Abstract
Individual differences in impulsive choice and rearing in differential environments are factors that predict vulnerability to drug abuse. The present study determined if rearing influences impulsive choice, and if d-amphetamine or methylphenidate alters impulsive choice in differentially-reared rats. Male Sprague-Dawley rats were raised from 21 days of age in either an enriched condition (EC) or an isolated condition (IC) and were tested as young adults on an adjusting delay task. In this task, two levers were available and a response on one lever yielded one 45 mg food pellet immediately, whereas a response on the other yielded three pellets after an adjusting delay. The delay was initially set at 6 sec, and it decreased or increased by 1 sec following responses on the immediate or delayed levers, respectively. A mean adjusted delay (MAD) was calculated upon completion of each daily session, and it served as the quantitative measure of impulsivity. Once MADs stabilized, rats were injected with saline, d-amphetamine (0.5, 1.0, or 2.0 mg/kg, s.c.), or methylphenidate (2.5, 5.0, or 10.0 mg/kg, s.c.) 15 min prior to adjusting delay sessions. EC rats had higher baseline MADs (were less impulsive) than IC rats. Additionally, administration of d-amphetamine, but not methylphenidate, dose-dependently increased impulsive choice (decreased MADs) in EC rats. In IC rats, d-amphetamine and methylphenidate dose-dependently decreased impulsivity (increased MADs). These results indicate that rearing environment influences impulsive choice and moderates the effect of psychostimulants on impulsive choice. Specifically, psychostimulants may decrease environment-dependent impulsive choice in individuals with high levels of impulsivity (e.g., those with ADHD), whereas they may increase impulsive choice in individuals with low levels of impulsivity.
Keywords: amphetamine, delay discounting, environmental enrichment, individual differences, impulsive choice, methylphenidate
Introduction
Impulsive choice has been measured in the laboratory using a delay discounting paradigm in which a subject must choose between a small reinforcer delivered immediately and a larger delayed reinforcer. Subjects who consistently choose the smaller immediate reinforcer are said to discount the value of the delayed reinforcer, and it is possible that drug abuse may occur, at least in part, because the beneficial value of drug abstinence is discounted compared to the immediate effects of a drug [21, 48]. Accordingly, compared to nonusers, delayed rewards are discounted to a greater extent in users of opioids [40, 41, 48], alcohol [89], cocaine [17, 34, 40], methamphetamine [37, 56], and cigarettes [4, 11, 36, 55, 58, 72, but see 47]. It is likely that the increased discounting in drug abusers compared with nonabusers arises from a combination of factors, including higher baseline levels of impulsivity in drug abusers, increases in impulsivity due to acute or chronic drug effects, and common genetic and environmental factors that predispose individuals to both drug abuse and impulsive choice.
High levels of impulsive choice may predict vulnerability to drug abuse; however, this concept is difficult to study in humans. In rodents, impulsive choice predicts acquisition of cocaine self-administration [62, 65] and cocaine-primed reinstatement of cocaine-seeking behavior [65]. Impulsive choice also predicts greater alcohol consumption in a two-bottle choice test [70], more self-administered nicotine infusions under a progressive ratio schedule [23], and greater resistance to extinction of nicotine-seeking behavior and cue-induced reinstatement of nicotine-seeking [23]. These preclinical results indicate that individual differences in impulsive choice and drug abuse vulnerability are linked biologically.
Heightened levels of impulsive choice in drug abusers may be attributed to the direct effects of the drug. In humans, acute administration of methylphenidate [67] or d-amphetamine [20] decreases impulsive choice; however, discounting does not change with administration of alcohol [59, 74], diazepam [71], or Δ9-tetrahydrocannabinol [53]. However, since these studies were conducted in individuals with prior drug experience, preclinical research may be better suited to determine the acute effects of drugs on impulsive choice. In rats, some reports have found impulsive choice to be decreased by amphetamine [87, 90, 93], methylphenidate [68, 87], atomoxetine [76], or methamphetamine [73]. However, other reports have found that impulsive choice was not altered by amphetamine [15, 27] or cocaine [46]. Several procedural factors may account for these discrepant findings, including the type of reinforcer offered (i.e., water or food), whether a cue was present during the delay to the larger reinforcer, and differences in baseline level of impulsivity [61].
Although it is known that genetic factors play a role in the relationship between impulsivity and drug abuse vulnerability [2, 14], environmental factors also play a role [54]. Rats reared in an enriched condition (EC) with novel objects and social cohorts self-administer less amphetamine than rats reared in an isolated condition (IC), without objects or social cohorts [7, 32, 82]. EC rats also have lower break points under a PR schedule maintained by a low dose of amphetamine compared to IC rats [32]. Compared to IC rats, EC rats also show a more rapid rate of extinction of responding maintained by amphetamine infusions, and show less reinstatement of amphetamine-seeking responses following an amphetamine priming injection [82].
To the extent that impulsive behavior and drug abuse vulnerability are linked, it is possible that the decreases in sensitivity to the reinforcing properties of amphetamine in EC rats compared to their IC counterparts may reflect an enrichment-induced reduction in impulsivity. Consistent with this, EC rats show superior response inhibition as measured by differential reinforcement of low rates of responding schedules [60] and show fewer premature anticipatory nose pokes before onset of a cue paired with sucrose availability in a sucrose-reinforced nose-poke task [94]. In contrast, using a delay discounting task, one report found that EC rats were more impulsive than IC rats [35]. In this latter study, however, EC rats initially showed a stronger preference for the small reinforcer over the large reinforcer compared to IC rats, even when there was no delay to the delivery of the large reinforcer. This initial preference for the small (vs large) reinforcer in EC rats makes it difficult to interpret the results obtained when the delay to the larger reinforcer increased. In addition, since this latter study first tested rats in a go/no-go task prior to the delay discounting task, the prolonged handling and testing of IC rats may have mitigated the isolation experience, as the effects of environmental enrichment are reversible [10, 77]. Thus, in the current study, we tested EC and IC rats in a delay discounting task that was designed to be acquired rapidly. Rather than using a procedure in which the delay to the larger reinforcer is increased incrementally across the session regardless of performance [35], we used an adjusting delay procedure in which the delay to the larger reinforcer is increased or decreased across the session depending on the subjects' performance. Moreover, since the effects of amphetamine and other stimulant drugs on impulsive choice in a delay discounting task have not been assessed in EC and IC rats, the present study also tested the effects of amphetamine and methylphenidate on impulsive choice in differentially-reared rats.
Materials and Methods
Subjects
Eighteen 21-day old male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN) were used as subjects. Animals initially had ad libitum access to food and water in their home cage, but they were food restricted to 18 g/day once operant sessions began on postnatal day 56. Thus, rats were differentially reared for 35 days prior to the onset of behavioral testing. Immediately after daily operant sessions, IC rats were fed in their home cages, and EC rats were fed while housed in individual plastic holding cages in order to assure that each rat received the same food restriction. Both groups were allowed approximately 1 h to consume their daily allotment of food, at which point any remaining food was removed from cages (this occurred infrequently) and EC rats were returned to their home cage. Rats were housed in rooms that were maintained on a light/dark cycle (lights on from 6:00 a.m. to 8:00 p.m.). Procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee, and recommended principles of animal care were followed [57].
Environmental Conditions
Rats were divided randomly into either the enriched (N= 10) or isolated (N= 8) condition at the beginning of each experiment. EC rats were housed together in a stainless steel cage (60 × 120 × 45 cm) with 14 plastic objects (e.g., commercially-available toys, plastic containers, etc) placed randomly throughout the cage. Each day, 7 objects were replaced with new objects and the remaining objects were rearranged into a novel configuration. IC rats were individually-housed in hanging stainless steel cages (17 × 24 × 20 cm) with wire mesh floors and front panels and solid metal side, back, and top walls.
Apparatus
Standard operant conditioning chambers (28 × 21 × 21 cm; ENV-001; MED Associates, St. Albans, VT) that had alternating aluminum and Plexiglas walls and a metal rod floor were located inside sound-attenuating chambers (ENV-018M; MED Associates, St. Albans, VT). A recessed food tray (5 × 4.2 cm) was located 2 cm above the floor in the center of one of the aluminum walls, and a retractable response lever was located 6 cm above the floor on each side of the food tray. A white stimulus light (28 v; 3 cm diameter) was mounted 6 cm above each lever. Responses were recorded and programmed consequences were controlled by a personal computer equipped with Med-PC software (Med Associates, St. Albans, VT).
Procedure
Adjusting delay sessions began at 08:00 daily, and sessions ended upon completion of 60 trials or after 2 h, whichever occurred first. As described previously [62], each session was divided into 15 4-trial blocks and began with illumination of the house light. The first and second trials of each block were forced-choice left and forced-choice right trials, and the order of these two trials alternated randomly within- and between-sessions. Forced-choice trials were signaled by extension of the response-appropriate lever and the illumination of the stimulus light above it. On forced choice trials, levers were retracted immediately following a lever press response. The third and fourth trials in each block were free-choice trials, and they were signaled by illumination of the stimulus lights above both levers. Levers were retracted at the end of each 4-trial block. A single response on one lever yielded one grain-based 45 mg pellet (PJA1-0045, Research Diets Inc., New Brunswick, NJ) delivered immediately, and a response on the other lever yielded three pellets delivered after an adjusting delay. The lever associated with the immediate or delayed reinforcer alternated daily.
Following each lever press, an inter-trial interval (ITI) was imposed so that each trial would last 60 s. The stimulus and house lights were turned off and responses on the levers had no programmed consequences during the ITI. The initial delay to the delivery of the larger reinforcer was 6 s, and subsequently the delay changed depending on responding during the free-choice trials. A response on the immediately-reinforced lever resulted in a 1 s decrease in the delay, and a response on the delayed reinforcement lever resulted in a 1 s increase in the delay (however, the lower and upper limits on the delay were 0 and 45 s, respectively). The delay was adjusted only after the third and fourth trials in each block (i.e., the free-choice trials). During the delay, the stimulus lights above each lever were turned off; however, the house light remained on until delivery of the 3 food pellets. The final delay for each session was used as the initial delay in the next session. A mean adjusted delay (MAD) was calculated at the end of each session by averaging all adjusting delays on the free-choice trials, and this procedure was repeated until the MAD stabilized (varying by less than 5 s across 5 days with no consistently increasing or decreasing trends). MAD values were used as a quantitative measurement of impulsive choice, with lower MADs indicating higher levels of impulsive choice.
After meeting stability criterion, rats were injected with saline, d-amphetamine (0.5, 1.0, 2.0 mg/kg, s.c.), or methylphenidate (2.5, 5.0, 10.0 mg/kg, s.c.) 15 min prior to adjusting delay sessions. Each injection day was followed by two testing days in which no drug was given prior to adjusting delay sessions. Half of the rats received all doses of d-amphetamine, followed by saline and then all doses of methylphenidate, while the other half received these drugs in reverse order. Within-drug dosing was conducted in random order. Behavioral training and testing took place 7 days/week over 7 weeks.
Data Analysis
Mean response latencies were generated by averaging the latencies from free-choice trials only. Nonreinforced responses (all responses that occurred during the delay to the larger reinforcer and during the ITI) were also calculated. The number of days to acquire the adjusting delay task, number of days until stable performance of the adjusting delay task, and baseline MADs, response latencies, and nonreinforced responses were compared using a student's t-test. MADs, response latencies, and nonreinforced responses during the first 5 post-acquisition days of testing were compared using a 2-way repeated measures analysis of variance (ANOVA; environmental condition x day). MADs, response latencies, and nonreinforced responses following administration of d-amphetamine or methylphenidate were compared using 2-way repeated measures ANOVA (environmental condition x dose). Post hoc comparisons were made using Fisher's LSD protected t-tests. Results were considered statistically significant if p ≤ 0.05. Data are presented as mean (± SEM).
Results
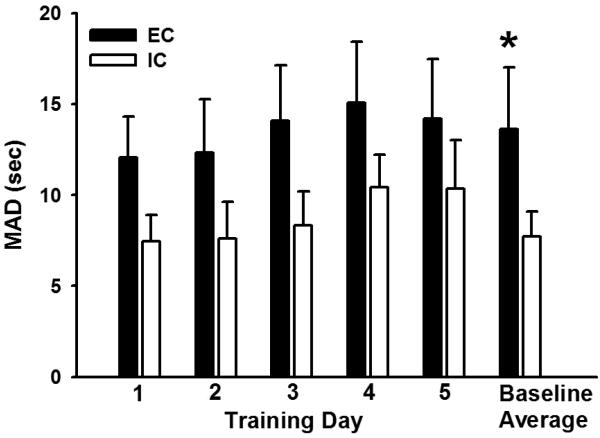
There were no between-group differences in the number of days to acquire the adjusting delay task (animals were considered to have acquired the task once they completed 60 trials within the 2 h time limit). EC rats took 2.4 (± 0.1) days to acquire, and IC rats took 3.0 (± 0.3) days to acquire. Additionally, there were no between group differences in the number of days to meet stability criteria (MADs differing less than 5 s over 5 days, with no steadily increasing or decreasing trends). EC rats reached the stability criteria in 11.7 (± 0.3) days, and IC rats reached the stability criteria in 12.0 (± 2.6) days. In order to illustrate the relative consistency of the MADs across training, Figure 1 shows MADs on the first through the fifth days after acquisition of the adjusting delay task and mean baseline MADs over the 5 days in which stability criterion was met. There were no significant differences in MADs between EC and IC rats on Days 1-5 following acquisition. However, EC rats had significantly higher baseline MADs than IC rats over 5 days of stable behavior (p < 0.05). There were no between-group differences in response latencies or non-reinforced responses on Days 1-5 of acquisition or over the 5-day stable baseline.
Figure 1.
Mean (± SEM) adjusted delay (MAD; sec) in EC and IC rats on the first through the fifth days after acquisition of the adjusting delay task and mean baseline MADs over the 5 days in which stability criterion was met. There were no significant differences on Days 1-5 following acquisition, however, EC rats had significantly higher baseline MADs than IC rats (*p < 0.05).
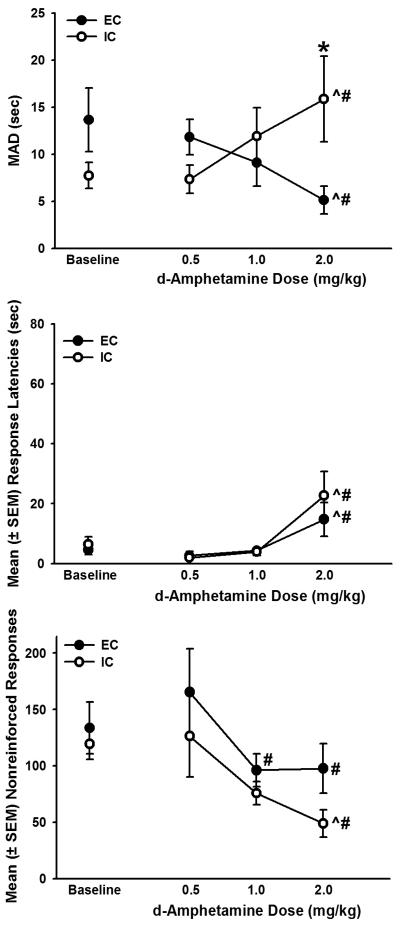
Figure 2 shows the mean MADs (top panel), response latencies (middle panel), and nonreinforced responses (bottom panel) for EC and IC rats at baseline and after administration of d-amphetamine (there were no significant differences between baseline data and data taken after administration of saline; results not shown). There was a significant dose by environmental condition interaction (F3,71 = 6.02, p < 0.05) on MADs. IC rats had significantly higher MADs after 2.0 mg/kg d-amphetamine compared to EC rats (p < 0.05). Additionally, EC rats had significantly lower MADs after administration of 2.0 mg/kg d-amphetamine compared to baseline (p < 0.05) and after administration of 0.5 mg/kg d-amphetamine. MADs in IC rats were increased after administration of 2.0 mg/kg d-amphetamine compared with baseline (p < 0.05) and after administration of 0.5 mg/kg d-amphetamine (p < 0.05).
Figure 2.
Top panel: MAD (sec) in EC and IC rats during baseline and after administration of 0.5, 1.0, or 2.0 mg/kg d-amphetamine. IC rats had significantly higher MADs than EC rats after administration of 2.0 mg/kg d-amphetamine (*p < 0.05). Additionally, IC rats MADs after 2.0 mg/kg d-amphetamine were significantly higher than baseline (^p < 0.05) and after 0.5 mg/kg d-amphetamine (#p < 0.05). EC rats had lower MADs after 2.0 mg/kg d-amphetamine compared with baseline (^p < 0.05) and 0.5 mg/kg d-amphetamine (#p < 0.05). Middle panel: Mean (± SEM) response latencies in EC and IC rats were higher after administration of 2.0 mg/kg d-amphetamine than under any other condition tested (#p < 0.05). Bottom Panel: Mean (± SEM) nonreinforced responses decreased as a function of dose in EC and IC rats. In EC rats, nonreinforced responses were reduced after 1.0 and 2.0 mg/kg d-amphetamine compared to 0.5 mg/kd d-amphetamine (#p < 0.05). Nonreinforced responses were reduced in IC rats after administration of 2.0 mg/kg d-amphetamine compared with baseline (^p < 0.05) and 0.5 mg/kg d-amphetamine (#p < 0.05).
There was a significant effect of dose on response latencies (F4,89 = 10.96, p < 0.05) such that both EC and IC rats had longer response latencies after 2.0 mg/kg d-amphetamine than under any other condition tested (p < 0.05; Fig 2, middle panel). There was also a main effect of dose on nonreinforced responses (F4,89 = 3.61, p < 0.05; Fig 2, lower panel). EC rats had significantly fewer nonreinforced responses after administration of 1.0 and 2.0 mg/kg d-amphetamine compared to 0.5 mg/kg d-amphetamine. IC rats also had significantly fewer nonreinforced responses after 2.0 mg/kg d-amphetamine than 0.5 mg/kg d-amphetamine and baseline.
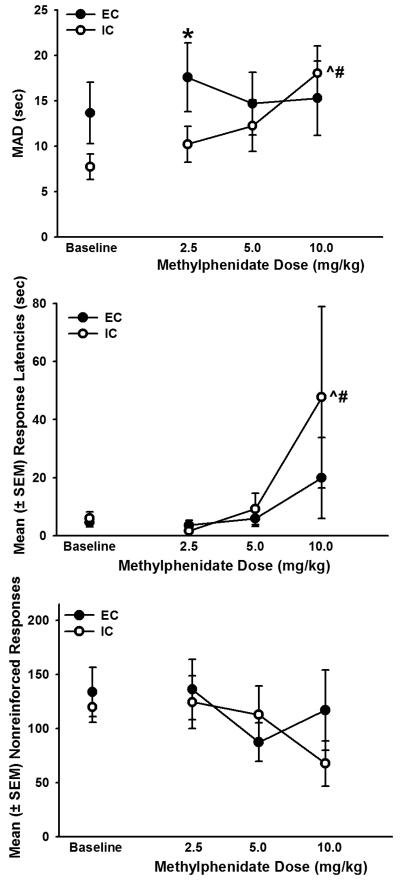
Figure 3 shows MADs (top panel), response latencies (middle panel), and nonreinforced responses (bottom panel) during baseline and after administration of methylphenidate (there were no significant differences between baseline data and data taken after administration of saline; results not shown). There were no significant overall effects of methylphenidate on MADs in EC and IC rats; however planned comparisons showed that EC rats had significantly higher MADs than IC rats after administration of 2.5 mg/kg methylphenidate. Additionally, IC rats had significantly higher MADs after administration of 10.0 mg/kg methylphenidate compared with baseline and following administration of 2.5 mg/kg methylphenidate.
Figure 3.
Top panel: MAD (sec) in EC and IC rats during baseline and after administration of 2.5, 5.0, or 10.0 mg/kg methylphenidate. EC rats had higher MADs after administration of 2.5 mg/kg methylphenidate than IC rats (*p < 0.05). MADs in IC rats were higher after administration of 10.0 mg/kg methylphenidate than baseline (^p < 0.05) and after 2.5 mg/kg methylphenidate (#p < 0.05). Middle Panel: Response latencies dose-dependently increased, and IC rats had higher response latencies following 10.0 mg/kg than baseline (^p < 0.05) and any other dose tested (#p < 0.05). Bottom Panel: There were no significant differences in nonreinforced responses after administration of any dose of methylphenidate.
There was a main effect of methylphenidate dose on response latencies (F4,89 = 3.37, p < 0.05; Fig 3, middle panel). IC rats had significantly higher response latencies following 10.0 mg/kg methylphenidate than any other condition tested. There were no significant differences in nonreinforced responses after administration of any dose of methylphenidate (Fig 3, lower panel).
Discussion
In the present study, EC and IC rats rapidly acquired the adjusting delay task (within an average of 2-3 days) and once acquired, behavior on the adjusting delay task stabilized quickly (in approximately 12 days). There were no differences in MADs, response latencies, or number of non-reinforced responses during acquisition. Following acquisition, however, when MAD scores were stable, EC rats showed higher MADs compared to IC rats, indicating that enrichment reduced impulsive choice. This finding corroborates previous work showing that EC rats are less impulsive compared to IC rats on both a DRL schedule [60] and a sucrose-reinforced nose-poke task [94]. To the extent that impulsive behavior and drug abuse vulnerability are linked, these results suggest that the decrease in amphetamine self-administration in EC rats compared to IC rats noted previously [7, 32] may reflect, at least in part, an increase in inhibitory control. This conclusion is consistent with recent hypotheses that have assigned a prominent role for inhibition as a protective factor in reducing drug abuse vulnerability [6, 9, 21, 29, 38, 39].
The current results are incongruent with a previous report by Hellemans and colleagues [35] in which, compared to IC rats, EC rats initially showed a decreased preference for the delayed larger reinforcer that dissipated with extended training. A major difference between the present study and that of Hellemans et al. [35] relates to the task procedures used to measure impulsive choice. Hellemans and colleagues [35] used a response-independent discrete delay procedure, whereas the current study used a response-dependent adjusting delay procedure. A number of other procedural differences beyond the discrete vs. adjusting delay procedures prevent any firm conclusions about whether this task-related difference was a critical factor in obtaining the contrasting results. For example, compared to the current study, Hellmans et al. [35] used a more prolonged differential rearing period (12 weeks), trained rats to perform a go/no-go task prior to assessment of delay discounting, and used a more extensive initial training period in acquisition of delay discounting. Moreover, it is not likely that the procedure difference related to using a discrete vs. adjusting delay was the only factor to explain the contrasting results across studies, because results from another study in our laboratory using a response-independent discrete delay procedure also found that EC rats were less impulsive compared to IC rats [42]. In any case, the current results are consistent with other results showing that environmental enrichment also decreases impulsivity measured by a discrete delay procedure in rats undergoing neonatal asphyxia [1].
In the present study, 2 manipulations differed between EC and IC groups: the presence of novel objects that were changed daily, and the presence of social cohorts. Both of these manipulations likely influenced impulsive behavior. For example, after undergoing neonatal asphyxia, animals double-housed in a larger cage and provided with novel objects showed decreases in impulsive choice compared to double-housed rats in a small cage with no novel objects [1], suggesting that the presence of novel objects influences impulsivity. We are unaware of studies that have examined the impact of group- vs. single-housing on impulsivity; however, there are data to suggest that the social hierarchies that develop when animals are group-housed influence impulsivity. Subordinate animals may experience chronic stress due to defeat in repeated social confrontations [e.g., 86], and because stress has been related to impulsivity [66], subordinate EC rats may show increased impulsivity relative to dominant EC rats. Alternatively, variations in impulsivity may predict social rank, as female cynomolgus monkeys that are more impulsive are also more likely to become subordinate when subsequently housed in same-sex groups [18]. Regardless of the relationship between impulsivity and social status, the presence of both novel objects and social cohorts likely influenced impulsivity in the present study.
In addition to the enrichment-induced decrease in impulsive choice, another key finding of this study was that both amphetamine and methylphenidate had differential effects in EC and IC rats. Specifically, amphetamine increased impulsive choice in EC rats, whereas it decreased impulsive choice in IC rats; methylphenidate did not significantly alter impulsive choice in EC rats, but similar to amphetamine it decreased impulsive choice in IC rats. It is notable that previous reports have yielded mixed results about the effects of amphetamine and methylphenidate on impulsive choice in rats, with some showing decreased impulsive choice [68, 87, 90, 93], and others reporting no effect [15, 27]. The current study suggests that one potential reason for these discrepant findings is that environmental factors may modulate the baseline levels of impulsivity, and thus alter the sensitivity to amphetamine and methylphenidate. Other studies using the stop-signal reaction time task to measure impulsivity have also found baseline dependent effects of amphetamine and methylphenidate on impulsivity [24, 25, 28]. Further, in humans, individuals with high baseline levels of impulsivity, such as those with attention deficit/hyperactivity disorder (ADHD), show stimulant-induced decreases in impulsivity [69, 84, 85], further illustrating the importance of preexisting differences in impulsivity on the subsequent effects of stimulant drugs.
Differences in dopamine transporter (DAT) function in the medial prefrontal cortex (mPFC) may have contributed to the discrepant psychostimulant-induced changes in impulsive choice in the differentially-reared groups. The mechanism of action of both amphetamine and methylphenidate includes increasing dopamine (DA) in the synapse by binding to DAT. Amphetamine reverses primarily DAT function and inhibits DA uptake, whereas methylphenidate inhibits both DAT and norepinephrine transporter (NET) function [31]. Moreover, compared to IC rats, EC rats show diminished DAT functioning in the mPFC, but not the striatum or nucleus accumbens, suggesting that EC rats may have higher levels of extracellular DA in the mPFC than IC rats [95, 96]. Recent results from our laboratory using standard-housed rats have shown that injection of indirect DA agonists into the mPFC decreases impulsivity (Perry et al., unpublished data) and others have shown that DA depletion in the mPFC increases impulsivity [81]. Thus, the higher level of extracellular DA in EC rats may play a role in explaining why EC rats are less impulsive than IC rats. It also follows that when administered indirect DA agonists, IC rats show reduced impulsivity. However, it is less clear why amphetamine would increase impulsivity in EC rats, although some have theorized that moderate amounts of DA modulation in the prefrontal cortex produce optimal cognitive performance, while low or high DA modulation produce sub-optimal performance [75]. Therefore, perhaps amphetamine-induced performance deficits (i.e., impulsivity) are due to enhanced DA modulation in the mPFC. Based on this hypothesis, we would expect that methylphenidate would also increase impulsivity in EC rats; however, in the present study, methylphenidate produced no significant change in EC rats. Future studies of the effects of methylphenidate on neurotransmission in EC and IC rats may clarify this issue.
The ability of both amphetamine and methylphenidate, two stimulants used widely to treat ADHD [e.g., 45], to decrease impulsive choice in IC rats suggests that isolation rearing may represent a novel preclinical model of ADHD. A number of different models have been used to model ADHD, including DAT knockout mice [79], genetically inbred spontaneously hypertensive (SHR) rats [78, 79] and rats selected for poor performance on the Five-Choice Serial Reaction Time Task [79]. Each of these models has both strengths and limitations; for example, SHR rats exhibit many of the behavioral characteristics of ADHD, but hypertension is a confounding factor in this model [79]. Isolation rearing may be a useful model because, in addition to the increased impulsivity noted in IC rats in the current study, IC rats are also hyperactive [13, 26, 47, 80] and show attentional deficits [5, 16, 19, 30, 33, 88, 92] compared to EC rats. Further, in the current study, amphetamine decreased preservative non-reinforced responding in IC rats. In addition to this face validity, isolation rearing appears to have predictive validity because stimulant drugs known to have clinical utility in the treatment of ADHD were shown to decrease impulsivity.
If IC rats are a model of ADHD, then the present results, combined with previous results showing increased psychostimulant self-administration in IC rats [7], may suggest that the use of stimulant medications can reduce the likelihood of substance abuse problems in those with ADHD. In humans, the use of psychostimulant treatment for ADHD may protect against the development of substance use disorders [12, 49, 52, 91], especially if treatment is started in childhood [for a review, see 43]. Conversely, the present results may also suggest that use of psychostimulants by individuals without ADHD could increase the risk of substance abuse problems. Indeed, in college students, nonmedical use of stimulants is associated with use of other illicit drugs [3, 8, 51]; however, these studies are correlational in nature and do not definitively indicate that nonmedical use of stimulants increases subsequent drug use.
In summary, the results of this study suggest that the rearing environment influences both impulsive choice and the response to psychostimulants, thus adding to the literature showing the importance of individual differences on both impulsive choice and drug abuse. The relationship between impulsivity and drug abuse vulnerability is known to involve genetic factors, as Lewis rats and rats that have been selectively bred for high saccharin intake (HiS) show both enhanced vulnerability to drug abuse and increased impulsive choice compared to Fischer 344 [2, 44, 50, 83] and rats selectively bred for low saccharin intake (LoS) [14, 22, 63, 64]. The present findings, combined with previous findings showing that IC rats are more vulnerable to drug abuse than EC rats [7, 32, 82], indicate that environmental factors also influence both impulsivity and drug abuse. Future studies should attempt to determine what critical environment-dependent neural mechanisms may explain the connection between impulsivity and drug abuse vulnerability.
Acknowledgements
The authors would like to thank Blake Dennis, Josh Cutshall, and Jason Ross for technical assistance, and Jason Ross for helpful comments on earlier drafts of this manuscript. This research was funded by USPHS grants DA05312 (MTB) and DA007304 (JLP).
References
- 1.Adriani W, Giannakopoulou D, Bokulic Z, Jernej B, Alleva E, Laviola G. Response to novelty, social and self-control behaviors, in rats exposed to neonatal anoxia: modulatory effects of an enriched environment. Psychopharmacol. 2006;184:155–165. doi: 10.1007/s00213-005-0223-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KG, Woolverton WL. Effects of comipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arria AM, Caldeira KM, O'Grady KE, Vincent KB, Johnson EP, Wish ED. Nonmedical use of prescription stimulants among college students: Associations with Attention-Deficit-Hyperactivity Disorder and polydrug use. Pharmacotherapy. 2008;28:156–169. doi: 10.1592/phco.28.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- 5.Bakshi VP, Swerdlow NR, Braff DL, Geyer MA. Reversal of isolation-rearing-induced deficits in prepulse inhibition by seroquel and olanzapine. Biol Psychiatry. 1998;43:436–445. doi: 10.1016/s0006-3223(97)00246-1. [DOI] [PubMed] [Google Scholar]
- 6.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacol. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 8.Barrett SP, Darredeau C, Bordy LE, Pihl RO. Characteristics of methylphenidate misuse in a university students sample. Can J Psychiatry. 2005;50:457–461. doi: 10.1177/070674370505000805. [DOI] [PubMed] [Google Scholar]
- 9.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 10.Bennett EL, Rosenzweig MR, Diamond MC, Morimoto H, Heblert H. Effects of successive environments on brain measures. Phys Behav. 1974;12:621–631. doi: 10.1016/0031-9384(74)90212-1. [DOI] [PubMed] [Google Scholar]
- 11.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacol. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 12.Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactvity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 13.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacol. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 14.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 15.Charrier D, Thiebot MH. Effects of psychotropic drugs on rat responding in an operant paradigm involving choice between delayed reinforcers. Pharmacol Biochem Behav. 1996;54:149–157. doi: 10.1016/0091-3057(95)02114-0. [DOI] [PubMed] [Google Scholar]
- 16.Cilia J, Reavill C, Hagen JJ, Jones DNC. Long-term evaluation of isolation-reared induced prepulse inhibition deficits in rats. Psychopharmacol. 2001;156:327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- 17.Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Czoty PW, Riddick NV, Gage HD, Icenhower M, Bounds MC, Kaplan JR, Bennett AJ, Pierre PJ, Nader MA. Neurobiological and behavioral predictors of social rank in female monkeys; College of Problems on Drug Dependence Meeting; Quebec City, Quebec, Ontario. June 2007. [Google Scholar]
- 19.Dalley JW, Theobald DE, Pereira EAC, Li PMMC, Robbins TW. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacol. 2002;164:329–340. doi: 10.1007/s00213-002-1215-y. [DOI] [PubMed] [Google Scholar]
- 20.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacol. 2002;28:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 21.de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- 22.Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 23.Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, de Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: Effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 25.Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacol. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- 26.Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–196. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacol. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 28.Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- 29.Fillmore MT. Drug abuse as a problem of impaired control: Current approaches and findings. Behav Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- 30.Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- 31.Grace AA. Psychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. University Press; Oxford: 2001. pp. 31–72. [Google Scholar]
- 32.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive- ratio schedules. Psychopharmacol. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 33.Heidbreder CA, Foxton R, Cilia J, Hughes ZA, Shah AJ, Atkins A, Hunter AJ, Hagen JJ, Jones DNC. Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacol. 2001;156:338–351. doi: 10.1007/s002130100760. [DOI] [PubMed] [Google Scholar]
- 34.Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–220. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behav Pharmacol. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacol. 2006:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 38.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacol. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 39.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 40.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 42.Klein ED, Bardo MT. Environmental enrichment produces a long-lasting decrease in impulsive behavior: Preclinical implications for drug abuse prevention; College of Problems on Drug Dependence Meeting; Orlando, FL. June 2005. [Google Scholar]
- 43.Kollins SH. A qualitative review of issues arising in the use of psychostimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24:1345–1357. doi: 10.1185/030079908x280707. [DOI] [PubMed] [Google Scholar]
- 44.Kosten T, Miserendino M, Haile C, DeCaprio J, Jatlow P, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- 45.Levin FR, Kleber HD. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- 46.Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: a preliminary report. Psychopharmacol. 1992;109:245–247. doi: 10.1007/BF02245509. [DOI] [PubMed] [Google Scholar]
- 47.Lore RK, Levowitz A. Differential rearing and free versus forced exploration. Psychonomic Science. 1966;5:21–42. [Google Scholar]
- 48.Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- 49.Mannuzza S, Klein RG, Moulton JL., III Does stimulant treatment place children at risk for adult substance abuse? A controlled, prospective follow-up study. J Child Adolesc Psychopharmacol. 1999;13:273–282. doi: 10.1089/104454603322572606. [DOI] [PubMed] [Google Scholar]
- 50.Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- 51.McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among U.S. college students: Prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 52.McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38:43–56. doi: 10.1080/02791072.2006.10399827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacol. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- 54.McGue M, Sharma A, Benson P. Parent and sibling influences on adolescent alcohol use and misuse: evidence from a U.S. adoption cohort. J Stud Alcohol. 1996;57:8–18. doi: 10.15288/jsa.1996.57.8. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell S. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacol. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 56.Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Research Council . Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 58.Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacol. 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- 59.Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol. 2003;38:151–156. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- 60.Ough BR, Beatty WW, Khalili J. Effects of isolated and enriched rearing on response inhibition. Psychonomic Science. 1972;27:293–294. [Google Scholar]
- 61.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacol. 2008 doi: 10.1007/s00213-008-1173-0. in press. [DOI] [PubMed] [Google Scholar]
- 62.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of iv cocaine self-administration in female rats. Psychopharmacol. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 63.Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of iv cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacol. 2006;186:235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- 64.Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry JL, Nelson SE, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of iv cocaine self-administration and reinstatement of cocaine-seeking behavior in male (v female) rats. Exp Clin Psychopharmacol. 2008 doi: 10.1037/1064-1297.16.2.165. in press. [DOI] [PubMed] [Google Scholar]
- 66.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 67.Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacol. 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- 68.Pitts RC, McKinney AP. Effects of methyphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacol. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- 70.Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- 71.Reynolds B, Richards JB, Dassinger M, de Wit H. Therapeutic doses of diazepam do not alter impulsive behavior in humans. Pharmacol Biochem Behav. 2004;79:17–24. doi: 10.1016/j.pbb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 73.Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacol. 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- 74.Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robbins TW. Chemistry of the mind: Neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- 76.Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacol. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- 77.Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: A replication and extension. J Comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- 78.Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- 79.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioral effects of cocaine and d-amphetamine. Psychopharmacol. 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- 81.Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994;642:20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- 82.Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988;245:164–170. [PubMed] [Google Scholar]
- 84.Tannock R, Schachar R, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- 85.Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: Dissociated dose effects in hyperactive children. J Abnorm Child Psychol. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- 86.Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: Differential protection by clonidine and metoprolol. Psychopharmacol. 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- 87.van Gaalen MM, van Koten R, Schoffelmeer ANM, Vanderschuren L. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of pre-pulse inhibition as models to screen antipsychotic drugs. Psychopharmacol. 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- 89.Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 90.Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacol. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- 91.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 92.Wilkinson LS, Killcross AS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacol. 1994;10:61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- 93.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacol. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- 94.Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Phys Behav. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 95.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- 96.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]