2000), it is possible that DA in either region compensates for loss in the other. Depletion of prefrontal DA is associated with upregulation of DA activity in the caudate (Roberts et al., 1994), which may explain why selective DA lesions in the caudate had little impact on shifting performance. This possibility is supported by evidence that the D2/D3 anatagonist sulpiride, which targets DA receptors in the PFC and NoGo pathway of the striatum, impairs set shifting performance in healthy volunteers (Mehta et al., 1999; 2004; 2005). Thus, rule shifting in PD may vary with the degree to which prefrontal dopaminergic activity and medication can compensate for striatal losses. This may explain why rule shifting impairment in PD patients may persist among optimally medicated patients (see Table 3).
In addition to the involvement of the dopaminergic system, accumulating evidence indicates a role for the noradrenergic (NA) system in supporting rule shifting performance. Noradrenergic projections from the locus ceruleus target diverse forebrain sites, including thalamus, PFC and ACC. Pharmacological manipulations of prefrontal NA have been shown to alter set shifting performance in rats (Lapiz & Morilak, 2006; Lapiz et al., 2007; Newman et al., 2008; Tait et al., 2007). Although the mechanism by which cortical NA supports set shifting is unclear, it is possible that repeated failure to achieve positive reinforcement results in a switch towards explorative behaviors, which would include shifting away from the rule governing classification responses. This switch is postulated to depend upon changes in the ceruleo-cortical noradrenergic system (Aston-Jones, Rajkowski & Cohen, 1999). Patients with PD typically experience significant degeneration of locus ceruleus(Rommelfanger & Weinshenker, 2007 for), which would result in altered NA activity and may cause a failure to switch towards more explorative behaviors). Further, given that many of the pharmacological treatments for PD, including L-dopa, increase NA production in locus ceruleus (Fornai, di Poggio, Pellegrini, Ruggieri & Paparelli, 2007), medication may improve shifting performance to the extent that locus ceruleus integrity is preserved.
In summary, rule shifting performance may reflect activity of the dopaminerigc and/or noradrenergic systems, both of which are compromised in PD. Among PD patients off medication, decreased DA and NA availability appears to disrupt rule shifting and this impairment may be ameliorated by pharmacological treatment.
Rule Selection
The preceding section addressed the neural architecture of rule shifting, which should occur following negative feedback. The receipt of negative feedback must not only trigger a shift away from the rule in use, but the selection of a new rule to guide responding. The possible impact of PD on rule selection was initially suggested by Downes et al. (1989), who argued that PD deficits on the ID/ED task reflect difficulty identifying the newly relevant dimension rather than an inability to abandon the previous rule. The specific process of rule selection has received relatively little attention in the category learning literature, although other lines of research offer evidence relevant to the present discussion. Table 4 summarizes evidence relevant to rule selection in PD.
Table 4.
Summary of studies examining rule selection in PD
| Source | Task | Patient sample | Result |
|---|---|---|---|
| Channon, 1997 | Unidimensional classification learning | 21 PD | PD < NC d=.62 |
| Channon, Jones, & Stephenson, 1993 | Unidimensional classification learning | 12 PD; Stages 2–3, M=2.75 | PD < NC; (d=.64) PD tested fewer correct hypotheses |
| Filoteo, Maddox, Ing, & Song, 2007 | Classification learning (uni-dimensional, conjunctive or disjunctive rules) | 12 PD; M=1.8 | unidimensional rule: PD < NC other rules: PD = NC |
| Filoteo, Maddox, Salmon, Song, 2005 | Unidimensional classification learning tasks | 19 PD; M=1.7 | <2 irrelevant dimensions: PD = NC >2 irrelevant dimensions: PD < NC |
| Gauntlett-Gilbert, Roberts & Brown, 1999 | Learned irrelevance shift on ID/ED task | 10 PD Stages 1–3; M=2.25 | PD < NC |
| Lewis, Slabosz, Robbins, Barker, & Owen, 2004 | Learned irrelevance shift on ID/ED task | 20 PD; M = 1.9 Compared ON and OFF |
PDON < NC PDOFF < NC |
| Owen et al., 1993 | Learned irrelevance shift on ID/ED task | 26 PDOFF (stages 1–3) 23 PDON (stages 1–4) |
PDON < NC PDOFF < NC |
| Price, 2006 | Multidimensional classification learning | 17 PD Stages 1–4, M = 2.3 | PD < NC |
| Slabosz et al., 2006 | Learned irrelevance shift on ID/ED task | 20 PD M = 1.9 compared ON and OFF | PDON < NC PDOFF < NC |
| Swainson, et al., 2006 | Five Dimension Task (Swainson & Robbins, 2001) | 11 mild PDOFF 27 mild PDON 14 severe PDON |
Mild PDOFF = NC Mild PDON < NC Severe PDON < NC |
Arguably, the first examination of rule selection processes was completed by Owen, Roberts, et al. (1993), who modified the traditional ID/ED task to include an additional shift stage, termed the learned irrelevance shift. In this shift, the relevant dimension was replaced with a novel dimension and participants had to sort according to the previously irrelevant dimension. PD patients have difficulty with this “learned irrelevance” shift, regardless of whether they were on medication (Owen, Roberts, et al., 1993; Gauntlett-Gilbert et al., 1999; Lewis et al., 2005; Slabosz et al., 2006). This deficit was interpreted as reflecting enhanced latent inhibition (LI) (Owen, Roberts, et al., 1993; Slabosz et al., 2006), which refers to slowed attention to previously ignored stimuli relative to novel stimuli (De La Casa, Ruiz & Lubow, 1993; Lubow, Dressler & Kaplan, 1999; Lubow & Gewirtz, 1995).
Enhanced LI is likely among PD patients off medication, given previously discussed evidence of reduced distractibility and set shifting ability. The inability to appropriately update the contents of WM may similarly impair the capacity to disinhibit previously irrelevant rules. This deficit may be especially pronounced if a rule had previously been fully irrelevant to classification and thus not represented in WM (Slabosz et al., 2006). Among medicated PD patients, however, enhanced LI is unlikely. As previously detailed, medication may reduce inhibitory processes and medicated PD patients exhibit diminished LI in other paradigms (Filoteo, Rilling & Strayer, 2002; Grande, et al., 2006, but see Wylie & Stout, 2002). Further, medicated PD patients have difficulty selecting the appropriate rule even when conditions are manipulated to favor enhanced LI (Gauntlett-Gilbert et al., 1999). Finally, if rule selection deficits in medicated PD primarily reflect enhanced LI, then selection should be unimpaired on category learning tasks that involve a single rule. However patients perform poorly on such tasks, even though the relevant stimulus dimension would not have been inhibited at any point in the task (Channon et al., 1993; Filoteo et al., 2005; 2007; Price, 2006). Taken together, these data suggest that difficulty on the learned irrelevance shift among medicated PD patients is not due to enhanced LI.
Instead, medicated PD patients may have difficulty with the learned irrelevance shift because they have difficulty identifying which stimulus dimension is most relevant for categorization. Category learning performance is normal among medicated PD patients when all dimensions are equally relevant to classification, but impaired when tasks include stimulus dimensions of varying relevance to classification (Channon et al., 1993; Channon, 1997; Filoteo et al., 2005; 2007; Knowlton, Mangels, & Squire, 1996; Maddox & Filoteo, 2001; Maddox, Aparicio, Marchant & Ivry, 2005; Price, 2006; Swainson et al., 2006). Moreover, when medicated PD patients identify the relevant dimension, they have difficulty selecting an appropriate decisional criterion along that dimension (Filoteo et al., 2007; Maddox et al., 2005).
Recent evidence suggests that selection deficits among medicated PD patients may reflect an inability to make proper use of negative feedback to identify the relevant dimension for classification (Cools et al., 2006; Frank et al., 2004; Schott, Niehaus & Wittman, 2007). Feedback-mediated learning is driven by reinforcement prediction errors, which are known to rely upon midbrain DA signals (Hollerman & Schultz, 1998). Whereas unexpected positive feedback (positive prediction error) triggers a phasic DA burst, unexpected negative feedback (negative prediction error) triggers a phasic dip. These signals are thought to drive learning by biasing activity in a number of target regions (Schultz, 2007; Schultz & Dickinson, 2000), including the NAc (Aron et al., 2004; Rodriguez et al., 2006) and ACC (Holroyd & Coles, 2002 Holroyd & Coles, 2005; Ridderinkhof, Ullsperger, Crone & Nieuwenhuis, 2004). Functional neuroimaging examinations of category learning have indicated that unexpected negative feedback is associated with increased activation of NAc and ACC (Aron et al., 2004; Rodriguez, Aron & Poldrack, 2006). This activity is thought to integrate reinforcement values of consecutive events to bias changes in cognitive or overt responding (DePisapia, Slomski, & Braver, 2007; Mogenson, 1987).
At present, it is unclear whether ACC activation varies as a function of NAc activity or if the two regions respond in parallel to reinforcement prediction errors. It is possible that a negative prediction error triggers increased NAc activation, which would briefly inhibit the GPi, resulting in increased ACC activity. In this way, error-driven changes within NAc may govern the relative activation of ACC neurons associated with a given rule representation. Alternatively, since midbrain DA signals directly bias ACC activation, it is possible that reinforcement-mediated changes in the NAc and ACC occur independently. Regardless, ACC is commonly believed to support response selection, including cognitive responses (Barch, Braver & Noll, 2000; Botvinick, Braver, Carter, Barch & Cohen, 2001; Buckner, Raichle, Miezin & Petersen, 1996; Corbetta, et al., 1992; Elliot & Dolan, 1998; Petersen, Fox, Posner, Mintan & Raichle, 1988; Raichle et al., 1994).
The role of ACC in supporting rule selection is supported by several lines of evidence. First, functional neuroimaging with healthy controls performing the WCST found ACC activation following negative feedback, when rule selection must occur, but not following positive feedback, when the rule in use can simply be maintained (Monchi et al., 2001; 2004). Further, evidence from other decision making tasks indicates that ACC activation is highest on learning conditions where feedback must be integrated over a series of trials, pointing to the role of ACC in the temporal integration of feedback (Rushworth, Buckley, Behrens, Walton, & Bannerman, 2007; Yarkoni, Braver, Gray & Green, 2005). Finally, ACC activity occurs with category learning only when stimuli vary along multiple dimensions; uni-dimensional variation is not associated with ACC activation (Corbetta et al., 1992). These data led Ashby et al. (1998) to conclude that the ACC facilitates rule selection on multi-dimensional tasks by biasing the relative strength of rule representations within prefrontal cortex.
To summarize, rule selection appears to depend upon reinforcement-mediated activation of the ACC, which biases the relative activation of rule representations in PFC such that the most consistently reinforced rule is selected to guide responding. Recent evidence suggests that medication in early PD disrupts reinforcement-mediated activity within ACC (Holroyd, Praamstra, Plat & Coles, 2002; Stemmer et al., 2007; Willemssen et al., 2008), which may explain findings of impaired rule selection performance in this population (Channon et al., 1997; Price, 2006; Swainson et al., 2006). Medication in early PD likely results in an excessive level of tonic DA activity in the ventral cortico-striatal circuit, including NAc and ACC, an area that is typically spared until advanced PD (Cools, 2006; Farley et al., 1977; Kish et al., 1988). Increased tonic DA stimulation associated with PD medication may “fill in” the phasic dip in DA release that is triggered by a negative prediction error (Frank et al., 2004; Hollerman & Schultz, 1998). If this is the case, then negative feedback would fail to trigger the learning signal within ACC that should weaken the associated rule representation in PFC. Thus, insensitivity to negative feedback among medicated PD patients may stem from excessive DA activity in the ventral cortico-striatal circuit (Cools, 2006).
Alternatively, as detailed in the Rule Maintenance section, medication in early PD may disrupt dorsal striatal function in certain patients. According to the PBWM model (Frank et al., 2001; Frank & O’Reilly, 2006), feedback shapes the strength of individual rule representations through patterns of activity along the “Go” and “NoGo” striatal pathways. Medication in PD may lead to abnormally high levels of tonic DA stimulation such that the phasic dip triggered by negative feedback is insufficient to counteract tonic excitation of the “Go” pathway. Consonant with this prediction, a pharmacologically-induced increase in DA release in healthy volunteers was associated with greater inhibition of the “NoGo” pathway and impairments in learning from negative feedback (Frank & O’Reilly, 2006). Consequently, selection deficits among medicated PD patients may reflect decreased sensitivity to negative feedback due to excessive DA in the dorsal striatum. It is possible that decreased response to negative feedback may explain observed deficits in rule shifting among medicated PD patients. However, rule shifting and selection processes have been dissociated in several studies; although medicated patients have no difficulty shifting away from an inappropriate rule they tend to select a new rule that should have been ruled out by negative feedback (Channon, 1997; Price, 2006). This would suggest that although negative feedback triggers rule shifting and selection processes, the two depend upon distinct mechanisms.
In contrast to medicated PD patients, PD patients off medication have no difficulty making use of negative feedback to identify the relevant stimulus dimension (Cools et al., 2006; Frank et al., 2004; Holroyd et al., 2002; Swainson et al., 2006). Among PD patients off medication, DA levels within the NAc are relatively normal and the pattern of NAc and ACC activation during the period following feedback is normal (Cools et al., 2007; Monchi et al., 2004). Despite this, PD patients off medication have difficulty with tasks designed to assess rule selection, specifically the learned irrelevance phase of the ED/IDS task (Owen, Roberts et al., 1993). Such deficits may stem from an insensitivity to positive feedback Frank et al., 2004; Schott et al., 2007; but see Cools et al., 2006; Frank et al., 2007). Normally, positive feedback is associated with phasic increases in DA within the dorsal stratum but DA depletion minimizes the size and efficacy of such bursts (Schott et al., 2007; Pizzigalli et al., 2008), thereby reducing sensitivity to positive feedback among PD patients off medication
Although limited research exists on the impact of PD on rule selection, the evidence we have reviewed here suggests impairment among patients on and off medication. It appears that PD patients off medication may be less sensitive to positive feedback, whereas medicated PD patients are less sensitive to negative feedback. In either case, rule selection fails to proceed normally.
Summary and Conclusions
In this review, we explored PD patient performance on rule based category learning with specific focus on the component processes of rule generation, maintenance, shifting and selection. This neuropsychological evidence was then integrated with neuroimaging and computational modeling research to clarify the neural architecture of explicit rule-based category learning (see Figure 2).
Successful rule-based category learning demands the generation of one or more viable rules to guide responding. The data reviewed here suggests that rule generation relies upon activity within the ventral cortico-striatal circuit, namely ACC and OFC. This circuit also appears to support rule selection through feedback-mediated changes in the relative activation of rule representations within WM. Specifically, reinforcement prediction errors trigger midbrain DA signals that govern activity within the ACC. The pattern of ACC activation biases activity within PFC towards those rules that have not received recent negative feedback. Whereas rule generation and selection appear to rely on ventral cortico-striatal circuitry, rule maintenance and shifting rely primarily on dorsal circuitry, namely head of the caudate and dorsolateral PFC. Individual rule representations are maintained in WM via distinct loops of recurrent excitation between PFC and thalamus. Rule maintenance is most likely governed by a DA-mediated gating mechanism that insures WM access only to those cognitive responses that are consistently reinforced by positive feedback; irrelevant responses are reinforced only intermittently and are therefore not maintained. Receipt of unexpected negative feedback triggers a phasic dip in DA release, which destabilizes the rule representation in WM and increases the likelihood of a shift away from that rule. Normally, coordinated activity of these cortico-striatal systems supports successful learning of rule-based category structures.
Among patients with PD, however these systems become dysfunctional due to disruption of the dopaminergic and, possibly, the noradrenergic systems. When not on medication, early PD patients largely suffer rule-based category learning deficits that stem from a lack of dorsal striatal DA availability and cortical norepinephrine activity. Because of these changes, patients off medication have difficulty updating WM, failing to readily shift away from unsuccessful rules. Although the tendency to perseverate with an unsuccessful rule limits the necessity of selecting new rules, early PD patients off medication may have little difficulty generating potential rules and making use of feedback to identify the most relevant among them. As PD advances and the ventral striatum begins to suffer a significant loss of DA availability, advanced PD patients off medication may begin to experience difficulty with those processes mediated by the ventral striatum and its cortical targets, specifically rule generation and rule selection.
The nature of rule-based category learning deficits in medicated PD patients, specifically those in the earlier stages of disease, is quite different from those observed in patients off medication. Medication should improve patients’ ability to update WM, thereby remediating rule shifting deficits. However, it appears that medication may also disrupt the gating mechanism that supports WM updating, causing increased distractibility among medicated PD patients. Presently, it is unclear if increased distractibility occurs in all medicated PD patients or if it stems from particular courses of treatment. Pharmacological therapies tend to also impact the ventral cortico-striatal circuits, which are relatively spared until advanced PD. Among earlier PD patients, medication may create a mild “DA overdose” within the ventral striatum and its cortical targets (e.g. OFC; ACC). Consistent with this overdose hypothesis, rule generation and selection processes, which we argue depend upon ventral cortico-striatal circuitry, are often impaired in PD patients when they are on, but not off, medication.
In summary, the available data indicate that rule-based category learning depends upon a network of striatal structures and frontal cortical regions. Learning to classify novel stimuli depends upon multiple processes, including the generation, maintenance, shifting and selection of classification rules. Dysfunction in any of these components, due to PD or its common treatments, will disrupt category learning, albeit in slightly different ways.
Footnotes
The COVIS model also details implicit category learning but we focus only on the components devoted to explicit, rule -based learning.
We use the general abbreviation PD when discussing research that did not specifically examine the impact of PD medication. When addressing research that specifically examined the impact of medication, we distinguish between PD patients on and off medication.
The present discussion focuses specifically on rule shifting within category learning tasks and does not address the substantial literature related to task switching. Task switching differs from rule shifting in that it does not emphasize learning and involves changing stimulus-response mappings. In addition, task switching is more consistently affected by DA withdrawal than rule shifting, which suggests the two processes are sufficiently different to not be discussed as relying on the same mechanisms.
Contributor Information
Amanda Price, Elizabethtown College.
J. Vincent Filoteo, University of California, San Diego.
W. Todd Maddox, University of Texas, Austin.
References
- Agid Y, Javoy-Agid F, Ruberg M. Biochemistry of neurotransmitters in Parkinson’s disease. Movement Disorders. 1987;2:166–230. [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neuroscience. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. Journal of Neurophysiology. 2004;92:1144–1152. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ennis JM. The role of the basal ganglia in category learning. The Psychology of Learning and Motivation. 2006;46:1–36. [Google Scholar]
- Ashby FG, Ell SE, Valentin VV, Casale MB. FROST: A distributed neurocomputational model of working memory maintenance. Journal of Cognitive Neuroscience. 2005;17:1728–1743. doi: 10.1162/089892905774589271. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Valentin VV. Multiple systems of perceptual category learning: theory and cognitive tests. In: Cohen H, Lefebvre C, editors. Categorization in cognitive science. New York: Elsevier; 2005. [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Noll DC. Anterior cingulate and the monitoring of response conflict: Evidence from an fMRI study of overt verb generation. Journal of Cognitive Neuroscience. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain Dopamine Neurons Encode a Quantitative Reward Prediction Error Signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W, Monson N. Problem Solving in Parkinson’s Disease: Comparison of Performance on the Wisconsin and California Card Sorting Tests. Journal of Geriatric Psychiatry and Neurology. 1990;3:163–171. doi: 10.1177/089198879000300308. [DOI] [PubMed] [Google Scholar]
- Beatty W, Staton R, Weir W, Monson N, Whitaker H. Cognitive disturbances in Parkinson’s disease. Journal of Geriatric Psychiatry & Neurology. 1989;2:22–33. doi: 10.1177/089198878900200106. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Carter CS, Barch DM, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bowen F, Kamienny R, Burns M, Yahr M. Parkinsonsim: effects of levodopa on concept formation. Neurology. 1975;25:701–704. doi: 10.1212/wnl.25.8.701. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: The gating model. Progress in Brain Research. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Attention and Performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 713–737. [Google Scholar]
- Brown R, Marsden C. Cognitive function in Parkinson’s disease: from description to theory. Trends in the Neurosciences. 1990;13:21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- HBrück AH, Aalto S, HNurmi EH, HVahlberg TH, HBergman JH, HRinne JOH. Striatal subregional 6-[18F]fluoro-L-dopa uptake in early Parkinson’s disease: a two-year follow-up study. Movement Disorders. 2006;21:958–963. doi: 10.1002/mds.20855. [DOI] [PubMed] [Google Scholar]
- Bruner J, Goodnow J, Austin G. A study of thinking. Oxford, England: Wiley; 1956. [Google Scholar]
- Burgess PW. Strategy application disorder: the role of the frontal lobes in human multitasking. Psychological Research. 2000;63:279–288. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. Journal of Neuroscience. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, Birdi S, Rajput AH, Hornykiewicz O, Bedard PJ, Di PT. Increase of preproenkephalin mRNA levels in the putamen of Parkinson disease patients with levodopa-induced dyskinesias. Journal of Neuropathology & Experimental Neurology. 2002;61:186–196. doi: 10.1093/jnen/61.2.186. [DOI] [PubMed] [Google Scholar]
- Canavan A, Passingham R, Marsden C, Quinn N, Wyke M, Polkey C. The performance on learning tasks of patients in the early stages of Parkinson’s disease. Neuropsychologia. 1989;27:141–156. doi: 10.1016/0028-3932(89)90167-x. [DOI] [PubMed] [Google Scholar]
- Channon S, Jones M-C, Stephenson S. Cognitive strategies and hypothesis testing during discrimination learning in Parkinson’s disease. Neuropsychologia. 1993;31:75–82. doi: 10.1016/0028-3932(93)90082-b. [DOI] [PubMed] [Google Scholar]
- Channon S. Impairment in deductive reasoning and working memory in Parkinson’s disease. Behavioural Neurology. 1997;10:1–8. doi: 10.3233/BEN-1997-10101. [DOI] [PubMed] [Google Scholar]
- Charbonneau D, Riopelle RJ, Beninger RJ. Impaired incentive learning in treated Parkinson’s disease. Canadian Journal of Neurological Sciences. 1996;23:271–278. doi: 10.1017/s031716710003821x. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, O’Reilly RC. A computational approach to prefrontal cortex, cognitive control and schizophrenia: Recent developments and current challenges. Philosophical Transactions of the Royal Society, Series B. 1996;346:1515–1527. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychological Review. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Collins P, Wilkinson LS, Everitt BJ, Robbins TW, Roberts AC. The effect of dopamine depletion from the caudate nucleus of the common marmoset (Callithrix jacchus) on tests of prefrontal cognitive function. Behavioral Neuroscience. 2000;114:3–17. doi: 10.1037//0735-7044.114.1.3. [DOI] [PubMed] [Google Scholar]
- Cools AR, van den Bercken JH, Horstink MW, van Spaendonck KP, Berger HJ. Cognitive and motor shifting aptitude disorder in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1984;47:443–53. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function – Implication for L-DOPA therapy in Parkinson’s disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–34. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cerebral Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or Impaired Cognitive Function in Parkinson’s Disease as a Function of Dopaminergic Medication and Task Demands. Cerebral Cortex. 2003;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Lewis S, Clark L, Barker R, Robbins TW. L-DOPA Disrupts Activity in the Nucleus Accumbens during Reversal Learning in Parkinson’s Disease. Neuropsycholopharmacology. 2007;32:180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]
- Cooper J, Sagar H, Doherty S, Jordan N, Tidswell P, Sullivan E. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in parkinson’s disease: a follow-up study of untreated patients. Brain. 1992;115:1701–1725. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- Cooper J, Sagar H, Jordan N, Harvey N, Sullivan E. Cognitive impairment in early, untreated Parkinson’s dsiease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed: Functional anatomy by positron emission tomography. Journal of Neuroscience. 1992;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford J, Kaldters A, Jones R, Watson R. A central executive deficit in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57:360–367. doi: 10.1136/jnnp.57.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Casa LG, Ruiz G, Lubow RE. Latent inhibition and recall/recognition of irrelevant stimuli as a function of preexposure duration in high and low psychotic-prone normals. British Journal of Psychology. 1993;84:119–132. doi: 10.1111/j.2044-8295.1993.tb02467.x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Bihrle A, Janowsky JS, Squire LR, Shimamura AP. Fractionation of problem-solving deficits in frontal-lobe patients. Journal of Clinical & Experimental Neuropsychology. 1989;11:50. [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cognitive, Affective & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- DeLong M. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Deogaonkar M, Piallat B, Subramanian T. Effect of l-dopa on neuronal activity of subthalamic nucleus and substantia nigra in hemiparkinsonian monkeys. Movement Disorders. 2005;20:S75. [Google Scholar]
- DePisapia N, Slomski JA, Braver T. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cerebral Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Dimitrov M, Grafman J, Soares AH, Clark K. Concept formation and concept shifting in frontal lesion and Parkinson’s disease patients assessed with the California card sorting test. Neuropsychology. 1999;13:135–143. doi: 10.1037//0894-4105.13.1.135. [DOI] [PubMed] [Google Scholar]
- Dominey P, Arbib M, Joseph JP. A model of corticostriatal plasticity for learning oculomotor associations and sequences. Journal of Cognitive Neuroscience. 1995;7:311–336. doi: 10.1162/jocn.1995.7.3.311. [DOI] [PubMed] [Google Scholar]
- Downes J, Roberts A, Sahakian B, Evenden J, Morris R, Robbins T. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: Evidence for a specific attentional dysfunction. Neuropsychology. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Degreef JF, Rogelet P, Defebvre L, Destee A. Impairment of the supervisory attentional system in early untreated patients with Parkinson’s disease. Journal of Neurology. 1999;246:783–788. doi: 10.1007/s004150050455. [DOI] [PubMed] [Google Scholar]
- Elliot R, Dolan RJ. Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage. 1998;8:17–29. doi: 10.1006/nimg.1998.0344. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rule-based extrapolation in perceptual categorization. Psychonomic Bulletin & Review. 2002;9:160–168. doi: 10.3758/bf03196273. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rules and exemplars in category learning. Journal of Experimental Psychology: General. 1998;127:107–140. doi: 10.1037//0096-3445.127.2.107. [DOI] [PubMed] [Google Scholar]
- Farina E. Frontal dysfunction in early Parkinson’s disease. Acta Neurologica Scandinavica. 1994;90:34–38. doi: 10.1111/j.1600-0404.1994.tb02676.x. [DOI] [PubMed] [Google Scholar]
- Farley I, Price K, Hornykiewicz O. Dopamine in the limbic regions of the human brain: normal and abnormal. Advances in Biochemical Psychopharmacology. 1977;16:57–64. [PubMed] [Google Scholar]
- Feredoes E, Tononi G, Postle BR. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. Proceedings of the National Academy of Science. 2006;103:19530–19534. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Research. 1991;547:152–161. [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Ing AD, Zizak V, Song DD. The impact of irrelevant dimensional variation on rule-based category learning in patients with Parkinson’s disease. Journal of the International Neuropsychological Society. 2005;11:503–513. doi: 10.1017/S1355617705050617. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT, Ing AD, Song D. Characterizing rule-based category learning deficits in patients with Parkinson’s disease. Neuropsychologia. 2007;45:305–320. doi: 10.1016/j.neuropsychologia.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Maddox TW. Quantitative modeling of visual attention processes in patients with Parkinson’s disease: Effects of stimulus integrality on selective attention and dimensional integration. Neuropsychology. 1999;13:206–222. doi: 10.1037//0894-4105.13.2.206. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Rilling LM, Strayer DL. Negative priming in patients with Parkinson’s disease: Evidence for a role of the striatum in inhibitory processes. Neuropsychology. 2002;16:230–241. doi: 10.1037//0894-4105.16.2.230. [DOI] [PubMed] [Google Scholar]
- Fimm B, Barti G, Zimmerman P, Wallesch CW. Different mechanisms underlying shifting set on external and internal cues in Parkinson’s disease. Brain & Cognition. 1994;25:287–304. doi: 10.1006/brcg.1994.1037. [DOI] [PubMed] [Google Scholar]
- Flowers K, Robertson C. The effect of Parkinson’s disease on the ability to maintain a mental set. Journal of Neurology, Neurosurgery & Psychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S, Morrison J. Extrathalamic modulation of cortex. Annual Review of Neuroscience. 1987;10:67–85. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- Fornai F, di Poggio AB, Pellegrini A, Ruggieri S, Paparelli A. Noradrenaline in Parkinson’s disease: from disease progression to current therapeutics. Current Medicinal Chemistry. 2007;14:2330–2334. doi: 10.2174/092986707781745550. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of Cognitive Neuroscience. 2004;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and non-medicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational account. Cognitive, Affective & Behavioral Neuroscience. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in human cognition: Psychopharmacological studies with cabergoline and haloperidol. Behavioral Neuroscience. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;23:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger L, O’Reilly RC. By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47:495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J, Roberts R, Brown V. Mechanisms underlying attentional set-shifting in Parkinson’s disease. Neuropsychologia. 1999;37:605–616. doi: 10.1016/s0028-3932(98)00049-9. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Anatomical segregation of component processes in an inductive inference task. Journal of Cognitive Neuroscience. 2000;12:1–10. doi: 10.1162/08989290051137639. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J. The role of the right prefrontal cortex in ill-structured problem solving. Cognitive Neuropsychology. 2000;17:415–436. doi: 10.1080/026432900410775. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Friedman HR. The circuitry of working memory revealed by anatomy and metabolic imaging. In: Levin H, Eisenberg H, Benton A, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Grande L, Crosson B, Heilman K, Bauer R, Kilduff P, McGlinchey R. Visual selective attention in Parkinson’s disease: Dissociation of exogenous and endogenous inhibition. Neuropsychology. 2006;20:370–382. doi: 10.1037/0894-4105.20.3.370. [DOI] [PubMed] [Google Scholar]
- Green J, McDonald W, Vitek J, Evatt M, Haber M, Bakay R, Triche R, Sirockman B, Delong M. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- Greve KW, Smith MC. A comparison of the Wisconsin Card Sorting Test with the Modified Card Sorting Test for use with older adults. Gerontology & Geriatrics Education. 1991;12:241–259. [Google Scholar]
- Hanes KR, Andrewes DG, Pantelis C. Cognitive flexibility and complex integration in Parkinson’s disease, Huntington’s disease, and schizophrenia. Journal of the International Neuropsychological Society. 1995;1:545–553. doi: 10.1017/s1355617700000679. [DOI] [PubMed] [Google Scholar]
- Hart R, Kwentus J, Wade J, Taylor J. Modified Wisconsin Card Sorting Test in elderly normal, depressed and demented patients. Clinical Neuropsychologist. 1988;2:49–56. [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Toward an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society - B. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Helkala E, Laulumaa V, Soininen H, Riekkinen PJ. Different error pattern of episodic and semantic memory in Alzheimer’s disease and Parkinson’s disease with dementia. Neuropsychologica. 1989;27:1241–1248. doi: 10.1016/0028-3932(89)90036-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier D, Reyes A, Galarraga E. D1 receptor activation enhancesevoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca conductance. Journal of Neuroscience. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17 doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nature Neuroscience. 1998;1:304–308. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Holroyd C, Coles M. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Praamstra P, Plat E, Coles MG. Spared error-related potentials in mild to moderate Parkinson’s disease. Neuropsychologia. 2002;40:2116–2124. doi: 10.1016/s0028-3932(02)00052-0. [DOI] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cerebral Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Marder K, Cote LJ, Sano M, Stern Y, Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson’s disease. Neurology. 1995;45:1691–1696. doi: 10.1212/wnl.45.9.1691. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: A community-based, 4-year longitudinal study. Journal of Geriatric Psychiatry and Neurology. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 2007;10:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A. Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain. 1996;119:2121–2132. doi: 10.1093/brain/119.6.2121. [DOI] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-Dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology. 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lees A, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Levy G, Jacobs DM, Tang MX, Cote LJ, Louis ED, Alfaro B, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Movement Disorders. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- Lewis S, Cools R, Robbins T, Dove A, Barker R, Owen A. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lewis S, Dove A, Robbins T, Barker R, Owen A. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Neuroscience. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S, Salbosz A, Robbins T, Barker R, Owen A. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Gewirtz JC. Latent inhibition in humans: Data, theory, and implications for schizophrenia. Psychological Bulletin. 1995;117:87–103. doi: 10.1037/0033-2909.117.1.87. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Dressler R, Kaplan O. The Effects of Target and Distracter Familiarity on Visual Search in De Novo Parkinson’s Disease Patients: Latent Inhibition and Novel Pop-Out. Neuropsychology. 1999;13:415–423. doi: 10.1037//0894-4105.13.3.415. [DOI] [PubMed] [Google Scholar]
- Maddox TW, Filoteo JV, Delis DC, Salmon DP. Visual selective attention deficits in patients with Parkinson’s disease: A quantitative model based approach. Neuropsychology. 1996;10:197–218. [Google Scholar]
- Maddox WT, Aparicio P, Marchant NL, Ivry RB. Rule-based Category Learning is Impaired in Patients with Parkinson’s Disease but not in Patients with Cerebellar Disorders. Journal of Cognitive Neuroscience. 2005;17:707–723. doi: 10.1162/0898929053747630. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG. Dissociating Explicit and Procedural-Learning Based Systems of Perceptual Category Learning. Behavioral Processes. 2004;66:309–332. doi: 10.1016/j.beproc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Filoteo JV. Striatal contribution to category learning: Quantitative modeling of simple linear and complex non-linear rule learning in patients with Parkinson’s disease. Journal of the International Neuropsychological Society. 2001;7:710–727. doi: 10.1017/s1355617701766076. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Filoteo JV, Delis DC, Salmon DP. Visual selective attention deficits in patients with Parkinson’s disease: A quantitative model-based approach. Neuropsychology. 1996;10:197–218. [Google Scholar]
- Maddox WT, Filoteo JV, Zeithamova D. Computational Models Inform Clinical Science and Assessment. An Application to Category Learning in Striatal-Damaged Patients. Journal of Mathematical Psychology. doi: 10.1016/j.jmp.2009.01.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahieux F, Fenelon G, Flahault A, Manifacier MJ, Michelet D, Boller F. Neuropsychological prediction of dementia in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64:178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell SA, Harris J. Irrelevant peripheral visual stimuli impair manual reaction times in Parkinson’s disease. Vision Research. 1997;37:3549–3558. doi: 10.1016/S0042-6989(97)00188-0. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Hinton EC, Montgomery AJ, Bantick RA, Grasby PM. Sulpiride and mnemonic function: effects of a dopamine D2 receptor antagonist on working memory, emotional memory and long-term memory in healthy volunteers. Journal of Psychopharmacology. 2005;19:29–38. doi: 10.1177/0269881105048889. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology. 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson’s disease. Psychopharmacology. 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Miller LA, Tippett LJ. Effects of focal brain lesions on visual problem-solving. Neuropsychologica. 1996;34:387–398. doi: 10.1016/0028-3932(95)00116-6. [DOI] [PubMed] [Google Scholar]
- Milner B. Effect of Different Brain Lesions on Card Sorting. Archives of Neurology. 1963;9:90–100. [Google Scholar]
- Mogenson G. Limbic-motor integration. Progress in Psychobiology & Physiological Psychology. 1987;12:117–169. [Google Scholar]
- Monchi O, Taylor JG, Dagher A. A neural model of working memory processes in normal subjects, Parkinson’s disease and schizophrenia for fMRI design and predictions. Neural Networks. 2000;13:953–973. doi: 10.1016/s0893-6080(00)00058-7. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma R, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. The Journal of Neuroscience. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S, Wise S, di Pellegrino G. A model that accounts for activity in primate frontal cortex during a delayed matching-to-sample task. Journal of Neuroscience. 1998;18:399–410. doi: 10.1523/JNEUROSCI.18-01-00399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology. 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola S, Surmeier J, Malenka R. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annual Review of Neuroscience. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM, Johansen MK. Exemplar-based accounts of “multiple-system” phenomena in perceptual categorization. Psychonomic Bulletin & Review. 2000;7:375–402. [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, DeLong MR, Olanow CW. Pathophysiology of levodopa-induced dyskinesias in Parkinson’s disease: problems with the current model. Annals of Neurology. 2000;47:S22–S32. [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: A computational model of learning in the frontal cortex and basal ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Munakata Y. Computational Explorations in Cognitive Neuroscience: Understanding the Mind by Simulating the Brain. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- O’Reilly RC, Braver TS, Cohen JD. A Biologically Based Computational Model of Working Memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. New York: Cambridge University Press; 1999. pp. 375–411. [Google Scholar]
- Owen AM, Bekinska M, James M, Leigh P, Summers B, Marsden C, et al. Visuospatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia. 1993;31:627–644. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts A, Hodges J, Summers B, Polkey C, Robbins T. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Annals of Neurology. 1999;46:732–738. doi: 10.1002/1531-8249(199911)46:5<732::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Paolo A, Troster A, Axelrod B, Koller W. Construct validity of the WCST in normal elderly and persons with Parkinson’s disease. Archives of Clinical Neuropsychology. 1995;10:463–473. [PubMed] [Google Scholar]
- Peterson SE, Fox PT, Posner MI, Mintan M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single word processing. Nature. 1988;331:585–588. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Picirilli M, D’Alessandro P, Finali G, Piccinin GL, Agostini L. Frontal lobe dysfunction in Parkinson’s disease prognostic value for dementia? European Neurology. 1989;29:71–76. doi: 10.1159/000116381. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M. Single dose of a dopamine agonist impairs reinforcement learning in humans: Behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology. 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck M. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Price A. Explicit category learning in Parkinson’s disease: Deficits related to impaired rule generation and selection processes. Neuropsychology. 2006;20:249–257. doi: 10.1037/0894-4105.20.2.249. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videnn TO, MacLeod MA, Pardo JV, Fox PT, Petersen SE. Practice related changes in human brain functional anatomy during non-motor learning. Cerebral Cortex. 1994 Jan/Feb;:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Parallel brain systems for learning with and without awareness. Learning & Memory. 1994;1:217–229. [PubMed] [Google Scholar]
- Reverberi C, D’Agostini S, Skrap M. Generation and recognition of abstract rules in different frontal lobe subgroups. Neuropsychology. 2005;43:1924–1937. doi: 10.1016/j.neuropsychologia.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reverberi C, Lavaroni A, Gigli GL, Skrap M, Shallice T. Specific impairments of rule induction in different frontal lobe subgroups. Neuropsychologia. 2005;43:460–472. doi: 10.1016/j.neuropsychologia.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Richards M, Cote L, Stern Y. Journal of Clinical and Experimental Neuropsychology. 1993;15:266–279. doi: 10.1080/01688639308402562. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. Journal of Neuroscience. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA. Evidence that the substantia nigra is a site of action for L-DOPA. Neuroscience Letters. 1988;89:204–208. doi: 10.1016/0304-3940(88)90382-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez PF, Aron AR, Poldrack RA. Ventral-striatal/nucleus-accumbens sensitivity to prediction errors during classification learning. Human Brain Mapping. 2006;27:306–313. doi: 10.1002/hbm.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E, Thorpe S, Boytim M, Szabo I, Perrett D. Responses of striatal neurons in the behaving monkey: 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neuroscience. 1984;12:1201–1212. doi: 10.1016/0306-4522(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochemical Pharmacology. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rougier NP, Noelle DC, Braver TS, Cohen JD, O’Reilly RC. Prefrontal cortex and flexible cognitive control: Rules without symbols. Proceedings of the National Academy of Sciences. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau I, Imbault H, Laframboise M, Bedard MA. Pattern of intrusions in verbal recall: Comparison of Alzheimer’s disease, Parkinson’s disease, and frontal lobe dementia. Brain & Cognition. 2001;46:15–17. doi: 10.1016/s0278-2626(01)80076-2. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Current Opinion in Neurobiology. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Salmon D, Lineweayer T, Heindel W. Non-declarative memory in neurodegenerative disease. In: Troester A, editor. Memory in neurodegenerative disease: Biological, cognitive, and clinical perspectives. New York: Cambridge University Press. 210–225; 1998. [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Scatton B, Worms P, Lloyd K, Bartholini G. Cortical modulation of striatal function. Brain Research. 1982;232(2):331–342. doi: 10.1016/0006-8993(82)90277-3. [DOI] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann B. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in Neurosciences. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sharpe MH. Distractibility in early Parkinson’s disease. Cortex. 1990;26:239–246. doi: 10.1016/s0010-9452(13)80353-x. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers C, Kalanithi J. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience & Biobehavioral Reviews. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: Evidence from Parkinson’s disease. Behavioral Brain Research. 2005;156:191–199. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Slabosz A, Lewis S, Smigasiewicz K, Szymura B, Barker R, Owen A. The role of learned irrelevance in attentional set-shifting impairments in Parkinson’s disease. Neuropsychology. 2006;20:578–588. doi: 10.1037/0894-4105.20.5.578. [DOI] [PubMed] [Google Scholar]
- Smith E, Grossman M. Multiple systems of category learning. Neuroscience and Biobehavioral Reviews. 2008;32:249–264. doi: 10.1016/j.neubiorev.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Patalano A, Jonides J. Alternative strategies of categorization. Cognition. 1998;65:167–196. doi: 10.1016/s0010-0277(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Starkstein S, Preziosi T, Berthier M, Bolduc P, Mayberg H, Robinson R. Depression and cognitive impairment in Parkinson’s disease. Brain. 1989;112:1141–1153. doi: 10.1093/brain/112.5.1141. [DOI] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Dywan J, Panisset M, Melmed C. The error negativity in nonmedicated and medicated patients with Parkinson’s disease. Clinical Neuropsychologist. 2007;118:1223–1229. doi: 10.1016/j.clinph.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Swainson R, Rogers R, Sahakian B, Summers B, Polkey C, Robbins Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lesions: Possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Swainson R, SenGupta D, Shetty T, Watkins LHA, Summers B, Sahakian B, et al. Impaired dimensional selection but intact use of reward feedback during visual discrimination learning in Parkinson’s disease. Neuropsychologia. 2006;44:1290–1304. doi: 10.1016/j.neuropsychologia.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. European Journal of Neuroscience. 2007;25:3719–3124. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109:845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Taylor JG, Taylor NR. Analysis of recurrent cortico-basal ganglia–thalamic loops for working memory. Biological Cybernetics. 2000;82:415–432. doi: 10.1007/s004220050595. [DOI] [PubMed] [Google Scholar]
- Tomer R, Aharon-Peretz J, Tsitrinbaum Z. Dopamine asymmetry interacts with medication to affect cognition in Parkinson’s disease. Neuropsychologia. 2007;45:357–367. doi: 10.1016/j.neuropsychologia.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Turner MS, Cipolatti L, Yousry T, Shallice T. Qualitatively different memory impairments across frontal lobe subgroups. Neuropsychologia. 2007;45:1540–1552. doi: 10.1016/j.neuropsychologia.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hedreen JC, Price DL. Parkinson’s disease: loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology. 1985;35:1215–1218. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- van Spaendonck KPM, Berger HJC, Horstink MWIM, Brom GF, Cools AR. Card sorting performance in Parkinson’s disease: a comparison between acquisition and shifting performance. Journal of Clinical and Experimental Neuropsychology. 1995;17:918–925. doi: 10.1080/01688639508402440. [DOI] [PubMed] [Google Scholar]
- Vartanian O, Goel V. Right Ventral Lateral Prefrontal Cortex Mediates Hypothesis Generation in an Unconstrained Anagram Task. NeuroImage. 2005;27:927–933. doi: 10.1016/j.neuroimage.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Vriezen E, Moscovitch M. Memory for temporal order and conditional associative learning in patients with Parkinson’s disease. Neuropsychologia. 1990;28:1283–1293. doi: 10.1016/0028-3932(90)90044-o. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Müller T, Schwarz M, Hohnsbein J, Falkenstein M. Error processing in patients with Parkinson’s disease: the influence of medication state. Journal of Neural Transmissoin. 2008;115:461–468. doi: 10.1007/s00702-007-0842-1. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;17:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Woods SP, Troster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson’s disease. Journal of the International Neuropsychological Society. 2003;9:17–24. doi: 10.1017/s1355617703910022. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Stout JC. Enhanced negative priming in Parkinson’s Disease. Neuropsychology. 2002;16:242–250. [PubMed] [Google Scholar]
- Yarkoni T, Gray JR, Chrastil ER, Barch DM, Green L, Braver TS. Sustained neural activity associated with cognitive control during temporally extended decision making. Cognitive Brain Research. 2005;23:71–84. doi: 10.1016/j.cogbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Zakzanis K, Freedman M. A neuropsychological comparison of demented and nondemented patients with Parkinson’s disease. Applied Neuropsychology. 1999;6:129–146. doi: 10.1207/s15324826an0603_1. [DOI] [PubMed] [Google Scholar]
- Zipser D. Recurrent network model of the neural mechanism of short-term active memory. Neural Computation. 1991;3:179–193. doi: 10.1162/neco.1991.3.2.179. [DOI] [PubMed] [Google Scholar]