Abstract
Context: The risk of many conditions, including prostate cancer, breast cancer, and osteoporosis, is associated with serum levels of sex steroids.
Objective: The aim of the study was to identify genetic variations in sex steroid-related genes that are associated with serum levels of estradiol (E2) and/or testosterone in men.
Design: Genotyping of 604 single nucleotide polymorphisms in 50 sex steroid-related candidate genes was performed in the Gothenburg Osteoporosis and Obesity Determinants (GOOD) study (n = 1041 men; age, 18.9 ± 0.6 yr). Replications of significant associations were performed in the Osteoporotic Fractures in Men (MrOS) Sweden study (n = 2568 men; age, 75.5 ± 3.2 yr) and in the MrOS US study (n = 1922 men; age, 73.5 ± 5.8 yr). Serum E2, testosterone, and estrone (E1) levels were analyzed using gas chromatography/mass spectrometry.
Results: The screening in the GOOD cohort identified the single nucleotide polymorphism rs2470152 in intron 1 of the CYP19 gene, which codes for aromatase, responsible for the final step of the biosynthesis of E2 and E1, to be most significantly associated with serum E2 levels (P = 2 × 10−6). This association was confirmed both in the MrOS Sweden study (P = 9 × 10−7) and in the MrOS US study (P = 1 × 10−4). When analyzed in all subjects (n = 5531), rs2470152 was clearly associated with both E2 (P = 2 × 10−14) and E1 (P = 8 × 10−19) levels. In addition, this polymorphism was modestly associated with lumbar spine BMD (P < 0.01) and prevalent self-reported fractures (P < 0.05).
Conclusions: rs2470152 of the CYP19 gene is clearly associated with serum E2 and E1 levels in men.
The single nucleotide polymorphisms rs2470152 of the CYP19 gene are strongly associated with serum estradiol 2 and estradol 1 levels in men.
Serum levels of sex hormones have been associated with several clinical conditions such as breast cancer (1), prostate cancer (2), and osteoporosis (3,4). Both genetic and environmental factors may influence serum levels of sex steroids (5). Several studies investigating different mouse models or human subjects with inactivations of either estrogen receptors or CYP19, coding for aromatase, which is responsible for the final step in the biosynthesis of both estradiol (E2) and estrone (E1), as well as clinical association studies have clearly demonstrated that not only testosterone (T) but also estrogens are important for several physiological functions in men (3,6,7,8). Actually, some of the effects of T, previously believed to be exerted directly via an activation of androgen receptors, are mediated via an aromatization to E2, followed by a stimulation of estrogen receptors in men. For instance, estrogen receptor activation is required for growth plate closure in men (7), and both human cross-sectional observational and prospective studies have, in general, shown that serum E2 is a stronger predictor of bone mineral density (BMD) than serum T in men (3,4,9).
There are several reports describing polymorphisms in sex steroid-related genes of possible impact on serum E2 levels in women (10,11,12,13). In general, findings have been difficult to replicate in other cohorts, but some promising findings have been made in the CYP19 gene (11,14).
Data on the impact of variations in sex steroid-related genes on serum E2 levels in men are scarce. We have previously reported that the H268Y polymorphism of the uridine diphosphate glucuronosyltransferase 2B7 (UGT2B7), which glucuronidates sex steroids and their metabolites, is associated with E2 levels in young adult men (15). Furthermore, one study including rather few subjects (n = 300) has shown associations between a polymorphism in the CYP19 gene and E2 levels in men (16).
The androgen receptor is the most investigated gene regarding the impact of genetic variations on serum T levels in men (17). Some, but not all, studies have shown that the length of a CAG repeat in exon 1 of the androgen receptor is associated with serum T levels (17,18,19). Furthermore, we have shown that an SHBG promoter polymorphism is clearly associated not only with serum SHBG but also serum T in two large (n = 3988) male Swedish cohorts (20), and that the H268Y polymorphism of UGT2B7 is associated with serum T levels in young adult Swedish men (15).
Most previous studies evaluating the impact of sex steroid-related polymorphisms on serum sex steroid levels, except the large-sized Swedish SHBG study (20), have investigated serum E2 and/or T levels using immunochemical assays with a questionable specificity at lower concentrations (21,22). Furthermore, the general strategy of these previous studies has been to determine the associations between one polymorphism in one candidate gene and sex steroid levels in men (15,16,17).
Possible genetic differences in serum levels of E2 and T could be caused by polymorphisms in genes involved in synthesis or degradation of sex steroids, sex steroid receptors and carrier proteins, or genes involved in central regulation of sex steroids (see Table 2). Furthermore, to capture the major genetic variations of each gene, several tagging SNPs have to be analyzed in each gene. The aim of the present study was to screen for most of the common genetic variation in 50 sex steroid-related genes to identify genetic variants that, in a reproducible manner, have an impact on serum E2 and/or T levels in men using three large (n = 5531) well-characterized male cohorts in which serum sex steroids have been analyzed by the specific gas chromatography/mass spectrometry (GC-MS) technique (23,24).
Table 2.
Sex steroid-related genes investigated in the GOOD cohort
| Genes | |
|---|---|
| Enzymes in synthesis | AKR1C3(1), CYP 17(5), CYP19(6), HSD17B (types 1(23), 2(24), 3(25), 4(26), 6(27), 7(28), 8(29), 10(30), 12(31), and 13(32)), HSD3B (types 1(33) and 2(34)), 5α-reductase type-1(41), 5α-reductase type 2(42) |
| Enzymes in degradation | COMT(4), CYP1A1(7), CYP1A2(8), CYP1B1(9), CYP2C9(10), CYP3A4(11), CYP3A5(12), STS(43), SULT1A1(44), SULT1E1(45), SULT2A1(46), SULT2B1(47), UGT1A(48), UGT2B4(49), UGT2B7(50) |
| Receptors and carrier proteins | AR(2), ESR1(13), ESR2(14), ESRRA(15), ESRRB(16), GPR30(22), SHBG(40) |
| Central regulation | CGA(3), FSHB(17), FSHR(18), GNRH1(19), GNRH2(20), GNRHR(21), INHA(35), INHBA(36), INHBB(37), LHB(38), LHCGR(39) |
Numbers within parentheses correspond to gene numbers in Fig. 1.
Subjects and Methods
Study subjects: young adult men
The population-based Gothenburg Osteoporosis and Obesity Determinants (GOOD) study was initiated to determine environmental and genetic factors involved in the regulation of bone and fat mass. Men aged 18.9 ± 0.6 yr (n = 1068) from the greater Gothenburg area in Sweden were included. A total of 1041 subjects with both successful genotyping and available serum sex steroid levels were included in the initial screening of sex steroid-related gene variations of possible importance for serum sex steroid levels (see Table 1).
Table 1.
Characteristics of the study subjects
| Variables | GOOD (n = 1041) | MrOS Sweden (n = 2568) | MrOS US (n = 1922) |
|---|---|---|---|
| Age (yr) | 18.9 ± 0.6 | 75.5 ± 3.2 | 73.5 ± 5.8 |
| Height (cm) | 181.4 ± 6.8 | 174.7 ± 6.5 | 173.6 ± 7.1 |
| Weight (kg) | 73.7 ± 11.8 | 80.5 ± 12.0 | 82.6 ± 13.4 |
| BMI (kg/m2) | 22.4 ± 3.2 | 26.4 ± 3.5 | 27.4 ± 3.8 |
| Serum sex steroids | |||
| E2 (pg/ml) | 18.7 ± 6.3 | 21.0 ± 8.0 | 22.6 ± 8.4 |
| E1 (pg/ml) | 22.7 ± 7.7 | 34.3 ± 15.0 | 33.7 ± 15.5 |
| T (ng/ml) | 4.7 ± 1.5 | 4.5 ± 1.9 | 4.0 ± 1.7 |
| SHBG (nmol/liter) | 20.5 ± 7.4 | 44.0 ± 22.4 | 49.2 ± 19.9 |
Values are given as mean ± sd. n, Number of subjects with both successful genotyping and available serum sex steroid levels.
Study subjects: elderly men
The major findings in the initial screening study were replicated in two large cohorts of elderly men [Osteoporotic Fractures in Men (MrOS) Sweden and MrOS US], with available serum sex steroid levels as analyzed by GC/MS (see Table 1).
Study subjects of the population-based MrOS Sweden study (n = 3014; men aged 69–81 yr) were randomly identified using national population registers, contacted, and asked to participate (25). A total of 2568 subjects with both successful genotyping and available serum sex steroid levels were included in the first replication analyses.
The MrOS US cohort consists of 5995 community-dwelling, ambulatory U.S. men aged 65 yr or older (26,27). The total sample size was 2048. For the replication analyses, 1922 MrOS US subjects with both successful genotyping and available serum sex steroids analyzed by the GC/MS technique were included.
For more information on participants and assessment of covariates, see supplemental data, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.
Assessment of sex hormones
Serum samples were collected in the morning after an overnight fast in the MrOS US cohort and in the majority of individuals in MrOS Sweden (Gothenburg, all study subjects; Malmö and Uppsala, most study subjects). In the GOOD study and in the remainder of MrOS Sweden study subjects, nonfasting samples were drawn throughout the day. The validated GC-MS system (23,24) was used for the analysis of serum sex steroids in the GOOD study and in the MrOS Sweden study [T: limit of detection, 0.05 ng/ml; intraassay coefficient of variation (CV), 2.9%; interassay CV, 3.4%; E2: limit of detection, 2.00 pg/ml; intraassay CV, 1.5%; interassay CV, 2.7%; E1: limit of detection, 8.00 pg/ml; intraassay CV, 1.8%; interassay CV, 1.7%], and all these analyses were performed in one laboratory (Laboratory of Molecular Endocrinology and Oncology, Laval University, Québec, Canada).
Serum sex steroid levels in the MrOS US study were analyzed using a gas chromatographic negative ionization tandem mass spectrometry (Taylor Technology, Princeton NJ) (T: limit of detection, 0.025 ng/ml; intraassay CV, 2.5%; interassay CV, 6.0%; E2: limit of detection, 0.625 pg/ml; intraassay CV, 6.4%; interassay CV, 10.1%; E1: limit of detection, 1.56 pg/ml; intraassay CV, 5.2%; interassay CV, 12.9%). See supplemental data for more information. Cross-calibration of 50 samples between the Québec and Taylor laboratories revealed a rather strong correlation for E2, E1, and T between the two laboratories (E2: r = 0.96; Québec mean value, 19.8 pg/ml; Taylor mean value, 21.8 pg/ml; E1: r = 0.91; Québec mean value, 28.2 pg/ml; Taylor mean value, 31.3 pg/ml; T: r = 0.98; Québec mean value, 45.3 ng/ml; Taylor mean value, 43.7 ng/ml).
For analysis of gonadotropins and SHBG, see supplemental data.
Genotyping: GOOD study
A total of 673 single nucleotide polymorphisms (SNPs) with minor allele frequencies of at least 5% in 52 different sex steroid-related genes were selected from HapMapData Rel 21/phase II using a pairwise correlation method (r2 ≥ 0.80) including 10 kb upstream and 5 kb downstream of each gene. The SNPs were genotyped using the Golden Gate Assay (28) from Illumina Inc. (San Diego, CA). The genotyping was performed by the SNP Technology Platform in Uppsala (www.genotyping.se). Of the genotyped SNPs, 615 had a genotype call rate of at least 95% in the study subjects. Eleven of these SNPs were not polymorphic, leaving 604 SNPs in 50 genes for further analysis. The reproducibility of the genotyping was 100% according to duplicate analysis of 5% of the genotypes. The SNP rs2470152 in CYP19 and the SNP rs4953616 in LH/choriogonadotropin receptor (LHCGR) were in Hardy-Weinberg equilibrium (P > 0.05).
For information on genotyping in MrOS Sweden and MrOS US studies, see supplemental data.
Assessments of BMD and self-reported previous fractures in GOOD and MrOS Sweden
BMD of the lumbar spine and femoral neck were measured using dual energy x-ray absorptiometry. Information on previous fractures was obtained using standardized questionnaires. For more information, see supplemental data.
Statistics
Bonferroni correction was used to adjust for multiple comparisons in the analysis of candidate SNPs in the GOOD cohort (Fig. 1). Serum levels of E2 and T adjusted for age and body mass index (BMI) were calculated using linear regression analyses. For the transformation of serum E2, E1, and T levels to normally distributed parameters in each of the cohorts investigated, an empirical distribution function was made which then was applied for the calculation of the inverse of the standardized normal distribution, and these normally distributed standardized values were then used in the subsequent evaluations of the associations between SNPs and serum sex steroid levels. Univariate correlation analyses between SNPs (coded 1, 2, 3 in alphabetical order for A, C, G, and T with 2 for the heterozygotes, which means that in the case of an A/G SNP the AA homozygotes will be coded as 1, the A/G heterozygotes will be coded as 2, and the GG homozygotes will be coded as 3) and age and BMI-adjusted serum sex steroid levels and gonadotropin levels in the comprehensive screening in the GOOD study were performed using Pearson’s correlation [Fig. 1 and Supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org]. Linear regression under an additive model (AA = 1, AG = 2, GG = 3) and corrected for race (in the MrOS US study and in all cohorts combined), age, and BMI was used to evaluate the associations between rs2470152 and serum estrogens (E2 and E1) in the three cohorts separately and combined (see Table 3). Per G allele effect size (regression coefficient) and se values are expressed in sd units of E2 or E1. Differences in serum levels of sex steroids between rs2470152 genotypes were evaluated using ANOVA with Tukey’s post hoc test (see Table 4). Age-adjusted logistic regression analyses were performed to determine the association between rs2470152 (coded AA = 1, AG = 2, and GG = 3) and prevalent fractures in the GOOD and MrOS Sweden cohorts, and the results are expressed as odds ratio (OR) with 95% confidence intervals (CI) per G allele. Values are given as mean ± sd. All tests were two-tailed and conducted at the 5% significance level.
Figure 1.
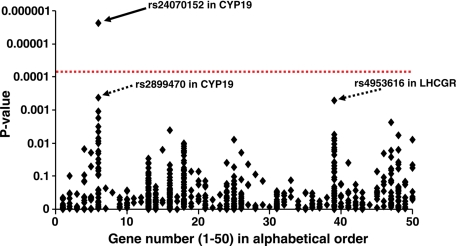
Screening of the associations between 604 genetic markers in 50 different sex steroid-related genes and serum E2 levels (adjusted for age and BMI) in the GOOD study (n = 1041; 18.9 yr of age). Univariate correlation analyses between SNPs (coded 1, 2, 3 in alphabetical order for A, C, G, and T, with 2 for the heterozygote) and age- and BMI-adjusted serum E2 levels in the comprehensive screening in the GOOD study were performed using Pearson’s correlation. P values on the y-axis are for associations between individual SNPs and serum E2 levels. Numbers on the x-axis indicate gene number (1–50) in alphabetical order (see Supplemental Table 1 for names of genes 1–50 and rs numbers for all the investigated SNPs). The number of genotyped SNPs per gene is 1–63. Dotted line indicates the significance level after conservative Bonferroni correction for multiple comparisons (1/604 × 0.05 = 8 × 10−5). The CYP19 SNP rs2470152, which remained significantly associated also after Bonferroni correction, is indicated by a solid arrow whereas two other SNPs (rs2899470 in CYP19 and rs4953616 in LHCGR), which did not fulfil this criterion but had unadjusted P values <0.001, are indicated by dotted arrows.
Table 3.
Associations of the SNP rs2470152 with serum estrogens in men
| No. of subjects | A allele frequency | Per G allele effect size in sd of E2 or E1 (sem) | P1 | P2 | |
|---|---|---|---|---|---|
| E2 | |||||
| Young adult men | |||||
| GOOD (Caucasians) | 1041 | 0.48 | 0.20 (0.04) | 5 × 10−6 | 2 × 10−6 |
| Elderly men | |||||
| MrOS Sweden (Caucasians) | 2568 | 0.46 | 0.14 (0.03) | 8 × 10−7 | 9 × 10−7 |
| MrOS US (75% Caucasians) | 1918 | 0.50 | 0.13 (0.03) | 1 × 10−4 | 1 × 10−4 |
| Caucasian | 1437 | 0.48 | 0.11 (0.04) | 3 × 10−3 | 2 × 10−3 |
| Non-Caucasian | 481 | 0.54 | 0.16 (0.06) | 0.01 | 0.01 |
| Combined elderly men | 4486 | 0.13 (0.02) | 4 × 10−10 | 4 × 10−10 | |
| Combined all three cohorts | 5527 | 0.15 (0.02) | 3 × 10−14 | 2 × 10−14 | |
| E1 | |||||
| Young adult men | |||||
| GOOD (Caucasians) | 1041 | 0.48 | 0.14 (0.04) | 1 × 10−3 | 9 × 10−4 |
| Elderly men | |||||
| MrOS Sweden (Caucasians) | 2568 | 0.46 | 0.18 (0.03) | 2 × 10−10 | 2 × 10−10 |
| MrOS US (74% Caucasians) | 1922 | 0.50 | 0.17 (0.03) | 2 × 10−7 | 2 × 10−7 |
| Caucasian | 1439 | 0.48 | 0.17 (0.04) | 5 × 10−6 | 3 × 10−6 |
| Non-Caucasian | 483 | 0.54 | 0.16 (0.06) | 0.01 | 0.01 |
| Combined elderly men | 4490 | 0.17 (0.02) | 2 × 10−16 | 2 × 10−16 | |
| Combined all three cohorts | 5531 | 0.17 (0.02) | 1 × 10−18 | 8 × 10−19 |
Linear regression analyzes of the predictive role of rs2470152 for serum E2 and E1 in young adult men (GOOD) and elderly men (MrOS US and MrOS Sweden). Number of subjects = subjects with both successful genotyping and available sex steroid levels. P values are calculated using linear regression under an additive model, corrected for race (in the MrOS US study and in all cohorts combined), age, and BMI. P1 values are for models only adjusting for race, and P2 are for models adjusted for race, age, and BMI. Effect size (regression coefficient) and se values are expressed in sd units. Non-Caucasians in MrOS US with available sex steroid levels and successful genotyping include 40% African-American, 36% Asian, and 20% Hispanic subjects. No significant interaction effect was seen between cohort and rs2470152 genotype.
Table 4.
Serum levels of sex steroids according to CYP19 rs2470152 genotype
| AA | AG | GG | P | GG vs. AAa | |
|---|---|---|---|---|---|
| GOOD | n = 241 | n = 514 | n = 286 | ||
| E2 (pg/ml) | 17.5 ± 6.2b,c | 18.8 ± 6.1b | 19.8 ± 6.2c | 1 × 10−5 | + 13% |
| E1 (pg/ml) | 21.8 ± 7.4b | 22.5 ± 7.5b | 24.0 ± 8.2c | 3 × 10−3 | + 10% |
| T (ng/ml) | 4.5 ± 1.6 | 4.6 ± 1.5 | 4.7 ± 1.4 | NS | |
| SHBG (nmol/liter) | 20.4 ± 7.6 | 20.7 ± 7.1 | 20.1 ± 6.9 | NS | |
| MrOS Sweden | n = 551 | n = 1249 | n = 768 | ||
| E2 (pg/ml) | 19.7 ± 7.5b,c | 21.1 ± 8.0 | 21.9 ± 8.1 | 4 × 10−6 | +11% |
| E1 (pg/ml) | 31.8 ± 12.8b,c | 33.8 ± 14.2b | 36.8 ± 17.2c | 1 × 10−9 | +16% |
| T (ng/ml) | 4.4 ± 1.8 | 4.4 ± 1.7 | 4.7 ± 1.8 | NS | |
| SHBG (nmol/liter) | 43.2 ± 22.7 | 44.4 ± 21.3 | 43.9 ± 21.0 | NS | |
| MrOS US | n = 484 | n = 944 | n = 494 | ||
| E2 (pg/ml) | 21.6 ± 7.9b,c | 22.8 ± 8.6 | 23.4 ± 8.2 | 4 × 10−4 | +8% |
| E1 (pg/ml) | 31.3 ± 13.4b,c | 34.1 ± 15.7b | 35.8 ± 15.8c | 1 × 10−6 | +14% |
| T (ng/ml) | 3.9 ± 1.5 | 4.0 ± 3.9 | 3.9 ± 1.7 | NS | |
| SHBG (nmol/liter) | 48.3 ± 16.7 | 49.7 ± 19.1 | 49.2 ± 19.9 | NS |
Values are adjusted for race (in the MrOS US cohort), age, and BMI and are given as mean ± sd. P values are for comparison of the three genotypes (ANOVA with Tukey’s post hoc test). NS, Nonsignificant.
Difference between GG and AA genotype given in percent for significant differences.
P < 0.05 vs. GG.
P < 0.05 vs. AG.
Results
Characteristics of the study subjects included in the initial HapMap-based screening study (GOOD study) of sex steroid-related genetic variations and the subjects included in the replication studies (MrOS Sweden and MrOS US) are shown in Table 1. The GOOD study and the MrOS Sweden study are Swedish population-based Caucasian cohorts, whereas the subpopulation with available sex steroid levels and successful genotyping of the MrOS US study included both Caucasian (75%) and non-Caucasian (25%) subjects.
Screening of the impact of genetic variations in sex steroid-related genes on serum sex steroid levels in the GOOD cohort
The impact of genetic variations in 50 sex steroid-related genes (Fig. 1) on serum sex steroid levels in men was first analyzed in the GOOD study. The investigated genes included genes coding for enzymes involved in synthesis or degradation of sex steroids, sex steroid receptors, and carrier proteins or genes involved in central regulation of sex steroids (Table 2). The association between 604 successfully genotyped SNPs in 50 different sex steroid-related genes (1–63 SNPs/gene) and serum E2 levels are shown in Fig. 1 (rs numbers and P values for the associations with E2, as well as E1 and T levels, for all successfully genotyped SNPs are given in Supplemental Table 1). The comprehensive screening in the population-based GOOD study identified the SNP rs2470152 in intron 1 of the CYP19 gene on chromosome 15q21.1 to be the SNP most significantly associated with serum E2 (Fig. 2; P = 2 × 10−6) of all investigated SNPs in the GOOD study, and the association remained significant after a conservative Bonferroni correction for multiple comparisons (required P value after Bonferroni correction 0.05/604 = 8 × 10−5; this corrected P value level is indicated by a dotted red line in Fig. 1).
Figure 2.
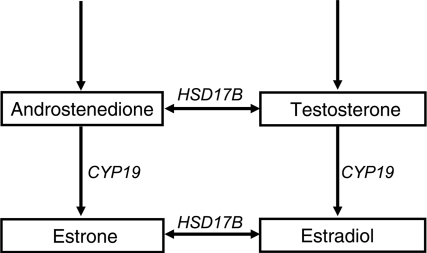
Final steps in biosynthesis of E1 and E2, CYP19, Cytochrome P-450 19 coding for aromatase; HSD17B, 17-β-hydroxysteroid dehydrogenase genes.
Two other SNPs, rs2899470 in CYP19 and rs4953616 in LHCGR, did not pass the P value required after Bonferroni correction but had unadjusted P values of P < 0.001. However, rs2899470 in CYP19 was clearly correlated with rs2470152 in CYP19 (r2 = 0.78), and thus it was not associated with serum E2 independently of rs2470152. Consequently, we did not evaluate this SNP in the subsequent replication studies. The SNP rs4953616 in LHCGR on chromosome 2p21 was associated with serum E2 levels (P < 0.001) independently of rs2470152. rs2470152 accounted for 2.1% and rs4953616 accounted for 1.1% of the variation in serum E2 levels, whereas both SNPs, when included in the same regression model, accounted for 3.3% of the variation in E2 levels. None of the analyzed SNPs in sex steroid-related genes were significantly associated with serum T levels after Bonferroni correction (Supplemental Table 1), and therefore we decided to focus the subsequent replication analyses on the associations between serum E2 and rs2470152 in CYP19 and rs4953616 in LHCGR.
Replication of findings in MrOS Sweden and MrOS US
The SNP rs2470152 in CYP19 (P = 9 × 10−7; Table 3), but not the SNP rs4953616 in LHCGR (nonsignificant, data not shown), was significantly associated with serum E2 levels in the elderly Caucasian subjects of the population-based MrOS Sweden study (n = 2568).
We finally replicated the association between serum E2 and rs2470152 in the MrOS US study (n = 1918, with available serum E2 levels and genotype data). rs2470152 was significantly associated with serum E2 levels in this cohort as well (P = 1 × 10−4; Table 3). A significant association was seen both in the Caucasian (n = 1437; P < 0.01) and in the non-Caucasian (n = 481; P < 0.01) subpopulations of the investigated MrOS US cohort.
Both when analyzed in the two replication cohorts of elderly subjects (n = 4486, MrOS Sweden and MrOS US; P = 4 × 10−10) and when analyzed in all three cohorts (n = 5527, GOOD, MrOS Sweden, and MrOS US; P = 2 × 10−14), rs2470152 in CYP19 was significantly associated with E2 levels (Table 3).
To avoid the problems with multiple comparisons in the screening, only E2 and T were included there. But because CYP19 is also involved in the biosynthesis of E1 (Fig. 2), the associations between rs2470152 and E1 levels were also analyzed in the subsequent investigations where the problem of multiple comparisons could be avoided to a much larger extent.
rs2470152 was significantly associated with serum E1 levels in all three cohorts investigated (Table 3), and when the three cohorts (n = 5531) were combined, the association signal was highly significant (P = 8 × 10−19; Table 3).
SHBG, which is involved in the regulation of levels of bioactive sex steroids, was not associated with rs2470152 (Table 4).
In the screening cohort (GOOD), neither serum FSH (AA, 2.3 ± 1.3; AG, 2.4 ± 4.4; GG, 2.1 ± 1.1 mIU/ml) nor LH (AA, 7.8 ± 8.0; AG, 8.5 ± 9.1; GG, 7.8 ± 6.6 mIU/ml) was significantly associated with rs2470152.
Estimation of the effect size of the associations between the SNP rs2470152 and serum estrogen levels
In the young adult men of the GOOD study, subjects with the GG genotype of rs2470152 in CYP19 had 13% higher E2 levels than subjects with the AA genotype, whereas the AG subjects had intermediate E2 levels (Table 4). In the elderly subjects of MrOS Sweden and MrOS US, the difference between GG and AA was slightly less pronounced (8–11%; Table 4). For E1, the GG subjects had 14–16% higher serum levels than AA subjects in the elderly subjects (MrOS Sweden and MrOS US), whereas they had 10% higher levels in the young adult men (Table 4).
The effect size per G allele was for E2 0.20, 0.14, and 0.13 sd in the GOOD, MrOS Sweden, and MrOS US studies, respectively, and for all three cohorts combined it was 0.15 sd (n = 5527; 95% CI, 0.11–0.18; Table 3). The effect size per G allele for E1 was 0.14, 0.18, and 0.17 sd in the GOOD, MrOS Sweden, and MrOS US studies, respectively, and for all three cohorts combined, it was 0.17 sd (n = 5531; 95% CI, 0.13–0.20; Table 3).
The association between SNP rs2470152 and skeletal phenotypes
Because it is well known that serum E2 is associated with BMD in males, the possible impact of rs2470152 on skeletal phenotypes was evaluated in the GOOD and MrOS Sweden cohorts. The SNP rs2470152 was significantly associated with lumbar spine BMD in the initial screening study of young adult men in the GOOD cohort (P = 0.03; Table 5). Both E2 levels (Table 4) and lumbar spine BMD (Table 5) were highest in subjects with the GG genotype. A similar nonsignificant trend (P = 0.07) of an association between rs2470152 and lumbar spine BMD was seen for the elderly men in MrOS Sweden. When the two cohorts were combined, the association was clearly significant (P = 0.008; Table 5). Femoral neck BMD was not significantly associated with rs2470152 (Table 5). The SNP rs2470152 was significantly associated with self-reported previous fractures in both the GOOD (OR per G allele, 0.82; 95% CI, 0.68–0.99) and MrOS Sweden (OR per G allele, 0.84; 95% CI, 0.73–0.96) cohorts.
Table 5.
Associations of the SNP rs2470152 with BMD in men
| AA | AG | GG | Per G allele effect size in sd of BMD (sem) | P1 | P2 | |
|---|---|---|---|---|---|---|
| Lumbar spine BMD | ||||||
| GOOD (g/cm2) | n = 241 | n = 517 | n = 288 | |||
| 1.23 ± 0.14 | 1.23 ± 0.13 | 1.26 ± 0.13 | 0.09 (0.04) | 0.03 | 0.03 | |
| MrOS Sweden (g/cm2) | n = 610 | n = 1366 | n = 816 | |||
| 1.17 ± 0.21 | 1.17 ± 0.20 | 1.19 ± 0.13 | 0.05 (0.03) | 0.15 | 0.07 | |
| Combined | 0.06 (0.02) | 0.008 | ||||
| Femoral neck BMD | ||||||
| GOOD (g/cm2) | n = 241 | n = 517 | n = 288 | |||
| 1.16 ± 0.14 | 1.16 ± 0.14 | 1.18 ± 0.15 | 0.07 (0.04) | 0.17 | 0.09 | |
| MrOS Sweden (g/cm2) | n = 603 | n = 1320 | n = 787 | |||
| 0.85 ± 0.14 | 0.85 ± 0.14 | 0.86 ± 0.13 | 0.02 (0.03) | 0.67 | 0.43 | |
| Combined | 0.04 (0.02) | 0.12 |
The predictive role of rs2470152 for BMD of the lumbar spine and the femoral neck evaluated in young adult (GOOD) and elderly (MrOS Sweden) men. n, Number of subjects with both successful genotyping and available BMD. BMD was adjusted for age, BMI, smoking, and physical activity. P1, P value from ANOVA. P2 values are calculated using linear regression under an additive model, corrected for study cohort in the combined (GOOD + MrOS Sweden) analyses. Z-scored BMD values were used in the combined linear regression analyses. No significant interaction effect was seen between cohort and rs2470152 genotype. Effect size (regression coefficient) and se values are expressed in sd units.
Discussion
Genetic variations in sex steroid-related genes might influence serum E2 levels, which in turn could affect several sex steroid-related phenotypes and clinical conditions. From a screening of the impact of 604 genetic variations in 50 important sex steroid-related genes on serum sex steroid levels and validation in two other cohorts, we identified an SNP in CYP19 that is significantly associated with serum E2 (P = 2 × 10−14) and E1 (P = 8 × 10−19) levels in men.
The SNP rs2470152 in intron 1 of the CYP19 gene showed the most significant association with serum E2 levels, and it was also significantly associated with both serum E2 and E1 levels in two large replication studies. The organization of the CYP19 gene is rather complex. The gene is comprised of a 30-kb coding region and a 93-kb regulatory region, the latter containing 10 tissue-specific promoters. rs2470152 is located in the region of the I.4 promoter (29). Interestingly, the G → A transition of rs2470152 is predicted to alter a potential binding site for the transcription factor cAMP response element binding protein (www.genomatix.de), which is thought to be important in regulating aromatase expression (30). In our cohorts, the AA genotype was associated with lower serum levels of E2 and E1, and one can speculate that this is caused by a decreased expression of aromatase as a result of loss of the cAMP response element binding protein site in the A allele. Yet another possibility is that rs2470152 is in linkage disequilibrium with another genetic variation that either regulates aromatase expression or alters the peptide sequence of the gene product. Thus, of all investigated sex steroid-related SNPs, an SNP located in the vicinity of the promoter regions of the gene responsible for the final step of E2 biosynthesis was most significantly associated with serum E2 levels.
The finding from the GOOD study of an association between the SNP rs4953616 in LHCGR and E2 was not replicated in the MrOS Sweden cohort. The finding in the GOOD cohort could be false-positive due to multiple comparisons. It cannot be excluded though that the age discrepancy between the cohorts was a contributor and that LHCGR is of relevance for E2 levels in young men but not in elderly men. Further studies are needed to explore this.
The overall effect sizes per G allele of rs2470152 in the combined cohort (n = 5531) were 0.15 and 0.17 sd on serum E2 and E1, respectively. This resulted in approximately 11 and 13% higher serum levels of E2 and E1, respectively, in GG subjects than in AA subjects in the present study. When compared to other polymorphisms, which in adequately powered large-scale studies have been reported to be reproducibly associated with quantitative traits, we believe this is a robust and substantial effect (31,32).
Serum E2 level is an undoubtedly polygenic quantitative trait, and rs2470152 is obviously only one of many potentially contributing genetic factors underlying the heritability of serum E2 levels. Actually, in the present initial screening study we identified 66 and 55 SNPs (Supplemental Table 1) associated with serum E2 and T, respectively, with a P value <0.05 but not reaching statistical significance after conservative Bonferroni correction (P < 8 × 10−5). Some of these association signals are probably false-positive as a result of multiple testing. However, some of them might be true association signals that can be confirmed in large scale replication studies and would then contribute to explaining a larger proportion of the genetic variation in serum E2 levels. Although a substantial number of genes was included in this study, there are of course many more genes involved in the complex regulation of serum sex steroid levels. It is quite likely that a future more comprehensive approach, such as a genome-wide association (GWA) study, will reveal other genes of importance. However, to avoid the problems with multiple comparisons, a very large number of study subjects will then be needed in the screening.
High levels of E2 are known to lower T levels via a negative feedback on LH secretion in the experimental situation (33). No such effect on gonadotropin or T levels was seen among men with the rs2470152 genotype associated with the highest E2 levels in this study. However, one might speculate that the effect size of rs2470152 is not of this magnitude, but rather lies within the physiological range for most subjects.
The general strategy in genetic association studies has for more than one decade been hypothesis driven in the search for associations between candidate SNPs, mostly in one candidate gene, and the investigated phenotype. This is in contrast to the recent nonhypothesis-driven GWA studies of the impact of genetic variations for different diseases or phenotypes (34,35,36), taking advantage of the recent development of improved genotype technology. GWA studies have been successful for the identification of polymorphisms highly significantly associated with some major complex human diseases (37,38). However, due to the high number of SNPs (often 500,000) analyzed, requiring substantial adjustments of significance levels for multiple testing, these studies must include a rather high number of subjects, and still only the most significant associations can be validated in replication studies. The strategy of the present study was to take advantage of the recent advancement in genotype technology while still being focused and hypothesis driven (hypothesis = genetic variations in known sex steroid-related genes have an impact on serum sex steroid levels), partly avoiding the problems with loss of power due to adjustments for multiple testing associated with GWA studies (adjustment for 604 and not 500,000 multiple comparisons was required in the initial screening cohort of the present study). To our knowledge, the present study of 604 SNPs in 50 genes is the first report evaluating the impact of the genetic variations in this many sex steroid-related genes on serum sex steroid levels.
Genetic variations with an impact on serum E2 levels might have an effect on E2-responsive tissues and thereby on sex steroid-related disorders. In a first evaluation of the possible physiological consequences of the rs2470152 polymorphism, the associations between this polymorphism and skeletal phenotypes were evaluated. Interestingly, rs2470152 was significantly associated with lumbar spine BMD, and the subjects with the GG genotype had both the highest serum E2 levels and the highest lumbar spine BMD. The lack of a significant association between rs2470152 and femoral neck BMD is congruent with the general view that the lumbar spine is a more estrogen-responsive bone site than the femoral neck (3,39). In addition, the rs2470152 SNP was modestly associated with self-reported previous fractures in both young adult and elderly men, suggesting that men with the GG genotype had not only increased lumbar spine BMD but also reduced risk of fractures.
In addition to its comprehensive tagging SNP approach, a major strength of the present study is that serum sex steroids were analyzed using the specific GC-MS technique. This technique has not been associated with the questionable specificity at lower concentrations described for previously used immunoassay-based techniques (21,22). In addition, serum sex steroids of the subjects in the two included Swedish cohorts (GOOD and MrOS Sweden) were analyzed in one laboratory, and thorough cross-calibrations between this laboratory and the separate laboratory performing the analyses of the subjects in the MrOS US study were performed. Another strength of the present study was the high number (n = 5531) of subjects with available serum sex steroid analyses in three different cohorts investigated, ensuring the reproducibility of the present findings.
Our study has limitations. The results are based on single measurements of sex steroids and may underestimate the true associations between the investigated polymorphisms and serum levels of sex steroids. Moreover, serum samples in the GOOD study were collected throughout the day. This could have influenced measurements, especially of T, which is known to be subject to diurnal variation. Another limitation of the present finding is that most of the investigated subjects were of Caucasian origin, although similar associations also were seen in the minor non-Caucasian subpopulation of the MrOS US study.
In conclusion, we provide compelling statistical evidence that the SNP rs2470152 of the CYP19 gene is strongly associated with serum E2 and E1 levels in men. In addition, it is modestly associated with lumbar spine BMD and prevalent fractures in men. Further studies are required to test the functional significance of rs2470152 on the expression of the CYP19 gene in relevant tissues and to evaluate the impact of this polymorphism on sex steroid-related disorders.
Supplementary Material
Acknowledgments
We thank RSKC Malmö genotyping facility for performing the Sequenom genotyping.
Footnotes
This work was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Avtal om Läkarutbildning och Forskning/Läkarutbildningsavtalet research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg’s Foundation, the Novo Nordisk Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grant R01-AR051124. The SNP Technology Platform in Uppsala was established by funding from the Knut and Alice Wallenberg Foundation via Wallenberg Consortium North (WCN). The Osteoporotic Fractures in Men (MrOS) US Study is supported by National Institutes of Health funding. The following institutes provided support: NIAMS, the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 30, 2008
Abbreviations: BMD, Bone mineral density; BMI, body mass index; CI, confidence interval; CV, coefficient of variation; E1, estrone; E2, estradiol; GC-MS, gas chromatography/mass spectrometry; GOOD, Gothenburg Osteoporosis and Obesity Determinants; GWA, genome-wide association; LHCGR, LH/choriagonadotropin receptor; OR, odds ratio; SNP, single nucleotide polymorphism; T, testosterone; UGT2B7, uridine diphosphate glucuronosyltransferase 2B7.
References
- Key T, Appleby P, Barnes I, Reeves G 2002 Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94:606–616 [DOI] [PubMed] [Google Scholar]
- Hsing AW, Reichardt JK, Stanczyk FZ 2002 Hormones and prostate cancer: current perspectives and future directions. Prostate 52:213–235 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C 2004 Androgens and bone. Endocr Rev 25:389–425 [DOI] [PubMed] [Google Scholar]
- Ring HZ, Lessov CN, Reed T, Marcus R, Holloway L, Swan GE, Carmelli D 2005 Heritability of plasma sex hormones and hormone binding globulin in adult male twins. J Clin Endocrinol Metab 90:3653–3658 [DOI] [PubMed] [Google Scholar]
- Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C 2000 Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA 97:5474–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K 1995 Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL 1998 Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- Olson SH, Bandera EV, Orlow I 2007 Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am J Epidemiol 165:235–245 [DOI] [PubMed] [Google Scholar]
- Haiman CA, Dossus L, Setiawan VW, Stram DO, Dunning AM, Thomas G, Thun MJ, Albanes D, Altshuler D, Ardanaz E, Boeing H, Buring J, Burtt N, Calle EE, Chanock S, Clavel-Chapelon F, Colditz GA, Cox DG, Feigelson HS, Hankinson SE, Hayes RB, Henderson BE, Hirschhorn JN, Hoover R, Hunter DJ, Kaaks R, Kolonel LN, Le Marchand L, Lenner P, Lund E, Panico S, Peeters PH, Pike MC, Riboli E, Tjonneland A, Travis R, Trichopoulos D, Wacholder S, Ziegler RG 2007 Genetic variation at the CYP19A1 locus predicts circulating estrogen levels but not breast cancer risk in postmenopausal women. Cancer Res 67:1893–1897 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Wilson AL, Kardia SR, Chu J, McConnell DS 2006 CYP1A1 and CYP1B1 polymorphisms and their association with estradiol and estrogen metabolites in women who are premenopausal and perimenopausal. Am J Med 119:S44–S51 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Jannausch ML, McConnell DS, Kardia SR, Randolph Jr JF 2006 Endogenous estradiol and its association with estrogen receptor gene polymorphisms. Am J Med 119:S16–S22 [DOI] [PubMed] [Google Scholar]
- Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD, Easton DF, Day NE, Ponder BA 2004 Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst 96:936–945 [DOI] [PubMed] [Google Scholar]
- Swanson C, Lorentzon M, Vandenput L, Labrie F, Rane A, Jakobsson J, Chouinard S, Belanger A, Ohlsson C 2007 Sex steroid levels and cortical bone size in young men are associated with a uridine diphosphate glucuronosyltransferase 2B7 polymorphism (H268Y). J Clin Endocrinol Metab 92:3697–3704 [DOI] [PubMed] [Google Scholar]
- Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S, Lucani B, Gennari C, Brandi ML 2004 A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab 89:2803–2810 [DOI] [PubMed] [Google Scholar]
- Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM 2007 Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab 92:3604–3610 [DOI] [PubMed] [Google Scholar]
- Van Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM 2001 Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin Endocrinol (Oxf) 55:659–666 [DOI] [PubMed] [Google Scholar]
- Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM 2005 Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol 98:132–137 [DOI] [PubMed] [Google Scholar]
- Eriksson AL, Lorentzon M, Mellstrom D, Vandenput L, Swanson C, Andersson N, Hammond GL, Jakobsson J, Rane A, Orwoll ES, Ljunggren O, Johnell O, Labrie F, Windahl SH, Ohlsson C 2006 SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab 91:5029–5037 [DOI] [PubMed] [Google Scholar]
- Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS 2004 Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 89:534–543 [DOI] [PubMed] [Google Scholar]
- Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, Dowsett M, Grady D, Cummings SR 2006 Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab 91:3791–3797 [DOI] [PubMed] [Google Scholar]
- Vandenput L, Labrie F, Mellstrom D, Swanson C, Knutsson T, Peeker R, Ljunggren O, Orwoll E, Eriksson AL, Damber JE, Ohlsson C 2007 Serum levels of specific glucuronidated androgen metabolites predict BMD and prostate volume in elderly men. J Bone Miner Res 22:220–227 [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, Gomez J, Candas B, Castiel I, Chaussade V, Deloche C, Leclaire J 2006 Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol 99:182–188 [DOI] [PubMed] [Google Scholar]
- Mellstrom D, Johnell O, Ljunggren O, Eriksson A, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C 2006 Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men—MrOs Sweden. J Bone Miner Res 21:529–535 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR 2005 Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Hansen M, Steemers F, Butler SL, Deloukas P, Galver L, Hunt S, McBride C, Bibikova M, Rubano T, Chen J, Wickham E, Doucet D, Chang W, Campbell D, Zhang B, Kruglyak S, Bentley D, Haas J, Rigault P, Zhou L, Stuelpnagel J, Chee MS 2003 Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol 68:69–78 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M 2003 The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 86:219–224 [DOI] [PubMed] [Google Scholar]
- Sofi M, Young MJ, Papamakarios T, Simpson ER, Clyne CD 2003 Role of CRE-binding protein (CREB) in aromatase expression in breast adipose. Breast Cancer Res Treat 79:399–407 [DOI] [PubMed] [Google Scholar]
- Weedon MN, Lettre G, Freathy RM, Lindgren CM, Voight BF, Perry JR, Elliott KS, Hackett R, Guiducci C, Shields B, Zeggini E, Lango H, Lyssenko V, Timpson NJ, Burtt NP, Rayner NW, Saxena R, Ardlie K, Tobias JH, Ness AR, Ring SM, Palmer CN, Morris AD, Peltonen L, Salomaa V, Davey Smith G, Groop LC, Hattersley AT, McCarthy MI, Hirschhorn JN, Frayling TM 2007 A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet 39:1245–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, Song K, Yuan X, Johnson T, Ashford S, Inouye M, Luben R, Sims M, Hadley D, McArdle W, Barter P, Kesaniemi YA, Mahley RW, McPherson R, Grundy SM, Bingham SA, Khaw KT, Loos RJ, Waeber G, Barroso I, Strachan DP, Deloukas P, Vollenweider P, Wareham NJ, Mooser V 2008 LDL-cholesterol concentrations: a genome-wide association study. Lancet 371:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven G, de Jong FH, Kaufman JM, de Ronde W 2006 In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 91:3324–3328 [DOI] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindstrom S, Adami HO, McDonnell SK, Schaid DJ, Cunningham JM, Wang L, Cerhan JR, St Sauver JL, Isaacs SD, Wiley KE, Partin AW, Walsh PC, et al. 2008 Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet 40:281–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, et al. 2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336 [DOI] [PubMed] [Google Scholar]
- Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, Masson G, Jakobsdottir M, Thorlacius S, Helgason A, Aben KK, Strobbe LJ, Albers-Akkers MT, Swinkels DW, Henderson BE, Kolonel LN, Le Marchand L, Millastre E, Andres R, Godino J, Garcia-Prats MD, Polo E, Tres A, Mouy M, Saemundsdottir J, Backman VM, Gudmundsson L, Kristjansson K, Bergthorsson JT, Kostic J, Frigge ML, Geller F, Gudbjartsson D, et al. 2007 Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 39:865–869 [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT 2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H 2007 Genomewide association analysis of coronary artery disease. N Engl J Med 357:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB 2003 Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 290:1729–1738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.