Abstract
Adipose tissue inflammation causes metabolic disturbances, including insulin resistance and hepatic steatosis. Exercise training (EX) may decrease adipose tissue inflammation, thereby ameliorating such disturbances, even in the absence of fat loss. The purpose of this study was to 1) compare the effects of low-fat diet (LFD), EX, and their combination on inflammation, insulin resistance, and hepatic steatosis in high-fat diet-induced obese mice and 2) determine the effect of intervention duration (i.e., 6 vs. 12 wk). C57BL/6 mice (n = 109) fed a 45% fat diet (HFD) for 6 wk were randomly assigned to an EX (treadmill: 5 days/wk, 6 or 12 wk, 40 min/day, 65–70% V̇o2max) or sedentary (SED) group. Mice remained on HFD or were placed on a 10% fat diet (LFD) for 6 or 12 wk. Following interventions, fat pads were weighed and expressed relative to body weight; hepatic steatosis was assessed by total liver triglyceride and insulin resistance by HOMA-IR and glucose AUC. RT-PCR was used to determine adipose gene expression of MCP-1, F4/80, TNF-α, and leptin. By 12 wk, MCP-1, F4/80, and TNF-α mRNA were reduced by EX and LFD. Exercise (P = 0.02), adiposity (P = 0.03), and adipose F4/80 (P = 0.02) predicted reductions in HOMA-IR (r2 = 0.75, P < 0.001); only adiposity (P = 0.04) predicted improvements in hepatic steatosis (r2 = 0.51, P < 0.001). Compared with LFD, EX attenuated increases in adiposity, hepatic steatosis, and adipose MCP-1 expression from 6 to 12 wk. There are unique metabolic consequences of a sedentary lifestyle and HFD that are most evident long term, highlighting the importance of both EX and LFD in preventing obesity-related metabolic disturbances.
Keywords: obesity, insulin resistance, hepatic steatosis, macrophage
obesity results from an energy imbalance attributable to our current lifestyle, which includes little physical activity and excessive intake of foods high in saturated and trans fats and refined carbohydrates. Consumption of a high-fat Western diet (HFD) and physical inactivity are potential triggers of chronic diseases such as type 2 diabetes and cardiovascular disease. Insulin resistance (IR) and lipid accumulation in the liver are two common metabolic changes that increase the risk of such chronic diseases. It is now recognized that hepatic steatosis (the initial stage of nonalcoholic fatty liver disease) and insulin resistance should be targeted early to prevent complications associated with these conditions.
Visceral white adipose tissue (WAT) is currently believed to be the key depot linked with obesity-related systemic metabolic disturbances (6). WAT becomes inflamed during adipose tissue hypertrophy due to an influx of macrophages (MΦs) that secrete proinflammatory cytokines, including tumor necrosis factor (TNF)-α. The cause of MΦ influx into WAT is not completely understood, but an increase in the gene expression of monocyte chemoattractant protein-1 (MCP-1) in WAT has been shown to precede MΦ entry, suggesting that this chemokine plays an important role in WAT MΦ accumulation (15).
Attenuating inflammation in WAT beneficially modifies (15) these metabolic disturbances and reduces disease risk, even in the absence of obesity reduction (29). Exercise training (EX) has been shown to decrease chronic low-level systemic inflammation in humans (23, 35, 38) as well as improve insulin resistance and hepatic steatosis (8). Unfortunately, the direct effects of EX, with or without low-fat diet (LFD), on WAT inflammation in HFD-induced obesity have not been adequately investigated. Recently, EX was reported to reduce human subcutaneous WAT inflammation (5); however, a limitation of that study was that the visceral depot was not assessed (10). Animal models have shown that EX (treadmill running) lowers WAT inflammation in the viscera of nonobese animals (9, 26), suggesting that exercise may be a useful therapy. Furthermore, Bradley et al. (3) demonstrated that voluntary wheel running improved insulin resistance and decreased visceral WAT inflammation in mice simultaneously fed a HFD. Although that study suggests that exercise is effective in improving insulin resistance and decreasing WAT, those animals ran more than 10 km/day, which is difficult to translate to humans. Therefore, it is unknown whether moderate levels of EX improve visceral WAT inflammation in a model of HFD-induced obesity. To date, no studies have investigated the duration effects (i.e., short-term vs. long-term effects) of caloric reduction and exercise in terms of fat loss, metabolic complications, and WAT inflammation.
The purpose of the present study was to investigate the effects of 6 and 12 wk of EX and LFD on WAT inflammation and metabolic disturbances using HFD-induced obese C57BL/6 mice. We hypothesized that LFD would more robustly reduce body weight and fat content compared with a moderate-intensity exercise program. Yet the effects of the interventions on WAT inflammation and metabolic complications would be similar. We hypothesized that the effects of a LFD would occur earlier than the effects of EX (i.e., at 6 wk) but that the effects of EX will be evident by 12 wk. Moreover, we hypothesized that the combination of treatments would be better than either individual treatment at both intervention durations.
METHODS
Animals and diets.
Male C57BL/6 mice (n = 109) were purchased from Jackson Laboratories (Bar Harbor, ME) at 4 wk of age. After a 1-wk acclimation period, all mice were individually caged and switched to a 45% fat diet (HFD) for 6 wk to induce obesity and then were randomly assigned to HFSED (HFD, sedentary), HFEX (HFD, exercised), LFSED [10% fat diet (LFD), no sedentary], or LFEX (LFD, exercise) groups for a 6- or 12-wk period. The HFD and LFD were purchased from Research Diets (New Brunswick, NJ); more specific information regarding nutrient composition can be found in Table 1. All groups were allowed to eat ad libitum, and food intake and body weight were recorded twice/wk for the duration of the study. The animals were housed on a reverse light-dark cycle (lights on at 2100 and off at 0900) at 25°C. National Institutes of Health guidelines for the care and use of laboratory animals were strictly followed, and all experiments were approved by the Animal Care and Use Committee at the University of Illinois at Urbana-Champaign.
Table 1.
Diet composition
|
HFD |
LFD
|
|||
|---|---|---|---|---|
| %g | %kcal | %g | %kcal | |
| Protein | 24 | 20 | 19.2 | 20 |
| CHO | 41 | 35 | 67.3 | 70 |
| Fat | 24 | 45 | 4.3 | 10 |
| kcal/g | 4.73 | 3.85 | ||
|
HFD |
LFD
|
|||
|---|---|---|---|---|
| g | kcal | g | kcal | |
| Sucrose | 172.8 | 691 | 350 | 1,400 |
| Soybean oil | 25 | 225 | 25 | 225 |
| Lard | 177.5 | 1,598 | 20 | 180 |
| Total | 858.15 | 4,057 | 1,055.05 | 4,057 |
HFD, high-fat diet; LFD, low-fat diet; CHO, carbohydrate. Rodent diets D12450B and D12451; Research Diets (New Brunswick, NJ).
EX protocol.
Animals were exercise trained (EX) during their dark cycle (i.e., during their active period) between 0900 and 1200 on a motorized treadmill (Jog-a-Dog, Ottawa Lake, MI) for 40 min/day at 12 m/min, 12% grade, 5 times/wk, for 6 or 12 wk; this intensity corresponds to ∼65–70% of maximal oxygen uptake (27). A foam sponge was placed in the back of each treadmill lane to prevent the animal from being injured. Negative reinforcement (e.g., electrical shock) was never used to force running, and all animals complied with the exercise protocol. The duration and intensity were increased gradually so that the animals were running at the prescribed level by the eighth training session. To control for any stress associated with the training protocol, our control animals were exposed to the same noise and handling as the exercisers. Spleens were extracted and weighed following the intervention to assess splenomegaly, an indicator of immune dysfunction or infection.
Real-time quantitative PCR.
Epididymal and retroperitoneal fat pads were harvested immediately after euthanization, weighed, flash-frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions and assessed for purity using the NanoDrop system (NanoDrop Technologies, Wilmington, DE). Total mRNA (500 ng) was reverse transcribed to cDNA using AffinityScript (Stratagene, Agilent Technologies, Palo Alto, CA) according to the manufacturer's instructions. RT-PCR was performed using the Mx3000P real-time PCR system using Brilliant II SYBR Green Master Mix kits (Stratagene, Agilent Technologies). The thermal profiles consisted of 10 min at 95°C for denaturing followed by 40 cycles of 95°C for 30 s, annealing at 60°C for 1 min, and 72°C for 30 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene, and all data are represented relative to its expression (i.e., using the ΔΔCT method) as fold change from HFSED. All duplicate or triplicate CT values were within 0.5 CT units of each other. All primer sequences were designed using IDT (Skokie, IL), verified using the National Center for Biotechnology Information (NCBI) BLAST feature, and purchased from MWG Biotech (Huntsville, AL). Primer sequences, product sizes, and NCBI accession numbers can be supplied upon request.
Plasma analytes.
Two days after the last exercise bout, all mice were fasted for 12 h and then euthanized via CO2 inhalation. Blood was collected from the vena cava immediately after euthanization, centrifuged for 15 min at 2,400 rpm at 4°C, and plasma was aliquotted and stored at −80°C until use. Glucose was measured using an automated analyzer (YSI 2300; YSI Life Sciences, Yellow Springs, OH) via the glucose oxidase method. Insulin was measured using commercial ELISA kits (R&D Systems, Minneapolis, MN) with a sensitivity of 0.2 ng/ml, an interassay percent coefficient of variation (%CV) of 6.03–17.9, and an intra-assay %CV of 0.92–8.35. Plasma serum amyloid A (SAA) and adiponectin levels were measured using commercial ELISA kits (SAA, sensitivity of 12.5 ng/ml: Alpco Immunoassays, Salem, NH; adiponectin, sensitivity of 0.1 ng/ml, inter-assay %CV of 3.8–7.7: BioVendor, Candler, NC) according to the manufacturers’ instructions. All samples were run in duplicate.
Assessment of IR.
IR was estimated using the homeostasis model assessment (HOMA) method (19) using postintervention serum samples. This method is routinely used for assessment of IR in humans and rodents and is strongly correlated with the glucose clamp method (the gold standard for determination of insulin sensitivity) (32). Intraperitoneal (ip) glucose tolerance tests (GTT) were also performed during the last week of training (e.g., after 5 or 11 wk, for the 6- and 12-wk interventions, respectively) to assess glucose tolerance. Following a 12-h fast, tail vein blood was collected before and 30, 60, 90, and 120 min following an ip injection of glucose (2 g/kg). Blood glucose concentrations were measured at each time point using a glucometer (OneTouch Ultra2; Johnson & Johnson, Langhorne, PA), and the total area under the glucose curve (AUC) was calculated using the trapezoidal rule (16).
Assessment of hepatic triglyceride content.
Whole livers were extracted after euthanization, weighed, and stored at −80°C until use. Liver tissue (50–200 mg) was homogenized for 7–10 min in 4 ml of isopropanol with a polytron disrupter. The homogenate was centrifuged at 4,600 rpm for 10 min, and 5 μl of the resulting supernatant was used to determine triglyceride (TG) content using the microtiter procedure of the L-type TG H test (Wako Diagnostics, Richmond, VA).
Statistics.
Body weight (BW) changes throughout the 6- and 12-wk interventions were analyzed using a one-way analysis of variance (ANOVA) with repeated measurements; post hoc Tukey HSD comparisons were used to determine individual group differences when a significant F ratio was obtained. Differences in initial BW, final BW, final organ weights, fasting glucose and insulin, glucose AUC, hepatic TG content, HOMA-IR, plasma SAA, plasma adiponectin, food intake, and WAT mRNA levels were determined using one-way ANOVA with post hoc Tukey comparisons where appropriate. Prevalence of HS was assessed using chi-square analysis. Duration effects were assessed using a univariate three-way ANOVA with EX (EX or SED), LFD (HF or LF), and duration (6 vs. 12 wk) as between-subjects factors; interaction effects were also determined. To determine whether treatment effects were independent of relative epididymal adiposity, the epididymal fat pad/BW ratio was used as a covariate. Correlation values were Pearson's bivariate. Where applicable, linear regression analyses were used. All analyses were done using SPSS V15 (SPSS, Chicago, IL), and data are presented as means ± SE unless noted otherwise. α-Level for main effects was set at P ≤ 0.05.
RESULTS
EX and LFD attenuate BW and WAT gain in obese mice.
After 6 wk of HFD feeding, there were no preintervention group differences in BW between mice randomized to the 6-wk or 12-wk intervention (Table 2). Compared with all other groups, HFSED gained more weight over the course of either the 6- (P < 0.001) or 12-wk (P < 0.001) interventions. Among the three interventions, LFEX gained less weight than HFEX (P < 0.05) but not LFSED after either length intervention. Epididymal fat pad weight was also reduced by the interventions (P < 0.05). Only after the 12-wk intervention was there an additive benefit of the combined treatment, as LFEX exhibited significantly lower epididymal fat pad weight compared with HFEX or LFSED (Table 2). Similar results were found when epididymal fat pad weight was expressed as relative to BW (Table 2) and for retroperitoneal fat pad weight (data not shown). Heart weight/BW ratio [a general indicator for an aerobic training effect (14)] tended (P = 0.08) to be elevated in the intervention groups after 12 wk of intervention. At 6 wk, there was a group main effect for caloric intake; HFSED consumed more calories than both LFD groups. In response to the 12-wk intervention, HFSED consumed more calories than both LFD groups, and the HFEX group consumed more calories than LFSED but not LFEX (Table 2). In no instance did any mouse exhibit overt splenomegaly or symptoms (e.g., reduced weight, food intake, grooming behavior, or nest building) indicative of acute infection, nor were there any visible tumors upon necropsy.
Table 2.
Body weight, fat pad weight, and caloric intake in response to 6- or 12-wk interventions
|
6-Wk Intervention |
12-Wk Intervention
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HFSED | HFEX | LFSED | LFEX | HFSED | HFEX | LFSED | LFEX | |
| n | 14 | 13 | 13 | 15 | 13 | 13 | 14 | 14 |
| Initial BW, g | 25.8±0.4 | 24.8±0.4 | 25.5±0.4 | 25.0±0.5 | 24.7±0.7 | 24.8±0.6 | 25.0±0.8 | 25.0±0.7 |
| Final BW, g | 32.1±0.9a | 28.2±0.7b | 26.9±0.3b,c | 24.9±0.4c | 38.5±1.2a* | 34.0±1.2b* | 30.7±0.8b,c* | 29.4±0.6c* |
| Epididymal fat weight, g | 1.8±0.2a | 1.0±0.1b | 0.7±0.03b,c | 0.5±0.03c | 2.1±0.08a* | 1.5±0.2b* | 1.1±0.1b* | 0.6±0.05c |
| REA, % | 5.4±0.3a | 3.6±0.4b | 2.6±0.1b,c | 2.1±0.1c | 5.6±0.2a* | 4.3±0.4b | 3.6±0.2b* | 2.0±0.1c |
| Heart weight/BW | 0.65±0.08 | 0.84±0.2 | 0.65±0.05 | 0.75±0.07 | 0.40±0.04* | 0.51±0.05 | 0.54±0.04 | 0.60±0.06 |
| Cumulative caloric intake, kcal | 512±16a | 474±12a,b | 455±14b | 426±8b | 1,039±23a* | 994±20a,b* | 916±18c* | 936±13b,c* |
Values are means ± SE. HFSED, HFD, sedentary; HFEX, HFD, exercised; LFSED, 10% fat diet (LFD), no sedentary; LFEX, LFD, exercise; n, no. of animals; BW, body weight; REA, epididymal fat pad weight/BW. Different letters represent significant between-group differences within time point based on ANOVA with post hoc Tukey's test.
Significant duration effect within group.
Differential treatment effects on insulin sensitivity.
After the 6-wk intervention, fasting blood glucose was reduced in HFEX compared with HFSED (Table 3). By 12 wk, both EX groups exhibited lower fasting blood glucose compared with HFSED, and the combined intervention resulted in lower glucose compared with LFD alone. In contrast, 6-wk fasting insulin values were lower in response to all interventions compared with HFSED (P < 0.05). By 12 wk, both LFD groups, but not HFEX, had lower fasting insulin vs. HFSED (P < 0.05). As a result, HOMA-IR was lower in all intervention groups after the 6-wk intervention but only in the LFD groups after 12 wk of intervention. There was a duration effect (P = 0.009), but not a group × duration effect (P = 0.57), such that HOMA-IR increased over time in all groups. GTT revealed that after 6 wk of intervention both LFD groups, but not HFEX, were better able to reduce glucose AUC (P < 0.001). LFEX was also more efficacious compared with EX alone (i.e., HFEX). After 12 wk of intervention, both LFD interventions reduced glucose AUC (P < 0.001) compared with groups ingesting HFD (Table 3). There were no duration or treatment × duration effects for glucose AUC. Regression analyses revealed that F4/80 WAT gene expression was most strongly related to HOMA-IR at 12 wk (β = 0.83, P < 0.001; regression r2 = 0.75; F4,42 = 29.1, P < 0.001). Adiposity (β = 0.56, P < 0.001), HFD (β = 0.27, P = 0.05), and lack of EX (i.e., SED) (trend β = 0.22, P = 0.06) were also associated with HOMA-IR.
Table 3.
Fasting blood glucose, insulin, HOMA-IR, and glucose AUC
|
6-Wk Intervention |
12-Wk Intervention
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HFSED | HFEX | LFSED | LFEX | HFSED | HFEX | LFSED | LFEX | |
| n | 5 | 7 | 5 | 6 | 12 | 13 | 14 | 14 |
| Glucose, mmol/l | 13.27±0.6a | 10.51±0.30b | 11.50±0.66a,b | 11.43±0.85a,b | 12.87±0.43a | 10.88±0.29b,c | 11.75±0.45a,b | 9.52±0.34c* |
| Insulin, μIU/ml | 15.95±1.8a | 10.23±0.4b | 10.78±1.1b | 7.90±0.7b | 24.52±4.7a | 20.91±3.4a,b | 13.96±1.1a,b* | 12.61±1.3b* |
| HOMA-IR | 9.41±1.2a | 4.77±0.2b | 5.39±0.6b | 4.07±0.5b | 14.08±2.8a | 10.15±1.7a,b* | 7.34±0.7b | 5.4±0.6b |
| Glucose AUC | 49,563±800a | 42,210±2,243a,b | 35,019±2,235b,c | 30,555±2,805c | 47,182±1,925a | 46,648±2,608a | 37,733±1,941b | 35,418±2,439b |
Values are means ± SE. HOMA-IR, homeostatic model of insulin resistance; AUC, area under the curve. All variables except glucose AUC were measured in the fasted state (12 h of no food). Different letters represent significant between-group differences within time point based on ANOVA with post hoc Tukey's test.
Significant duration effect within group.
Exercise, but not LFD, attenuates obesity-related hepatic steatosis.
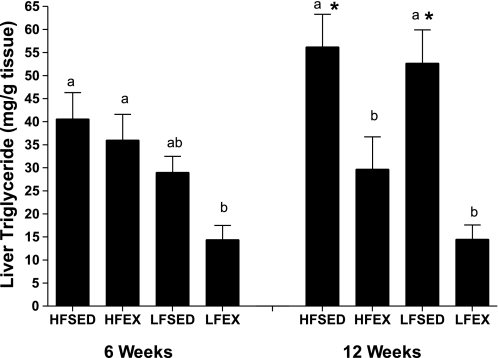
There were no between-group differences in liver weight in response to 6-wk intervention (0.82 ± 0.06, 0.83 ± 0.06, 0.83 ± 0.06, and 0.72 ± 0.06 for HFSED, HFEX, LFSED, and LFEX, respectively). In response to the 12-wk intervention, although all interventions reduced liver weight (1.2 ± 0.10, 0.91 ± 0.05, 0.96 ± 0.04, and 0.92 ± 0.06 for HFSED, HFEX, LFSED, and LFEX, respectively; P = 0.02), only HFEX and LFEX did so significantly relative to HFSED. At 6 wk, there were differences in liver triglycerides (Fig. 1) such that LFEX was lower than HFSED and HFEX (P = 0.001). In contrast, at 12 wk, both exercise groups (HFEX and LFEX) exhibited lower liver TG values compared with the SED groups (P < 0.001). Regression analysis revealed adiposity (β = 0.73, P = 0.001; regression r2 = 0.63; F5,42 = 12.6, P < 0.001) to be the strongest correlate of hepatic steatosis, independent of F4/80 (P = 0.70), SED (P = 0.69), and HOMA-IR (P = 0.12). Consumption of LFD (β = 0.53, P = 0.004) was also positively related to steatosis.
Fig. 1.
Effect of a 6- or 12-wk exercise or low-fat diet intervention on liver triglyceride values in obese mice. Different letter designations represent significant (P < 0.05) group differences within 6- or 12-wk intervention. *Significant within-treatment group difference at 12- vs. 6-wk intervention. Data are means ± SE; n = 12–15 mice/group. HFSED, high-fat diet, sedentary; HFEX, high-fat diet, exercised; LFSED, 10% fat diet (low-fat diet), no sedentary; LFEX, low-fat diet, exercise.
When comparing outcomes between the 6- and 12-wk durations, we found a treatment by duration effect (P = 0.03), indicating that HFD feeding from 6 to 12 wk resulted in an additional increase in hepatic steatosis that was completely eliminated when mice were exercised, regardless of diet (Fig. 1). Clinically significant hepatic steatosis is defined as liver TG exceeding 5% of liver weight (2). Based upon this definition, when we compared the percentages of steatotic mice between treatment groups, we found that EX (χ2 = 9.5, P = 0.002), but not LFD (χ2 = 2.2, P = 0.12), significantly reduced the incidence of hepatic steatosis in response to the 12- but not 6-wk intervention.
Exercise and diet intervention reduces systemic inflammation, and exercise reduces adiponectin in HFD-fed mice.
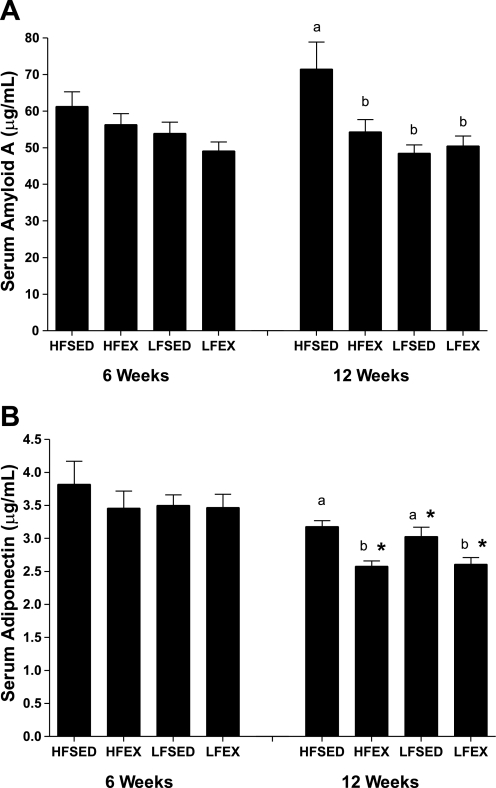
In a random subset of mice (n = 9–10/group), we found that the systemic inflammatory marker SAA was lowered by all interventions (P = 0.003), with no differences between interventions at the 12-wk time point (Fig. 2A). There were no differences between groups at 6 wk, and there was no effect of intervention duration on SAA.
Fig. 2.
Effects of 6- or 12-wk intervention on serum amyloid A (A) and serum adiponectin (B). Different letters represent significant between-group differences within 6- or 12-wk intervention. *Significant within-treatment group difference at 12- vs. 6-wk intervention. Data are means ± SE; n = 9–10/group.
Adiponectin levels were not different between groups at the 6-wk time point. At 12 wk, we found a group effect such that both EX groups were lower than both SED groups (P < 0.001). There was a duration effect on adiponectin levels such that adiponectin levels decreased over time in the intervention groups (P < 0.001; Fig. 2B).
Effects of exercise and LFD intervention on WAT adipocytokine gene expression.
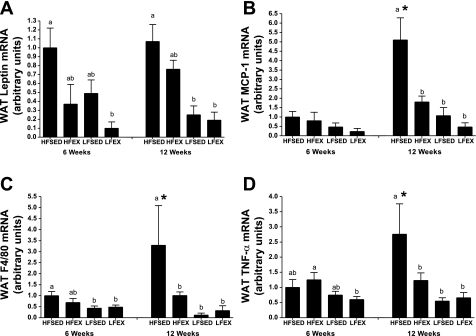
The housekeeping gene GAPDH was used to correct mRNA expression values for all genes examined, and there were no intervention-induced differences in GAPDH (data not shown). At 6 wk, there was a difference (P = 0.003) in WAT leptin gene expression such that LFEX was lower than HFSED (P < 0.05; Fig. 3A). In contrast, by 12 wk both LFSED and LFEX leptin mRNA levels were lower than HFSED (P < 0.05). Exercising while on a HFD tended to result in lower WAT leptin mRNA expression (P = 0.09). There were no intervention duration effects for WAT leptin mRNA expression.
Fig. 3.
Effects of 6- or 12-wk interventions on white adipose tissue (WAT) adipocytokine leptin (A), monocyte chemoattractant protein-1 (MCP-1; B), F4/80 (C), and TNF-α (D) gene expression in obese mice. Different letters represent significant between-group differences within time point based on ANOVA with post hoc Tukey's test. *Significant within-treatment group difference at 12- vs. 6-wk intervention. Values are means ± SE; n = 8–15/group.
WAT MCP-1 mRNA expression was not significantly (P = 0.06) affected by the 6-wk intervention; however, EX and LFD interventions resulted in lower MCP-1 expression in WAT by 12 wk (P < 0.001) (Fig. 3B). Moreover, there was an intervention duration interaction effect (P < 0.001) such that WAT MCP-1 mRNA expression was higher at 12 wk compared with 6 wk in the HFSED but not the other groups (Fig. 3B).
WAT F4/80 mRNA expression (indicative of MΦ presence) was affected by the 6-wk intervention such that both LFD groups expressed less F4/80 mRNA compared with HFSED (P = 0.004) but not HFEX (Fig. 3C). By 12 wk of intervention, all EX and LFD interventions exhibited lower (P = 0.001) F4/80 mRNA compared with HFSED. As was the case with WAT MCP-1 expression, F4/80 expression also increased (P = 0.001) at the 12-wk time point (vs. 6 wk) in the HFSED but not the other groups (Fig. 3C).
In response to 6 wk of intervention, LFD, but not EX, reduced WAT TNF-α gene expression; LFEX had lower (P = 0.04) TNF-α than HFEX (Fig. 3D). In stark contrast, after 12 wk of intervention both LFD and EX lowered TNF-α mRNA expression in visceral WAT (P < 0.001). Similar to MCP-1 and F4/80 gene expression, continued HFD feeding from 6 to 12 wk led to further increases in TNF-α mRNA (duration effect, P = 0.002). This increase was abolished in response to either EX, LFD, or their combination (Fig. 3D).
DISCUSSION
The most important finding of this study was that moderate exercise (EX), LFD, or their combination resulted in a significant attenuation of HFD-induced increases in systemic and WAT inflammation. These effects were stronger after 12 wk compared with 6 wk. At 6 wk, only the combined treatment reduced adiposity, leptin gene expression, and hepatic steatosis. Both LFD interventions reduced adipose F4/80 gene expression, and all three interventions improved insulin resistance. By 12 wk, all interventions reduced adiposity, circulating SAA, and adipose F4/80, MCP-1, and TNF-α gene expression. Interestingly, LFD had a more robust ability to reduce adiposity and insulin resistance, yet the effects of the individual treatments on inflammatory outcomes were similar. These findings demonstrate that both EX and LFD play important roles in reducing WAT inflammation and the associated metabolic disturbances and that the mechanisms responsible for their effects may be different.
It has recently been reported that accumulation of visceral WAT is causally related to metabolic outcomes (6), a relationship presumably due to the inflammatory processes that take place in obese WAT. In this study, both LFD and EX attenuated body weight (BW) gain and adiposity after the initial 6-wk HFD feeding period, although the LFD had a more vigorous effect. Interestingly, by 12 wk, LFD and EX had equal and additive effects on adiposity. Furthermore, the only significant increase in relative adiposity from 6 to 12 wk was in the LFSED group, suggesting that EX effectively attenuated adipose tissue accretion in the viscera, whereas LFD in isolation did not. Studies have concluded that EX has important benefits over diet alone, specifically in terms of its effects on preferentially reducing visceral adipose (30), which our findings support. Combining the moderate exercise program with a reduction in caloric intake was more robust than either single intervention, supporting the current public health guidelines suggesting that Americans perform at least moderate amounts of physical activity (1) and reduce their saturated fat intake to less than 10% of total calories (31).
Similar to the stronger fat-reducing effects of LFD compared with EX at 6 wk, only LFD resulted in lower epididymal WAT F4/80 and TNF-α gene expression. However, the more robust fat-reducing effects of LFD early on may have resulted in this intervention having a more profound effect on WAT inflammation. By 12 wk both LFD and EX reduced MCP-1, F4/80, and TNF-α, suggesting that by 12 wk EX was just as effective as LFD in decreasing relative adiposity and WAT inflammation. Furthermore, it seems that, by the end of the extended intervention, both LFD and EX lowered WAT inflammation by mechanisms that were not fully explained by reduction in visceral fat. That is, when statistical adjustments were made for the effect of adiposity reduction, both interventions still lowered inflammation (data not shown). To determine the kinetics of WAT inflammation in obesity and how EX and LFD may mitigate this, we examined the effect of intervention duration on inflammatory markers in the WAT. Compared with HFSED, all three interventions completely prevented the inflammatory exacerbation from 6 to 12 wk (MCP-1, F4/80, TNF-α), suggesting that all interventions were equally effective at attenuating adipose tissue inflammation.
The increase in WAT inflammation in obesity contributes to systemic inflammation (18), as measured by C-reactive protein (4) and SAA (39). Indeed, we found significant correlations between SAA and F4/80 (r = 0.8, P < 0.001), MCP-1 (r = 0.4, P < 0.01), and TNF-α (r = 0.7, P < 0.001). These findings support other work showing WAT MΦ infiltration to be a causative factor in systemic inflammation and its metabolic consequences (22). Although there were no group differences in circulating levels of SAA at 6 wk, all treatments effectively reduced SAA by 12 wk, an effect that was reduced when adiposity was adjusted (data not shown), suggesting that fat loss contributed to this reduction in circulating inflammation.
Adiponectin levels are usually inversely related to insulin resistance and inflammation and increase with weight loss and/or exercise (21). Quite surprisingly, in this study, EX decreased circulating levels of adiponectin at 12 wk, which remained significant after the effect of adiposity was adjusted, suggesting that EX lowered adiponectin levels at 12 wk by a mechanism unrelated to changes in visceral adipose tissue. Moreover, adiponectin levels decreased from 6 to 12 wk in all interventions. Studies on the effects of EX on circulating adiponectin have been equivocal (24), with most studies showing a beneficial (i.e., increasing) effect of fat loss, but not exercise per se (13), and some showing additional benefits of vigorous exercise (7, 25). No previous studies have reported an adiponectin-reducing effect of exercise, but no studies have investigated how adiponectin levels differ at different-length exercise interventions in mice. More research is necessary to definitively determine the unique effects of EX and LFD on adiponectin as well as the implications and mechanisms behind these effects.
At 6 wk, all interventions were equally effective at improving HOMA-IR, but only LFD significantly improved glucose tolerance. By 12 wk, LFD (but not EX) improved HOMA-IR and glucose tolerance. This may have been attributed to the better fat-reducing effect of LFD compared with EX. Cytokines produced by WAT (11) as well as the MΦs that reside there (22) have been shown to interfere with insulin signaling, causing insulin resistance. In support of this idea, regression analyses revealed F4/80 WAT gene expression to be most strongly related to HOMA-IR at 12 wk.
In contrast to the results on insulin resistance, although both treatments reduced hepatic steatosis at 6 wk, only EX effectively did so by 12 wk. Previous studies have shown that weight loss (20) and EX (8) reduce hepatic steatosis, whereas high-carbohydrate diets (such as the LFD used here, which is 70% sucrose) may increase hepatic steatosis by promoting de novo lipogenesis (12). Here we also report that continued consumption of the LFD was related to an increase in hepatic steatosis, because there was a significant effect of intervention duration such that only the LFSED group experienced an increase from 6 to 12 wk. This is unlike studies that have shown that switching from a HFD to a normal rodent chow diet reverses hepatic steatosis; the lack of such an effect in this study is probably due to the high sucrose content of the LFD compared with standard rodent chow. The LFD used in this study has been used as a high-carbohydrate diet in other studies (37). Importantly, the current study illustrates that EX, regardless of diet, is an effective means to prevent hepatic steatosis. The fact that EX did not affect insulin resistance, but did improve hepatic steatosis at 12 wk, may suggest that hepatic steatosis is affected before insulin resistance. This is an important finding because no studies have yet identified the time course with which these metabolic disturbances occur and/or are reduced with behavioral interventions. Our findings are in support of recent human studies showing visceral adipose accumulation to be predictive of hepatic steatosis (33). Regression analysis at 12 wk revealed that relative visceral adiposity was the most important correlate of hepatic steatosis, independent of HOMA-IR and WAT expression of F4/80, MCP-1, and TNF-α. This suggests that other factors residing in, or related to, WAT that were not measured in this study may also be important mediators of hepatic steatosis.
It has been shown that diets high in saturated fats directly cause WAT inflammation, and the mechanism is thought to involve Toll-like receptor signaling (36) and/or MΦ phenotype switching toward an inflammatory profile (17), which may help explain the independent effects of LFD on the inflammatory and metabolic outcomes reported here. The finding that EX has unique anti-inflammatory effects is novel and likely will have important implications, given the strong independent relationship between WAT inflammation and obesity-related comorbidities (33) and the lack of long-term success of dietary interventions without exercise (34). Mechanisms by which EX reduces WAT inflammation may involve improvements in the “health” of the WAT, including reduced adipocyte size, increased blood flow, increased mitochondrial function and facilitated fatty acid oxidation, decreased cellular stress, and/or improved resistance to cell stress. Recent studies have implicated hypoxia as a cause of adipose tissue inflammation in obesity (40). Although no studies have yet determined whether exercise decreases hypoxia in obese WAT, it is plausible that an exercise-related increase in WAT blood flow may mediate its anti-inflammatory effects on WAT.
The findings presented here should be considered along with this study's limitations. The mode of EX used in this study was treadmill running, which has the disadvantage of the stress associated with its forced nature (although our control animals were yoked). Treadmill training has significant advantages over voluntary wheel running (which could be considered an observational intervention) because the intensity and duration of the running can be accurately dosed. When given a running wheel, some mice partake in extreme physical activity (>10 km/day), and the variability in the amount run between mice is high and difficult to control. Thus, the public health generalizability of such doses (e.g., 10 km) of exercise could be questioned. We feel that treadmill running is an appropriate model to examine the influence of exercise. More studies where fat loss is strictly matched in LFD and EX groups to definitively test the hypothesis that EX and LFD have adipose tissue loss-independent effects are necessary. Also, because tissues needed to be extracted for the studies to be carried out, the ideal repeated-measures design, where the same animals were used at both time points, was not an option. Thus, the analyses of intervention duration were done using different sets of mice. Finally, although F4/80 gene transcription is often used as an indicator of MΦ presence (28), direct assessment of WAT MΦ content is needed.
In summary, the current findings confirm previous data regarding the potential anti-inflammatory effect of EX and contribute insight regarding the time course with which these interventions improve obesity and its related metabolic complications. Although at 6 wk of intervention improvements in metabolic disturbances and WAT inflammation were likely attributed to reductions in relative adiposity, by 12 wk independent treatment effects were observed such that both EX and LFD independently reduced WAT inflammation. EX improved hepatic steatosis, whereas LFD improved glucose tolerance and HOMA-IR. LFD caused more robust reductions in relative adiposity early on, but EX was equally effective by 12 wk. EX also powerfully attenuated the increase in hepatic steatosis that occurred in the absence of obesity interventions over time, whereas hepatic steatosis increased on a LFD. Taken together, these findings suggest that LFD, EX, and visceral WAT reduction all impart anti-inflammatory properties to the WAT and improve metabolic outcomes.
GRANTS
This research was supported by National Institute on Aging Grant AG-023580 and an American College of Sports Medicine doctoral student research grant to V. J. Vieira.
Acknowledgments
We thank Drs. Rodney Johnson and Kimberly Huey for assistance with the conduct of the study.
REFERENCES
- 1.No authors listed. Physical activity guidelines for Americans. Okla Nurse 53: 25, 2008. [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 295: E586–E594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning LM, Jebb SA, Mishra GD, Cooke JH, O'Connell MA, Crook MA, Krebs JD. Elevated sialic acid, but not CRP, predicts features of the metabolic syndrome independently of BMI in women. Int J Obes Relat Metab Disord 28: 1004–1010, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290: E961–E967, 2006. [DOI] [PubMed] [Google Scholar]
- 6.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 54: 945–955, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson M, Johnson O, Boman K, Hallmans G, Hellsten G, Nilsson TK, Soderberg S. Improved fibrinolytic activity during exercise may be an effect of the adipocyte-derived hormones leptin and adiponectin. Thromb Res 122: 701–708, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol 94: 2127–2134, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Merino D, Drogou C, Guezennec CY, Chennaoui M. Effects of chronic exercise on cytokine production in white adipose tissue and skeletal muscle of rats. Cytokine 40: 23–29, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2: 367–373, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest 94: 1543–1549, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Dedousis N, O'Doherty RM. Hepatic steatosis and plasma dyslipidemia induced by a high-sucrose diet are corrected by an acute leptin infusion. J Appl Physiol 102: 2260–2265, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs DR, Sluik D, Rokling-Andersen MH, Anderssen SA, Drevon CA. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: results from the 1-y randomized Oslo Diet and Exercise Study. Am J Clin Nutr 89: 509–517, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Kamiñski KA, Oledzka E, Bialobrzewska K, Kozuch M, Musial WJ, Winnicka MM. The effects of moderate physical exercise on cardiac hypertrophy in interleukin 6 deficient mice. Adv Med Sci 52: 164–168, 2007. [PubMed] [Google Scholar]
- 15.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care 13: 172–175, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord 28: 993–997, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Moschen AR, Tilg H. Nutrition in pathophysiology and treatment of nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care 11: 620–625, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Palomer X, Pérez A, Blanco-Vaca F. [Adiponectin: a new link between obesity, insulin resistance and cardiovascular disease]. Med Clin (Barc) 124: 388–395, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8: 301–309, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen BK The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 42: 105–117, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr 138: 2293–2296, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DA, McMurray RG, Harrell JS, Thorpe DE, Hackney AC. Vigorous physical activity and cytokines in adolescents. Eur J Appl Physiol 103: 495–500, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai T, Izawa T, Kizaki T, Ogasawara JE, Shirato K, Imaizumi K, Takahashi K, Ishida H, Ohno H. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem Biophys Res Commun 379: 605–609, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol 31: 387–392, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology 148: 2753–2763, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhausl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia 49: 2109–2119, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Tsai AC, Sandretto A, Chung YC. Dieting is more effective in reducing weight but exercise is more effective in reducing fat during the early phase of a weight-reducing program in healthy humans. J Nutr Biochem 14: 541–549, 2003. [DOI] [PubMed] [Google Scholar]
- 31.US Department of Agriculture. Dietary Guidelines for Americans, 2005. Washington, DC: USDA, 2005.
- 32.Vaccaro O, Masulli M, Cuomo V, Rivellese AA, Uusitupa M, Vessby B, Hermansen K, Tapsell L, Riccardi G. Comparative evaluation of simple indices of insulin resistance. Metabolism 53: 1522–1526, 2004. [DOI] [PubMed] [Google Scholar]
- 33.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, Peduto T, Chisholm DJ, George J. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 48: 449–457, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Van Dorsten B, Lindley EM. Cognitive and behavioral approaches in the treatment of obesity. Endocrinol Metab Clin North Am 37: 905–922, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Vieira V, Hu L, Valentine RJ, McAuley E, Evans EM, Baynard T, Woods JA. Reduction in trunk fat predicts cardiovascular exercise training-related reductions in C-Reactive protein. Brain Behav Immun. In press. [DOI] [PubMed]
- 36.Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, Freedman J, Gokce N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 16: 932–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams EA, Perkins SN, Smith NC, Hursting SD, Lane MA. Carbohydrate versus energy restriction: effects on weight loss, body composition and metabolism. Ann Nutr Metab 51: 232–243, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Neurol Clin 24: 585–599, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg AP, Shuldiner AR, Fried SK, Gong DW. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med 3: e287, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye J Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33: 54–66, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]