Abstract
Spinal microglia play a key role for creating exaggerated pain following tissues inflammation or injury. Electroacupuncture (EA) can effectively control the exaggerated pain both in humans with inflammatory disease and animals with experimental inflammatory pain. However, little is known about the relationship between spinal glial activation and EA analgesia. Using immunohistochemistry, RT-PCR analysis, and behavioral testing, the present study demonstrated that (1) Unilateral intra-articular injection of CFA produced an robust microglial activation and the up-regulation of the tumor necrosis factor (TNF)-α, interleukin (IL-1β), and IL-6 mRNA levels in the spinal cord; (2) Repeated intrathecal (i.t.) injection of minocycline (100 μg), a microglial inhibitor, or EA stimulation of ipsilateral “Huantiao”(GB30) and “Yanglingquan” (GB34) acupoints significantly suppressed CFA-induced nociceptive behavioral hypersensitivity and spinal microglial activation; (3) Combination of EA with minocycline significantly enhanced the inhibitory effects of EA on allodynia and hyperalgesia. For the first time, these data provide direct evidence for the involvement of spinal microglial functional state in anti-nociception of EA. Thus, anti-neuroinflammatory effect of EA might be considered as one of the mechanisms of its anti-arthritic pain effects, and thereby a multidisciplinary integrated approach to treating symptoms related to arthritis might be raised.
Keywords: Glia, Microglia, Astrocyte, Minocycline, Electroacupuncture, Monoarthritis, Hyperalgesia, Allodynia, RT-PCR, Rat
1. Introduction
Arthritis is a major health care burden, with increasing incidence. Pain is its predominant clinical feature, yet therapy is ineffective for many patients. Nearly all symptomatic patients have use-related pain, 50 % or less describe rest pain, and about 30 % report night pain. Current recommendations for managing arthritis (rheumatoid, or osteoarthritis), including guidelines published by the American College of Rheumatology and European League of Associations of Rheumatology, focus on relieving pain and maintaining or improving physical function. So controlling pain is an important goal of both pharmacological and non-pharmacological management. However, pharmacological management of arthritis is often ineffective and agents such as non-steroidal anti-inflammatory drugs (NSAIDs) may cause unwanted and dangerous side effects (Berman et al., 2004). Therefore, effective and safe therapeutic strategy in arthritis is currently an unmet need (Curatolo and Bogduk, 2001).
Complete Freund’s adjuvant (CFA) has been utilized to induce rat arthritic immunopathological disease that displays many of the pathological features of human rheumatoid arthritis (Colpaert, 1987; Schaible et al., 2002). Following intra-articular injection of CFA, hyperalgesia (augmented pain response to noxious stimulation), allodynia (pain produced by normally non-painful stimulation), and spontaneous pain develop within hours and last for more than 2 weeks in the injected paw (Sluka et al., 1998; Schaible et al., 2002). It is thought that the exaggerated pain results from peripheral sensitization (increase of sensitivity of nociceptive primary afferent neurons) and central sensitization (hyperexcitability of nociceptive neurons in the central nervous system, CNS) (Ji and Woolf, 2001). It is well known now that spinal cord glia (microglia and astrocytes) importantly contribute to the development and maintenance of central sensitization (Deleo and Yezierski, 2001; Watkins et al., 2001; Wieseler-Frank et al., 2004). Peripheral nerve or spinal cord injury, peripheral nerve or tissues inflammation, and bone cancer produce hyperalgesia and allodynia, as well as activation of glia in the spinal cord (Colburn et al., 1999; Ledeboer et al., 2005; Zhang et al., 2005; Sun et. al., 2006b). Following inflammation or injury, microglia that are often considered resident macrophages in the CNS, rapidly become less ramified and begin to proliferate. Upon activation, microglia can release a variety of algesic substances that enhance pain transmission (Watkins, 2001; Watkins and Maier, 2003; Deleo et al., 2004). Furthermore, blocking the activation of spinal cord microglia with minocycline (a microglial inhibitor) blocks the development of exaggerated pain states, suggesting that microglial activation might be necessary for the initiation of pain facilitation (Ledeboer et al., 2005).
Electroacupuncture (EA), an acupuncture therapy, has been used for several decades in the treatment of acute and chronic pain. Currently it has been employed as an alternative treatment for the chronic pain syndromes related to rheumatoid arthritis in humans (Berman et al., 2004). In experimental animals, arthritis-induced hyperalgesia and allodynia can be successfully suppressed by repeated EA as well (Luo F, 1996). Several processes have been proposed to explain effects of EA, primarily those on pain. One of the main mechanisms is to stimulate the CNS to release neurotransmitters or neuromodulators including opioid peptides, serotonin and noradrenaline into the muscles, spinal cord, and brain. These substances either change the experience of pain or promote release of other chemicals, such as neurohormones that influence neuroimmune function (Han, 2003). Whether EA-induced changes in spinal chemistry influence spinal immunelike glial activation and resultant release of algesic substances under conditions of pain enhancement is unknown. In the present study, we attempt to examine whether the EA antagonizes early activation of spinal microglia, and whether suppression of spinal microglial activation enhances anti-allodynic and anti-hyperalgesic effects of EA in CFA-induced rat ankle joint monoarthritis (MA) model.
2. Materials and methods
2.1 Animals
Experiments were performed on adult male Sprague–Dawley rats (Experimental Animal Center, Shanghai Medical College of Fudan University, China) weighing 200–250 g. Rats were housed in temperature-controlled (22 ± 2 °C) and light-controlled (12: 12 h light-dark cycle) room with free access to food and water. Prior to experimental manipulation, rats were allowed to acclimate to the housing facilities and were handled daily at least for 3 days. All experimental protocols and animal handling procedures were approved by Animal Care and Use Committee (ACUC) of Fudan University, and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Induction of monoarthritis (MA)
Monoarthritis (MA) was induced by an injection of complete Freund’s adjuvant (CFA) into the unilateral ankle articular cavity. The rat was briefly anesthetized with isoflurane. The skin around the site of injection was sterilized with 75 % alcohol. The left leg of the rat was held and the fossa of the lateral malleolus of the fibula was located. A 28 gauge needle was inserted vertically to penetrate the skin, and turned distally to insert into the articular cavity from the gap between the tibiofibular and tarsus bone until a distinct loss of resistance was felt. A volume of 50 μl CFA was then injected. Sham MA control animals were similarly injected with sterile normal saline (NS).
2.3 Intrathecal surgery and drug injection
An intrathecal catheter (PE-10 tube) was inserted through the gap between the L4 and L5 vertebrae and extended to the subarachniod space of the lumbar enlargement (L4 and L5 segments) under Chloral Hydrate (300 mg/kg, intraperitoneal (i.p.)) anesthesia. The catheter was filled with sterile NS (approximately 4 μl), and the outer end was plugged. The cannulated rats were allowed to recover for 3–4 days. Rats that showed any neurological deficits resulting from the surgical procedure were excluded from the experiment.
Minocycline hydrochloride (20, or 100 μg in 10 μl; Sigma), a semisynthetic second-generation tetracycline that has emerged as a potent inhibitor of microglial activation without direct effects on astrocytes, oligodendroglia, and neurons (Amin et al., 1996; Tikka, 2001), was freshly dissolved daily in NS. Drug or vehicle (NS) was injected over a period of 1 min via the catheter at a volume of 10 μl, followed by 5 μl NS for flushing. The location of the distal end of the intrathecal (i.t.) catheter was verified at the end of each experiment by the injection of pontamine sky blue via the i.t. catheter.
2.4 Electroacupuncture (EA) treatment
Rats were loosely immobilized in a special made restrainer with the head, hind legs and tail protruding. A pair of stainless steel pins of 0.34 mm diameter were inserted with a depth of 5 mm into the left (ipsilateral to CFA-injected joint) “Huantiao” (GB30, located the lateral 1/3 and medial 2/3 of the distance between the the sacral hiatus and the greater trochanter of femur), and “Yanglingquan” (GB34, located in the depression anterior and inferior to the fibula capitulum) acupoints. The two pins were connected with the output terminals of the H.A.N.S. Acupoint Stimulator (LH-202H, Huawei Co., Ltd., Beijing, China). The electroacupuncture parameters were set as follows: square wave current output (pulse width: 0.2 ms); intensities ranging from 1-2-3 mA (each intensity for 10 min, totaling 30 min); at a 100 Hz and 2 Hz alternating frequencies (automatically shifting between 100 Hz and 2 Hz stimulation for three seconds each) (Zhang et al., 2002). Sham EA control animals received needle insertion into GB30 and GB34 similar to EA treatment, but without electrical stimulation.
2.5 Behavioral testsing
von Frey test for mechanical allodynia
The hindpaw withdrawal threshold (PWT) was determined using a calibrated series of von Frey hairs (Stoelting, IL, USA) ranging from 1 to 26 g. Animals were placed individually into Plexiglas chambers with customized platform that contains 1.5 mm diameter holes in a 5 mm grid of perpendicular rows throughout the entire area of the platform (Pitcher et al., 1999). The protocol used in this study was a variation of that described by Takaishi et al., (1996). After acclimation to the test chambers, a series of 9 calibrated von Frey hairs were applied to the central region of the plantar surface of one hind paw in ascending order (1, 1.4, 2, 4, 6, 8, 10, 15, and 26 g). A particular hair was applied until buckling of the hair occurred. This was maintained for approximately 2 s. The hair was applied only when the rat was stationary and standing on all four paws. A withdrawal response was considered valid only if the hind paw was completely removed from the customized platform. Each hair was applied 5 times at 5 s intervals. If withdrawal responses did not occur more than twice during five applications of a particular hair, the next ascending hair in the series was applied in a similar manner. Once the hindpaw was withdrawal from a particular hair three out of the five consecutive applications, the rat was considered responsive to that hair. The PWT was defined as the lowest hair force in grams that produced at least three withdrawal responses in five tests. After the threshold was determined for one hindpaw, the same testing procedure was repeated on the other hindpaw at 5-min interval. Second and third testing trials were run for both hindpaws, respectively. If the withdrawal threshold in the second or third trial did not match the withdrawal threshold of the previous testing trial (s) in a given hindpaw, the next large hair in the series was tested. This was done until the withdrawal threshold in the three successive trials matched.
Hargreaves’ test for thermal hyperalgesia
After acclimation to the test chambers, thermal hyperalgesia was assessed by measuring the latency of paw withdrawal in response to a radiant heat source. Rats were placed individually into Plexiglas chambers on an elevated glass platform, under which a radiant heat source (model 336 combination unit, IITC/life Science Instruments, Woodland Hill, CA, USA) was applied to the glabrous surface of the paw through the glass plate. The heat source was turned off when the rat lifted the foot, allowing the measurement of time from onset of radiant heat application to withdrawal of the rat’s hindpaw. This time was defined as the hindpaw withdrawal latency (PWL). The heat was maintained at a constant intensity, which produced a stable PWL of approximately 10–12 s in the absence of arthritis. A 20 s cut-off was used to prevent tissue damage in the absence of a response. Both hindpaws were tested for three trials at each time period with 10 min intervals between each trial. The average of the three trials was then determined.
2.6 Immunohistochemistry
After defined survival times, rats were given an overdose of urethane (2 g/kg, i.p.) and perfused intracardially with saline followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The L4–5 segments of spinal cord were then removed, post-fixed in the same fixative for 4 h at 4 °C, and immersed from 10 – 30 % gradient sucrose in PB for 24–48 hrs at 4 °C for cryoprotection. Transverse spinal sections (free-floating, 30 μm) were cut in a cryostatand processed for immunofluorescence. All the sections were blocked with 10 % normal donkey serum in 0.01 M phosphate buffered saline (PBS, pH 7.4) with 0.3 % Triton-X-100 for 1 h at room temperature (RT) and incubated over night at 4 °C with, mouse anti-OX-42 (1:3000, Serotec) or mouse anti-glial fibrillary acidic protein (GFAP, 1: 1000, Sigma) primary antibody. The sections were then incubated for 120 min at 4°C with fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (1:200, Jackson Immunolab). Omission of primary antibody served as negative control. The stained sections were examined with a Leica fluorescence microscope, and images were captured with a CCD spot camera. Because the morphology of astrocytes and microglia is complex and the immunoreactive staining includes both cell bodies and their processes, cell counts may not sufficiently quantify activation. Therefore, the integrated densities (pixelwise integration of density) of OX-42-IR and GFAP-IR within the spinal dorsal horn including laminae I–VI were measured with the Leica Qwin 500 image analysis system (Germany) at six randomly selected sections for each animal. The relative density of images was determined by subtracting the background density (the dorsal funiculus of the white matter) in each image (Zhang et al., 2005).
2.7 RT-PCR analysis
After defined survival times, rats were killed by overdose of urethane. The lumbar spinal cord from naive, MA, and EA-treated MA rats were rapidly removed and frozen in liquid nitrogen, then stored at −70 °C until further processing. Total RNA was extracted by the Trizol reagent (Invitrogen, CA, USA) according manufacturer’s protocol. Extracted RNA was treated with DNase I (DNA-free, Ambion, TX, USA) to remove genomic DNA. A 2 μg sample of total RNA was used for cDNA synthesis with random decamers using the Ambion RETROscript reverse transcription kit according to the manufacturer’s instructions. Negative control reactions were run without RNA to test for contamination of reagents. Polymerase chain reaction (PCR) amplification was performed with 1 U of Taq DNA polymerase (Invitrogen), 2.5 mM dNTP mix and 1 μM of each primer, using 1μl of the RT reaction product as template. PCR reaction conditions involved denaturation at 94°C for 45 s, annealing at 58°C for 45 s, and extension at 72°C for 1 min, with an initial denaturation at 94 °C for 10 min and a final extension step at 72°C for 10 min. Appropriate number of PCR cycles for each specific cDNA was determined in the linear range of cycles, and the linearity of the RT-PCR assays was ascertained with respect to the amount of cDNA products (Ito, et al., 1998). Thirty-five cycles were run for IL-1β and IL-6, 33 cycles for TNFα, and 26 cycles for β-actin. After amplification, the products were electrophoresed in 2 % agarose gel, visualized by ethidium bromide staining and scanned with ultraviolet transilluminator (GDS 8000, Gene Tools from Syngene Software, UK). Bands were analyzed with Quantity one (Bio-Rad). Relative levels of target fragment were normalized against β-actin (internal control), and data are presented ration of the signal intensity of examined gene vs. that of β-actin. The sequences of primers were as follows: IL-1β forward: 5′CAGGATGAGGACATGAG-3′, reverse: 5′-CTCTGCAGACTCAAACTCCAC-3′; IL-6 forward: 5′-GACAAAGCCAGAGTCCTTCAGAGAG-3′, reverse: 5′-CTAGGTTTGCCGAGTA- GATCCTC-3′; TNFα forward: 5′-ATGAGCACAGAAAGCATGATC-3′, reverse: 5′- TACAGGCTTGTCACTCGAATT-3′; β-actin forward: 5′-CACCATGTACCCTGGCATTG-3′, reverse: 5′-TAACGCAACTAAGTCATAGT-3′ (Shanghai Institute of Biochemistry, China).
2.8 Statistical analysis
Data were expressed as mean ± S.E.M. Both Pre-MA baseline and pre-minocycline treatment measures were analyzed by one-way analysis of variance (ANOVA) for von Frey and Hargreaves tests. Post-drug time course measures for hyperalgesia and allodynia were analyzed by two-way ANOVA (treatment × time) followed by Newman-Keuls post-hoc test. Immunohistochemical analysis was performed by student’s t-test when comparing two groups, or one-way ANOVA followed by Dunnett multiple comparisons when comparing more than two groups. P<0.05 was considered statistically significant.
2.9 Experimental design
Effects of MA on spinal microglial activation
After baseline behavioral assessments, rats received an intra-articular injection of 50 μl CFA (day 0). On day 1, 3, and 10 after CFA, rats were perfused with fixative and the lumbar spinal cords were removed for immunohistochemistry analysis. Sham MA rats received an intra-articular injection of sterile NS (50 μl) and were killed at day 3 after NS injection. In order to assess the development of allodynia and hyperalgesia, behavioral testing was performed immediately before sacrifice.
Effects of EA, i.t. minocycline or minocycline together with EA on MA-induced behavioral hypersensitivity and spinal microglial activation
After baseline behavioral assessments, rats received an intra-articular injection of 50 μl CFA (day 0). Repeated EA, i.t. injection of minocycline (20, or 100 μg)/vehicle (NS), or minocycline together with EA were carried out once daily for 5 days after behavioral testing, with the first application 30 min before intra-articular injection of CFA (preemptive treatment). Behaviors were reassessed from day 1 to 10. This administration schedule was based on the finding that microglial activation occurred within one day, and peaked at day 3 post-MA. As a combination of minocycline and EA was given, rats received i.t. minocycline 30 min prior to EA stimulation, when minocycline was effective according to the previous study (Ledeboer et al., 2005). Upon completion of testing on day 10, rats were perfused for immunohistochemistry analysis. Some additional rats were rapidly sacrificed at above time points and the lumbar spinal cord tissues were collected for RT-PCR analysis.
Effects of i.t. minocycline on existing behavioral hypersensitivity
After baseline behavioral assessments, rats received an intra-articular injection of CFA (day 0). Behaviors were reassessed on alternating days to confirm development of behavioral hypersensitivity. Directly after testing on day 3, rats received repeated i.t. injections of minocycline (100 μl) once daily for 5 consecutive days (post-MA treatment). Behaviors were then assessed every other day to day 10 post-MA.
All the testing (including behavior, western blot, immunohistochemistry, and RT-PCR) was performed blinded.
3. Results
3.1 Time course of MA-induced microglial activation in the spinal cord
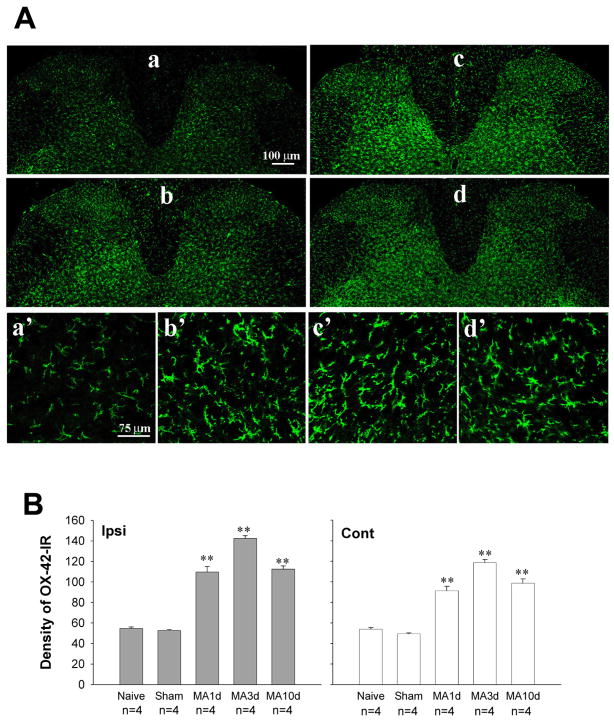
To examine the time course of microglial activation induced by MA, protein level of CD 11b (OX-42) was assessed in the lumbar spinal cord in naive, sham MA, and MA rats. Sporadic moderate microglia labeled by OX-42, with classic ramified morphology, antler-like fine processed and slender cytoplasm, were seen in both the gray and white matters of the spinal cord in naive and sham MA rats (Fig. 1Aa, a′). Following intra-articular injection of CFA, a robust microglial activation was observed across all time points examined with maximum at 3 days post-MA (Fig. 1A, B). Activated microglia exhibited large cell bodies and short or thick processes (Fig. 1Ab,b′-d,d′). The mean density of OX-42-IR on both sides of the spinal dorsal horn was significantly greater in MA rats than that obtained in either sham MA or naive rats (Fig. 1B) (ipsilateral: F4, 15 = 158.6, p<0.01; contralateral: F4, 15 = 91.54, p<0.01).
Fig. 1.
Time course of OX-42 (microglial marker) expression on the lumbar spinal cord following monoarthritis (MA). (A) Photomicrographs showing OX-42-immunoreactivity (OX-42-IR) on both sides of the spinal dorsal horn on day 3 post-sham MA (a, a′), and day 1(b, b′), day 3 (c, c′), day 10 (d, d′) post-MA. (Aa–d) 10 ×; (Aa′–d′) 40 ×. (B) Quantification of OX-42-IR showing significant up-regulation in all time-point examined. ** p<0.01 vs. sham MA.
3.2 Time course of MA-induced behavioral hypersensitivity
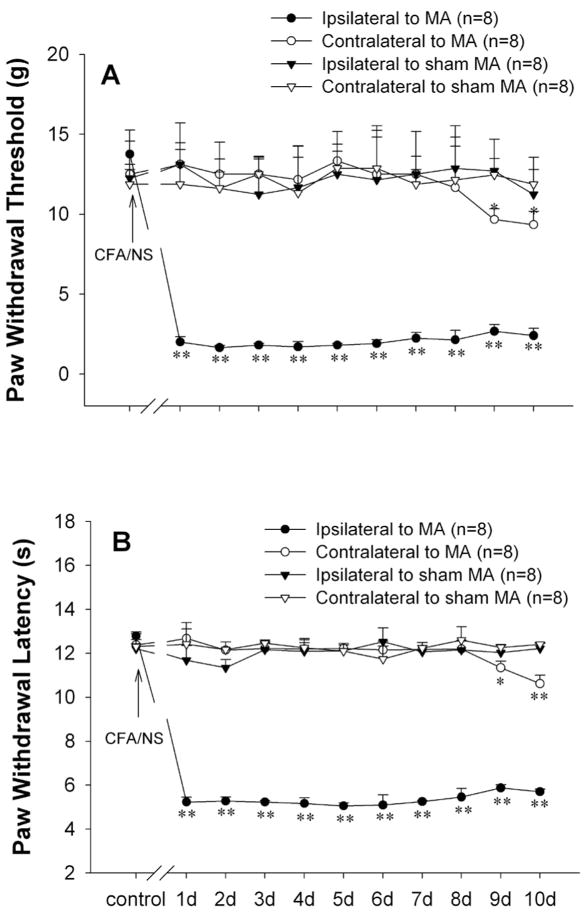
Baseline measures of PWT to von Frey hairs and PWL to radiant heat stimulation on both hindpaws did not differ between MA and sham MA groups prior to intra-articular injection. Following unilateral intra-articular injection of CFA, strong mechanical allodynia and thermal hyperalgesia developed within one day and persisted over 10 days in the ipsilateral hindpaw (One-way RM ANOVA, PWT: F8, 10, 71 = 78.72, p<0.01; PWL: F 8, 10, 71 = 213.74, p<0.01). The PWTs and the PWLs in contralateral hindpaws remained stable for 7 days after CFA injection. From day 9, contralateral hindpaws showed significant decrease in the PWTs and PWLs compared to baseline (PWT: F8, 10, 71 = 5.84, p<0.01; PWL: F8, 10, 71 = 6.978, p<0.01) (Fig. 2A, B).
Fig. 2.
Unilateral intra-articular injection of complete Freund’s adjuvant (CFA) induced significant mechanical allodynia (A) and thermal hyperalgesia (B) initially in the ipsilateral and subsequently in the contralateral hindpaw. **p<0.01 vs. before CFA injection.
3.3 Blockade of repeated i.t. minocycline on the development of behavioral hypersensitivity
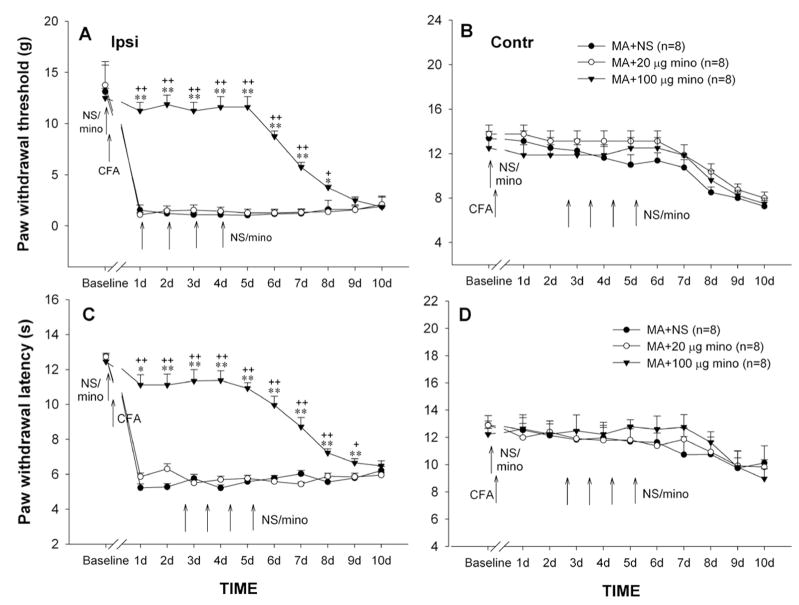
Based on the finding of an early microglial activation after MA, repeated minocycline treatments were earlier applied to examine the role of microglia in the development of MA-induced behavioral hypersensitivity. Before i.t. and intra-articular injection, all groups exhibited comparable baseline responses on the von Frey and Hargeaves’ tests on both hindpaws. I.t. injections of minocycline (100 μg) once daily for 5 days with the first injection 30 min before intra-articular CFA (preemptive treatment) completely blocked the development of mechanical allodynia and thermal hyperalgesia on days 1–5. Four days after cessation of i.t. injection, ipsilateral allodynia and hyperalgesia were still reliably suppressed (Fig. 3A, C). Two-way ANOVA analysis revealed significant effect of minocycline (100 μg) treatment (PWT: F1, 182 = 628.25, p<0.01; PWL: F1, 182 = 613.92, p<0.01) and interaction between minocycline treatment and time (PWT: F12, 182 = 28.58, p<0.01; PWL: F12, 182 = 26.104, p<0.01). MA-induced mild mechanical allodynia and thermal hyperalgesia on the contralateral hindpaw were not obviously affected by daily i.t. 100 μg minocycline (Fig. 3B, D). Neither low dose minocycline (20 μg) nor NS altered PWTs and PWLs of both hindpaws in MA rats (Fig. 3).
Fig. 3.
Effects of repeated intrathecal (i.t.) injections of minocycline (mino) at different doses on PWT (A & B) and PWL (C & D) of hindpaws ipsilateral (Ipsi, A & C) and contralateral (Cont, B & D) to MA. Repeated i.t. normal saline (NS) or minocycline were given once daily for 5 days with the first injection 30 min before MA (preemptive treatment), and behavioral testes were performed immediately before i.t. injection every day. * p<0.05, ** p<0.01 vs. NS; + p<0.05, ++ p<0.01 vs. 20 μg minocycline.
3.4 Inefficiency of repeated i.t. minocycline on existing behavioral hypersensitivity
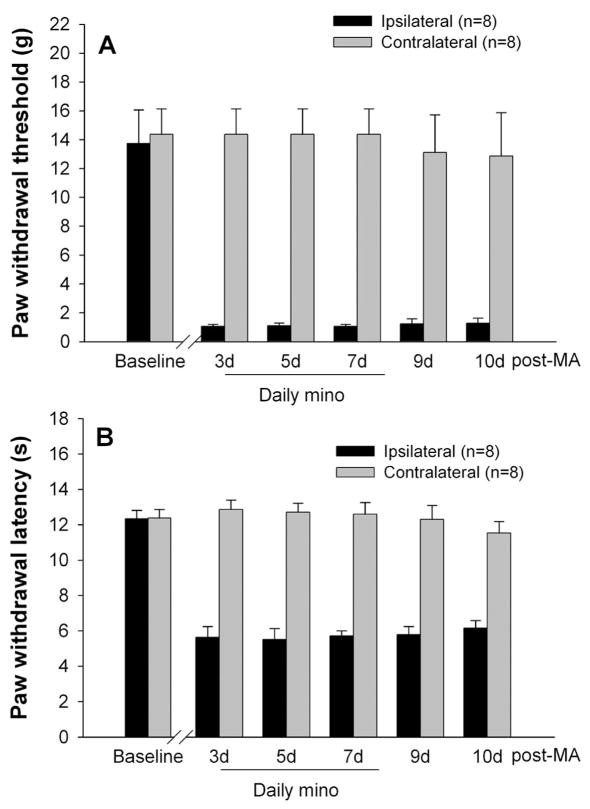
To examine whether minocycline reverses established mechanical allodynia and thermal hyperalgesia in MA rats, repeated i.t. injections of minocycline (100 μg) were given once daily for 5 successive days starting on day 3 after MA (post-MA treatment). As shown in Fig. 4, MA-induced allodynia and hyperalgesia were not affected by repeated post-MA treatment of minocycline (100 μg). No detectable changes in PWTs and PWLs were found on the hindpaw contralateral to MA (Fig. 4).
Fig. 4.
Effects of repeated intrathecal (i.t.) injections of 100 μg minocycline (mino) on PWT (A) and PWL (B) of both hindpaws. Repeated i.t. minocycline were administered once daily for 5 days starting on day 3 after monoarthritis (MA) (post-MA treatment), and behavioral tests were performed immediately before i.t. injections.
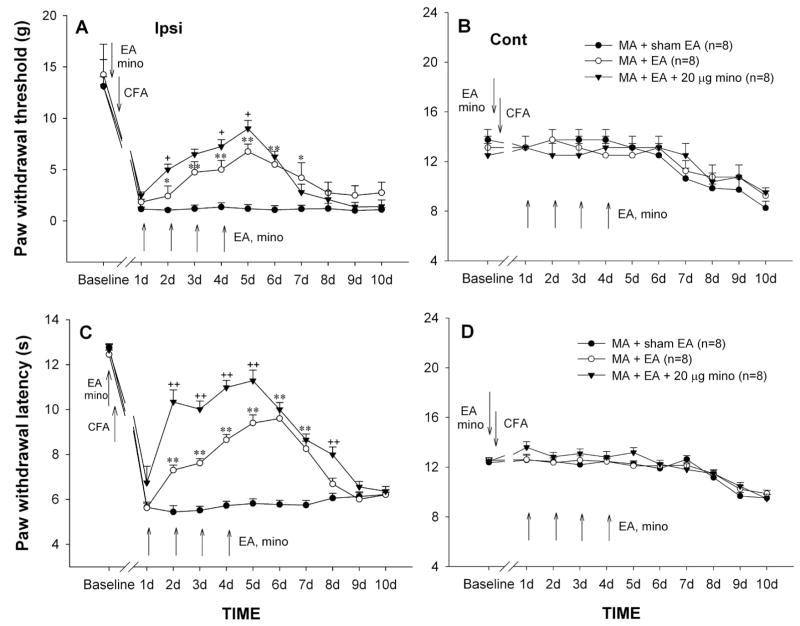
3.5 Cumulative effect of repeated EA on MA-induced behavioral hypersensitivity
As indicated above, the mechanical allodynia and thermal hyperalgesia on the ipsilateral hindpaw stably occurred in one day after CFA injection. EA delivered to the ipsilateral hindlimb at the “GB 30” and “GB 34” acupoints was repeatedly given once daily for 5 days with the first application 30 min before intra-articular CFA. Single application of EA before MA failed to prevent the development of mechanical allodynia and thermal hyperalgesia that reliably occurred on day 1 post-MA (Fig. 5). Following subsequent daily applications, MA-induced ipsilateral allodynia and hyperalgesia were markedly decreased from day 2 and lasting 3–4 days after cessation of EA (Fig. 5A, C). Two-way ANOVA analysis revealed a significant effect of EA treatment (PWTs: F1,154 = 286.50, p<0.01; PWLs: F1,154 = 210.816, p<0.01) and interaction between EA treatment and time (PWTs: F10,154 = 9.26, p<0.05; PWLs: F10,154 = 19.855, p<0.01). Neither the ipsilateral nor the contralateral PWTs and PWLs were influenced by repeatedly inserting the stimulation electrodes into the acupoints (sham EA) in MA rats (Fig. 5A–D).
Fig. 5.
Effects of repeated sham electroacupuncture (EA), EA, or EA plus i.t. minocycline (mino, 20 μg) on PWT (A & B) and PWL (C & D) of the hindpaws ipsilateral (Ipsi, A & C) and contralateral (Cont, B & D) to monoarthritis (MA). Sham EA, EA, and EA plus i.t. minocycline were given once daily for 5 days with the first injection 30 min before MA, and behavioral tests were performed immediately before EA or EA plus i.t. minocycline every day. * p<0.05, ** p<0.01 vs. sham EA; + p<0.05, ++ p<0.01 vs. EA alone.
3.6 Potentiation of minocycline on anti-allodynic and anti-hyperalgesic effects of EA
Although low dose of minocycline (20 μg) failed to prevent MA-induced mechanical allodynia and thermal hyperalgesia, co-applications of EA and minocycline significantly enhanced inhibitory effect of EA on behavioral hypersensitivity. Compared to EA or 20 μg minocycline alone group, EA together with 20 μg minocycline group exhibited a significant increase in PWTs and PWLs of the handpaw ipsilateral to MA (PWT: F2, 210 = 254.18, p<0.01; PWL: F2, 210 = 283.95, p<0.01) (Fig. 3 & 5A, C). Repeated co-application of EA and minocycline did not obviously alter the contralateral PWTs and PWLs (Fig. 5B, D).
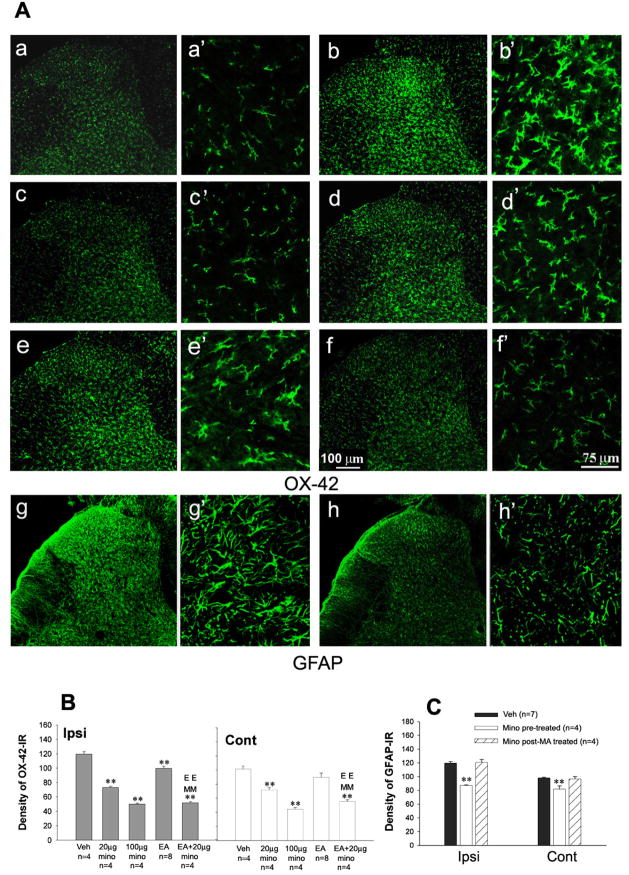
3.7 Inhibition of i.t. minocycline, EA, or EA together with minocycline on MA-induced spinal microglial activation
Correlated to behavioral responses, repeated i.t. injections of minocycline (20, or 100 μg) once daily for 5 days with the first administration 30 min prior intra-articular CFA dose-dependently reduced OX-42-IR on the both sides of spinal dorsal horn by day 10 post-MA (Ipsilateral: F2, 9 = 235.76, p<0.01; contralateral: F2, 9 = 74.44, p<0.01) (Fig. 6Ac,c′, d,d′, B). In addition, the effects of minocycline (100 μg) on MA-induced spinal astrocytic activation were assessed. GFAP-IR on the both sides of spinal dorsal horn was increased by intra-articular CFA at the same time-point. Only preemptive treatment of minocycline suppressed GFAP, whereas post-MA treatment failed to effect of GFAP expression in MA rats. (Ipsilateral: F2, 12 = 40.132, p<0.01; contralateral: F2, 12 = 11.892, p<0.01) (Fig. 6Ag,g′, h,h′, C).
Fig. 6.
Effects of repeated intrathecal (i.t.) injections of minocycline (mino), electroacupuncture (EA), co-application of EA and 20 μg minocycline on increase in OX-42-IR (and GFAP-IR) expression on the spinal dorsal horn induced by monoarthritis (MA). (A) Photomicrographs showing OX-42-IR (Aa–f) and GFAP-IR (Ag & Ah) on the ipsilateral spinal dorsal horn by 10 days post-MA. (Aa, a′) Naïve; (Ab,b′ & Ag, g′) Vehicle (Veh); (Ac,c′ & h,h′) 100 μg minocycline; (Ad, d′) 20 μg minocycline; (Ae,e′) EA; (Af, f′) 20 μg minocycline plus EA. (Aa–h) 10 ×; (Aa′–h′) 40 ×. (B) Quantification of OX-42-IR showing that repeated i.t. minocycline and EA suppressed OX-42-IR expression on the spinal dorsal horn. Repeated EA, i.t. minocycline and minocycline plus EA were given once daily for 5 days with the first administration 30 min before MA on day 0 (days 0–4). (C) Quantification of GFAP-IR showing that repeated i.t. minocycline (pre-treatment) suppressed GFAP-IR expression on the spinal dorsal horn. Rats were perfused for immunohistochemistry analysis on day 10 after behavioral tests. * p<0.05, ** p<0.01 vs. vehicle control; EE p<0.01 vs. EA alone; MM p<0.01 vs. 20 μg minocycline alone. mino pre-treated, repeated i.t. minocycline was given once daily for 5 days with the first administration 30 min before MA on day 0; mino post-MA treated, repeated i.t. minocycline was administered once daily for 5 days starting on day 3 afterMA.
When repeated EA were given once daily for 5 successive days with the initial application 30 min before intra-articular CFA, MA-induced spinal microglial activation was significantly suppressed by day 10 post-MA (Student’s t-test, Ipsilateral, t= 4.123, p<0.01; Contralateral, t=1.343, p>0.05) (Fig. 6Ae,e′, B).
When a combination of EA with 20 μg minocycline exhibited a robust decrease in OX-42-IR on both sides of the spinal dorsal horn. The mean density of OX-42-IR was markedly less in group with combination of EA and 20 μg minocycline than in group with EA or 20 μg minocycline alone (Ipsilateral: F2, 9 = 112.95, p<0.01; contralateral: F2,9 = 19.34, p<0.01) (Fig. 6Af,f′, B).
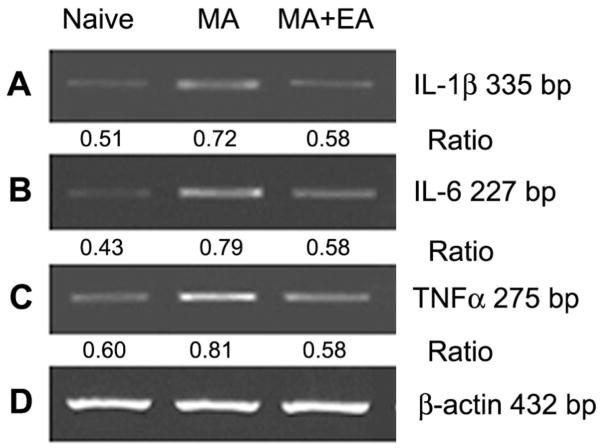
3.8 Suppression of EA on MA-induced up-regulation of IL-1β, IL-6, and TNFα mRNA expression in the spinal cord
To determine functional changes of spinal glia, the levels of IL-1β, IL-6, and TNFα mRNA in the spinal cord were assessed using semi-quantitative RT-PCR. Low, but detectable, levels of IL-1β, IL-6, and TNFα mRNA were presented in the normal rat spinal cord. Intra-articular injection of CFA produced a marked elevation in the levels of IL-1β, IL-6, and TNFα mRNA in the spinal cord by day 10 post-MA. Repeated EA once daily for 5 successive days with the initial application 30 min before MA markedly reduced MA-induced up-regulation of IL-1β, IL-6, and TNFα mRNA levels (Fig. 7).
Fig. 7.
Semiquantitative RT-PCR analysis showing the effects of repeated electroacupuncture (EA) on monoarthritis (MA)-induced increase in IL-1β (A), IL-6 (B), and TNFα mRNA (C) levels of the spinal cord. Repeated EA were applied once daily for 5 days with the first administration 30 min before MA on day 0. Rats were killed for RT-PCR analysis on day 10. β-actin mRNA was used as internal control. Ratio indicates the signal intensity of examined gene vs. that of β-actin.
4. Discussion
Our study provides the first identification that repeated EA significant reduced MA-induced activation of spinal microglia and up-regulation of IL-1β, IL-6, and TNFα mRNA levels on the spinal cord. Suppression of spinal microglial activation with minocycline strongly enhanced inhibition of EA on MA-induced mechanical allodynia and thermal hyperalgesia, suggesting that anti-allodynic and anti-hyperalgesic effects of EA in CFA-induced rat monoarthritic model might be associated with its counter-regulation to spinal microglial activation.
4.1 Microglial activation contributes to the initiation of exaggerated pain in monoarthritic rats
In agreement with the previous studies on various animal models of pathological pain (Sweitzer et al., 2001; Milligan et al., 2003; 2004; Raghavendra et al., 2003; 2004; Ledeboer et al., 2005), the present results showed an early, robust and sustained microglial activation on the spinal cord during the acute (1 day), subacute (3 day), and chronic (10 day) phases of CFA-induced MA, which associated with development of behavioral hypersensitivity. Alternatively, the significant up-regulation of astrocytic GFAP expression in the spinal cord was observed 3 days after MA (Sun et al., 2006a). Similar observations also reported after injury to peripheral nerves or spinal cord (Svensson et al., 1993; Popovich et al., 1997; Raghavendra et al., 2003; Tanga et al., 2004). These suggest that microglia might be the first responding glial cells in the spinal cord, and products released from activated microglia in turn lead to astrocytic activation. Our recent study showed that the expression of CX3CR1, a fractalkine receptor on microglia, in the spinal cord was up-regulated within one day after MA, which paralleled the activation of microglia. Blockade of CX3CR1 prevented MA-induced microglial and astrocytic activation (Sun et al., 2006a). Furthermore, the present data demonstrated that repeated i.t. minocycline, a microglial inhibitor, with the first injection 30 min before intra-articular CFA (preemptive treatment) not only blocked microglial activation, but also reduced GFAP expression in MA rats. Minocycline has been reported to inhibit microglial activation without direct effects on astrocytes or neurons (Yrjanheikki et al., 1999). Thus, the inhibition of minocycline on astrocytic activation might be mediated by blocking microglial activation, which is supported by Reghavendra’s study that only preemptive treatment but not postinjury administration of minocycline suppressed GFAP expression on the lumbar spinal cord in spinal nerve transection rats (Reghavendra et al., 2003). Either activated microglia or astrocytes can release proinflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor α (TNFα), and other algesic substances to enhance neuronal transmission of nociception, leading to exaggerated pain (Raghavendra et al., 2003; Wieseler-Frank et al., 2004).
In our previous study, i.t. injection of fluorocitrate, a non-selective glial metabolic inhibitor, significantly suppressed established thermal hyperalgesia and mechanical allodynia 48 hrs after MA (Sun et al., 2006b). Differently, in the present study, preemptive treatment of minocycline completely prevented the development of mechanical allodynia and thermal hyperalgesia, whereas post-MA treatment failed to reverse existing behavioral hypersensitivity. This is consistent with the observations that minocycline prevented, but not reversed allodynia and hyperalgesia induced by spinal nerve transection (Raghavendra et al., 2003), sciatic nerve inflammation and spinal HIV-1 (Ledeboer et al., 2005). Unlike to fluorocitrate that can disrupt both microglial and astrocytic activation, minocycline dose not inhibit activated astrocytes, it thereby failed to reverse the established hyperalgesia and allodynia. Together these data suggest that spinal microglial activation contributes to the initiation of MA-induced pain facilitation.
4.2 Combination of EA with minocycline enhances anti-hyperalgesia and anti-allodynia in monoarthritic rats
Acupuncture and EA as complementary and alternative medicines have been accepted worldwide mainly for the treatment of acute and chronic pain (Ezzo et al., 2001; Han, 2003; Berman et al., 2004). Several processes were proposed to explain effects of EA on pain. However, little is known about relationship between EA and glia. The present study demonstrated for the first time that repeated EA significantly suppressed MA-induced activation of spinal microglia, and EA combined with a sub-effective dose of minocycline (20 μg) enhanced EA-produced anti-allodynic and anti-hyperalgesic effects.
As mentioned above, the release of proinflammatory cytokines and other algesic substances from activated microglia in the spinal cord contributes to the development of exaggerated pain associated with peripheral inflammation. Minocycline has been reported to inhibit the synthesis and/or release of the proinflammatory cytokines, such as IL-1β and TNFα, and/or affect their metabolism, indicating the anti-allodynic and anti-hyperalgesic actions of minocycline might be attributed to its ability to suppress central proinflammatory immune responses (Raghavendra et al., 2003; Ledeboer et al., 2005). Studies on the mechanisms of EA revealed that endogenous opioid peptides in the CNS play an essential role in mediating analgesia effects of EA. Both μ and δ opioid receptor antagonists blocked the anti-hyperalgesia of 10 and 100 Hz EA in a CFA-induced peripheral inflammatory pain rat model, suggesting that the anti-hyperalgesia produced by high and low frequency EA is the result of the activation of endorphin/endomorphin (for μ receptor) and enkephalin (for δ receptor) systems at the spinal level during persistent pain (Zhang et al., 2004). Consistently, the analgesic effects of μ and δ opioid receptor agonists were potentiated during persistent inflammation (Hylden et al., 1991; Przewlocka et al., 1992; Hurley et al., 2000). Also, μ and κ opioid receptor mRNAs expression is increased in laminae I–II of the spinal dorsal horn in the arthritic rats (Maekawa et al., 1995). All three classes of opioid receptors are located on primary nociceptive afferent terminals and on the dendrites of pain sensitive neurons in the dorsal horn of the spinal cord. Presynaptic activation of opioid receptors on the primary afferent terminals results in a direct reduction of excitatory transmitters release (such as excitatory amino acids and substance P, and CGRP, etc.) (Dickenson and Sullivan, 1986; Malmberg and Yaksh, 1995; Ulett et al., 1998; Cheunsuang et al., 2002). It has been reported that spinal glia express an array receptors including NMDA, AMPA/KA, NK-1, ATP, and CGRP receptors (Reddington et al., 1995; Araque et al., 1999; Svensson et al., 2003; Tsuda et al., 2003; Marriott, 2004). All the neurotransmitters/neuromodulators can activate microglia and astrocytes, leading to pain facilitation (Milligan et al., 2003). Thus, minocycline and EA synergistically block activation of spinal microglia and subsequent astrocytes, reduce release of proinflammatory cytokines and other pain enhancing substances in the spinal cord, and disrupt neuron- to- glia-to-neuron excitatory circuit, thereby leading to a reinforcing inhibitory action on MA-induced thermal hyperalgesia and mechanical allodynia.
Acknowledgments
The authors wish to thanks professor R.Y. Wei (UCLA) and Dr. R.R. Ji (Harvard Medical School) for their critical reading of the manuscript and helpful criticism. This work was supported by National Natural Science Fund of China (NSFC) (30570594 and 30425022), National Basic Research Program of China (2006CB500807), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT), Research Fund for Doctoral Program of Higher Education (20050246017), and National Institute of Health Grants FIRCA (TW 07180).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramso SB. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA. 1996;93:14014–1419. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2004;141:901–910. doi: 10.7326/0003-4819-141-12-200412210-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cheunsuang O, Maxwell D, Morris R. Spinal lamina I neurons that express neurokinin I receptors: II Electrophysiological characteristics, responses to primary afferent stimulation and effects of a selective mu-opioid receptor agonist. Neurosci. 2002;111:423–434. doi: 10.1016/s0306-4522(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 5.Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- 6.Colpaert FC. Evidence that adjuvant arthritis in the rat is associated with chronic pain. Pain. 1987;28:201–222. doi: 10.1016/0304-3959(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 7.Curatolo M, Bogduk N. Pharmacologic pain treatment of musculoskeletal disorders: current perspectives and future prospects. Clin J Pain. 2001;17:25–32. doi: 10.1097/00002508-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Deleo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 9.Deleo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 10.Dickenson AH, Sullivan AF. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurons in the rat dorsal horn. Pain. 1986;24:211–222. doi: 10.1016/0304-3959(86)90044-8. [DOI] [PubMed] [Google Scholar]
- 11.Ezzo J, Hadhazy V, Birch S. Acupuncture for osteoarthritis of the knee: a systematic review. Arthritis Rheum. 2001;44:819–825. doi: 10.1002/1529-0131(200104)44:4<819::AID-ANR138>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends in Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 13.Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Yamamoto M, Li M, Doyu M, Tanaka F, Mutch T, Mitsuma T, Sobue G. Differential temporal expression of mRNAs for ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), interleukin-6 (IL-6), and their receptors (CNTFR alpha, LIFR beta, IL-6R alpha and gp130) in injured peripheral nerves. Brain Res. 1998;793:321–327. doi: 10.1016/s0006-8993(98)00242-x. [DOI] [PubMed] [Google Scholar]
- 16.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 17.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Luo F. A study on the cumulative effect of repeated electroacupuncture on chronic pain. Prog Physiol Sci(Chinese) 1996;27:241–244. [PubMed] [Google Scholar]
- 19.Maekawa K, Minami M, Masuda T, Satoh M. Expression of μ- and κ-, but not δ-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. Pain. 1995;64:365–371. doi: 10.1016/0304-3959(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 20.Malmberg AB, Yaksh TL. The effect of morphine on formalin-evoked behavior and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in conscious rats. Br J Pharmacol. 1995;114:1069–1075. doi: 10.1111/j.1476-5381.1995.tb13315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marriott I. The role of tachykinins in central nervous system inflammatory responses. Front Biosci. 2004;9:2153–2165. doi: 10.2741/1377. [DOI] [PubMed] [Google Scholar]
- 22.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Method. 1999;87:185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 25.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Przewlocka B, Dziodzicka M, Lason W, Przewlocki R. Differential effects of opioid receptor agonists on nociception and cAMP level in the spinal cord of monoarthritic rats. Life Sci. 1992;50:45–54. doi: 10.1016/0024-3205(92)90196-v. [DOI] [PubMed] [Google Scholar]
- 27.Raghavendra V, Tanga F, Deleo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 28.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 29.Reddington M, Priller J, Treichel J, Haas C, Kreutzberg GW. Astrocytes and microglia as potential targets for calcitonin gene related peptide in the central nervous system. Can J Physiol Pharmacol. 1995;73:1047–1049. doi: 10.1139/y95-148. [DOI] [PubMed] [Google Scholar]
- 30.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 31.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and Carrageenan into the knee joint. Pain. 1998;77:97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 32.Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2006a doi: 10.1016/j.pain.2006.09.035. in press. [DOI] [PubMed] [Google Scholar]
- 33.Sun S, Chen WL, Wang PF, Zhao ZQ, Zhang YQ. Disruption of glial function enhances electroacupuncture analgesia in arthritic rats. Exp Neurol. 2006b;198:294–302. doi: 10.1016/j.expneurol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Sweitzer SM, Schubert P, Deleo JA. Propentofyline, a glial modulating agent, exhibits antiallodynia properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- 35.Svensson M, Eriksson P, Persson JK, Molander C, Arvidsson J, Aldskogius H. The response of central glia to peripheral nerve injury. Brain Res Bull. 1993;30:499–506. doi: 10.1016/0361-9230(93)90284-i. [DOI] [PubMed] [Google Scholar]
- 36.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 37.Takaishi K, Eisele JH, Jr, Carstens E. Behavioral and electrophysiological assessment of hyperalgesia and changes in dorsal horn responses following partial sciatic nerve ligation in rats. Pain. 1996;66:297–306. doi: 10.1016/0304-3959(96)03023-0. [DOI] [PubMed] [Google Scholar]
- 38.Tanga FY, Raghavendra V, Deleo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Tikka T, Fiebich BI, Goldsteins G, Keinanen R, Koistinabo J. Minocycline, a tetracycline derivative, a neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 42.Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiaty. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- 43.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 44.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 45.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Yrjanhelkki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Zhang RX, Lao LX, Wang LB, Liu B, Wang XY, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 49.Zhang YQ, Ji GC, Wu GC, Zhao ZQ. Excitatory amino acid receptor antagonists and electroacupuncture synergistically inhibit Carrageenan-induced behavioral hyperalgesia and spinal Fos expression in rats. Pain. 2002;99:525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]