Abstract
Context: In Graves’ disease, thyroid-stimulating antibodies (TSAb) activate the TSH receptor (TSHR) causing hyperthyroidism. Serum polyclonal TSAb are difficult to study because of their extremely low serum levels.
Objective: Our objective was to determine whether monoclonal TSAb possess characteristics previously reported for polyclonal autoantibodies in Graves’ sera.
Design: We studied monoclonal TSAb from three laboratories: six generated from mice with induced hyperthyroidism; and one, M22, a human autoantibody obtained from Graves’ B cells.
Results: All TSAb with one exception were potent activators of TSHR-mediated cAMP generation, with relatively similar half-maximal stimulatory concentrations. Like polyclonal autoantibodies, monoclonal TSAb were largely neutralized by conformationally “active” (but not “inactive”) recombinant TSHR A subunits (the N-terminal cleavage product of the TSHR). Chimeric substitutions of TSHR amino acids 25–30 (the extreme N terminus after removal of the 21 residue signal peptide) abrogated the binding and function of all monoclonal TSAb but with one antibody (TSAb4) revealing a nonidentical epitope. Remarkably, these residues are uninvolved in the M22 epitope determined by x-ray analysis. Finally, flow-cytometric dose-response analyses, not previously possible with polyclonal TSAb, revealed that all monoclonal TSAb, human and murine, bound with lower affinity to their in vivo target, the TSH-holoreceptor, than to the isolated TSHR ectodomain.
Conclusions: TSAb function does not require antibodies with identical epitopes, and human autoantibody M22 may, therefore, not represent the full epitopic repertoire of polyclonal TSAb in Graves’ disease. Most important, we provide strong evidence that the shed ectodomain (primarily the A subunit) is the primary antigen driving affinity maturation of TSAb producing B cells.
Strong evidence is provided that the shed ectodomain (primarily the A-subunit) is the primary antigen driving affinity maturation of thyroid stimulating antibody-producing B-cells.
Among the many autoimmune diseases affecting humans, Graves’ disease is one of the most common and best understood. IgG class autoantibodies mimic the action of TSH by activating the TSH receptor (TSHR), leading to goiter and hyperthyroidism (reviewed in1). Nevertheless, numerous important questions remain regarding the genetic and environmental factors underlying the development of thyroid-stimulating autoantibodies (TSAb), as well as the molecular mechanism by which TSAb interact with and stimulate the TSHR. Answers to these questions may ultimately lead to improved therapy and, perhaps, prevention of Graves’ disease. The study of the interaction between the TSHR and polyclonal TSAb is difficult because of the very low serum concentration of the latter (2,3). Fortunately, the generation of monoclonal TSAb in recent years has provided a major impetus to this endeavor. Murine monoclonal TSAb have been obtained by fusion of splenocytes from mice made hyperthyroid by immunization with vectors expressing the cDNA for the human TSHR (4,5,6,7,8). Of particular importance, a human monoclonal TSAb, M22, the only such antibody obtained to date, was isolated from peripheral B cells of a Graves’ patient (9). Indeed, the three-dimensional structure of the M22 Fab bound to TSHR amino acids 22–260 has been determined from crystals of this complex (10).
Previous studies in our laboratory of polyclonal TSAb in Graves’ patients’ sera have identified a number of intriguing properties. Using a chimeric TSH-LH receptor (LHR) (TSH-LHR-6A1), we observed that the activity of many, but not all, polyclonal TSAb was reduced by substitution of TSHR amino acids 25–30, the extreme N terminus of the ectodomain (amino acids 1–21 being the signal peptide) (11). A second observation regarding polyclonal TSAb in Graves’ sera was their preferential recognition on flow cytometry of the TSHR ectodomain attached to the plasma membrane by a glycosylphosphatidyl inositol (GPI) anchor compared with the same ectodomain in the holoreceptor with its serpentine membrane-spanning region (12,13). Finally, TSHR A subunits generated in eukaryotic Chinese hamster ovary (CHO) cells (14) exist in two conformational forms. One (termed “active”) neutralizes polyclonal TSAb in Graves’ patients’ sera but is not recognized by murine monoclonal antibody (mAb) 3BD10 (15,16). The second A-subunit form (“inactive”) has the reciprocal properties: recognition by 3BD10 but not by Graves’ TSAb. Inactive A subunits retain their native state, at least in part, because denaturation abrogates 3BD10 binding (15).
In the present study, we examined whether human monoclonal TSAb M22 and murine TSAb generated in three different laboratories reflect the foregoing properties of polyclonal TSAb in Graves’ patients’ sera. These powerful new tools, permitting more precise and detailed analysis, corroborate the previous observations and provide challenging new insight into the pathogenesis of Graves’ disease. Most important among these, we report the first evidence that all monoclonal TSAb studied have a lower affinity for their in vivo target in disease, the wild-type TSHR, than for the isolated TSHR ectodomain. These data provide strong evidence that, in Graves’ disease, the shed ectodomain (primarily the A subunit) is the primary antigen driving affinity maturation of B cells producing TSAb.
Materials and Methods
Monoclonal TSHR antibodies
Monoclonal TSAb were kindly provided by the following laboratories: Human M22 (9) and murine TSAb4 (17) (Dr. B. Rees-Smith, Cardiff, UK); murine TSAb IRI-SAb1 (6), IRI-SAb2, and IRI-SAb3 (7) (Drs. S. Costagliola and G. Vassart, Brussels, Belgium); and murine KSAb1 and KSAb2 (8) (Dr. J. P. Banga, London, UK). mAb were provided as purified IgG, except for KSAb1 and KSAb2, which were present in conditioned culture medium. IgG concentrations were determined by mouse IgG ELISA (Bethyl Laboratories, Montgomery, TX). Controls were murine mAb lacking TSAb: CS-17 (18); and 2C11 and 4C1 (19,20) (the latter two from Serotec, Oxford, UK).
TSHR-expressing cells
We used the following stably transfected CHO cell lines:
TSHR-GPI: the TSHR ectodomain tethered by a GPI anchor (plasmid kindly provided by Dr. A. P. Johnstone (London, UK) (21).
TSHR-0, and
TSHR-10,000 cells express the wild-type TSHR with and without transgenome amplification with 10,000 nm methotrexate, respectively (22). For TSHR-0, the H601 polymorphism was mutated to Y601 (23).
Cells expressing chimeric receptors TSH-LHR-6 and TSH-LHR-6A1 were generated after transferring cDNAs for these receptors (11) into the pcDNA5/FRT (Invitrogen Corp., Carlsbad, CA).
The wild-type TSHR inserted into pcDNA5/FRT after correction of the H601 polymorphism to wild-type Y601 (QuickChange site-directed mutagenesis kit; Stratagene, La Jolla, CA).
Plasmids were stably transfected using FuGENE HD (Roche Applied Science, Indianapolis, IN) into Flp-In CHO cells (Invitrogen) according to the manufacturer’s protocol except using reduced hygromycin B concentrations (50–100 μg/ml, increasing up to 200 μg/ml when tolerated). CHO cells were cultured in F12 supplemented with 10% fetal calf serum, 10 mm HEPES, 2 mm glutamine, and standard antibiotics.
Cultured cell cAMP assays
TSHR-expressing CHO cells were transferred into 96-well plates (∼4 × 104 per well) 24 h before assay. For bioassay, culture medium was replaced with DMEM/F12 (1:1), 1 mm isobutyl-methylxanthine, 10 mm HEPES, 5% fetal calf serum, and the indicated concentrations of monoclonal TSAb or bTSH (Sigma-Aldrich Corp., St. Louis, MO). Because KSAb1 and KSAb2 were only available in conditioned cell culture medium, the maximum concentration that could be tested was 0.33 μg/ml. After completing these studies, we obtained purified KSAb1 and KSAb2 IgG that provided similar data to those obtained with conditioned medium. For TSAb neutralization, TSAb (0.1 μg/ml) were preincubated for 45 min at room temperature with 2 μg/ml purified, recombinant A subunits (16). After incubation with the cells for 60 min at 37 C, medium was aspirated, and intracellular cAMP was extracted with 0.2 ml ethanol. The extracts were evaporated to dryness, resuspended in 0.2 ml PBS (pH 7.5), and samples were assayed using the LANCE cAMP kit (PerkinElmer, Shelton, CT).
Flow cytometry
TSHR-expressed CHO cells were harvested from 10-cm plates using 1 mm EDTA, 1 mm EGTA in PBS. After washing twice with PBS containing 10 mm HEPES (pH 7.4), and 2% fetal bovine serum, the cells were incubated for 30 min at room temperature in 100 μl of the same buffer with or without 10 μg/ml mAb, or the indicated concentration in experiments examining dose responses. After rinsing, the cells were incubated for 45 min with 100 μl fluorescein isothiocyanate-conjugated goat antimouse IgG (1:100) (Caltag, Burlingame, CA) or fluorescein isothiocyanate-conjugated antihuman IgG (1:20) (BD PharMingen, San Diego, CA). After rinsing, fluorescence was measured using a Beckman FACScan flow cytofluorimeter (Beckman Coulter, Inc., Fullerton, CA). Cells stained with propidium iodide (6 μg/ml final concentration) were excluded from analysis.
Results
Monoclonal TSAb potencies
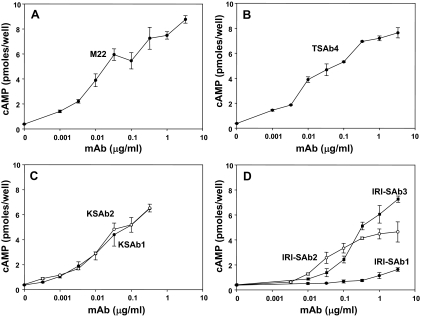
We compared the potencies of monoclonal TSAb generated in different laboratories. The majority were murine mAb, namely IRI-SAb1, IRI-SAb2, and IRI-SAb3 (6,7), KSAb1 and KSAb2 (8), and TSAb4 (17). M22 is a human monoclonal TSAb, the only one of this type generated to date (9). All except IRI-SAb1 were potent stimulators of intracellular cAMP production in CHO cells expressing the human TSHR (Fig. 1). Stimulation by M22, TSAb4, KSAb1, and KSAb2 was clearly evident under physiological conditions at 1 ng/ml (7 × 10−12 m). Although maximal stimulation could not be attained for all mAb because of the limited amounts of these reagents, the approximate effective doses producing half-maximal stimulation (ED50 values) were relatively similar, in the 0.01–0.1 μg/ml range (∼7 × 10−11 to 7 × 10−10 m), with the exception of IRI-SAb3 (ED50 ∼10−9 m) and IRI-SAb1 (Fig. 1D), whose very low potency precluded further study.
Figure 1.
Relative potencies of monoclonal TSAb measured under physiological conditions. CHO cells expressing the human TSHR were incubated with the indicated concentrations of human mAb M22 (A) and murine mAb TSAb4 (B), KSAb1 and KSAb2 (C), and IRI-SAb1, IRI-SAb2, and IRI-SAb3 (D). After 1 h the medium was removed, and intracellular cAMP levels were measured. Each point represents the mean plus the range of cAMP values in duplicate wells. Similar data were obtained in two separate experiments.
Recognition by monoclonal TSAb of conformationally different forms of TSHR A subunits
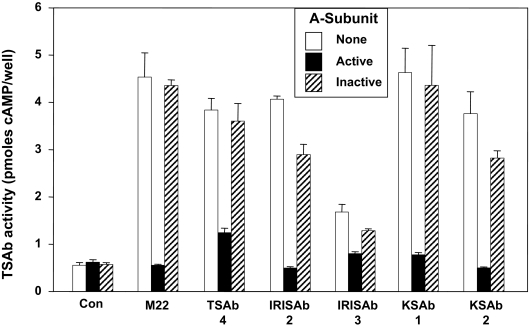
Polyclonal Graves’ TSAb and murine mAb 3BD10 reciprocally recognize two conformational forms of recombinant TSHR A subunits, termed “active” and “inactive,” respectively (15,16). Therefore, we tested the relative abilities of purified active and inactive TSHR A subunits to neutralize monoclonal TSAb functional activities. Monoclonal TSAb (0.1 μg/ml) were preincubated for 45 min with 2 μg/ml A subunits before addition to TSHR-expressing CHO cells. Without exception, TSAb stimulation of intracellular cAMP levels was largely neutralized by active but not inactive A subunits (Fig. 2). In this regard, the monoclonal TSAb behaved identically to polyclonal TSAb in Graves’ sera.
Figure 2.
Neutralization of monoclonal TSAb activity by “active” TSHR A subunits. Monoclonal TSAb (0.1 μg/ml) were preincubated for 45 minutes with 2 μg/ml of either “active” or “inactive” TSHR A subunits before addition to TSHR-expressing CHO cells. After 1 h the medium was removed, and intracellular cAMP levels were measured. Each point represents the mean plus the range of cAMP values in duplicate wells. Similar data were obtained in two separate experiments. Con, Control.
Role of the extreme N terminus of the TSHR in monoclonal TSAb activity
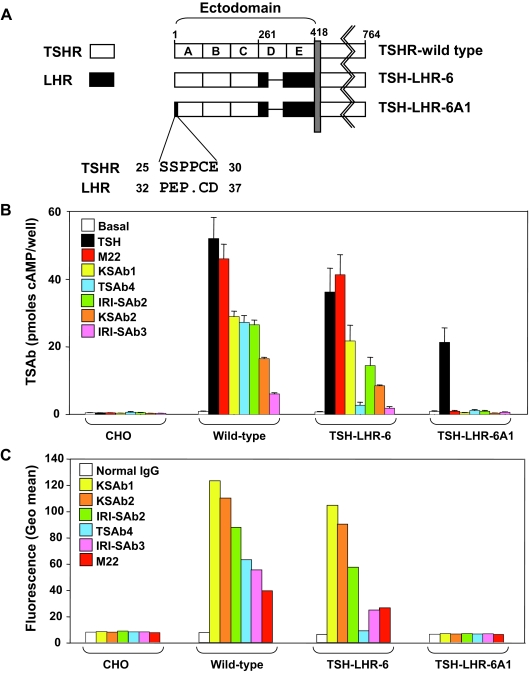
Evidence that polyclonal TSAb interact with the TSHR terminus is that the chimeric substitution of human TSHR amino acids 25–30 with the corresponding segment of the rat LHR reduces TSAb activity in a large proportion of Graves’ sera (11). Therefore, we examined whether murine and human monoclonal TSAb activities were influenced by this chimeric substitution (TSH-LHR-6A1) (Fig. 3A). As controls, we tested the chimeric receptor TSH-LHR-6 and the wild-type TSHR, all stably expressed using the same vector (see Materials and Methods). Monoclonal TSAb were ranked in terms of their functional activities as tested with the wild-type TSHR (Fig. 3B). With chimera TSH-LHR-6, TSAb4 was a disproportionately weak stimulator of cAMP generation. None of the monoclonal TSAb stimulated chimera TSH-LHR-6A1, even though this receptor responded vigorously to TSH, as reported previously (11).
Figure 3.
Role of the extreme N terminus of the TSHR in monoclonal TSAb activity. A, Schematic representation of chimeric receptors in which portions of the human TSHR were replaced with the corresponding segments of the rat LHR. The ectodomain of the wild-type TSHR was divided into five arbitrary segments (A–E). In chimeric receptor TSH-LHR-6, only the A–C components of the ectodomain contain the TSHR (amino acid residues 1–261). On this background, substitution of TSHR amino acid residues 25–30 with the homologous LHR component generates chimeric receptor TSH-LHR-6A1. The dot in the LHR sequence represents the absence of one residue relative to the TSHR. The difference between TSHR and LHR residue numbering reflects the longer LHR signal peptide. B, Monoclonal TSAb stimulation of cAMP generation by CHO cells stably expressing the wild-type TSHR and chimeric receptors TSH-LHR-6 and TSH-LHR-6A1. Monoclonal TSAb were tested at 1 μg/ml, except KSAb1 and KSAb2, which were tested at 0.3 μg/ml because of their lower concentration in conditioned medium (Materials and Methods). TSAb were ranked in terms of their functional activities as tested with the wild-type TSHR As controls, we used medium without additive (“basal”) or containing 10 mU/ml TSH. After 1 h the medium was removed, and intracellular cAMP levels were measured. The bars indicate the mean + se of three separate experiments (each measured in duplicate), except for KSAb2, which was tested in two experiments. C, Flow-cytometric recognition by the monoclonal TSAb of chimeric TSH-LHRs. Antimouse and antihuman second antibodies were used for the mouse and human mAb, respectively (Materials and Methods). Fluorescence signals (geometric means) are ranked from highest to lowest on the CHO cells stably expressing the wild-type TSHR. The same rank order is maintained when tested with CHO cells expressing chimeric receptors TSH-LHR-6 and TSH-LHR-6A1.
On flow cytometry, all monoclonal TSAb recognized the wild-type TSHR relative to CHO cells not expressing the TSHR and normal IgG as a control (Fig. 3C). Compared with the ranked fluorescence signals with the wild-type TSHR, TSAb4 again gave a disproportionately low signal with CHO cells expressing TSH-LHR-6, consistent with its weak functional activity with this receptor (Fig. 3B). As for TSAb stimulation, none of the monoclonal TSAb detected TSH-LHR-6A1. It is noteworthy that the rank order of TSAb potencies differed between the functional experiments and flow cytometry, in part because different second antibodies were used for mouse and human IgG. However, each assay is internally controlled, with the different TSHR types being tested with the same TSAb in the same experiment.
TSAb recognition of the TSHR ectodomain differentially anchored to the plasma membrane
We observed previously that polyclonal TSAb in Graves’ sera provided a higher flow-cytometric signal when tested with cells expressing a GPI-anchored TSHR ectodomain than with the identical ectodomain linked to the TSHR heptahelical transmembrane domain, i.e. the wild-type TSHR (12,13). However, in initial experiments, human monoclonal TSAb M22 (10 μg/ml) yielded the opposite result, a stronger flow-cytometric signal with the wild-type TSHR (data not shown). We then repeated and confirmed our previous findings with affinity enriched polyclonal TSAb from Graves’ sera (data not shown). A possible explanation for the discrepancy between the M22 and polyclonal TSAb data was that very low polyclonal TSAb serum concentrations with consequent weak flow-cytometric signals, as well as limited quantities of affinity enriched material, precluded dose-response analyses. In contrast, such studies were feasible with monoclonal TSAb and are reported herein.
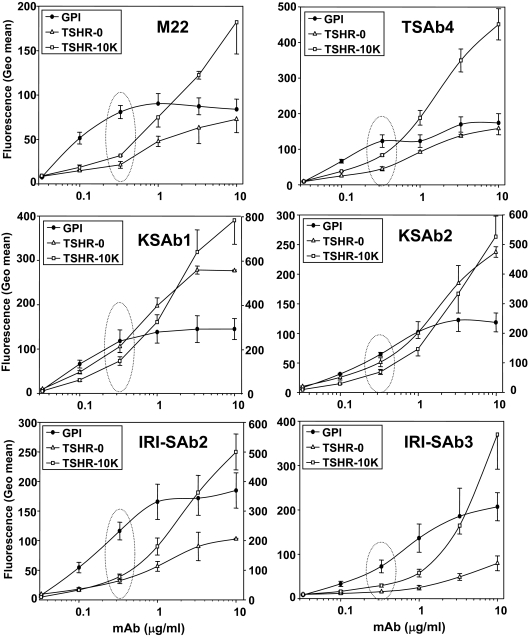
We performed flow cytometry with serially diluted monoclonal TSAb and the following CHO cell lines: 1) the TSHR ectodomain-GPI (TSHR-GPI), 2) wild-type holoreceptor (TSHR-0), and 3) wild-type holoreceptor overexpressed by transgenome amplification (TSHR-10,000). These cells express approximately 105, 105, and 106 receptors per cell, respectively. At low concentrations (<1 μg/ml) relative to high concentrations (10 μg/ml) all monoclonal TSAb preferentially recognized the TSHR-GPI cells (Fig. 4, dotted oval). As expected, at higher TSAb concentrations, binding to TSH-10,000 cells surpassed that to the TSHR-GPI and TSHR-0 cells, consistent with the greater number of receptors on the TSHR-10,000 cells. The very large number of receptors on the latter cells is evident by the inability to attain saturation, even at the highest IgG concentration tested (10 μg/ml). The disparity at low IgG concentrations between binding to the TSHR-GPI and wild-type TSHR-expressing cells was greatest for the human monoclonal TSAb M22, and for murine TSAb IRI-SAb2 and IRI-SAb3 (Fig. 4). Remarkably, these data indicate that all monoclonal TSAb, both human and murine, have a lower affinity for the in vivo functional TSHR than for the GPI-anchored TSHR ectodomain.
Figure 4.
TSAb recognition of the TSHR ectodomain differentially anchored to the plasma membrane. Flow cytometry was performed with indicated concentrations of six monoclonal TSAb: one human (M22); and the five murine (TSAb4, KSAb1, KSAb2, IRI-SAb2, and IRI-SAb3). TSAb were tested with CHO cell lines expressing: 1) the TSHR ectodomain-GPI (TSHR-GPI); 2) wild-type holoreceptor (TSHR-0; ∼1.5 × 105 receptors per cell); and 3) wild-type holoreceptor (TSHR-10,000; ∼2 × 106 receptors per cell). Flow-cytometric data are expressed as the geometric mean fluorescence (Geo mean). In panels with two scales on the ordinate (KSAb1, KSAb2, and IRI-SAb2), the left scale indicates values for TSHR-GPI and TSHR-0 cells, and the right scale indicates values for the TSHR-10,000 cells. Each point represents the mean + se of values obtained in three separate experiments, except for the TSHR-0 cells, which were tested twice. The dotted ovals highlight relative binding to the different cell types at low TSAb concentrations (0.33 mg/ml), a semiquantitative reflection of antibody affinity.
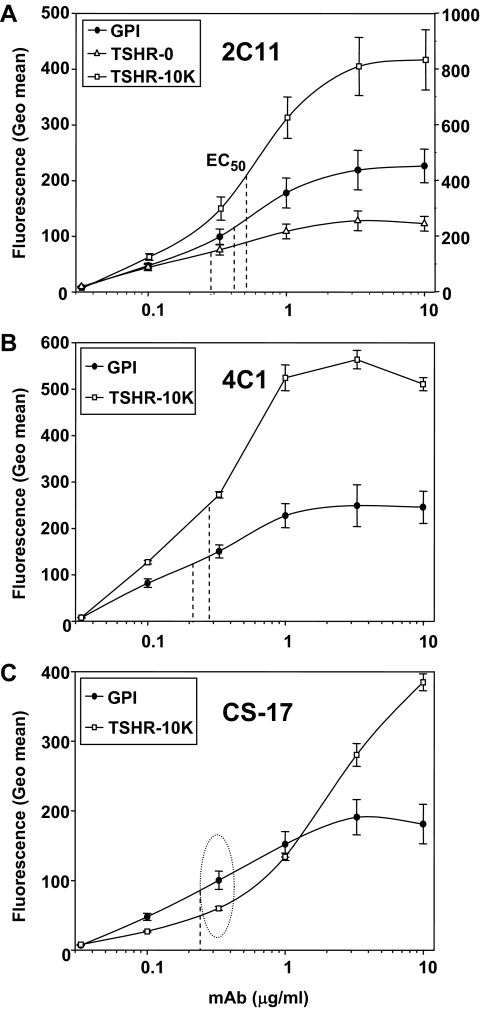
As controls for the monoclonal TSAb, we studied dose responses with three nonfunctional mAb with epitopes in different regions of the TSHR. In contrast to the monoclonal TSAb, mAb 2C11 (epitope including amino acids 355–358 within the hinge region) (19,20) had similar affinities for the TSHR-GPI and the wild-type TSHR (TSHR-0 and TSHR-10,000 cells) (Fig. 5), i.e. IgG concentrations required for half-maximal binding (ED50) were between 0.2 and 0.4 μg/ml. A mAb to the TSHR B subunit (4C1; epitope including amino acids 381–384) (20) behaved similarly to 2C11, with ED50 values of 0.2–0.3 μg/ml for the TSHR-GPI and wild-type TSHR-10,000 cells (Fig. 5). In contrast to 2C11 and 4C1, at low concentrations (<0.33 μg/ml), mAb CS-17 recognized the wild-type TSHR less well than the GPI-anchored TSHR ectodomain (Fig. 5, dotted oval). Indeed, the affinity for the former was too low to attain saturation and compare ED50 values. Therefore, though CS-17 does not activate the TSHR (18), it nevertheless resembles the monoclonal TSAb in terms of this property. Of note, in contrast to 2C11 and 4C1 (19,20), CS-17 was generated by immunization with the free TSHR A subunit, amino acids 22–289 (18,24).
Figure 5.
Flow cytometry with nonfunctional mAb with epitopes in different regions of the TSHR. Antibodies were tested with the TSHR-GPI and wild-type TSHR-10,000 cells. 2C11 was also tested with TSHR-0 cells. Flow-cytometric data are expressed as the geometric mean fluorescence (Geo mean). A, 2C11; epitope includes amino acid residues 355–358 within the hinge region (19,20). The left ordinate scale is for TSHR-GPI and TSHR-0 cells; the right ordinate scale is for TSHR-10,000 cells. B, 4C1; epitope includes residues 381–384(B subunit) (20). C, CS-17; epitope restricted to TSHR amino acid residues 22–289 within the A subunit (signal peptide residues 1–21) (18,24). IgG concentrations required for half-maximal binding (EC50) are indicated by the vertical dashed lines. The CS-17 affinity was too low for the wild-type TSH to attain saturation and accurate calculation of its EC50. The dotted oval depicts the greater signal of CS-17 at 0.33 μg/ml for the TSHR-GPI than for the wild-type TSHR-10,000 cells despite overexpression of the wild-type TSHR in the latter. Each point represents the mean + se of values obtained in six separate experiments for 2C11, and three experiments for 4C1 and CS-17.
Discussion
The development in recent years of mAb capable of activating the TSHR is a major (and long sought) advance, the feasibility of which was even questioned before their advent (25). The obvious importance of these critical reagents is their potential to advance the understanding of the pathogenesis of Graves’ disease, to provide improved diagnostic tools and, perhaps, novel therapeutic agents. In the present study, surveying properties of a wide spectrum of monoclonal TSAb from three different laboratories provides a unique opportunity to compare their potencies as well as their relationship with polyclonal TSAb in Graves’ patients’ sera. We tested six murine (6,7,8,17) and one human (9) monoclonal TSAb. The human TSAb is the only one presently available and is remarkable in that the three-dimensional structure of its Fab has been resolved, on its own (17) as well as complexed with TSHR amino acids 22–260, essentially the entire TSHR A subunit (10). The latter information, a major technical feat, has aroused optimism for developing “new strategies to understand and control both glycoprotein hormone receptor activation and the autoimmune response to the TSHR” (10).
In agreement with the reports from their respective laboratories, we found all monoclonal TSAb, with the exception of IRI-SAb1 (7), to be highly potent in terms of increasing TSHR-mediated cAMP generation with detectable stimulation evident at IgG concentrations as low as 1 ng/ml (7 × 10−12 m) and ED50 values in the range of 10−10 to 10−9 m; limited amounts of IgG precluded attaining plateaus in TSHR activation. A caveat in interpreting published TSAb ED50 values is the assay conditions under which the TSAb are tested. Omission of NaCl from cell culture medium decreases the ED50 (increased sensitivity) (26). This modification is of value for increasing the sensitivity of clinical assays for polyclonal TSAb in Graves’ patients’ sera but does not permit assessment of the ED50 under more physiological conditions. To our knowledge there are no published data on human M22 and murine TSAb4 potency in medium containing NaCl. When tested in the absence of NaCl, M22 was nearly two orders of magnitude more potent than TSAb4 (Fig. 1 in Ref. 17). It is interesting that in the presence of NaCl (present report), the ED50 values of M22 and TSAb4 appear to be quite similar.
Three features of polyclonal Graves’ TSAb were generally shared by all monoclonal TSAb. First, “active,” but not “inactive,” recombinant human TSHR A subunits (amino acids 22–289) (14,15,16) more effectively neutralized monoclonal TSAb activity. As mentioned previously, “active” A subunits neutralize TSAb but not mAb 3BD10, whereas “inactive” A subunits neutralize 3BD10, but not TSAb. Neither type of antibody recognizes denatured A subunits. Reciprocal recognition by TSAb and 3BD10 of active and inactive TSHR A subunits provides evidence that their epitopic locations overlap. The 3BD10 epitope is, in large part, localized to the cysteine-rich extreme N terminus of the TSHR (amino acids 22–41; amino acids 1–21 representing the signal peptide) (27).
Second, monoclonal TSAb resemble polyclonal TSAb in that the substitution of TSHR amino acids 25–30 (the “A1” domain) superimposed on chimeric receptor TSH-LHR-6 (Fig. 3A) greatly reduces TSAb, but not TSH, activation of the TSHR. These data support the concept that TSAb interact with the extreme N terminus of the TSHR. However, of note, TSAb4 recognition and activation of TSH-LHR-6 were much impaired even without the added A1 substitution, indicating that its epitope is not identical to those of the other monoclonal TSAb. This observation is consistent with the finding that TSAb activity in nearly half of Graves’ sera tested was unaffected by the A1 substitution (11). Subsequent evidence supports the concept that polyclonal Graves’ TSAb can activate the TSHR despite subtle differences in their epitopes (7,8,28,29,30).
With this background, the TSAb M22 epitope, precisely determined by x-ray diffraction analysis (10), may not represent the full repertoire of polyclonal TSAb in Graves’ disease sera. Because TSAb contact with a precise site on the TSHR is not necessary for activation, a portion of the antibody distinct from its antigen binding site may impact, or cause torsion, in another TSHR component, possibly the hinge region. Excluding the TSAb antigen binding site and the IgG Fc component (reviewed in Ref. 31), we suggest that it is the lateral facet of the Fab that alters TSHR conformation, thereby activating the receptor. Remarkably, TSHR amino acids 25–30 (the “A1” substitution) have a profound effect on M22 binding and function yet are not contact points in the M22/A-subunit complex. One possibility is that the A1 substitution introduces an allosteric conformational change further downstream in the leucine-rich repeat region of the TSHR. In our view this likelihood is reduced because TSHR amino acids 25–30 are a relatively discrete segment at the extreme N terminus of the receptor. An alternative consideration is that the M22 binds to a cryptic epitope on TSHR amino acids 22–260 (only a part of the TSHR ectodomain) that may be nonidentical to the M22 binding site on the holoreceptor, as suggested by Moyle and colleagues (32), for the FSH binding site on the equivalent portion of the FSH receptor. This issue will be difficult to resolve until a monoclonal TSAb is crystallized with the TSH holoreceptor, a daunting task.
Finally and most important in our view is that dose-response flow-cytometric studies with monoclonal TSAb, not previously feasible with polyclonal TSAb in Graves’ sera, clearly indicate the lower affinity of TSAb for the wild-type TSHR than for the GPI-anchored TSHR ectodomain. This is the case for all monoclonal TSAb tested, including human autoantibody M22, as previously suggested for a monoclonal hamster TSAb (29). Different methodological approaches have established that the TSAb concentration in the majority of Graves’ patients is less than 1 μg/ml (14,15,33), far lower than autoantibodies to thyroid peroxidase (2,3). Fortuitously, had the polyclonal TSAb concentration been higher, the differential recognition of the wild-type TSHR and the GPI-anchored ectodomain would not have been observed (12,13).
That TSAb have a lower affinity for the holoreceptor than for the isolated ectodomain has implications for the pathophysiology of Graves’ disease. The isolated TSHR ectodomain does not exist in vivo. However, the TSHR undergoes intramolecular cleavage within the ectodomain to generate an A subunit linked by disulfide bonds to a B subunit with seven membrane-spanning helices (reviewed in Ref. 1). Moreover, TSHRs shed their A subunits, and it is clearly established that TSAb in Graves’ sera bind to the A subunit. Generation of very high affinity antibodies requires antigen-driven B-cell affinity maturation associated with mutations within the complementarity determining regions in the IgG heavy and light-chain germline genes, as established for M22 (17). With this background, the present data provide strong evidence that the shed TSHR A-subunit component of the ectodomain is the immunogen underlying TSAb affinity maturation in Graves’ disease. Unlike the TSHR, the homologous glycoprotein hormone receptors do not undergo cleavage and shedding of part of their ectodomains, and there is no Graves’ disease of the gonads.
Support for the concept that the TSHR A subunit is the immunogen in Graves’ disease is provided by studies on induced hyperthyroidism in mice. Injection of adenovirus expressing the free A subunit is more effective than expression of a TSHR mutated so as not to cleave into subunits and, therefore, incapable of shedding its A subunit (34). The high efficacy of A-subunit immunization has been confirmed in other laboratories (8,35,36,37). Indeed, monoclonal KSAb1 and KSAb2 were generated in this manner. Of note, unlike nonfunctional TSHR mAb 2C11 and 4C1, murine monoclonal CS-17 was also generated by immunization with A-subunit adenovirus (18). Of these mAb, only CS-17 resembles TSAb in having a lower affinity for the TSH holoreceptor than for its isolated ectodomain. Therefore, although an A-subunit immunogen may favor TSAb generation, it is not sufficient. CS-17 lacks TSHR stimulatory activity; indeed, it is an inverse agonist and TSH antagonist (18). It is logical that A-subunit immunization will generate antibodies with a spectrum of epitopes, only some of which are associated with TSHR stimulation. As demonstrated in mouse immunization studies, the shed TSHR A subunit may also be an immunogen in the generation of some TSH-blocking antibodies (38). TSH-blocking autoantibodies in humans, a rare cause of hypothyroidism (39,40), have more diverse epitopes than TSAb and are not limited to the A subunit (12).
In summary, the present study represents the first comparison between functional characteristics of polyclonal TSAb and a compendium of monoclonal TSAb, one being a human autoantibody. The evidence obtained indicates that TSAb need not have identical epitopes, but the TSHR extreme N terminus appears to be involved in their activity. Most important, TSAb bind with lower affinity to the TSHR holoreceptor, their natural autoantigen in Graves’ disease, than to the isolated TSHR ectodomain. Therefore, antigen-driven affinity maturation of B cells producing TSAb is likely to involve the shed TSHR A subunit.
Acknowledgments
We are grateful to Drs. B. Rees Smith, J. Furmaniak, S. Costagliola, G. Vassart, and J. P. Banga for providing aliquots of their valuable monoclonal antibodies. We also thank Dr. Boris Catz, Los Angeles, CA, for his contributions to our laboratory.
Footnotes
This work was supported by National Institutes of Health Grants DK 19289 (to B.R.) and DK 54684 (to S.M.M.).
Present address for F.L.: Department of Endocrinology, University Hospital of Pisa, 56100 Pisa, Italy.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 9, 2008
Abbreviations: CHO, Chinese hamster ovary; GPI, glycosylphosphatidyl inositol; LHR, LH receptor; mAb, monoclonal antibody; TSAb, thyroid-stimulating antibody; TSHR, TSH receptor.
References
- Rapoport B, McLachlan SM 2007 The thyrotropin receptor in Graves’ disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- De Forteza R, Smith CU, Amin J, McKenzie JM, Zakarija M 1994 Visualization of the thyrotropin receptor on the cell surface by potent autoantibodies. J Clin Endocrinol Metab 78:1271–1273 [DOI] [PubMed] [Google Scholar]
- Jaume JC, Kakinuma A, Chazenbalk GD, Rapoport B, McLachlan SM 1997 Thyrotropin receptor autoantibodies in serum are present at much lower levels than thyroid peroxidase autoantibodies: analysis by flow cytometry. J Clin Endocrinol Metab 82:500–507 [DOI] [PubMed] [Google Scholar]
- Sanders J, Jeffreys J, Depraetere H, Richards T, Evans M, Kiddie A, Brereton K, Groenen M, Oda Y, Furmaniak J, Rees Smith B 2002 Thyroid stimulating monoclonal antibodies. Thyroid 12:1043–1050 [DOI] [PubMed] [Google Scholar]
- Ando T, Latif R, Pritsker A, Moran T, Nagayama Y, Davies TF 2002 A monoclonal thyroid-stimulating antibody. J Clin Invest 110:1667–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola S, Franssen JD, Bonomi M, Urizar E, Willnich M, Bergmann A, Vassart G 2002 Generation of a mouse monoclonal TSH receptor antibody with stimulating activity. Biochem Biophys Res Commun 299:891–896 [DOI] [PubMed] [Google Scholar]
- Costagliola S, Bonomi M, Morgenthaler NG, Van Durme J, Panneels V, Refetoff S, Vassart G 2004 Delineation of the discontinuous-conformational epitope of a monoclonal antibody displaying full in vitro and in vivo thyrotropin activity. Mol Endocrinol 18:3020–3024 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Gianoukakis AG, Salehi S, Moorhead J, Rao PV, Khan MZ, McGregor AM, Smith T, Banga JP 2006 Monoclonal pathogenic antibodies to the TSH receptor in Graves’ disease with potent thyroid stimulating activity but differential blocking activity activate multiple signaling pathways. J Immunol 176:5084–5092 [DOI] [PubMed] [Google Scholar]
- Sanders J, Evans M, Premawardhana LD, Depraetere H, Jeffreys J, Richards T, Furmaniak J, Rees Smith B 2003 Human monoclonal thyroid stimulating autoantibody. Lancet 362:126–128 [DOI] [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Depraetere H, Jeffreys J, Richards T, Furmaniak J, Rees SB 2007 Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17:395–410 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Rapoport B 1992 Thyroid stimulatory autoantibodies in different patients with autoimmune thyroid disease do not all recognize the same components of the human thyrotropin receptor: selective role of receptor amino acids Ser25-Glu30. J Clin Endocrinol Metab 75:1425–1430 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Pichurin P, Chen CR, Latrofa F, Johnstone AP, McLachlan SM, Rapoport B 2002 Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest 110:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latrofa F, Chazenbalk GD, Pichurin P, Chen CR, McLachlan SM, Rapoport B 2004 Affinity-enrichment of thyrotropin receptor autoantibodies from Graves’ patients and normal individuals provides insight into their properties and possible origin from natural antibodies. J Clin Endocrinol Metab 89:4734–4745 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B 1997 Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves’ patients’ sera. J Biol Chem 272:18959–18965 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Wang Y, Guo J, Hutchison JS, Segal D, Jaume JC, McLachlan SM, Rapoport B 1999 A mouse monoclonal antibody to a thyrotropin receptor ectodomain variant provides insight into the exquisite antigenic conformational requirement, epitopes and in vivo concentration of human autoantibodies. J Clin Endocrinol Metab 84:702–710 [DOI] [PubMed] [Google Scholar]
- Chazenbalk G, McLachlan S, Pichurin P, Rapoport B 2001 A prion-like shift between two conformational forms of a recombinant thyrotropin receptor A-subunit module: purification and stabilization using chemical chaperones of the form reactive with Graves’ autoantibodies. J Clin Endocrinol Metab 86:1287–1293 [DOI] [PubMed] [Google Scholar]
- Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Nunez MR, Blundell TL, Furmaniak, Rees SB 2004 Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid 14:560–570 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]
- Johnstone AP, Cridland JC, DaCosta CR, Harfst E, Shepherd PS 1994 Monoclonal antibodies that recognize the native human thyrotropin receptor. Mol Cell Endocrinol 105:R1–R9 [DOI] [PubMed] [Google Scholar]
- Johnstone AP, Cridland JC, Da Costa CR, Nussey SS, Shepherd PS 2003 A functional site on the human TSH receptor: a potential therapeutic target in Graves’ disease. Clin Endocrinol (Oxf) 59:437–441 [DOI] [PubMed] [Google Scholar]
- Da Costa CR, Johnstone AP 1998 Production of the thyrotropin receptor extracellular domain as a glycosylphosphatidylinositol-anchored membrane protein and its interaction with thyrotropin and autoantibodies. J Biol Chem 273:11874–11880 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B 1996 Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology 137:4586–4591 [DOI] [PubMed] [Google Scholar]
- Chen C-R, Chazenbalk GD, McLachlan SM, Rapoport B 2003 Targeted restoration of cleavage in a noncleaving thyrotropin receptor demonstrates that cleavage is insufficient to enhance ligand-independent activity. Endocrinology 144:1324–1330 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2008 Identification of key amino acid residues in a thyrotropin receptor monoclonal antibody epitope provides insight into its inverse agonist and antagonist properties. Endocrinology 149:3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola S, Vassart G 2002 Monoclonal antibodies with thyroid stimulating activity, at last. Thyroid 12:1039–1041 [DOI] [PubMed] [Google Scholar]
- Kasagi K, Konishi J, Iida Y, Ikekubo K, Mori T, Kuma K, Torizuka K 1982 A new in vitro assay for human thyroid stimulator using cultured thyroid cells: effect of sodium chloride on adenosine 3′,5′-monophosphate increase. J Clin Endocrinol Metab 54:108–114 [DOI] [PubMed] [Google Scholar]
- Schwarz-Lauer L, Pichurin PN, Chen C-R, Nagayama Y, Paras C, Morris JC, Rapoport B, McLachlan SM 2003 The cysteine-rich amino terminus of the thyrotropin receptor is the immunodominant linear antibody epitope in mice immunized using naked deoxyribonucleic acid or adenovirus vectors. Endocrinology 144:1718–1725 [DOI] [PubMed] [Google Scholar]
- Ban Y, Greenberg DA, Davies TF, Jacobson E, Concepcion E, Tomer Y 2008 Linkage analysis of thyroid antibody production: evidence for shared susceptibility to clinical autoimmune thyroid disease. J Clin Endocrinol Metab 93:3589–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk GD, Latrofa F, McLachlan SM, Rapoport B 2004 Thyroid stimulation does not require antibodies with identical epitopes but does involve recognition of a critical conformation at the N terminus of the thyrotropin receptor A-subunit. J Clin Endocrinol Metab 89:1788–1793 [DOI] [PubMed] [Google Scholar]
- Sanders J, Bolton J, Sanders P, Jeffreys J, Nakatake N, Richards T, Evans M, Kiddie A, Summerhayes S, Roberts E, Miguel RN, Furmaniak J, Smith BR 2006 Effects of TSH receptor mutations on binding and biological activity of monoclonal antibodies and TSH. Thyroid [Erratum (2007) 17:188] 16:1195–1206 [DOI] [PubMed] [Google Scholar]
- Vieland VJ, Huang Y, Bartlett C, Davies TF, Tomer Y 2008 A multilocus model of the genetic architecture of autoimmune thyroid disorder, with clinical implications. Am J Hum Genet 82:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Bernard MP, Cao D, Myers RV, Kerrigan JE, Moyle WR 2007 Follitropin receptors contain cryptic ligand binding sites. Mol Cell Endocrinol 260–262:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatake N, Sanders J, Richards T, Burne P, Barrett C, Pra CD, Presotto F, Betterle C, Furmaniak J, Smith BR 2006 Estimation of serum TSH receptor autoantibody concentration and affinity. Thyroid 16:1077–1084 [DOI] [PubMed] [Google Scholar]
- Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutori Y, Saitoh O, Eguchi K, Nagayama Y 2006 Adenovirus encoding the thyrotropin receptor A-subunit improves the efficacy of dendritic cell-induced Graves’ hyperthyroidism in mice. J Autoimmun 26:32–36 [DOI] [PubMed] [Google Scholar]
- Land KJ, Moll JS, Kaplan MH, Seetharamaiah GS 2004 Signal transducer and activator of transcription (Stat)-6-dependent, but not Stat4-dependent, immunity is required for the development of autoimmunity in Graves’ hyperthyroidism. Endocrinology 145:3724–3730 [DOI] [PubMed] [Google Scholar]
- Kaneda T, Honda A, Hakozaki A, Fuse T, Muto A, Yoshida T 2007 An improved Graves’ disease model established by using in vivo electroporation exhibited long-term immunity to hyperthyroidism in BALB/c mice. Endocrinology 148:2235–2244 [DOI] [PubMed] [Google Scholar]
- Chen C-R, Pichurin P, Chazenbalk GD, Aliesky A, Nagayama Y, McLachlan SM, Rapoport B 2004 Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves’ disease. Endocrinology 145:228–233 [DOI] [PubMed] [Google Scholar]
- Endo K, Kasagi K, Konishi J, Ikekubo K, Tatsuyo O, Takeda Y, Mori T, Torizuka K 1978 Detection and properties of TSH-binding inhibitor immunoglobulins in patients with Graves’ disease and Hashimoto’s thyroiditis. J Clin Endocrinol Metab 46:734–739 [DOI] [PubMed] [Google Scholar]
- Matsuura N, Yamada Y, Nohara Y, Konishi J, Kasagi K, Endo K, Kojima K, Wataya K 1980 Familial neonatal transient hypothyroidism due to maternal TSH-binding inhibitor immunoglobulins. N Engl J Med 303:738–741 [DOI] [PubMed] [Google Scholar]