Abstract
Context: Although diurnal variation of testosterone and other hormones in men has been well documented, the effect of this variation on sampling during typical clinic hours has not been examined.
Objective: Our objective was to examine temporal variation in serum testosterone and five other hormones in men over normal clinic hours.
Design: Blood samples were collected at six separate visits, three morning visits 1–3 d apart and three afternoon visits 1–3 d apart.
Setting and Participants: In Boston, MA, 66 men participated, 30–80 yr of age, randomly selected from the Boston Area Community Health Survey who completed at least five visits.
Main Outcome Measures: The age-specific ratio of hormone level at times ranging from 0801–1600 h to hormone level at 0800 h was calculated. Ratios were calculated from parameter estimates obtained from cosinor models.
Results: In men 30–40 yr old, testosterone levels were 20–25% lower at 1600 h than at 0800 h. The difference declined with age, with a 10% difference at 70 yr. 17 men with at least one of three measurements less than 300 ng/dl (10.4 nmol/liter) after 1200 h had normal testosterone levels at all three visits before 1200 h (five of eight men 30–47 yr old, four of nine men 66–80 yr old). Much lower levels of diurnal variation were found for dihydrotestosterone, SHBG, LH, FSH, and estradiol at all ages.
Conclusions: Our results support the recommendation of restricting testosterone measurements to morning hours in both young and older men. Limited diurnal variation in other hormones indicates that sampling through the day is appropriate.
Although diurnal variation of testosterone declines with age, testosterone measurements should be restricted to morning hours at all ages.
Diurnal variation of testosterone (T) has been well documented (1,2,3,4,5,6,7,8,9,10,11,12,13,14). Levels peak between 0530 and 0800 h, depending on the study, with trough levels occurring approximately 12 h later. The amplitude of variation of total T, which is usually estimated as the difference between peak and mean hormone levels on a fitted cosinor model, was 6–12% of mean hormone level in most studies (1,6,7,10,11,12,13). A few reports of amplitudes as high as 24% of the mean have been published (2,4,14). Some of the variation in amplitude is attributable to reduced diurnal variation in older men (1,4,7,13). Other sources of variation have not been identified. The amplitude and acrophase of the diurnal variations in free and bioavailable T levels are similar to that of total T (2,4,7,10). Diurnal variation of other sex hormones has received less attention (2,3,4,5,7,8,9,10,12,13,14,15,16).
The effect of diurnal variation on clinical measurement of hormones is unclear. For example, if a clinic opens at 0800 h and closes at 1600 h, then the 8-h interval during which it is open will represent only 67% of the 12-h interval between peak and trough T levels. Thus, the morning-afternoon differences seen in the clinic will likely represent only a fraction of the total amplitude of variation over 24 h. Morning-afternoon differences should be even smaller in older men for hormones with reduced amplitude of variation with advancing age. Furthermore, the reported amplitudes of diurnal variation in hormone levels are all considerably smaller than the intra-individual variation observed when samples are collected at the same time of day over several days (17). Still, diurnal variation in hormone levels will lead to systematic differences between values collected in the morning and afternoon, and it is important to understand the magnitude of the differences in evaluating hormone measurements.
We compared morning and afternoon levels of total, free, and bioavailable T, dihydrotestosterone (DHT), SHBG, LH, FSH, and estradiol (E2) in young, middle-age, and older men.
Subjects and Methods
Study participants and recruitment
The Hormone Variation Study took place in two phases. In phase I, we characterized day-to-day and 3-month intra-individual variation in levels of T and several other hormones (17). Subjects for the study were selected from among 2301 male respondents to the Boston Area Community Health survey (18). Subjects for the Boston Area Community Health survey were randomly selected from Boston residents who were 30–79 yr old, using a weighted sampling scheme to recruit approximately equal numbers of Hispanics, non-Hispanic Black Americans and non-Hispanic Caucasians. Recruitment was also stratified on decade of age to provide approximate balance over the target age range. A total of 134 subjects enrolled in phase I of the Hormone Variation Study.
Subjects enrolled in the present study, phase II, were randomly selected from among the 121 men in phase I who completed all six visits, after excluding two additional men who were determined to be ineligible for phase I after that phase was completed. For phase II, subjects were randomly selected within each of five age strata (30–39, 40–49, 50–59, 60–69, and 70–80 yr) to provide approximate balance on age. A potential subject who refused or was found to be ineligible was replaced with another randomly selected subject from the same stratum.
Potential subjects for phase II were screened to determine whether they still met the phase I eligibility criteria. Men were excluded if they had medical conditions or were taking medications that altered levels of the hormones of interest, if they had problems with blood draws, or if they had compromised immune systems caused by HIV/AIDS, chemotherapy, radiation, or other conditions. Details on the exclusions are provided elsewhere (17).
Subjects were enrolled after written informed consent was obtained. The consent form, protocol, telephone scripts, and contact documents were approved by the Institutional Review Board of the New England Research Institutes.
Sample and data collection
The usual approach to examining diurnal variation, collecting hourly samples for 24 h, fails to reflect the intra-individual variability caused by day-to-day and longer-term variation in hormone levels (17). We chose a different sampling schedule that more closely reflected the latter variations because these sources of variation also affect clinical measurement.
Blood samples were obtained from each subject on 6 d. Samples were collected at three consecutive visits in the morning and three consecutive visits in the afternoon, with the two sets of visits separated by 1–2 wk. Within each set of three visits, blood samples were obtained 1–3 d apart (median, 2 d). Men were randomly assigned to have the morning or afternoon set of visits first. Specific sampling times were arranged to accommodate each subject’s schedule; thus, sampling times varied within each set of three visits in individual subjects and among different subjects. Study visits generally took place in the subject’s home. If the subject so requested, the visits took place in other locations, such as the subject’s place of employment or study headquarters at the New England Research Institutes.
At each visit, two blood samples were drawn 20 min apart to reduce the effects of pulsatile secretion on hormone levels. The two samples were placed in an ice-filled cooler for transport back to study headquarters, where they were centrifuged. Equal aliquots were pooled, and the pooled samples were stored in scintillation vials at −70 C until they were assayed for hormone levels. Assays were performed in the Endocrine Laboratory of the Department of Physiology at the University of Massachusetts Medical School, Worcester, MA.
At visits 1 and 4, a questionnaire was administered regarding recent changes that could affect hormone levels. In particular, subjects who had started taking medications or had developed conditions that would have made them ineligible at the start were excluded from further participation.
Hormone assays
Total T was measured by a RIA from Diagnostic Products Corp., Los Angeles, CA [intraassay coefficient of variation (CV) 4.3%, interassay CV 9.8%]. E2 was measured by a RIA from Diagnostic Systems Laboratories, Webster, TX (intraassay CV 2.3%, interassay CV 7.4%). DHT was measured by a RIA that was developed at the Endocrine Laboratory of the Department of Physiology at the University of Massachusetts (21) (intraassay CV 2.1%; interassay CV 3.1%). LH, FSH, and SHBG were measured by chemiluminescent immunoassays from Diagnostic Products (LH, intraassay CV 4.2% and interassay CV 5.5%; FSH, intraassay CV 3.5% and interassay CV 3.7%; SHBG, intraassay CV 3.1% and interassay CV 4.1%). Free and bioavailable T were calculated from total T and SHBG levels using the Södergard equation using a constant albumin concentration of 4.3 g/dl (19,20).
All samples obtained from a subject were assayed in the same run for each hormone to exclude interassay variation from changes in hormone level within subjects.
Statistical analysis
All hormone values were transformed to base 10 logarithms for data analysis (17). Without the logarithmic transformation, men with higher average hormone levels also had more variable hormone levels. This relationship violates the assumption of homogeneous intra-individual variation underlying the statistical model used for data analysis. Diurnal variation was examined using a cosinor model. The approach to modeling differed from those used in the numerous previous studies of diurnal variation of hormone levels in which hourly samples were collected from each subject for 24 h. Some investigators fit the cosinor model separately to the data from each subject and averaged the parameter estimates across subjects, whereas others averaged the observations each hour across subjects and fit the cosinor model to the set of 24 average values. Neither approach is suitable given the nature of the data set in this study. Instead, a single cosinor model was fit to a data set that included all observations from subjects who completed at least five of six study visits. A random effect for subject was included in the model; thus, hormone levels for an individual were assumed to vary diurnally around that individual’s average level. The amplitude of variation was assumed to change linearly with age. The model took the form:
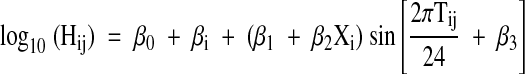 |
where Hij is hormone level at the jth time point in subject i, bi is a normally distributed random variable with mean zero, Xj is the age of subject i, and Tij is the decimal time for the jth time point in subject i. Under this model, β0 is overall mean log-transformed hormone level, β0 + βi is mean hormone level for subject i, (β1+ β2Xi) is the amplitude of diurnal variation as a function of age and β3 is a phasing parameter that determines the time of peak hormone level.
Models with age-related change expressed as (β1+ β2Xi + β4Xi2) and [β1 + β2 log(Xi)] were also considered, but neither of these fit the data better than the cosinor model with a linear change in amplitude with age. Therefore, only the results from the model with the linear expression are presented here.
The effect of diurnal variation on clinical sampling was quantified by computing the ratio of hormone level at times between 0800 and 1600 h to hormone level at 0800 h using the parameter estimates from the fitted cosinor models. Ratios were computed for ages 30, 40, 50, 60, 70, and 80 yr as:
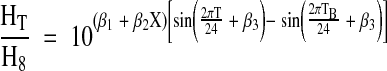 |
where T is the time point corresponding to the hormone level in the numerator of the ratio.
To illustrate the clinical impact of diurnal variation on T measurements in the context of agated dichotomized afternoon (after 1200 h) T levels against morning (before 1200 h) T levels.
All model fitting took place using the nonlinear mixed-model procedure of SAS/STAT software.
Results
A total of 99 men were invited to participate in the study, and 67 did so. Twelve of 99 men were excluded at screening because they were no longer eligible, 11 could not be contacted because they had moved or had disconnected telephones, five refused to participate, and four did not respond or were not available until after enrollment closed. One man who dropped out after completing three morning visits was excluded from the analysis. Two men who completed five visits each and 64 men who completed all six visits were included in the analysis. Of these 66 men, two subjects had four morning visits and two afternoon visits each, whereas the remaining 64 men had three morning and three afternoon visits. As a result, the data set included hormone levels from 199 morning visits and 195 afternoon visits. Eleven men were 30–39 yr old, 13 were 40–49 yr old, 15 were 50–59 yr old, 11 were 60–69 yr old, and 16 were 70–80 yr old.
The morning blood draws took place between 0505 and 1155 h (median, 0757 h; 10th and 90th percentiles, 0540 and 1035 h), whereas afternoon blood draws took place between 1300 and 1948 h (median, 1510 h; 10th and 90th percentiles, 1310 and 1810 h). Morning and afternoon sampling times were generally consistent within subjects. The median time between the earliest and latest of an individual’s two to four morning or afternoon blood draws was approximately 32 min (10th and 90th percentiles, 5 and 202 min). The interval between the morning and afternoon intra-subject median sampling times ranged from 2.4–13.2 h (median, 7.6 h; 10th and 90th percentiles, 4.1 and 10.9 h). Intervals tended to be shorter in men 60–80 yr old than in men 30–59 yr old (median, 6.2 vs. 8.4 h) because morning visits were, on average, earlier and afternoon visits were, on average, later in the younger group. However, the distributions of visit times in the two groups overlapped considerably.
Descriptive statistics for morning and afternoon hormone levels are provided in Table 1. Results of the analysis of diurnal variation for each hormone are described below.
Table 1.
Descriptive statistics for morning and afternoon hormone levels in 66 men
| Hormone | Percentile
|
||
|---|---|---|---|
| 5th | 50th | 95th | |
| T (ng/dl) | |||
| Morning | 229 | 435 | 754 |
| Afternoon | 204 | 376 | 605 |
| Bioavailable T (ng/dl) | |||
| Morning | 81 | 174 | 316 |
| Afternoon | 66 | 151 | 259 |
| Free T (pg/ml) | |||
| Morning | 43 | 92 | 167 |
| Afternoon | 35 | 80 | 137 |
| DHT (ng/ml) | |||
| Morning | 0.22 | 0.47 | 1.14 |
| Afternoon | 0.18 | 0.44 | 0.98 |
| E2 (pg/ml) | |||
| Morning | 10 | 22 | 37 |
| Afternoon | 11 | 21 | 32 |
| FSH (mIU/ml) | |||
| Morning | 2.40 | 7.12 | 29.70 |
| Afternoon | 2.17 | 7.01 | 29.70 |
| LH (mIU/ml) | |||
| Morning | 1.79 | 5.18 | 14.10 |
| Afternoon | 1.82 | 5.61 | 13.90 |
| SHBG (nmol/liter) | |||
| Morning | 14.6 | 39.8 | 66.9 |
| Afternoon | 13.7 | 39.0 | 68.3 |
The morning blood draws took place between 0505 and 1155 h (median, 0757 h; 10th and 90th percentiles, 0540 and 1035 h), whereas afternoon blood draws took place between 1300 and 1948 h (median, 1510 h; 10th and 90th percentiles, 1310 and 1810 h). For T and bioavailable T, to convert to nmol/liter, multiply values by 0.0347; for free T, to convert to pmol/liter, multiply values by 3.47; for DHT, to convert to nmol/liter, multiply values by 3.44; for E2, to convert to pmol/liter, multiply values by 3.671.
Total testosterone
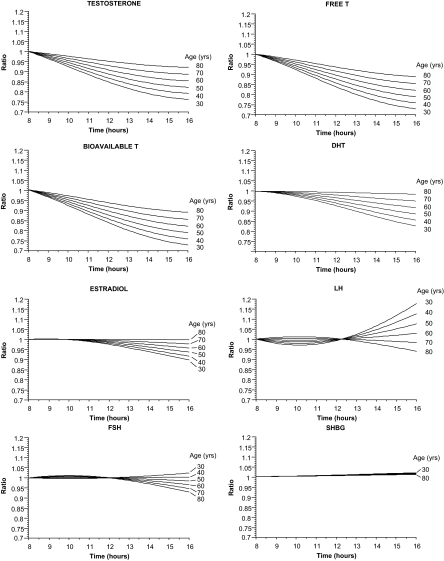
The amplitude of variation of T declined with age (P = 0.0174). The curves in Fig. 1 illustrate the effect of the changes with age on the differences between T levels in the morning and afternoon. For example, at 30 yr of age, T levels at 1600 h were, on average, about 76% of levels at 0800 h, whereas at 70 yr of age, T levels at 1600 h were about 89% of levels at 0800 h. The curves indicate a substantial decline in T levels among younger men even if attention is restricted to morning hours. For example, at 30 yr of age, T levels at 1200 h are about 85% of levels at 0800 h. On the other hand, at 70 yr of age, levels at 1200 h are 93% of those at 0800 h.
Figure 1.
The ratio of hormone level at 1600 h to hormone level at 0800 h as a function of age and the time of day at which the later measurement is obtained.
Bioavailable T
The amplitude of diurnal variation of bioavailable T also declined with age (P = 0.0223). The fitted curves indicated that the amplitude of variation of bioavailable T may be slightly greater than the amplitude of variation of total T. For example, parameter estimates indicate that at 30 yr of age, T levels at 1600 h were, on average, about 73% of levels at 0800 h compared with 76% for total T. At 70 yr of age, the difference is 86 vs. 89%.
Free T
The amplitude of diurnal variation declined with age (P = 0.0231). The age-related changes in the calculated ratios for free T were essentially the same as those for bioavailable T. This similarity makes sense given the way the values for the two fractions were calculated.
DHT
A borderline result was obtained in the test for age-related changes in amplitude (P = 0.0720). Diurnal variation was detected using the model without age-related change (P = 0.0145). Plots of the hormone ratios, taking account of age, indicate the possibility of relatively low-amplitude variation in younger men with essentially no diurnal variation in older men. In a 30-yr-old man, DHT at 1600 h were, on average, about 83% of levels at 0800 h, whereas at 70 yr of age, levels at 1600 h were 95% of levels at 0800 h. Both of these differences are considerably smaller than the corresponding differences for total, free, and bioavailable T.
E2
No diurnal variation was detected for E2 (age-related change in amplitude: P = 0.27; diurnal variation without age-related changes in amplitude: P = 0.1066). When age-related changes are taken into account, the fitted model indicates that hormone levels at 1600 h were about 90% of those at 0800 h at 30 yr of age but 98% of levels at 0800 h at 70 yr of age. When age was excluded from the model, values at 1600 h were 94% of values at 0800 h.
FSH
No age-related changes in diurnal amplitude were detected (P = 0.14). In a 30-yr-old man, FSH levels at 0800 and 1600 h were about the same, whereas in a 70-yr-old man, levels at 1600 h were about 93% of levels at 0800 h. A borderline result was obtained in the test for diurnal variation excluding age-related changes in amplitude (P = 0.0487). Without the term for age-related change in amplitude, FSH levels at 1600 h were about 97% of levels at 0800 h.
LH
A borderline result was obtained from the test for age-related changes in amplitude (P = 0.0511). No diurnal variation was detected in the model without age-related variation in amplitude (P = 0.13). From the model with age-related changes in amplitude, LH levels at 1600 h were about 18% higher than those at 0800 h in a 30-yr-old man but were about 94% of those at 0800 h in a 70-yr-old man.
SHBG
The model with age-related change in amplitude failed to converge to a solution. The likely explanation for this failure is the absence of any diurnal variation in SHBG. No diurnal variation was detected using the model without the term for age-related change (P = 0.23). From the parameter estimates, SHBG levels at 1600 h were, on average, 1% higher than levels at 0800 h.
Impact of diurnal variation in T levels on clinical decision making
The effect of diurnal variation on clinical decision making can be illustrated by comparing the number of morning vs. afternoon T values from an individual that were less than 300 ng/dl (10.4 nmol/liter), a value used to define hypogonadism. Only men with three morning and three afternoon samples were included in this part of the analysis. They were divided into three roughly equal-sized age groups to illustrate the joint effect of age and diurnal variation. Overall, these analyses indicate a considerable effect of diurnal variation on classification of men as abnormal/normal. For instance, Table 2 shows that 12 of 24 (50%) men with at least one of three measurements less than 300 ng/dl (10.4 nmol/liter) in the afternoon (after 1200 h) had normal T levels in all three of their assessments in the morning (before 1200 h). Data in Table 2 further indicate that despite the stronger effect of diurnal variation on T levels in younger men, the probability of subjects being classified as having low T was higher in samples obtained in the afternoon, regardless of age. For instance, five of eight younger (30–47 yr) men with at least one of three measurements less than 300 ng/dl (10.4 nmol/liter) after 1200 h had normal T levels in all three of their assessments before 1200 h. Similarly, in the oldest age group (66–80 yr), four of nine men with at least one of three measurements less than 300 ng/dl (10.4 nmol/liter) after 1200 h had normal T levels in all three of their assessments before 1200 h. Finally, Table 2 shows that a few subjects had low T in the morning (before 1200 h) but normal levels in the afternoon (after 1200 h). This occurred relatively rarely (four of 16 men) and included some variation by age. All three of the seven men 48–65 yr old who fit this description had one T value less than 300 ng/dl (10.4 nmol/liter) and five values more than 300 ng/dl (10.4 nmol/liter).
Table 2.
Afternoon vs. morning T measurements stratified by age group among 62 subjects with three morning and three afternoon measurements
| Morning, measurements before 1200 h | Afternoon, measurements after 1200 h
|
Total | |
|---|---|---|---|
| At least one of three measurements <300 ng/dl | All three measurements ≥300 ng/dl | ||
| Men 30–47 yr old | |||
| At least one of three measurements <300 ng/dl | 3 | 1 | 4 |
| All three measurements ≥300 ng/dl | 5 | 10 | 15 |
| Total | 8 | 11 | 19 |
| Men 48–65 yr old | |||
| At least one of three measurements <300 ng/dl | 4 | 3 | 7 |
| All three measurements ≥300 ng/dl | 3 | 15 | 18 |
| Total | 7 | 18 | 25 |
| Men 66–80 yr old | |||
| At least one of three measurements <300 ng/dl | 5 | 0 | 5 |
| All 3 measurements ≥300 ng/dl | 4 | 9 | 13 |
| Total | 9 | 9 | 18 |
Note that 300 ng/dl = 10.4 nmol/liter.
Discussion
In previous investigations of diurnal variation of hormone levels in men, investigators have typically obtained hourly samples from each subject over a 24-h interval. This design has been used to document diurnal changes in T and other hormones, but the implications of the identified variation for clinical sampling during typical daytime clinic hours have rarely been discussed in explicit quantitative terms. For this reason, we undertook an investigation to determine the extent to which hormone levels vary over clinic hours. In young men (30–40 yr old), we found that total, free, and bioavailable T levels at 0800 h were, on average, 30–35% higher than levels measured in the mid to late afternoon. The difference declined with age, dropping to approximately 10% at 70 yr of age. The amplitude of diurnal variation of DHT, FSH, LH, E2, and SHBG at all ages was either considerably smaller or not detectable.
In terms of amplitude and acrophase (not shown), the results for total, free, and bioavailable T are consistent with those of previous investigations (1,2,3,4,5,6,7,8,9,10,11,12,13,14). The large majority of previous studies of diurnal variation of FSH and LH in men produced negative results, whereas borderline significant results were obtained in this investigation (5,7,8,9,12,13,14). These discrepant results may be explained by a combination of relatively low amplitude of variation, as indicated by the available estimates, and generally much smaller sample sizes in previous investigations. An early morning acrophase for DHT and the absence of diurnal variation for E2 also match the results of previous studies (3,7). Diurnal variation of SHBG was found in three of four previous studies but not in this one (2,4,7,10). Although the variation was not statistically significant in this study, parameter estimates from the fitted curve indicate that acrophase, if there is one, occurs in the early to mid afternoon, as it did in previous studies.
The results of this study support the recommendation that clinicians limit sampling for total, free, and bioavailable T levels to the morning to establish biochemical androgen deficiency to limit the effects of diurnal variation. The relatively large declines in total, free, and bioavailable T between 0800 h and the afternoon certainly justify this practice in young and middle-aged men. A broader time window for sampling may be justified in older men, given the reductions in diurnal amplitude with advancing age. However, despite the blunting of circadian variation of serum total T levels observed among older men in this and other studies, we observed that afternoon T values less than 300 ng/dl (10.4 nmol/liter) occur quite often in the absence of low morning T values. This suggests that morning blood sampling for T would be preferred to afternoon sampling for confirmation of a diagnosis of biochemical androgen deficiency in both young and old men. Furthermore, morning samples are usually used to establish normal reference ranges for sex hormones, in particular T concentrations. Our results do not support restricting blood sampling to the morning hours for other hormones in this study. Results also indicated that half of the men with at least one of three measurements less than 300 ng/dl (10.4 nmol/liter) in the afternoon (after 1200 h) had normal T levels in all three of their assessments in the morning (before 1200 h). Likewise, we observed some variation in the other direction, i.e. with men having low morning (before 1200 h) but normal afternoon (after 1200) T levels. We believe the single low values can reasonably be interpreted as measurement variability in men with average T levels over 300 ng/dl (10.4 nmol/liter) (17). These findings support recommendations for performing more than one T measurement to diagnose hypogonadism. The results of this study do not necessarily apply to patients on T replacement for whom clinicians are making dose adjustments, but they should apply to middle-aged and older men with hypogonadal symptoms because there is no reason to believe that diurnal variation should vary according to presence of symptoms.
Footnotes
This study was supported by Grant AG23027 from the National Institute on Aging of the National Institutes of Health.
Disclosure Statement: D.J.B., A.B.A., and J.B.M. have nothing to declare. A.M.M. consults for GlaxoSmithKline, Solvay Pharmaceuticals, GTx, Amgen, and Quatrx and received grant support from GlaxoSmithKline (June 1, 2004 to May 31, 2008, and July 1, 2007 to June 30, 2010) and Ardana Bioscience (July 1, 2007 to June 30, 2010).
First Published Online December 16, 2008
Abbreviations: CV, Coefficient of variation; DHT, dihydrotestosterone; E2, estradiol; T, testosterone.
References
- Bremner WJ, Vitiello MV, Prinz PN 1983 Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab 56:1278–1281 [DOI] [PubMed] [Google Scholar]
- Cooke RR, McIntosh JE, McIntosh RP 1993 Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf) 39:163–171 [DOI] [PubMed] [Google Scholar]
- de la Torre B, Sjöberg B, Hedman M, Bártfai G, Diczfalusy E 1981 A study of the short-time variation and interrelationship of plasma hormone levels reflecting pituitary, adrenocortical and testicular function in fertile men. Int J Androl 4:532–545 [DOI] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD 2003 Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 58:710–717 [DOI] [PubMed] [Google Scholar]
- Faiman C, Winter JS 1971 Diurnal cycles in plasma FSH, testosterone and cortisol in men. J Clin Endocrinol Metab 33:186–192 [DOI] [PubMed] [Google Scholar]
- Lévi FA, Canon C, Touitou Y, Sulon J, Mechkouri M, Ponsart ED, Touboul JP, Vannetzel JM, Mowzowicz I, Reinberg A, Mathe G 1988 Circadian rhythms in circulating T lymphocyte subtypes and plasma testosterone, total and free cortisol in five healthy men. Clin Exp Immunol 71:329–335 [PMC free article] [PubMed] [Google Scholar]
- Montanini V, Simoni M, Chiossi G, Baraghini GF, Velardo A, Baraldi E, Marrama P 1988 Age-related changes in plasma dehydroepiandrosterone sulphate, cortisol, testosterone and free testosterone circadian rhythms in adult men. Horm Res 29:1–6 [DOI] [PubMed] [Google Scholar]
- Nicolau GY, Lakatua D, Sackett-Lundeen L, Haus E 1984 Circadian and circannual rhythms of hormonal variables in elderly men and women. Chronobiol Int 1:301–319 [DOI] [PubMed] [Google Scholar]
- Nicolau GY, Haus E, Lakatua DJ, Bogdan C, Sackett-Lundeen L, Popescu M, Berg H, Petrescu E, Robu E 1985 Circadian and circannual variations of FSH, LH, testosterone, dehydroepiandrosterone-sulfate (DHEA-S) and 17-hydroxy progesterone (17 OH-Prog) in elderly men and women. Endocrinologie 23:223–246 [PubMed] [Google Scholar]
- Plymate SR, Tenover JS, Bremner WJ 1989 Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl 10:366–37l [DOI] [PubMed] [Google Scholar]
- Reinberg A, Lagoguey M, Chauffournier JM, Cesselin F 1975 Circannual and circadian rhythms in plasma testosterone in five healthy young Parisian males. Acta Endocrinol (Copenh) 80:732–734 [DOI] [PubMed] [Google Scholar]
- Reinberg A, Lagoguey M 1978 Circadian and circannual rhythms in sexual activity and plasma hormones (FSH, LH, testosterone) of five human males. Arch Sex Behav 7:13–30 [DOI] [PubMed] [Google Scholar]
- Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ 1988 Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol 43:M163–M169 [DOI] [PubMed] [Google Scholar]
- Winters SJ 1991 Diurnal rhythm of testosterone and luteinizing hormone in hypogonadal men. J Androl 12:185–190 [PubMed] [Google Scholar]
- Del Ponte A, Di Monte MG, Graziani D, Guagnano MT, Menduni P, Vitullo F, Sensi S 1990 Changes in plasma DHEAS circadian rhythm in elderly men. Prog Clin Biol Res 341A:791–796 [PubMed] [Google Scholar]
- Zhao ZY, Xie Y, Fu YR, Li YY, Bogdan A, Touitou Y 2003 Circadian rhythm characteristics of serum cortisol and dehydroepiandrosterone sulfate in healthy Chinese men aged 30 to 60 years. A cross-sectional study. Steroids 68:133–138 [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, O'Donnell AB, Matsumoto AM, McKinlay JB 2007 Intra-individual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 67:853–862 [DOI] [PubMed] [Google Scholar]
- McKinlay JB, Link CL 2007 Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol 52:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman J 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Longcope C, Franz C, Morello C, Baker R, Johnston Jr CC 1986 Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas 8: 189–196 [DOI] [PubMed] [Google Scholar]