Abstract
Mutations in the human presenilin genes PS1 and PS2 cause early-onset Alzheimer’s disease. Studies in Caenorhabditis elegans and in mice indicate that one function of presenilin genes is to facilitate Notch-pathway signaling. Notably, mutations in the C. elegans presenilin gene sel-12 reduce signaling through an activated version of the Notch receptor LIN-12. To investigate the function of a second C. elegans presenilin gene hop-1 and to examine possible genetic interactions between hop-1 and sel-12, we used a reverse genetic strategy to isolate deletion alleles of both loci. Animals bearing both hop-1 and sel-12 deletions displayed new phenotypes not observed in animals bearing either single deletion. These new phenotypes—germ-line proliferation defects, maternal-effect embryonic lethality, and somatic gonad defects—resemble those resulting from a reduction in signaling through the C. elegans Notch receptors GLP-1 and LIN-12. Thus SEL-12 and HOP-1 appear to function redundantly in promoting Notch-pathway signaling. Phenotypic analyses of hop-1 and sel-12 single and double mutant animals suggest that sel-12 provides more presenilin function than does hop-1.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of the central nervous system involving loss of memory and cognitive function. Amyloid plaques, whose major component is the β-amyloid, or Aβ, peptide, are a neuropathological hallmark of AD. Dominant mutations in any of three genes, PS1, PS2, or APP, cause early-onset familial AD. PS1 and PS2 encode related proteins termed presenilins 1 and 2 (PS1 and PS2) (1–3), and APP encodes the amyloid precursor protein (APP), from which the Aβ peptide is generated by proteolytic processing (for review, see ref. 4).
Three presenilin genes, spe-4 (5), sel-12 (6), and hop-1 (7), have been identified in the nematode Caenorhabditis elegans. Rescue experiments using transgenes have shown that human PS1 and PS2 can substitute for SEL-12, demonstrating that at least some aspects of presenilin function have been conserved from nematodes to mammals (8, 9). Experiments by Levitan and Greenwald (6) indicate that sel-12 acts as a positive regulator of Notch-pathway signaling mediated by the C. elegans Notch receptor homologs GLP-1 and LIN-12: loss-of-function mutations in sel-12 suppress lin-12 gain-of-function phenotypes and enhance lin-12 and glp-1 partial loss-of-function phenotypes. A similar interaction has been proposed to occur in mice: the lethal phenotype of PS1 knockout mice resembles that seen in Notch ligand and receptor knockouts (10, 11).
sel-12 mutations do not cause strong Glp-1 or Lin-12 loss-of-function phenotypes, suggesting that sel-12 might act redundantly with other presenilin genes (6). To examine the function of hop-1 and to test this hypothesis, we used a reverse genetic strategy to generate hop-1 and sel-12 deletion mutations. Our analysis of hop-1; sel-12 double mutant phenotypes indicates that hop-1 functions redundantly with sel-12 to promote Notch-pathway signaling in C. elegans. This analysis extends previous findings by Li and Greenwald (7) based on RNA-mediated interference of hop-1 in a sel-12 mutant background. The availability of mutations in both hop-1 and sel-12 has allowed us to perform a detailed phenotypic analysis of animals lacking hop-1 and sel-12 function, indicating that sel-12 provides more presenilin function than does hop-1 and demonstrating a requirement for maternal expression of hop-1 and sel-12.
MATERIALS AND METHODS
General Methods and Strains.
Nematodes were cultured at 20°C (unless noted otherwise) by using standard techniques (12), except that strains used to generate deletion libraries were cultured in liquid, as described below. C. elegans variety Bristol strain N2 (12) is the parent of all strains used in these studies. Alleles used were as follows: for LGI, unc-73(e936), hop-1(nr2003), and dpy-5(e61); for LGIII, lin-12(n950sd), and glp-1(q231ts); for LGX, egl-17(e1313), pha-2(ad472), sel-12(nr2011), unc-1(e538), and dpy-3(e27). One rearrangement used was LGI: hDf7.
Generation of Deletion Libraries.
Late fourth-larval (L4) stage N2 hermaphrodites were mutagenized for 4 h with 0.25% ethyl methanesulfonate, 0.4 mM ethylnitrosourea, or 1 mM diepoxyoctane (ref. 13 and references within) or by exposure to UV light after incubation in trimethylpsoralen (30 μg/ml) (14). F1 eggs derived from mutagenized hermaphrodites were collected. For each library, hatched larvae were distributed to 48 96-well polystyrene microtiter plates at approximately 20 F1 animals per well in liquid NGM medium (15) containing 1% Escherichia coli HB101 as food. Each library thus contained ≈2 × 105 mutagenized genomes. Worms were cultured in wells until no food remained (about 5 days), generating approximately 100 F2 progeny per F1 animal. Fifty percent of the worms in each well were used to make genomic DNA; of these, half were transferred to wells of V-bottom 96-well microtiter plates and half were used to make a pool of worms from all wells of a single microtiter plate (plate pool). The remaining worms were frozen by using standard methods (15) and served as viable stocks.
Identification and Recovery of hop-1 and sel-12 Deletion Mutants.
Plate pools from multiple libraries were screened by PCR using nested primers specific for genomic sequences flanking the hop-1 or sel-12 coding regions. Primer pairs were chosen so that they amplified wild-type products ranging from 2.5 to 3.5 kb. Sequences of the primers are available on request from the authors. Extension times were adjusted so that a faint wild-type product was amplified reproducibly. Plate pools that gave rise to a smaller, presumptive deletion, amplicon were rescreened in quadruplicate. For plate pools confirmed as positive, DNA in wells from that pool were screened individually by PCR to identify the specific well containing the deletion. The corresponding well of worms was then thawed and survivors transferred clonally to agar plates. Hermaphrodites were cultured until they had laid eggs and then genotyped by single-worm PCR (16). Homozygous lines were established from the self progeny of hermaphrodites containing the deletions.
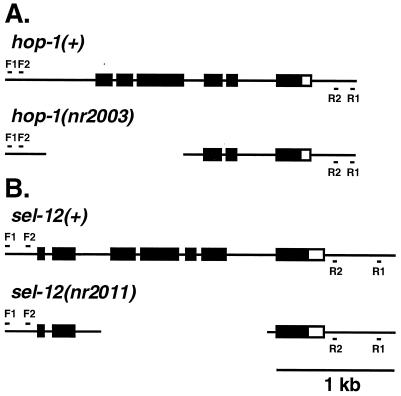
Sequence analysis demonstrated that the hop-1(nr2003) deletion is 1196 bp long, extending from bp 23,744 or 23,743 to 22,549 or 22,548 of cosmid C18E3 (accession number AF000265), and that the sel-12(nr2011) deletion is 1,426 bp in length, extending from bp 5,533 or 5,534 to 6,958 or 6,959 of cosmid F35H12 (accession number U41540). hop-1(nr2003) was backcrossed 10 times to an unc-73(e936) dpy-5(e61) strain and sel-12(nr2011) was backcrossed 7 times to N2 before the genetic analyses described herein were conducted.
Laser Killing of Cells in Embryos and Antibody Staining.
To visualize the intestinal valve cells, embryos were stained with the J126 antibody (17). As the intestine also stains with J126 (17) and makes identification of the valve cells difficult, either the EMS or E blastomere was laser-killed to eliminate the intestine. For cell killing, early embryos were dissected from gravid N2, glp-1(q231ts), or hop-1; sel-12 parents. glp-1(q231ts) hermaphrodites had been shifted from 15°C to 20°C [nonpermissive temperature for the maternal-effect embryonic lethal (Mel) phenotype (18)] 12–24 h before dissection. Laser microsurgery followed the technique of Bowerman et al. (19). After cell killing, embryos were incubated for 6 h at 22°C and then stained with the J126 antibody. To visualize pharyngeal tissue, a monoclonal antibody (9.2.1) specific for pharyngeal myosin (20) was used. For both antibodies, methanol/acetone fixation and freeze-crack permeabilization followed the method of Miller and Shakes (21) and antibody staining followed the method of Shi and Mello (22).
RESULTS
hop-1 and sel-12 Deletion Mutations Were Generated by a Reverse Genetic Approach.
hop-1 was identified by virtue of its sequence homology to other presenilin genes (7). To demonstrate that the gene is expressed, we (data not shown) and others (7) isolated hop-1 cDNAs and performed reverse transcriptase-coupled PCR analysis.
To identify a deletion mutation in the hop-1 gene, deletion libraries representing 460,000 mutagenized genomes were screened by PCR using primers flanking the hop-1 coding region. Animals containing the deletion nr2003 were recovered from a library mutagenized with UV-trimethylpsoralen. Sequence analysis revealed a 1,196-bp deletion beginning 434 bp upstream of the hop-1 translation start site and ending in the third intron (Fig. 1A). The same reverse genetic strategy was used to isolate a sel-12 deletion mutation. Deletion libraries representing 1,100,000 genomes were screened with sel-12-specific primers. Animals containing the deletion nr2011 were recovered from a library mutagenized with ethylnitrosourea. Sequence analysis revealed a 1,426-bp deletion starting in the second intron and ending into the sixth intron (Fig. 1B); splicing of the second exon to the seventh exon, if it occurred, would cause a frameshift. Thus, sel-12(nr2011) is predicted to encode a severely truncated protein containing the N-terminal 82 amino acids of SEL-12 followed by 23 novel amino acids.
Figure 1.
Schematic representation of the hop-1 and sel-12 loci in wild-type (+) and deletion-containing animals. Solid boxes represent coding sequences, open boxes represent 3′ untranslated sequences, and lines represent extragenic sequences (6, 7). (A) F1, F2, R1, and R2 denote the approximate locations of the nested PCR primers used to identify the nr2003 deletion. The extent of the nr2003 deletion is indicated by the gap. (B) Primers used to identify the nr2011 deletion are designated as in A. The extent of the nr2011 deletion is indicated by the gap. See text for deletion endpoints.
Like previously described sel-12 mutations (6), sel-12(nr2011) conferred a highly-penetrant egg-laying defective (Egl) phenotype and suppressed the Multivulva phenotype of a lin-12 gain-of-function allele (n950sd) (Table 1). By contrast, hop-1(nr2003) had no effect on egg laying and did not suppress the Multivulva phenotype of lin-12(n950sd) (Table 1). Moreover, hop-1(nr2003) homozygotes and hop-1(nr2003)/hDf7 hemizygotes (hDf7 is a large multilocus deletion that removes the hop-1 coding region) had no apparent morphological or behavioral defects. At 15°C, however, hop-1(nr2003) hermaphrodite brood size was reduced by nearly half (data not shown); this fertility defect has not been investigated further.
Table 1.
Interaction of hop-1 and sel-12 mutations with a lin-12(gf) allele
| Genotype | No. of vulvae or pseudovulvae*
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Wild type | 100 | 0 | 0 | 0 | 0 | 0 |
| lin-12(gf)† | 0 | 0 | 0 | 29 | 68 | 3 |
| hop-1; lin-12(gf)‡ | 0 | 0 | 0 | 33 | 64 | 3 |
| lin-12(gf); sel-12§ | 76 | 19 | 4 | 1 | 0 | 0 |
One hundred animals of each genotype were scored with a dissecting microscope.
Complete genotype is lin-12(n950gf).
Complete genotype is hop-1(nr2003); lin-12(n950gf).
Complete genotype is lin-12(n950gf); sel-12(nr2011).
hop-1 and sel-12 Function Redundantly to Promote Normal Embryogenesis and Germ-Line Development.
hop-1; sel-12 double mutants display phenotypes that are not observed in either single mutant and that vary depending on the maternal genotype. As shown in Table 2, strains of three different genotypes that segregate hop-1; sel-12 progeny were analyzed. hop-1/+; sel-12 animals segregate hop-1; sel-12 double homozygotes that are viable but sterile. By contrast, both hop-1; sel-12/+ animals and hop-1/+; sel-12/+ animals segregate hop-1; sel-12 homozygotes that are viable and have a normal germ line but produce dead embryos, a Mel phenotype. These animals are also Egl, as expected due to reduced sel-12 activity. These results show that absence of maternal and zygotic hop-1 and sel-12 function leads to embryonic lethality, consistent with either a strict maternal requirement for hop-1 and sel-12 function or a requirement for both maternal and zygotic hop-1 and sel-12 function. In the absence of hop-1 function, maternal expression of sel-12 is sufficient for normal embryogenesis and germ-line development. By contrast, in the absence of sel-12 function, maternal expression of hop-1 is sufficient for normal embryogenesis but not for normal germ-line development, resulting in sterility.
Table 2.
hop-1; sel-12 double mutant phenotypes
| Maternal genotype | Zygotic genotype | Glp-1-like defects
|
Lin-12-like defect (two ACs*) | |
|---|---|---|---|---|
| Sterile | Mel | |||
| hop-1/+; sel-12† | hop-1; sel-12 | Yes‡ | NA | 24/24 |
| hop-1; sel-12/+§ | hop-1; sel-12 | No | Yes¶ | 0/25 |
| hop-1/+; sel-12/+‖ | hop-1; sel-12 | No | Yes** | ND |
NA, not applicable; ND, not determined.
Number of hop-1; sel-12 animals with two ACs and the total number of animals scored are indicated. The number of ACs in non-Unc non-Dpy progeny of hop-1/unc-73 dpy-5; sel-12 parent animals or in non-Unc progeny of hop-1; sel-12/egl-17 unc-1 parent animals was determined in third- or fourth-larval stage animals by using differential interference contrast microscopy (see Fig. 2). After scoring, animals were allowed to develop to adulthood. Adult hop-1; sel-12 animals could be distinguished from their heterozygous siblings by their sterile or Mel phenotype. sel-12 single mutant animals have a single AC (ref. 6 and data not shown), indicating that in a hop-1(+) background, the absence of maternal and zygotic sel-12 activity does not cause defects in the AC/VU decision.
Complete genotype is hop-1(nr2003)/unc-73(e936) dpy-5(e61); sel-12(nr2011).
Non-Unc non-Dpy progeny (n = 147) of hop-1/+; sel-12 parent animals were cloned as L4 stage animals and incubated for 24–36 h. Of these, 60% were Egl and 40% were sterile. PCR analysis of 48 Egl and 44 sterile animals indicated that all of the Egl animals were heterozygous for the hop-1 deletion, whereas all of the sterile animals were hop-1 homozygotes. In a separate experiment, the germ-line phenotype of 59 young adult sterile animals was scored with differential interference contrast microscopy. Each gonad arm contained 50–100 sperm, no oocytes, and no undifferentiated germ cells (see Fig. 2), a phenotype resembling that of glp-1 reduction-of-function mutants (18).
Complete genotype is either hop-1(nr2003); sel-12(nr2011)/pha-2(ad472) dpy-3(e27) or hop-1(nr2003); sel-12(nr2011)/egl-17(e1313) unc-1(e538).
Non-Dpy progeny (n = 287) of hop-1; sel-12/pha-2 dpy-3 parent animals were cloned as L4 stage animals and incubated for 24–36 h. Of these, 64% were wild type, 29% were filled with dead eggs (the Mel phenotype), 5% were Egl with live progeny, and 2% were sterile. PCR analysis of 20 wild-type and 20 Mel animals indicated that all of the wild-type animals were heterozygous for the sel-12 deletion, whereas all of the Mel animals were sel-12 homozygotes. The Egl animals with live progeny were all sel-12 heterozygotes, indicating that, in a hop-1 mutant background, a single wild-type copy of sel-12 is not always sufficient for normal egg laying. The rare sterile animals did not appear to have a germ-like proliferation defect; the sterility was not characterized further.
Complete genotype is hop-1(nr2003)/unc-73(e936) dpy-5(e61); sel-12(nr2011)/+.
Twenty Egl, non-Unc non-Dpy progeny of hop-1/unc-73 dpy-5; sel-12/+ parent animals were cloned. Fourteen of these animals (presumed genotype hop-1/unc-73 dpy-5; sel-12) segregated both sterile animals with a germ-line proliferation defect and Unc Dpy animals. The remaining six animals had a Mel phenotype, producing only dead embryos; PCR analysis indicated that all of the Mel animals were of the genotype hop-1; sel-12.
hop-1; sel-12 Double Mutant Phenotypes Can Be Attributed to Defects in GLP-1/LIN-12 Signaling.
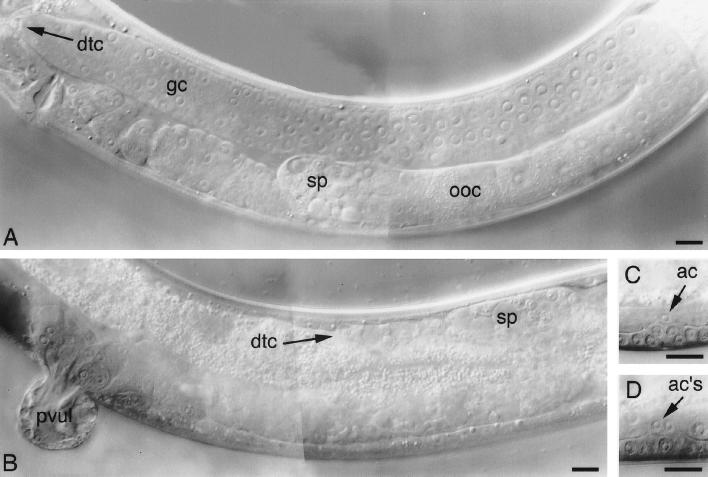
The sterile and Mel phenotypes displayed by hop-1; sel-12 progeny are reminiscent of defects seen in glp-1 loss-of-function mutants. In wild-type animals, a cell–cell interaction between the distal tip cell and germ cells, mediated by the GLP-1 receptor, induces germ cells to proliferate (18). Animals bearing strong glp-1 mutations produce a reduced number of sperm and no oocytes and are sterile due to the failure of germ cells to respond to this proliferative signal (18). Similarly, gonads of hop-1; sel-12 progeny that segregate from hop-1/+; sel-12 parents contain 50–100 sperm but neither oocytes nor undifferentiated germ cells (Table 2 and Fig. 2).
Figure 2.
Glp-1- and Lin-12-like phenotypes are observed in the germ line and somatic gonads of hop-1; sel-12 animals. Differential interference contrast photomicrographs of wild-type (A and C) or hop-1; sel-12 hermaphrodites from hop-1/+; sel-12 parent animals (B and D). (A) A gonad arm of a young adult hermaphrodite containing undifferentiated germ cells (gc) near the distal tip cell (dtc) and oocytes (ooc) and sperm (sp) proximally. (B) A Glp-1-like gonad arm (18) containing sperm (sp) distally and proximally but neither oocytes nor undifferentiated germ cells. This field of view also shows a protruding vulva (pvul). (C) An L4 stage hermaphrodite containing a single anchor cell (AC). (D) An L4 stage hermaphrodite containing two ACs. (Bars = 10 μm.)
Studies of weak glp-1 mutations have revealed roles for maternally contributed glp-1 in early embryonic development (18, 23, 24). At the four-cell embryo stage, glp-1 is required for proper specification of the fate of the blastomere ABp (24). In glp-1 mutants, ABp adopts the fate of its sister, ABa. One outcome of this change in cell fate is that glp-1 mutant embryos fail to make intestinal valve cells, which are descended from ABp (24). Similarly, we found that inviable hop-1; sel-12 mutant embryos derived from hop-1; sel-12/+ grandparents lack intestinal valve cells (Fig. 3 A–C).
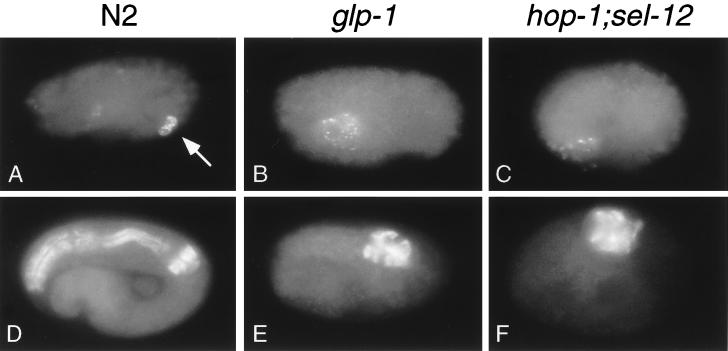
Figure 3.
Phenotypes similar to those observed in a conditional glp-1 mutant are observed in hop-1; sel-12 embryos derived from hop-1; sel-12/+ parent animals. (A–C) Immunofluorescence micrographs of embryos stained with the monoclonal antibody J126 to visualize the intestinal valve cells (17). Wild-type embryos exhibit staining from two intestinal valve cells (arrow in A), whereas neither glp-1(q231ts) (ref. 24 and B) nor hop-1; sel-12 (C) mutant embryos exhibit intestinal valve cell staining. The embryo in C is representative of 25 embryos scored. Either the E or EMS blastomere was laser-killed in each of the embryos shown to eliminate the intestine. The J126 antibody also stains pharyngeal gland cells (17); pharyngeal gland cell staining can be distinguished from valve cell staining based on cell morphology (19). Staining of pharyngeal gland cells is visible in A–C; in A most of this staining is in a different focal plane than that shown. (D–F) Immunofluorescence micrographs of embryos stained with the monoclonal antibody 9.2.1, which recognizes pharyngeal myosin C (20). glp-1 (ref. 23 and E) and hop-1; sel-12 (F) embryos have less pharyngeal tissue than does a wild-type embryo (D). The embryo depicted in F is representative of more than 50 embryos scored. C. elegans embryos are approximately 50 μm long.
A second requirement for glp-1 occurs at the 12-cell embryo stage (23, 24). At this stage, descendants of ABa are induced to produce anterior pharyngeal tissue as a result of a glp-1-dependent cell–cell interaction. In glp-1 mutant embryos, this interaction fails and the anterior lobe of the pharynx is not formed. glp-1 function, however, is not required for the formation of the posterior lobe of the pharynx, which is composed of cells descended from the MS blastomere (23). Like glp-1 mutant embryos, inviable hop-1; sel-12 mutant embryos lack anterior pharynx but form posterior pharynx. The amount of pharyngeal tissue seen in hop-1; sel-12 embryos stained with an antibody specific for pharyngeal myosin was reduced relative to that seen in wild-type embryos and was comparable to that seen in glp-1 mutant embryos (Fig. 3 D–F). Furthermore, as with glp-1 mutant embryos (23), no pharyngeal tissue was observed in hop-1; sel-12 embryos in which the posterior pharynx had been eliminated by killing descendants of the MS blastomere (data not shown). Taken together, these data suggest that cell-fate defects similar to those observed in glp-1 mutant embryos occur in inviable hop-1; sel-12 mutant embryos.
In addition to Glp-1-like defects, some hop-1; sel-12 mutants display Lin-12-like defects. One well-characterized cell-fate change in lin-12 mutants occurs in the hermaphrodite somatic gonad (25). In wild-type animals, lin-12-mediated signaling between the somatic gonad primordium cells Z1.ppp and Z4.aaa ensures that they develop into two distinct somatic gonadal cell types, an anchor cell (AC) and a ventral uterine precursor cell (VU). In lin-12 reduction-of-function mutants, both Z1.ppp and Z4.aaa develop into ACs (25). We found that hop-1; sel-12 mutant animals segregating from hop-1/+; sel-12 parents also have two ACs (Table 2 and Fig. 2). Furthermore, these hop-1; sel-12 mutant animals have a highly penetrant protruding vulva phenotype that closely resembles that seen in lin-12 reduction-of-function mutants (ref. 25 and Fig. 2. These defects were not seen in hop-1; sel-12 mutant animals segregating from hop-1; sel-12/+ parents.
Because of partial redundancy of lin-12 and glp-1 functions, lin-12 glp-1 double mutants display more severe defects than does either single mutant (26). The lin-12 glp-1 double mutant phenotype, termed Lag (lin-12 and glp-1), is a zygotic larval lethal with characteristic cell fate defects (26). We did not detect Lag-like animals among 485 progeny segregating from hop-1/+; sel-12 hermaphrodites. By contrast, Li and Greenwald (7) observed Lag-like progeny of sel-12 mutant animals injected with hop-1 antisense RNA. One possible explanation for this difference is that hop-1 activity in the gonads of hop-1/+; sel-12 hermaphrodites may not be reduced as much as in the gonads of sel-12 animals injected with hop-1 antisense RNA.
DISCUSSION
sel-12 and hop-1 Function Redundantly to Facilitate Notch-Family Receptor Signaling.
We used a reverse genetic strategy to isolate deletions in two C. elegans presenilin genes, hop-1 and sel-12. The hop-1(nr2003) deletion mutant does not cause an obvious mutant phenotype, whereas the sel-12(nr2011) deletion mutation, like sel-12 point mutations (6), confers an Egl phenotype and suppresses the Multivulva defect associated with lin-12(n950sd). hop-1; sel-12 double mutants display new phenotypes, sterility and Mel. Analysis of the cellular phenotypes of hop-1; sel-12 double mutants revealed specific changes in cell fates involved in the development of the embryo, germ line, and somatic gonad that are indicative of defects in signaling through the Notch-type receptors GLP-1 and LIN-12.
The finding that hop-1; sel-12 mutant animals display Glp-1- and Lin-12-like defects not observed in either single mutant indicates that hop-1 and sel-12 function redundantly to promote Notch-pathway signaling. Three lines of evidence argue that sel-12 plays a larger role to promote signaling than does hop-1. (i) sel-12 mutants exhibit an Egl phenotype that resembles that of lin-12 partial loss-of-function mutants (6), whereas hop-1 mutants lay eggs normally. (ii) sel-12 mutations suppress the Multivulva defect associated with lin-12(n950sd) (6), whereas the hop-1 allele described herein does not. (iii) Maternal expression of sel-12 is more potent than maternal expression of hop-1 in promoting glp-1 or lin-12 signaling. In strains homozygous for the sel-12 deletion, a single maternal copy of hop-1, although sufficient for normal embryonic development, is unable to prevent defects in two later glp-1 and lin-12 signaling events, germ-line proliferation, and the AC/VU decision. By contrast, in strains homozygous for the hop-1 deletion, a single maternal copy of sel-12 is sufficient not only for embryogenesis but also for germ-line proliferation and the AC/VU decision. In these animals, defects in glp-1 and lin-12 signaling are first manifested during the embryonic development of their progeny (the Mel phenotype).
Preliminary analysis of the frequencies with which phenotypes of sel-12 single mutant animals and hop-1; sel-12 double mutant animals can be suppressed also supports the notion that hop-1 and sel-12 function redundantly. Whereas we were able to find suppressors of the Egl defect of sel-12 mutants at a high frequency, we were unable to find suppressors of the sterility of hop-1; sel-12 mutants (D.P., B.W., and C.D.J., unpublished results). One interpretation of these results is that mutations that potentiate lin-12 signaling by activating hop-1 or another component of the signaling pathway might be unable to bypass the more severe reduction in glp-1 or lin-12 signaling associated with a lack of both hop-1 and sel-12 function.
Presenilins, APP, and Notch.
Since mutations in PS1 and PS2 were first identified as causing early-onset AD (1–3), much effort has been devoted to elucidating their biological function in normal and disease states. Substantial evidence suggests that presenilins regulate the proteolytic processing of APP, although their involvement in other types of disease-causing mechanisms has also been proposed (e.g., refs. 28 and 29). APP is cleaved in the extracellular and transmembrane domains, releasing the Aβ peptide (4). Cleavage in the transmembrane domain can occur at either of two sites, generating either a 40- or 42-amino acid peptide (Aβ40 or Aβ42). Presenilin mutations that cause familial AD are associated with an increased level of Aβ42, the more amyloidogenic form of the Aβ peptide (27–31). By contrast, both Aβ40 and Aβ42 levels are decreased in neurons derived from PS1 knockout mice due to reduced proteolytic cleavage at the two sites in the transmembrane domain (32). These results support a model in which presenilins facilitate APP cleavage in the transmembrane domain.
Experiments conducted in C. elegans [refs. 6 and 7 and this paper] and in mice (10, 11) have shown that presenilins promote Notch-receptor function. There is as yet no evidence that Notch-pathway signaling is involved in the pathophysiology of AD. Thus, the relationship between the roles of presenilins in proteolytic processing of APP and in facilitating Notch receptor function has been unclear. Intriguingly, recent evidence suggests that multiple proteolytic processing events are required for intracellular trafficking and signal transduction of the Notch receptor: two cleavage events are proposed to occur in the extracellular domain (33–35) and a third proposed cleavage occurs within or just carboxyl-terminal to the transmembrane region (36–38). The apparent similarities between the processing of APP and Notch, particularly the prospect that both are cleaved within the transmembrane domain, raise the possibility that presenilins affect proteolytic processing of APP and Notch in analogous ways. Presenilins might regulate proteolytic processing directly or might do so indirectly, for example, by promoting normal intracellular trafficking of APP or Notch. In support of a role for presenilins in processing or trafficking of Notch, Levitan and Greenwald (39) have recently demonstrated that LIN-12∷GFP levels at the plasma membrane are reduced in a sel-12 mutant background. An understanding of how presenilins affect Notch-receptor activity may be relevant to an understanding of the way in which presenilins affect APP cleavage and to the identification of targets for preventing the pathophysiological effects of presenilin dysfunction in AD.
Acknowledgments
We are grateful to Brian Reardon, Jill Spoerke, and Christy Harvey for excellent technical assistance; Chantale Guy for isolating hop-1 cDNAs; Bob Horvitz for strains; and Lisa Kadyk for comments on the manuscript. B.W. especially thanks Craig Mello for expert advice and all equipment and reagents pertaining to the experiments depicted in Fig. 3. Some strains were purchased from the C. elegans Genetic Stock Center, which is funded by a grant from the National Institutes of Health.
ABBREVIATIONS
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- Aβ
β-amyloid
- Mel
maternal-effect embryonic lethal
- Egl
egg-laying defective
- AC
anchor cell
- VU
ventral uterine precursor cell
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 2.Levy-Lahad E, Wasco W, Poorkaj P, Romano D M, Oshima J, Pettingell W H, Yu C E, Jondro P D, Schmidt S D, Wang K, et al. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 3.Rogaev E I, Sherrington R, Rogaeva E A, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. Nature (London) 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 5.L’Hernault S W, Arduengo P M. J Cell Biol. 1992;119:55–68. doi: 10.1083/jcb.119.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitan D, Greenwald I. Nature (London) 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Greenwald I. Proc Natl Acad Sci USA. 1997;94:12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitan D, Doyle T G, Brousseau D, Lee M K, Thinakaran G, Slunt H H, Sisodia S S, Greenwald I. Proc Natl Acad Sci USA. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C. Genes Function. 1997;1:149–159. doi: 10.1046/j.1365-4624.1997.00012.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong P C, Zheng H, Chen H, Becher M W, Sirinathsinghji D J, Trumbauer M E, Chen H Y, Price D L, Van der Ploeg L H T, Sisodia S S. Nature (London) 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 11.Shen J, Bronson R T, Chen D F, Xia W, Selkoe D J, Tonegawa S. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 12.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson P. In: Caenorhabditis elegans: Modern Biological Analysis of an Organism. Epstein H F, Shakes D C, editors. Vol. 48. San Diego: Academic; 1995. pp. 31–58. [Google Scholar]
- 14.Yandell M D, Edgar L G, Wood W B. Proc Natl Acad Sci USA. 1994;91:1381–1385. doi: 10.1073/pnas.91.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood W B. The Nematode Caenorhabditis elegans. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 16.Williams B D, Schrank B, Huynh C, Shownkeen R, Waterston R H. Genetics. 1992;131:609–624. doi: 10.1093/genetics/131.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mango S E, Thorpe C J, Martin P R, Chamberlain S H, Bowerman B. Development. 1994;120:2305–2315. doi: 10.1242/dev.120.8.2305. [DOI] [PubMed] [Google Scholar]
- 18.Austin J, Kimble J. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- 19.Bowerman B, Eaton B A, Priess J R. Cell. 1992;68:1061–1075. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- 20.Epstein H F, Miller D M I, Gossett L A, Hecht R M. In: Muscle Development. Pearson M, Epstein H, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. pp. 7–14. [Google Scholar]
- 21.Miller D M, Shakes D C. In: Caenorhabditis elegans: Modern Biological Analysis of an Organism. Epstein H F, Shakes D C, editors. Vol. 48. San Diego: Academic; 1995. pp. 365–394. [Google Scholar]
- 22.Shi Y, Mello C. Genes Dev. 1998;12:943–955. doi: 10.1101/gad.12.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priess J R, Schnabel H, Schnabel R. Cell. 1987;51:601–611. doi: 10.1016/0092-8674(87)90129-2. [DOI] [PubMed] [Google Scholar]
- 24.Mello C C, Draper B W, Priess J R. Cell. 1994;77:95–106. doi: 10.1016/0092-8674(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 25.Greenwald I S, Sternberg P W, Horvitz H R. Cell. 1983;34:435–444. doi: 10.1016/0092-8674(83)90377-x. [DOI] [PubMed] [Google Scholar]
- 26.Lambie E J, Kimble J. Development. 1991;112:231–240. doi: 10.1242/dev.112.1.231. [DOI] [PubMed] [Google Scholar]
- 27.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 28.Duff K, Eckman C, Zehr C, Yu X, Prada C M, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Nature (London) 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 29.Borchelt D R, Thinakaran G, Eckman C B, Lee M K, Davenport F, Ratovitsky T, Prada C M, Kim G, Seekins S, Yager D, et al. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 30.Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, et al. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 31.Tomita T, Maruyama K, Saido T C, Kume H, Shinozaki K, Tokuhiro S, Capell A, Walter J, Grunberg J, Haass C, et al. Proc Natl Acad Sci USA. 1997;94:2025–2030. doi: 10.1073/pnas.94.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 33.Pan D, Rubin G M. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- 34.Blaumueller C M, Qi H, Zagouras P, Artavanis-Tsakonas S. Cell. 1997;90:281–291. doi: 10.1016/s0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 35.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah N G, Israel A. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Struhl G, Adachi A. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 37.Schroeter E H, Kisslinger J A, Kopan R. Nature (London) 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 38.Lecourtois M, Schweisguth F. Curr Biol. 1998;8:771–774. doi: 10.1016/s0960-9822(98)70300-8. [DOI] [PubMed] [Google Scholar]
- 39.Levitan D, Greenwald I. Development. 1998;125:3599–3606. doi: 10.1242/dev.125.18.3599. [DOI] [PubMed] [Google Scholar]