Abstract
Weight gain induced by an energy-dense diet is hypothesized to arise in part from defects in the neuronal response to circulating adiposity negative feedback signals, such as insulin. Peripheral tissue insulin resistance involves cellular inflammatory responses thought to be invoked by excess lipid. Therefore, we sought to determine whether similar signaling pathways are activated in the brain of rats fed a high-fat (HF) diet. The ability of intracerebroventricular (icv) insulin to reduce food intake and activate hypothalamic signal transduction is attenuated in HF-fed compared with low-fat (LF)-fed rats. This effect was accompanied by both hypothalamic accumulation of palmitoyl- and stearoyl-CoA and activation of a marker of inflammatory signaling, inhibitor of κB kinase-β (IKKβ). Hypothalamic insulin resistance and inflammation were observed with icv palmitate infusion or HF feeding independent of excess caloric intake. Last, we observed that central IKKβ inhibition reduced food intake and was associated with increased hypothalamic insulin sensitivity in rats fed a HF but not a LF diet. These data collectively support a model of diet-induced obesity whereby dietary fat, not excess calories, induces hypothalamic insulin resistance by increasing the content of saturated acyl-CoA species and activating local inflammatory signals, which result in a failure to appropriately regulate food intake.
Keywords: lipotoxicity, fatty acid, adipose
the incidence of obesity has increased dramatically over the last 15 years, such that two-thirds of adults in the US are now overweight or obese, and the prevalence of childhood obesity has increased similarly (22). In light of clear evidence (37) that body adiposity in normal individuals is constrained by physiological mechanisms that protect against weight gain, dysfunction of biological mechanisms that control body weight is implicated in the rapid increase of obesity prevalence in human populations. Delineating the nature of this dysfunction is a high priority.
A current model of energy homeostasis proposes that body adiposity is regulated by an endocrine “adiposity” negative feedback loop involving the hormones insulin and leptin (33). Both hormones function in a negative feedback manner to reduce food intake while increasing energy expenditure via actions on target neurons found in the arcuate nucleus (arc) of the hypothalamus (33) and elsewhere in the brain (9). Such neurons express receptors for insulin and leptin (2, 41), respond directly to these hormones (38, 39), and project to key brain areas involved in energy homeostasis (9).
In the setting of positive energy balance (energy intake in excess of expenditure), this model predicts that increased circulating insulin and leptin levels should elicit neuronal responses that reduce food intake, increase energy expenditure, and protect against pathological expansion of fat mass. Indeed, this integrated response is well described in animal models in which food is delivered directly to the stomach in amounts that exceed ongoing energy needs (34) but appears to be disrupted in common forms of human obesity and in rodent models of diet-induced obesity (DIO) in which palatable food is voluntarily overconsumed. In these forms of obesity, increased fat mass is accompanied by the expected elevation of plasma insulin and leptin levels; however, food intake remains normal or elevated (19). These findings suggest that DIO is characterized by the acquisition of functional central nervous system (CNS) resistance to insulin (6) and leptin (23), which, in turn, contributes to pathological weight gain. Since insulin and leptin can utilize common intracellular signal transduction pathways (24, 26, 27), neuronal resistance to these hormones may involve the same or similar mechanisms. Here, we seek to extend emerging evidence (6, 44) that CNS insulin resistance involves hypothalamic inflammation induced by over nutrition via mechanisms analogous to those mediating peripheral insulin resistance in obese individuals.
In peripheral tissues such as muscle or liver, the effect of insulin to lower blood glucose levels depends on activation of the insulin receptor, insulin receptor substrate (IRS) proteins, and phosphatidylinositol 3-kinase (PI3K). Consistent with this hypothesis, acquired defects in intracellular PI3K signal transduction are implicated in the pathogenesis of insulin resistance in obesity and diabetes (13, 36). One mechanism for the pathogenesis of insulin resistance in peripheral tissues proposes that intracellular accumulation of long-chain fatty acyl-CoA molecules (or metabolites thereof) activates serine/threonine kinase signal transduction cascades (potentially involving PKCθ and/or IKKβ) that dampen insulin signal transduction via serine phosphorylation of the insulin receptor and IRS proteins.
We and others (3, 26) have demonstrated that insulin activates PI3K in the mediobasal hypothalamus and that the ability of intracerebroventricularly administered insulin to inhibit food intake requires intact neuronal PI3K signaling. Since obesity is associated with insulin resistance at the level of PI3K signaling in peripheral tissues (30), we hypothesized that a similar defect in insulin-stimulated PI3K activation also occurs in the hypothalamus and thereby favors further weight gain. Consistent with this hypothesis, De Souza et al. (6) recently reported that DIO in rodents induces inflammatory gene and cytokine expression in the hypothalamus and that these effects are associated with diminished hypothalamic insulin signal transduction. Further, genetic interventions designed to reduce neuronal IKKβ signaling in mice were recently reported to reduce intake of a high-fat (HF) diet, whereas interventions that increase neuronal IKKβ signaling had the opposite effect (44). Thus cellular inflammation induced in hypothalamic neurons by nutrient excess is implicated in the mechanism whereby HF diets promote obesity.
In the current work, we first sought to confirm previous reports (6) that obesity induced by HF feeding blunts the food-intake-lowering effects of insulin delivered directly into the brain and that this effect is accompanied by impaired activation of hypothalamic PI3K signaling. We then investigated 1) whether accumulation of intracellular long-chain acyl-CoAs occurs in mediobasal hypothalamus of rats with DIO, as described in peripheral insulin resistance; 2) whether an acute increase in local levels of the saturated fatty acid palmitate (by infusion directly into the adjacent third cerebral ventricle) induces hypothalamic insulin resistance; 3) whether consumption of a HF diet in an amount equal to that consumed by rats fed a low-fat (LF) diet is sufficient to impair insulin-stimulated PI3K signaling and induce inflammation in the hypothalamus; and 4) whether pharmacological inhibition of central IKKβ signaling selectively reduces intake of a HF diet.
MATERIALS AND METHODS
Animal Studies
The National Institutes of Health regulations for the use and care of animals were followed and studies were approved by the Institutional Animal Care and Use Committees at the University of Washington, University of Cincinnati, and Vanderbilt University. Long-Evans rats initially weighing 250–300 g (Harlan, Indianapolis, IN) were housed individually and fed micronutrient matched diets containing either 60% (HF) or 10% fat (LF); both fat sources from lard (Research Diets D12492 and D12450B, New Brunswick, NJ). Adiposity was determined by magnetic resonance spectrometry (Echo Medical Systems, Houston, TX). We defined DIO as a 10% increase of body weight in the HF vs. the LF group, typically achieved after 5–7 wk.
Study Protocols
Effect of intracerebroventricular insulin on food intake.
Third ventricle cannulation was performed (26, 27), and animals were placed on LF (10%) or HF (60%) diets. After DIO was established, animals were fasted for 5–6 h before dark cycle onset and received an injection of saline (1 μl) vehicle or insulin (8 mU in 1 μl) intracerebroventricular 1 h before lights off. Food intake was measured over a 24-h period after treatment.
Hormones/metabolites.
DIO rats were euthanized after a 4-h fast, and trunk blood was collected in EDTA tubes. Plasma hormones were assayed by the Vanderbilt Diabetes Center Hormone Assay Core (Nashville, TN). Total free fatty acids (FFA) were measured using Wako FFA kit (Wako Pure Chemical Industries, Osaka, Japan).
Glucose tolerance test.
Glucose tolerance testing was conducted by bolus intraperitoneal glucose injection (2.5 g/kg body wt, 50% dextrose solution) after a 6-h fast. Blood glucose was measured from the tip of the tail at 20-min intervals using a glucometer (Freestyle Flash; Abbott Laboratories, Abbott Park, IL), and the area under the glucose curve was calculated.
Biochemistry.
After a 24-h fast, an intracerebroventricular injection of insulin (10 mU in 1 μl saline) or saline vehicle (1 μl) was given and 20 min later animals were euthanized and the mediobasal hypothalamus rapidly removed and frozen (26, 27). Activation of PI3K signal transduction was assessed by measuring phosphoserine protein kinase B (PKB; serine residue 473) in hypothalamic extracts by ELISA assay (Biosource, Camarillo, CA) or by Western blot and densitometry quantification. The phosphoserine inhibitor of κB-kinase-β (IKKβ serine residue 181) and total IKBα were also assessed by Western blot and quantified by densitometry. All antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Long-chain acyl-CoA analysis.
Animals were euthanized after a 4-h fast, and the mediobasal hypothalamus was rapidly removed and frozen. Samples were prepared and CoA species purified on solid phase oligonucleotide purification columns, according to Deutsch et al. (7). Samples were derivatized with n-butylamine for gas chromatography-electron ionization-mass spectrometry analysis, using a 15-m DB-1 column (J&W Scientific, Folsom, CA) and selected ion monitoring, using a Hewlett-Packard (HP) 6890 gas chromatograph coupled to an HP 5973 mass detector operated in the positive EI mode. C16:0 (palmitoyl-CoA), C18:0 (stearoyl-CoA), and C18:1 (oleoyl-CoA) were quantified by calculating their respective peak areas.
Intracerebroventricular palmitate infusion.
A stock solution of 1 mM palmitic acid (Alltech Associates, Deerfield, IL) was prepared as described previously (14). The vehicle injectate was prepared equivalently but minus palmitic acid. After an overnight fast, male Long-Evans rats maintained on standard chow diet had primed injectors (Plastics One, Roanoke, VA) inserted into previously placed (7 days) third ventricular cannula. Intracerebroventricular vehicle or intracerebroventricular palmitate was infused at 0.5 ul/min, delivering 0.5 nmol palmitate (or vehicle)/min for a total dose of 120 nmol by micropump, while animals were in their home cages. The injectors were then removed and an intracerebroventricular injection of either 10 mU insulin or vehicle was performed, and the animals were euthanized 20 min later for analysis of hypothalami.
Pair-Feeding Study
Animals were divided into three treatment groups: LF, HF, and high-fat diet, pair-fed (PF). The LF and HF groups were given unrestricted access to diet containing 10 or 45% kcal fat (fat source from lard) and matched for micronutrient composition (D12450 and D12451; Research Diets). This study utilized the 45% instead of 60% fat diet to facilitate the matching of caloric intake. The PF group was given the same 45% HF diet but access was limited to match the caloric intake of the LF group; two-thirds of total daily calories were given to PF rats at lights off and the remaining one-third at lights on. A glucose tolerance test was performed as described except using a glucose dose of 3 g/kg lean mass of a 50% dextrose solution after 3 wk of diet administration. At the end of the study (4 wk of diet administration), 4-h fasted animals received an intraperitoneal injection of saline or glucose (3 g/kg lean mass, 50% dextrose). Blood glucose measurements were taken before and 15 min after the intraperitoneal injection. Animals were euthanized after 15 min blood glucose measurement, and tissues were collected for analysis of phosphoserine PKB, phosphoserine IKKβ, and total IκBα. Trunk blood was collected for plasma insulin measurements.
Intracerebroventricular IKK Inhibitor Study
For food intake studies, intracerebroventricular cannulated animals fed a LF (10%) or HF (45%) diet (Research Diets D12450 and D12451) ad libitum for 8 wk received an intracerebroventricular dose of vehicle or PS-1145 (10 μg in saline; Sigma-Aldrich, St. Louis, MO), an IKK inhibitor, after a 4-h fast. Food intake was measured over a 4- and 24-h period after treatment. During food intake studies, animals were also provided with kaolin pellets and kaolin consumption was measured to monitor any indications of visceral illness (nonspecific reduction of food intake) due to the intracerebroventricular treatments (21). For biochemical analysis of inhibitor activity, laboratory chow (LabDiet #5001; LabDiet, St. Louis, MO) fed animals were fasted and given an intracerebroventricular dose of vehicle or PS-1145 (3 μg) into the third ventricle 6 h before euthanasia. Hypothalami were collected for measurement of total IκBα levels, a measure of IKKβ activity. For biochemical analysis of the effect of intracerebroventricular PS-1145 infusion on hypothalamic insulin signal transduction, HF-fed animals (10 wk of diet) were fasted and given a dose of PS-1145 (3 μg) into the third ventricle 6 h before study termination. Animals then received an intracerebroventricular injection of either vehicle or 10 mU insulin 1 h before euthanasia. Hypothalami were collected for measurement of phosphoserine PKB.
Statistical Analysis
Data are means ± SE. Two-group comparisons were performed using Student's t-test and three-group comparisons by one-way ANOVA, with P values <0.05 considered significant. Correlation analysis was performed using Graphpad Prism's correlation analysis tool (Graphpad Software, San Diego, CA).
RESULTS
DIO
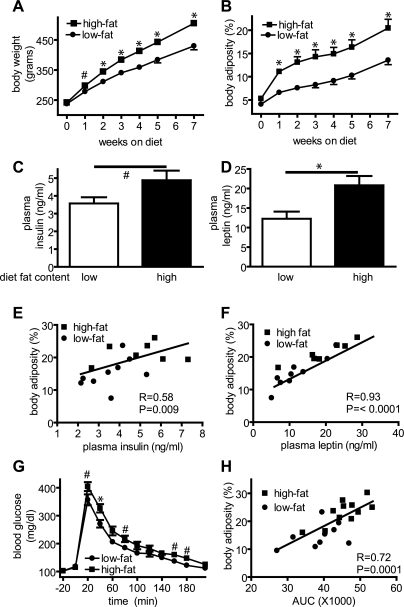
Rats fed a HF diet gained significantly more body weight and fat mass than LF-fed controls over time (Fig. 1, A and B). Plasma insulin (Fig. 1C) and leptin (Fig. 1D) levels were elevated after 7 wk of HF feeding in a manner that paralleled their increase of adiposity (r = 0.58, P = 0.009; r = 0.93, P < 0.0001, respectively; n = 8–10; Fig. 1, E and F). Similarly, 7-wk HF-fed animals exhibited impaired glucose tolerance compared with LF controls [Fig. 1G; area under the curve (AUC): 45,660 ± 1,634 vs. 38,770 ± 2,059; P = 0.016; n = 8–10/group] and the magnitude of this impairment also correlated significantly with body fat mass (r = 0.72; P = 0.0001; n = 8–10/group; Fig. 1H). DIO induced by HF feeding was also associated with elevated plasma levels of FFAs (LF: 0.92 ± 0.1 mmol/l and HF: 1.31± 0.1 mmol/l; P = 0.02; n = 7–9/group).
Fig. 1.
Effect of high-fat feeding on body weight and composition, plasma hormone levels, and glucose tolerance. Effects of consuming either a low- or high-fat diet on body weight (A) and body adiposity (B) and on plasma insulin (C) and leptin (D) levels. Differences in plasma concentrations of insulin (E) and leptin (F) across groups were significantly correlated with changes of body adiposity (r = 0.58 and r = 0.93, respectively; P < 0.01 for each), as were differences in the blood glucose concentration measured during an intraperitoneal glucose tolerance test and area under the curve (AUC) of glucose excursion was significantly correlated with adiposity (r = 0.72; P = 0.0001; G and H). #P < 0.05; *P < 0.01.
Responses to Intracerebroventricular Insulin
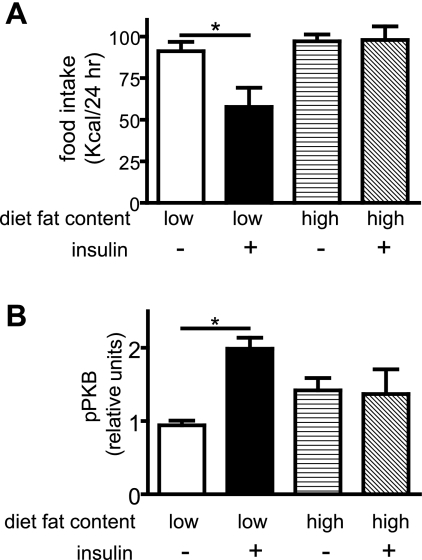
After infusion into the third cerebral ventricle, insulin induced a readily detectable food-intake-lowering effect in LF controls. In contrast, intracerebroventricular insulin had no effect on intake in HF-fed animals (Fig. 2A). To investigate the hypothesis that behavioral (food intake) resistance to insulin involves impaired activation of hypothalamic PI3K signal transduction, as occurs in peripheral tissues of obese animals and humans (reviewed in Ref. 30), we measured serine phosphorylation of PKB (a marker of PI3K activation) in mediobasal hypothalamic extracts 20 min after intracerebroventricular infusion of either insulin (10 mU) or vehicle. Whereas hypothalamic phosphoserine PKB was induced strongly by intracerebroventricular insulin administration in the LF group (Fig. 2B), this response was absent in the HF group (Fig. 2B), despite higher hypothalamic phosphoserine PKB levels at baseline. Collectively, these findings confirm previous evidence in rats (6), whereby DIO induces central insulin resistance when measured either in terms of its ability to reduce food intake or its ability to induce hypothalamic IRS-PI3K signal transduction.
Fig. 2.
Effect of diet-induced obesity (DIO) on the response to intracerebroventricular insulin. Effect of insulin (8 mU intracerebroventricular, just before lights off) on food intake in low- and high-fat fed male Long-Evans rats (A). Effect of insulin (10 mU intracerebroventricular) on phosphoserine PKB (residue 473, a marker of insulin receptor substrate proteins, and PI3K signaling) in low- and high-fat fed rats (B). *P < 0.05.
Hypothalamic Long-Chain Fatty Acyl-CoA Content and Inflammatory Signaling
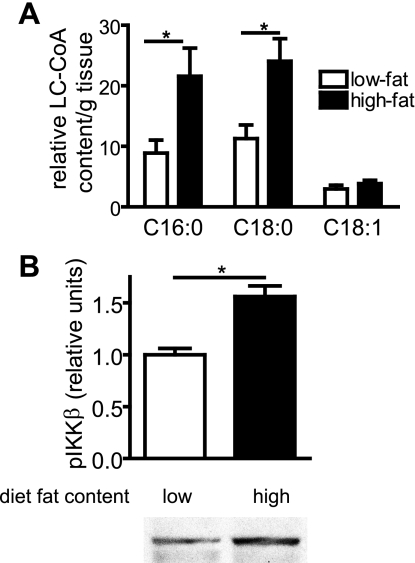
In peripheral tissues, pathological accumulation of intracellular long-chain acyl-CoA molecules (10, 36) is implicated in the pathogenesis of insulin resistance. To investigate whether a similar mechanism occurs in the CNS, we measured hypothalamic levels of three long-chain acyl-CoA species: palmitoyl (C16:0)-, stearoyl (C18:0)-, and oleoyl-CoA (C18:1). DIO was associated with markedly increased hypothalamic content of two saturated acyl-CoA species, palmitoyl- and stearoyl-CoA (Fig. 3A). Interestingly, levels of oleoyl-CoA, which is monounsaturated and hypothesized to exert antiobesity effects in mediobasal hypothalamus (29), were not elevated in the HF group (Fig. 3A). Furthermore, the level of phosphoserine IKKβ (a marker of activated IKKβ and cellular inflammation) was also elevated in hypothalami of HF-fed rats (Fig. 3B).
Fig. 3.
Effect of DIO on hypothalamic long-chain fatty acyl-CoA content and inflammatory signaling. Hypothalamic content of palmitoyl-, stearoyl-, and oleoyl-CoA (A) and IKKβ phosphorylation (B) in low- and high-fat fed rats. *P < 0.05.
Hypothalamic Response to Intracerebroventricular Palmitate
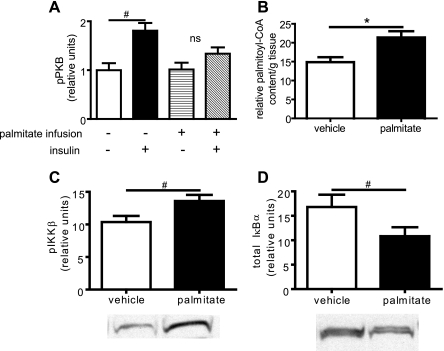
Because palmitate readily induces insulin resistance in peripheral tissues (15, 35) and because HF feeding increases hypothalamic palmitoyl-CoA levels (Fig. 3A), we asked whether intracerebroventricular infusion of palmitate in rats maintained on a standard chow diet mimics the effect of HF feeding on hypothalamic insulin signaling, palmitoyl-CoA accumulation, and IKKβ activity. After intracerebroventricular infusion of palmitate for 240 min (0.5 nmol/min; 120 nmol total), hypothalamic palmitoyl-CoA content was increased, while the ability of intracerebroventricular insulin to increase PI3K signaling, as detected by PKB serine 473 phosphorylation, was significantly blunted relative to intracerebroventricular vehicle-infused animals in which hypothalamic PI3K activation after subsequent intracerebroventricular insulin treatment was readily detected (Fig. 4, A and B). Concomitant with increased palmitoyl-CoA content and a reduction in activation of PKB, increased phosphorylation of IKKβ (Fig. 4C) and decreased IκBα content (an additional marker of IKKβ activity; Fig. 4D) were also observed in hypothalamic extracts.
Fig. 4.
Effect of intracerebroventricular palmitate on hypothalamic insulin signaling. After intracerebroventricular infusion of palmitate-BSA or BSA vehicle for 4 h (0.5 nmol/min), the effect of intracerebroventricular insulin (10 mU) on IRS-PI3K signaling measured as phosphoserine PKB was determined in the mediobasal hypothalamus (A), as was the effect on palmitoyl-CoA accumulation (B), IKKβ phosphorylation (C), and total IκBα level (D: when activated, IKKβ phosphorylates IκBα resulting in degradation). #P < 0.05; *P < 0.01.
Effects of Eucaloric HF Feeding
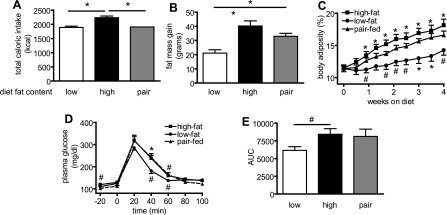
Since animals placed on the HF diet were hyperphagic compared with those fed the LF diet, the deleterious hypothalamic and peripheral metabolic effects of the former diet could arise from increased energy intake, a change in diet composition, or a combination of both. To investigate whether the weight gain, glucose intolerance, hypothalamic inflammation, and insulin resistance observed during HF feeding are dependent upon dietary caloric excess or, alternatively, on inherent physicochemical properties of dietary fat, we measured changes in body weight, adiposity, and glucose tolerance in a separate study that included a group of rats (PF) that were fed the HF diet in an amount that was matched to the caloric intake of LF controls (Fig. 5A). Interestingly, rats fed the HF diet (HF and PF) gained significantly more fat mass than LF controls, even when their intake was matched to that of animals on the LF diet (Fig. 5, B and C). Thus PF gained 60% more than LF and gained 82% of the fat mass accrued by the HF group.
Fig. 5.
Effects of eucaloric high-fat feeding (pair-feeding) on body composition, and glucose tolerance. The food intake of the pair-fed group was calorically matched to that of the low-fat diet fed group and the high-fat group consumed significantly more calories than both the low-fat and pair-fed groups (A; P < 0.01). Fat mass gain (B) and body adiposity (C) were elevated in pair-fed animals to nearly the level of ad libitum high-fat fed animals. Likewise, pair-fed animals had similar blood glucose excursion (D) and AUC (E) during an intraperitoneal glucose tolerance test as ad libitum high-fat fed animals. #P < 0.05; *P < 0.01. C: symbols below low-fat data points indicate statistical significance compared with pair-fed group and symbols above high-fat data points indicate statistical significance compared with low-fat group.
After 3 wk of diet treatment, HF-fed animals exhibited impaired glucose tolerance compared with LF controls as expected (Fig. 5, D and E), while the PF animals exhibited a similar impairment of glucose tolerance (PF AUC: 8,130 ± 1,023 and HF AUC: 8,430 ± 791, respectively; P = NS; n = 9–11/group). However, the difference in glucose AUC did not reach statistical significance between the PF and LF group (Fig. 5E; PF AUC: 8,130 ± 1,023 vs. LF AUC: 6,160 ± 519; P = 0.06; n = 9–11/group). As expected, fasting plasma glucose concentration was lower in the LF than the HF group (110 ± 3 vs. 119 ± 3 mg/dl; P < 0.05). Fasting plasma glucose levels in the PF group (102 ± 3 mg/dl) were not directly comparable with the other groups due to longer period of fasting necessitated by the pair-feeding regimen.
Effect of Dietary Fat on Hypothalamic Response to Endogenous Insulin
To measure hypothalamic sensitivity to endogenously secreted insulin, we performed an intraperitoneal injection of glucose. Mean blood glucose concentrations 15 min after glucose administration were identical between the groups, although baseline levels were slightly lower in the PF group (baseline: 109 ± 3 mg/dl PF group, 118 ± 3 mg/dl LF group, and 120 ± 3 mg/dl HF group; post intraperitoneal glucose: 283 ± 18 mg/dl PF group, 280 ± 9 mg/dl LF group, and 268 ± 17 mg/dl HF group). Likewise, baseline plasma insulin levels were slightly lower in the PF animals compared with LF and HF animals (0.7 ± 0.04 ng/ml in the PF group, 1.6 ± 0.3 ng/ml LF, and 1.5 ± 0.2 ng/ml HF), owing to the longer period of fasting imposed by the pair-feeding protocol. Nonetheless, glucose-stimulated plasma insulin levels were identical among all three groups (4.1 ± 0.6 ng/ml PF group, 4.4 ± 1.0 ng/ml LF group, and 4.3 ± 0.3 ng/ml HF group). Animals were euthanized 15 min after glucose injection and hypothalamic extracts assayed for both IKKβ activity and phosphoserine PKB. As previously observed (Fig. 2B), HF animals exhibited slightly higher baseline phosphoserine PKB levels that were not further increased in response to glucose-stimulated insulin secretion, whereas in the LF group, glucose-induced insulin secretion was associated with increased serine 473-PKB phosphorylation in hypothalamic extracts (Fig. 6A; P < 0.05; n = 6–7/group). Phosphorylation of PKB was also blunted in the hypothalamus of PF animals after intraperitoneal glucose, as was observed in the HF group (Fig. 6A). Moreover, when plotted vs. plasma insulin levels, PKB phosphorylation was significantly correlated with plasma insulin concentration in the LF group (Fig. 6D; r = 0.73; P = 0.01) but not in the HF or PF groups. Consistent with the hypothesis that HF feeding causes hypothalamic inflammation even in the absence of hyperphagia, consumption of a HF diet, even when limited to the caloric intake of the LF group (PF), was associated with increased phosphorylation (activation) of IKKβ (Fig. 6B) and reduced levels of IκBα (Fig. 6C). We next sought to compare insulin responses in key peripheral tissues to those observed in the hypothalamus of the three groups of rats. In the liver, activation of PKB was comparable after intraperitoneal glucose (Fig. 6E), and while resistance to insulin activation of PKB in skeletal muscle (gastrocnemius; Fig. 6F) was observed in the HF group, PF rats displayed intermediate sensitivity. After a relatively brief exposure to a HF diet (4 wk), therefore, the PF group displayed intact insulin signal transduction in liver, partially reduced insulin signaling in muscle, and a complete inhibition of insulin-induced PKB activation in hypothalamus. This observation raises the possibility that the CNS may be more susceptible than peripheral tissues to HF diet-induced insulin resistance.
Fig. 6.
Effect of pair-feeding on glucose-stimulated hypothalamic insulin signaling. Effects of dietary fat content on phosphoserine PKB (A) with 15-min vehicle (open bars) or glucose (3 g/kg lean mass; solid bars) treatment administered intraperitonally. Pair-feeding also resulted in increased phosphorylation of IKKβ (B) and decreased total IκBα (C). The relationship between phosphorylated PKB and peak plasma insulin levels was compared among groups in mediobasal hypothalamus (D), liver (E), and muscle (F). *P < 0.05.
Effect of Central IKKβ Inhibition on Food Intake, Inflammation, and Insulin Signaling
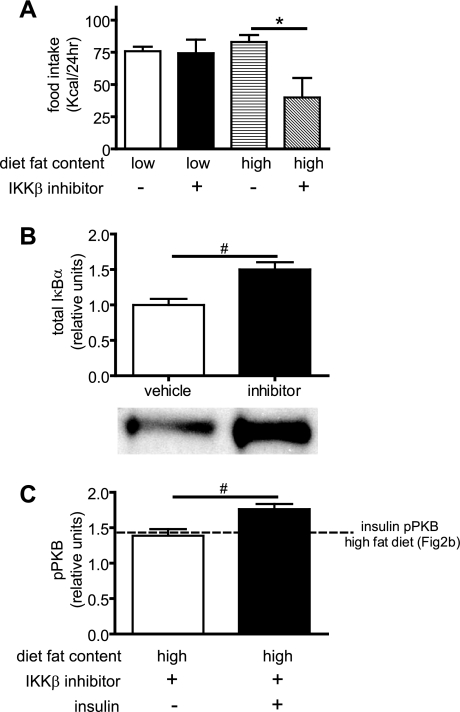
To determine whether increased hypothalamic IKKβ activity contributes to the effect of HF feeding on food intake and hypothalamic insulin action, we administered a pharmacological inhibitor of IKK (PS-1145; 10 μg) into the third cerebral ventricle of rats fed either a HF (45%) or a LF diet. As shown in Fig. 7A, this intervention had no effect on food intake in the LF group (animals that are characterized by relatively low hypothalamic IKKβ activity; Fig. 3B). In contrast, intracerebroventricular infusion of PS-1145 potently reduced intake for up to 24 h in rats fed the HF diet (Fig. 7A) in whom hypothalamic IKKβ activity is relatively increased (Fig. 3B). This effect was dose dependent, as a lower dose of PS1145 (3 μg intracerebroventricular) reduced food intake through 4 but not 24 h (data not shown). As expected, intracerebroventricular PS-1145 administration also inhibited hypothalamic IKK activity, as indicated by increased IκBα content compared with vehicle treatment (Fig. 7B), and when given as a pretreatment injection (3 μg, 5 h before 10 mU intracerebroventricular insulin), it modestly, but significantly, increased insulin activation of AKT in the hypothalamus of rats on the HF diet (Fig. 7C). That the feeding effect of PS-1145 could be due to “visceral” or nonspecific illness seems unlikely for two reasons. First, no such feeding effect was observed in animals consuming the LF diet, and second, drug treatment did not elicit an increase of kaolin intake (LF vehicle: 0.9 ± 0.2 g, LF inhibitor: 1.0 ± 0.3g, HF vehicle: 1.1 ± 0.4g, and HF inhibitor: 1.2 ± 0.3 g; P = NS; n = 6–7/group), a marker of visceral illness (21).
Fig. 7.
Effects of central IKK inhibitor on food intake, inflammation, and insulin signaling. Cumulative food intake measurements over a 24-h period after intracerebroventricular injection of vehicle or PS1145 (IKK inhibitor) in low-fat and high-fat fed animals are shown (A). PS1145 treatment results in increased total IκBα levels (B) and modestly increased insulin stimulated phosphoserine PKB (C). #P < 0.05; *P < 0.01.
DISCUSSION
The control of energy homeostasis depends on input to key brain areas from adiposity negative feedback signals such as insulin and leptin. We (25, 32) and others (6, 44) have hypothesized that as in peripheral tissues resistance to insulin in the CNS is a consequence of nutrient excess and that once it develops it contributes to the defense of an elevated body weight. Here, we sought to characterize the effect on hypothalamic insulin action of lipid in the form of dietary fat, fatty acid infusion, and dietary fat fed under eucaloric conditions. In line with previous observations (6), we found that obesity induced by a HF diet is accompanied by activation of inflammatory signaling involving IKKβ and both behavioral and biochemical resistance to insulin. In addition, we made the novel observation that HF feeding induced hypothalamic accumulation of saturated long-chain acyl-CoA, which prompted additional investigation into the hypothalamic consequences of lipid excess.
As expected (42), we found that compared with LF-fed controls, Long-Evans rats fed a HF, lard-based diet exhibited increases of body weight and body adiposity that, combined with elevated plasma levels of both insulin and leptin, are indicative of systemic resistance to both hormones. Impaired glucose tolerance is a consequence of insulin resistance (and inadequate β-cell compensation) in obese humans (16) and animal models (42) and was present in proportion to the degree of adiposity in HF-fed rats. To confirm previous evidence (1, 6) that central insulin resistance is also present in DIO, we compared the response to intracerebroventricular insulin between the HF and LF groups in terms of both food intake inhibition and activation of hypothalamic signaling via the IRS-PI3K pathway and observed a blunted insulin effect in both assays. Together these findings extend emerging evidence that DIO is associated with impaired activation by insulin of both food-intake-lowering pathways and IRS-PI3K signaling in the hypothalamus.
Animals placed on a HF diet are characteristically hyperphagic (18, 42) and are therefore exposed both to excess calories and excess dietary lipid. The extent to which hyperphagia in this setting is due to increased palatability vs. increased fat content per se remains uncertain. Also uncertain is how HF feeding causes the defended level of body weight to gradually increase, reflecting an attenuation of compensatory responses designed to prevent excess fat deposition. One potential explanation for this phenomenon is that dietary fats possess inherent physicochemical properties that impair central mechanisms governing energy homeostasis.
Saturated fatty acids such as palmitate are implicated in the development of peripheral insulin resistance via a mechanism hypothesized to involve intracellular accumulation of lipotoxic substrates such as long-chain acyl-CoA (or metabolites thereof; Ref. 36). In lean animals that were not previously exposed to high levels of dietary fat, we found that intracerebroventricular infusion of palmitate recapitulates the effect of DIO to cause both inflammation (as judged by IKKβ activation) and insulin resistance (as judged by reduced PI3K signaling) in the hypothalamus. As in peripheral tissues (8), therefore, exposure of the CNS to excess saturated fatty acids is sufficient to trigger cellular responses that reduce insulin action.
To investigate whether exposure to excess dietary fat causes CNS insulin resistance even in the absence of excess energy intake, we performed an additional study in which a third group of animals was included that were fed the HF diet but allowed to consume food only in amounts that matched the caloric intake of LF-fed controls. Intriguingly, despite consuming the same number of calories, the PF group accrued significantly more adipose tissue than the LF-fed animals (they gained 82% of the adipose of the HF-fed group) and developed impaired glucose tolerance similar to that observed in the HF group fed ad libitum. This finding suggests that, independently of total energy intake, increased dietary fat intake may undermine CNS responses to adiposity negative feedback signals such as insulin, thus leading to the defense of an elevated level of body fat mass. Consistent with this interpretation, we found that hypothalamic IKKβ signaling was increased in both HF and PF groups compared with LF-fed controls and that whereas hypothalamic PI3K signaling was increased by glucose-induced stimulation of endogenous insulin secretion in the LF group, this response was absent in HF-fed animals even when hyperphagia was prevented by pair-feeding. Thus HF feeding induced hypothalamic inflammation and insulin resistance irrespective of whether hyperphagia occurred, suggesting that dietary fats may possess inherent obesogenic properties, whether by acting directly on target tissues or by indirect, perhaps, metabolic mechanisms.
Abundant evidence in animals and humans implicates both exogenous (palmitate infusion) and endogenous (tissue long-chain acyl-CoA) fatty acids in the pathogenesis of insulin resistance triggered by activation of intracellular inflammatory signals. For example, long-chain acyl-CoA content and IKKβ signaling are elevated in skeletal muscle sampled from morbidly obese, insulin-resistant humans (10) and exposure of cultured cells to palmitate induces insulin resistance via activation of IKKβ, IκBα, and NF-κB signaling (11, 13, 43). Similarly, we observed that intracerebroventricular infusion of palmitic acid (in chow fed rats) increases hypothalamic IKKβ activation and impairs hypothalamic activation of the IRS-PI3K pathway by insulin. This observation supports a model in which consumption of a HF diet contributes to the development of CNS insulin resistance perhaps via a mechanism involving either elevated circulating FFA levels or increased de novo tissue synthesis of saturated fatty acids in key hypothalamic neurons. Whether such CNS resistance occurs early (23) in the course of HF feeding and actively contributes to increased food intake, adiposity, and peripheral insulin resistance characteristic of DIO is an additional important unanswered question.
Among the three long-chain acyl-CoA species measured in the hypothalamus of DIO rats and controls, increased levels were detected only in the saturated species, palmitoyl-CoA and stearoyl-CoA, whereas levels of the monounsaturated oleoyl-CoA were not significantly altered by HF feeding. One hypothesis to explain this outcome is that HF feeding reduces the activity of hypothalamic stearoyl-CoA desaturase-1/2 (SCD-1 or 2), the enzyme that desaturates palmitic and stearic acids to palmitoleic and oleic acids (28). In the liver, reduced SCD-1 activity is implicated as a mediator of leptin action on lipid metabolism (4) and SCD-1 deficiency reduces triglyceride accumulation, protects against weight gain, and increases insulin sensitivity (31). These observations raise the possibility that, as in related intracellular signaling systems (20), the impact of reduced SCD-1 (or 2) activity in the CNS opposes its effect in peripheral tissues.
Interestingly, although we found that total circulating free fatty acid levels were elevated in HF-fed rats, consistent with other DIO models, the plasma profiles of fatty acids are known to reflect the dietary fat source. In these studies, the source of lipid was lard, which contains roughly double the content of oleic acid vs. palmitic acid, and this ratio was reflected in the circulating fatty acid profiles in our animals (data not shown). Thus patterns of long-chain fatty acid accumulation in hypothalamus do not appear to be the result of “mass action” accumulation of plasma fatty acids in tissue and likely involve processes that are more complex, including changes in long-chain acyl-CoA oxidation and/or synthesis. Whether circulating free fatty acids or de novo synthesis supplies the majority of long-chain acyl-CoAs in nutrient-sensing hypothalamic neurons also remains to be addressed, as does the question of whether such processes are sensitive to changes of diet composition or energy balance (or both).
Insight into mechanisms linking HF feeding to inflammatory signaling in hypothalamus and other tissues also awaits further study. Kim et al. (12) reported a requirement for Toll-like receptor 4 (TLR4; a component of the innate immune system) in the effect of HF feeding to induce vascular inflammation in a mouse model of DIO, as well as in the effect of palmitate to activate IKKβ in cultured endothelial cells, and similar results have been reported in other tissues (5, 17, 35, 40; and others). Whether long-chain fatty acids activate inflammatory hypothalamic signals via TLR4 signaling is therefore an important unanswered question. Our finding that hypothalamic IKKβ signaling increases in response to both HF feeding (even in the absence of excess energy intake) and acute fatty acid infusion into the third ventricle supports our hypothesis of a direct link between exposure to excess saturated FFA and cellular inflammation within the hypothalamus. The significance of the latter observation with respect to feeding behavior was addressed recently by Zhang et al. (44), who reported that reduced neuronal IKKβ signaling lowers susceptibility to DIO in mouse models, whereas increased IKKβ signaling has the opposite effect, and implicated SOCS3 as a potential mediator of these responses. Our current finding that intracerebroventricular infusion of an IKKβ inhibitor reduces food intake in rats fed a HF, but not a LF, diet and that it at least partially reverses hypothalamic insulin resistance in the former group identifies neuronal IKKβ and related inflammatory molecules as interesting targets for obesity drug development.
In conclusion, we extend prior evidence that DIO is associated with central, as well as peripheral, insulin resistance and report the first evidence that HF feeding is characterized by the accumulation of potentially “inflammatory” lipid in the mediobasal hypothalamus. We also show that intracerebroventricular infusion of palmitate recapitulates the effect of a HF diet to induce hypothalamic inflammation and insulin resistance at the level of IRS-PI3K signaling and that the same responses occur even after consumption of a fat-rich diet at eucaloric levels. Finally, we demonstrate that activation of inflammatory signals such as IKKβ appear to contribute to the effect of a HF diet to increase food intake and to cause insulin resistance in a rat model of DIO. Combined with growing evidence of a role for hypothalamic signaling via both PI3K and JAK-STAT in energy homeostasis, and of resistance to leptin activation of JAK-STAT signaling in mediobasal hypothalamus of DIO rats (23), our results support a model in which cellular exposure to excess nutrients, particularly dietary fat, triggers cellular inflammation and insulin resistance that in turn contribute to the pathogenesis of obesity. Identification of the molecular basis for these effects may yield novel approaches to the treatment of this increasingly common and costly metabolic disorder.
GRANTS
This work was supported with resources of the Tennessee Valley Healthcare System (Nashville, TN); and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-064857 and DK-069927 (to K. D. Niswender); DK-52989, DK-68384, DK-083042, T32 Grant DK-07247, and the Clinical Nutrition Research Unit and Diabetes Endocrinology Research Center at the University of Washington (to M. W. Schwartz); supported in part by the Office of Research and Development Medical Research Service, Department of Veterans Affairs (to D. G. Baskin; Senior Research Career Scientist, Research and Development Service, Department of Veterans Affairs Puget Sound Health Care System, Seattle, WA); and Juvenile Diabetes Research Foundation 2-2003-149 (to S. Pennathur). Mass spectrometry experiments were performed by the Mass Spectrometry Resource, Department of Medicine Diabetes and Endocrinology Research Center at the University of Washington (National Heart, Lung, and Blood Institute Grant P01 HL030086) and the Mass Spectrometry Core of the Michigan Diabetes Research and Training Center (MDRTC) (National Institute of Diabetes and Digestive and Kidney Diseases Grant P60 DK-020572). We also acknowledge the Vanderbilt Diabetes Research and Training Center of the MDRICE (National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-20593) Hormone Assay and Analytical Services Core.
Acknowledgments
We thank Aileen Macatangay, Maxine Turney, Sanaz Saadat, and Le Zhang for providing superb technical support.
REFERENCES
- 1.Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol Regul Integr Comp Physiol 255: R974–R981, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Baskin DG, Hahn TM, Schwartz MW. Leptin sensitive neurons in the hypothalamus. Horm Metab Res 31: 345–350, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Carvalheira JB, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia 46: 1629–1640, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297: 240–243, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity 16: 1248–1255, 2008. [DOI] [PubMed] [Google Scholar]
- 6.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem 220: 321–323, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103: 253–259, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23: 2–40, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Houmard JA, Tanner CJ, Yu C, Cunningham PG, Pories WJ, MacDonald KG, Shulman GI. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes 51: 2959–2963, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Kim F, Gallis B, Corson MA. TNF-α inhibits flow and insulin signaling leading to NO production in aortic endothelial cells. Am J Physiol Cell Physiol 280: C1057–C1065, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Kim F, Tysseling KA, Rice J, Gallis B, Haji L, Giachelli CM, Raines EW, Corson MA, Schwartz MW. Activation of IKKbeta by glucose is necessary and sufficient to impair insulin signaling and nitric oxide production in endothelial cells. J Mol Cell Cardiol 39: 327–334, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 25: 989–994, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leahy JL Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36: 197–209, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 282: R46–R54, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Minokoshi Y, Kahn BB. Role of AMP-activated protein kinase in leptin-induced fatty acid oxidation in muscle. Biochem Soc Trans 31: 196–201, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiol Behav 17: 691–697, 1976. [DOI] [PubMed] [Google Scholar]
- 22.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 15: 362–369, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Niswender KD, Clegg DJ, Morrison CD, Morton GJ, Benoit SC. The human obesity epidemic–a physiological perspective. Curr Med Chem Immun Endoc Metab Agents 4: 91–104, 2004. [Google Scholar]
- 26.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW. Key enzyme in leptin-induced anorexia. Nature 413: 795–796, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Ntambi JM, Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol 14: 255–261, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 119: S10–S16, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci USA 100: 11110–11115, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz MW, Niswender KD. Adiposity signaling and biogical defense against weight gain: absence of protection or central hormone resistance? J Clin Endocrinol Metab 89: 5889–5897, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Seeley RJ, Matson CA, Chavez M, Woods SC, Dallman MF, Schwartz MW. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am J Physiol Regul Integr Comp Physiol 271: R819–R823, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulman GI Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sims EA, Danforth E Jr, Horton ES, Glennon JA, Bray GA, Salans LB. Experimental obesity in man. A progress report. Isr J Med Sci 8: 813–814, 1972. [PubMed] [Google Scholar]
- 38.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986–1998, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Wilcox BJ, Corp ES, Dorsa DM, Figlewicz DP, Greenwood MR, Woods SC, Baskin DG. Insulin binding in the hypothalamus of lean and genetically obese Zucker rats. Peptides 10: 1159–1164, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Yu C, Chen Y, Zong H, Wang Y, Bergeron R, Kim JK, Cline GW, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of IRS-1 associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]