Abstract
Calorie restriction (CR) alleviates insulin resistance and has a beneficial effect on numerous metabolic disorders, yet the underlying mechanism has not been fully elucidated. In the present study, we found that CR of mice (60% of the diet consumption compared with ad libitum mice) reduces the expression levels of Grb2 in skeletal muscle, an insulin target tissue that accounts for 85% of insulin-stimulated blood glucose clearance. To determine whether Grb2 downregulation contributes to increased insulin sensitivity in the regulation of glucose metabolism, we generated C2C12 cell lines in which the expression of Grb2 is suppressed by RNA interference. Suppressing Grb2 expression in C2C12 myoblasts enhances insulin-stimulated insulin receptor substrate (IRS)-1, tyrosine phosphorylation, and Akt phosphorylation, which is associated with decreased IRS-1 serine phosphorylation at residues 307, 612, and 636/639. In addition, reducing Grb2 expression levels increased insulin-stimulated glucose uptake in C2C12 myotubes. Reduced IRS-1 serine phosphorylation is also found in Grb2+/− heterozygous knockout mice, which is associated with enhanced insulin signaling and resistance to high-fat diet-induced glucose and insulin intolerance. All together, our results suggested that reducing the expression levels of Grb2 provides a mechanism by which CR increases insulin sensitivity in vivo.
Keywords: mitogen-activated protein kinase, phosphoinositide 3-kinase, insulin sensitivity
moderate caloric restriction (CR) has an insulin-sensitizing effect and attenuates the development of age-associated pathological and biological changes in many species, ranging from yeast, rodents, primates, and humans (4, 10, 19, 22, 26, 31, 32, 34, 35). CR improves insulin sensitivity in the liver of rodents by promoting gluconeogenesis and decreasing fatty acid synthesis and glycolysis (38). In skeletal muscle, CR enhances insulin signaling (28, 29), stimulates the expression levels of genes involved in gluconeogenesis and fatty acid biosynthesis (24), and potentiates insulin-stimulated glucose transport (7). Since skeletal muscle accounts for 85% of insulin-stimulated blood glucose clearance (16), improved glucose metabolism in skeletal muscle has been considered to play a key role in enhanced whole body insulin action seen with CR (29).
Insulin action is initiated by the binding of this hormone to its membrane receptor in insulin target cells, such as liver, fat, and muscle cells, which leads to autophosphorylation of the insulin receptor (IR) and subsequent activation of two major pathways, the phosphoinositide 3-kinase (PI3K) and the p44/42 mitogen-activated protein kinase (MAPK) pathways. Activation of the PI3K pathway, which is critical for regulating glucose homeostasis, is mediated by a series of cellular events including tyrosine phosphorylation of IR substrate (IRS) isoforms and serine phosphorylation of Akt at both Thr308 in the activation loop and Ser473 at the COOH terminus. Activation of the p44/42 MAPK signaling pathway, which has been implicated in insulin-mediated mitogenic events, is mediated by recruiting adaptor proteins, such as Shc and Grb2, to the tyrosine-phosphorylated IR. Through both the PI3K and the p44/42 MAPK pathways, insulin stimulates a variety of cellular responses, including glucose uptake, cellular growth, and differentiation.
The underlying mechanisms by which CR increases insulin sensitivity remain to be fully elucidated. Short-time (5–28 days) CR has been found to increase insulin sensitivity in rats but had no effect on either the number or the tyrosine kinase activity of the IR, suggesting that the increased insulin sensitivity is via a postreceptor mechanism (2, 9). In rhesus monkeys, the CR-induced increase in insulin sensitivity is unrelated to alterations in glucose transporter 4, PI3K, and IRS-1 abundance (19). On the other hand, CR has been found to increase the ratio of PI3K catalytic-to-regulatory subunits, which may contribute to increased Akt phosphorylation and insulin sensitivity (30). However, whether altering the ratio of PI3K catalytic-to-regulatory subunits is sufficient to mimic the insulin-sensitizing effect of CR remains unknown.
In the present study, we show that CR greatly reduced the expression levels of the adaptor protein Grb2. We also show that reducing Grb2 expression levels is sufficient to enhance insulin-stimulated PI3K signaling, both in cells and in vivo. Our results suggest a potential mechanism by which CR improves insulin sensitivity in vivo.
MATERIALS AND METHODS
Materials.
The following reagents were used: dextrose anhydrous (EM Science, Gibbstown, NJ), human recombinant insulin (Eli Lilly, Indianapolis, IN), UO126, antibodies to Grb2, Akt, and ERK1/2 and phosphospecific antibodies to AktT308, ERK1/2, IRS-1S307, IRS-1S612, and IRS-1S636/639 (Cell Signaling Technology, Beverly, MA), antibody to IRS-1 (Santa Cruz Biotechnology, Santa Cruz, CA) and to phosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY), high-fat diet (HFD; 45% fat, 35% carbohydrate, 20% protein) (D12451; Research Diets, New Brunswick, NJ), and horseradish peroxidase- or alkaline phosphatase-conjugated second antibody (Promega, Madison, WI).
Cell culture and treatment.
For generation of Grb2-suppressed RNA interference (RNAi) stable cell lines, C2C12 myoblasts were transfected with Grb2 RNAi construct or scrambled control and selected with 5 μg/ml puromycin as previously described (23). The sense and antisense sequences of the RNAi were synthesized and inserted into pSIREN-RetroQ (BDK knockout RNAi system; BD Biosciences, San Jose, CA). The sense sequence corresponds to nucleotides 939–958 of the mouse Grb2 (GCATGTTTCCCCGCAATTAT). The sequence (GCGGTAAGTCGATATGATCA) was used for the scramble control. C2C12 RNAi and scramble cells were maintained at subconfluent conditions in DMEM (ATCC) medium containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum. The differentiation of Grb2-suppressed and scramble C2C12 cells was performed as described previously (27). For insulin-stimulated Akt and MAPK phosphorylation, C2C12 myoblasts were serum starved for 5 h, pretreated with or without UO126 for 1 h, and then with or without insulin at different concentrations for 5 min. For IRS-1 phosphorylation experiments, C2C12 myoblasts were serum starved for 12 h and then treated with or without 50 nM insulin for 5 min. C2C12 cells were lysed in lysis buffer containing 0.02 M Tris, pH 7.6, 1% NP40, 0.15 M NaCl, 0.15 M NaF, 2 mM Na3VO4, 5 mM EDTA, and complete cocktail protease inhibitors. Cell lysates were prepared for Western blot or immunoprecipitation experiments.
Animal studies.
The CR and ad libitum (AL)-fed control mice were purchased from the NIA CR Colony (for more information, please see http://www.nia.nih.gov/ResearchInformation/ScientificResources/AgedRodentColoniesHandbook/CaloricRestrictedColony.htm). The CR mice were individually housed, and CR was started at 16 wk of age. The AL control mice were fed with NIH-31 standard diet, whereas the CR mice were fed with 60% of the diet consumed by the AL mice for 4 mo.
The Grb2+/− heterozygous knockout mice were a generous gift by Dr. Tony Pawson and have been described previously (11). The Grb2−/− homozygous knockout mice die before birth because of nonsufficient differentiation of endodermal cells and formation of the epiblast, but the Grb2+/− heterozygous mice are viable and show no obvious defects in normal physiology (11). The mice were backcrossed with C57BL/6 mice for six generations and genotyped by PCR using primers that identified both the neomycin cassette and genomic DNA (forward 1: 5′-aga,ccc,gca,ggt,aaa,gag-3′; forward 2:gga,cat,agc,gtt,ggc,tac-3′; reverse: 5′-cct, ggg,aag,cat,gag,ttg-3′). All mice were maintained on a normal light/dark cycle at the University of Texas Health Science Center at San Antonio. Animal protocols were approved by the University Animal Care and Use Committee.
Glucose tolerance tests.
Male Grb2+/− heterozygous knockout mice and wild-type littermates (8–10 mice/group; 4 wk old) were fed a HFD (45% kcal from fat; D12451; Research Diets) for 16 wk. The mice were fasted for 16 h and were then intraperitoneally injected with glucose (2 g/kg animal body wt). Tail venous blood glucose levels were withdrawn at 0, 15, 30, 60, and 120 min and were measured using a blood glucose monitor (Rightest GM300; Bionime, Dali City, Taiwan).
Insulin tolerance tests.
Male Grb2+/− heterozygous knockout mice and wild-type littermates (8–10 mice/group; 4 wk old) housed in a 12-h light/dark cycle (lights on from 0900 to 2100) were fed with the above mentioned HFD for 16 wk. Grb2+/− mice and wild-type littermates were intraperitoneally injected with human insulin (0.75 U/kg animal body wt) at 1230 and tail venous blood glucose levels were determined as described above.
Insulin signaling in vivo.
Male Grb2+/− heterozygous knockout mice and wild-type littermates (8–10 mice/group; 4 wk old) were fed with the above mentioned HFD for 16 wk. Mouse body weights were measured once a week. Mice were fasted overnight and intraperitoneally injected with insulin (10 U/kg body wt) or equal volume of saline (0.1 ml/10 g body wt). After 10 min, mice were euthanized via cervical dislocation, and mouse tissues were immediately excised, frozen in liquid nitrogen, and kept at −80°C until homogenization. To determine the potential effect of CR on insulin signaling, the 8-mo-old AL and CR mice were fasted overnight and injected intraperitoneally with insulin (5 U/kg body wt) for 5 min. The mice were euthanized, and tissues were excised immediately. Tissue samples were homogenized in ice-cold lysis buffer containing 50 mM HEPES (pH 7.6), 150 mM sodium chloride, 20 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10 mM sodium fluoride, 2 mM sodium orthovanadate, 2 mM EDTA, 1.0% Igepal, 10% glycerol, 2 mM PMSF, 1 mM magnesium chloride, 1 mM calcium chloride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Tissue homogenates were allowed to sit on ice for 20 min and clarified by centrifugation at 14,000 revolution/min for 10 min. The protein concentration in the supernatant was then determined using the Bradford assay. All the samples were aliquoted and stored at −80°C to prevent freeze-thaw cycles.
Immunoprecipitation and Western blot.
Immunoprecipitation and Western blot experiments were performed as described in our previous studies (36, 37).
2-Deoxyglucose uptake.
Glucose uptake was performed as described previously (27) with some modification. In brief, the Grb2-suppressed and scramble control C2C12 myoblasts were differentiated into myotubes. After serum starvation for 6 h, the cells were treated with or without 50, 100, or 200 nM insulin at 37°C for 30 min. Then cells were incubated in 0.5 ml transport solution containing 2-deoxyl-d-2-[3H]-glucose (0.5 μCi/ml; American Radiolabeled Chemicals, St. Louis, MO) and 10 μM 2-deoxyglucose (Sigma, St. Louis, MO) at 37°C for 10 min. Cells were subsequently washed with cold PBS and lysed in 0.5 ml of 0.1 M NaOH. The nonspecific uptake was measured in the presence of 10 μM cytochalasin B (Sigma).
Statistical analysis.
All data are presented as the means ± SE and were analyzed by either the Student's t-test or the ANOVA with post hoc Bonferoni test. P < 0.05 was considered statistically significant.
RESULTS
CR markedly decreases Grb2 expression and enhances insulin signaling in mouse skeletal muscle.
To determine the potential mechanism by which CR increases insulin sensitivity, we examined the effect of CR on the expression levels of components in the PI3K and p44/42 MAPK signaling pathways of mice. Several insulin target tissues, including rectus femoris muscle, liver, and fat, were isolated from AL-fed and CR C57BL/6 mice, and the expression of insulin signaling molecules in these tissues was determined by Western blot using specific antibodies. We found that CR had no effect on the expression levels of the IR, IRS-1, Akt, phosphoinositide-dependent protein kinase-1, and p44/42 MAPK (data not shown). On the other hand, CR significantly suppressed the expression of Grb2 in skeletal muscle (Fig. 1, A and B) and white adipose tissue (Fig. 1, A and C), a key signaling molecule functioning in the p44/42 MAPK signaling pathway. There was no significant difference in Grb2 levels in the liver between AL and CR mice (data not shown). Consistent with previous findings that CR increases insulin signaling in the skeletal muscle (28, 29), we found that insulin-stimulated Akt phosphorylation was significantly increased in skeletal muscle of the CR mice (Fig. 1, D and E). The insulin-stimulated p44/42 MAPK phosphorylation was also reduced in skeletal muscle of CR mice, but this difference did not reach significance (P = 0.09) (Fig. 1, D and F).
Fig. 1.
Effect of calorie restriction (CR) on Grb2 expression and insulin signaling in mouse tissues. A: overnight fasted mice were intraperitoneally injected with 5 U/kg insulin for 5 min. Rectus femoris skeletal muscle and white adipose tissue (WAT) homogenates (30 μg/lane) from ad libitum (AL) fed or CR C57BL/6 mice were separated by SDS-PAGE. The expression levels of Grb2 were examined by Western blot using an anti-Grb2 antibody. The protein levels of Grb2 in skeletal muscle (B) and in white adipose tissue (C) shown in A were quantified by densitometry analysis using the Scion Imaging program. Data are presented as means ± SE. *P < 0.05 (t-test). D: insulin-stimulated phosphorylation of p44/42 MAPK and Akt in mouse skeletal muscle were determined by Western blot using antibodies as indicated. The phosphorylation and protein levels of Akt (E) and p44/42 MAPK (F) were quantified, and the relative phosphorylation (Phospho-protein/protein) was presented as means ± SE. *P < 0.05 (t-test).
Reducing Grb2 expression levels inhibits IRS-1 serine phosphorylation and increases PI3K signaling.
To determine whether Grb2 deficiency is sufficient to affect insulin signaling, we generated stable C2C12 cell lines in which the expression levels of Grb2 are greatly suppressed by RNAi (Fig. 2A, bottom blot). Scramble and Grb2-suppressed C2C12 myoblasts were pretreated with or without the p44/42 MAPK inhibitor UO126 and then treated with or without insulin. Cells were lysed, and insulin-stimulated phosphorylation of Akt and p44/42 MAPK were determined by Western blot using phosphospecific antibodies. Suppression of Grb2 led to increased Akt phosphorylation (Fig. 2A, top blot, and Fig. 2B), which is associated with increased IRS-1 tyrosine phosphorylation (Fig. 2, E and F). No significant difference was observed in insulin-stimulated p44/42 MAPK phosphorylation between the scramble and the Grb2-suppressed cells (Fig. 2A, middle and lower middle blots, and Fig. 2C). Treating cells with the p44/42 MAPK inhibitor UO126 almost completely blocked insulin-stimulated phosphorylation of p44/42 MAPK (Fig. 2A, middle blot) but had no significant effect on insulin-stimulated Akt phosphorylation (Fig. 2A, top blot, and 2B). Consistent with increased PI3K/Akt signaling, the insulin-stimulated glucose uptake was significantly increased in the Grb2-suppressed C2C12 myotubes compared with scramble control cells (Fig. 2D).
Fig. 2.
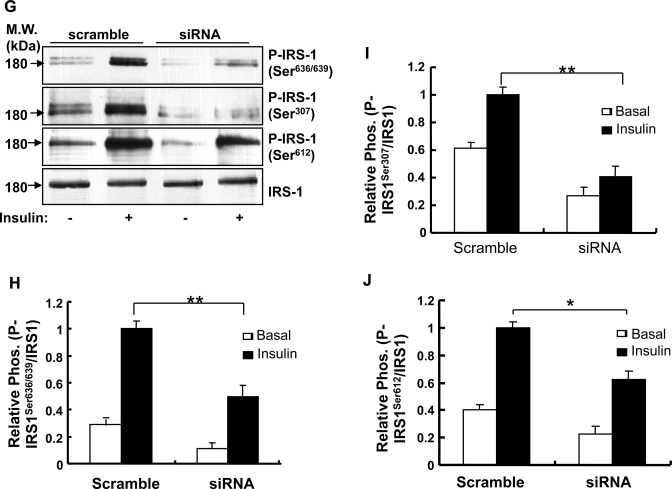
Effect of Grb2 suppression on insulin signaling. A: serum-starved scramble and Grb2-suppressed C2C12 myoblasts were pretreated with or without UO126 (UO) (μM) for 1 h, followed by insulin (Ins) at different concentrations for 5 min and lysed. Equal amounts of lysates from scramble and Grb2-suppressed C2C12 myoblasts were separated by SDS-PAGE and determined by Western blot with specific antibodies as indicated. B and C: 10 nM insulin-stimulated phosphorylation of Akt and p44/42 MAPK and the protein levels of these proteins in the scramble and Grb2-suppressed RNA interference (RNAi) cells were quantified by densitometry analysis of the Western blot signals shown in A using the Scion Imaging program. Data are presented as means ± SE from 3 independent experiments. *P < 0.05 (two-way ANOVA with post hoc Bonferroni test). D: Grb2-suppressed and scramble C2C12 cells were differentiated into myotubes. At differentiation day 4, cells were serum starved for 6 h, and insulin-stimulated glucose uptake in these cells was determined as described in materials and methods. Data are presented as means ± SE from 3 independent experiments, each with triplicate determinations. *P < 0.05 (two-way ANOVA with post hoc Bonferroni test). E: serum-starved scramble and Grb2-suppressed C2C12 myoblasts were treated with or without insulin for 5 min and lysed. Insulin receptor substrate 1 (IRS-1) was immunoprecipitated using an antibody to this protein, and the tyrosine phosphorylation and protein levels of IRS-1 were determined by Western blot using antibodies as indicated. F: phosphorylation and protein levels of IRS-1 were quantified by densitometry analysis of the Western blot signals shown in E using the Scion Imaging program. Data are presented as means ± SE from 3 independent experiments. **P < 0.01 (t-test). G: insulin-stimulated IRS-1 serine phosphorylation in scramble and Grb2-suppressed C2C12 cells myoblasts was determined by Western blot using phosphospecific antibodies as indicated. Data are representatives of 3 independent experiments with similar results. H–J: phosphorylation of IRS-1 was quantified by densitometry analysis of the Western blot signals shown in G using the Scion Imaging program. Data are presented as means ± SE from 3 independent experiments. *P < 0.05, **P < 0.01 (two-way ANOVA with post hoc Bonferroni test).
To elucidate the mechanism by which reducing Grb2 expression enhances PI3K signaling, we examined insulin-stimulated IRS-1 serine phosphorylation in scramble and Grb2-suppressed cells. We found that insulin treatment led to increased IRS-1 serine phosphorylation at several serine residues, including Ser307, Ser612, and Ser636/639 (Fig. 2G), which are known negative regulatory sites in insulin signaling (5, 13, 33). Insulin-stimulated serine phosphorylation at these sites was significantly reduced in the Grb2-suppressed cells compared with that in the scramble cells (Fig. 2, G–J). Taken together with the findings that IRS-1 tyrosine phosphorylation and Akt phosphorylation at Thr308 are enhanced in the Grb2-suppressed cells (Fig. 2), these results suggest that reducing the negative feedback role of IRS-1 serine phosphorylation may be the mechanism by which reducing Grb2 enhances insulin sensitivity.
Reducing Grb2 ameliorates HFD-induced insulin resistance in vivo.
To investigate whether reducing Grb2 expression levels affects insulin sensitivity in vivo, we fed the Grb2+/− heterozygous knockout mice, which showed reduced Grb2 expression in skeletal muscle and liver (Fig. 3, A and B), with a HFD for 16 wk. The Grb2+/− mice showed a reduced body weight increase compared with wild-type littermates under HFD feeding conditions (Fig. 3C). By performing the glucose tolerance test and insulin tolerance test, we found that the Grb2+/− mice were more insulin sensitive compared with wild-type littermates under HFD-fed conditions (Fig. 3, D and E).
Fig. 3.
Reducing Grb2 expression levels increases resistance to high-fat diet (HFD)-induced insulin resistance in vivo. A: Grb2 expression in the rectus femoris skeletal muscle and liver of the Grb2+/− heterozygous knockout and wild-type (wt) littermates was determined by Western blot using an antibody to Grb2. Tubulin was used as a loading control. B: expression levels of Grb2 in skeletal muscle were quantified by densitometry analysis using the Scion Imaging program. Data are presented as means ± SE. *P < 0.05 (two-way ANOVA with post hoc Bonferroni test). C: body weight of Grb2+/− heterozygous knockout and wild-type littermates was measured before and after being fed with HFD. Data are presented as means ± SE. *P < 0.05 (one-way ANOVA with post hoc Bonferroni test). Glucose tolerance test (GTT) (D) and insulin tolerance test (ITT) (E) on wild-type (⧫) and Grb2+/− (▵) mice after 16 wk on a HFD. Data are presented as means ± SE. *P < 0.05 (one-way ANOVA with post hoc Bonferroni test).
Reducing Grb2 expression inhibits IRS-1 serine phosphorylation and enhances insulin signaling in vivo.
To determine whether reducing Grb2 expression levels affects insulin signaling in vivo, we examined the effect of HFD feeding on insulin-stimulated phosphorylation of Akt, IRS-1, and p44/42 MAPK in skeletal muscle of Grb2+/− mice and their wild-type littermates. In agreement with the findings obtained from Grb2-suppressed C2C12 cells, insulin-stimulated IRS-tyrosine phosphorylation and Akt phosphorylation at Thr308 were significantly increased in the Grb2+/− knockout mice compared with their wild-type littermates (Fig. 4, A–C). Reducing the expression levels of Grb2 decreased insulin-stimulated p44/42 MAPK phosphorylation, but the difference was not statistically significant (P = 0.08) (Fig. 4, A and D). In addition, insulin-stimulated IRS-1 Ser612 phosphorylation was notably reduced in the Grb2+/− knockout mice (Fig. 4, E and F).
Fig. 4.
Insulin-stimulated phosphoinositide 3-kinase (PI3K) signaling is enhanced in skeletal muscle of Grb2+/− mice. A: Grb2+/− knockout mice and wild-type littermates fed with a HFD for 16 wk were fasted overnight and intraperitoneally injected insulin (10 U/kg body wt) or an equal amount of saline for 10 min. Rectus femoris skeletal muscle was isolated. IRS-1 was immunoprecipitated, and its tyrosine phosphorylation and protein levels in skeletal muscle were determined by Western blotting using specific antibodies as indicated. The phosphorylation and protein levels of Akt and p44/42 MAPK in cell lysates were detected by Western blotting using specific antibodies as indicated. B–D: phosphorylation and protein levels of IRS-1, Akt, and p44/42 MAPK were quantified by densitometry analysis of the Western blot signals shown in A using the Scion Imaging program; □, saline; ▪, insulin-stimulated. E: insulin-stimulated IRS-1 Ser612 phosphorylation in skeletal muscle of Grb2+/− heterozygous knockout mice and wild-type littermates was determined by Western blot using a phosphospecific antibody. F: relative IRS-1 Ser612 phosphorylation was quantified by densitometry analysis of the Western blot signals shown in E using the Scion Imaging program. Data are presented as means ± SE. *P < 0.05 (two-way ANOVA with post hoc Bonferroni test).
DISCUSSION
CR has a wide range of health benefits including anti-inflammation, anti-insulin resistance, antitumorigenesis, cardioprotection, and extension of lifespan (14, 26, 31, 32). CR reverses insulin resistance in the liver of aging rats by decreasing visceral fat (3). CR has also been shown to improve insulin sensitivity by insulin-stimulated glucose transport in rat skeletal muscle (8, 29, 30), an insulin target tissue that accounts for the majority of insulin-stimulated blood glucose clearance (16). However, although enhanced PI3K/Akt signaling has been implicated in improved insulin sensitivity on glucose metabolism in skeletal muscle of CR animals (15, 29, 30), the underlying biochemical mechanisms remain largely unknown.
We have found that CR greatly suppresses the expression levels of Grb2 in skeletal muscle and white adipose tissue (Fig. 1), a signaling molecule involved in the insulin-mediated p44/42 MAPK signaling pathway. On the basis of the findings that CR stimulates PI3K signaling in skeletal muscle (29, 30), we were interested in determining whether downregulation of Grb2 in mice provides a mechanism by which CR sensitizes insulin action to regulate glucose homeostasis. We found that insulin-stimulated IRS-1 tyrosine phosphorylation and Akt phosphorylation were increased in Grb2-suppressed C2C12 cells (Fig. 2) and in Grb2+/− mice (Fig. 4). In addition, we found that reducing Grb2 expression levels enhanced insulin-stimulated Akt phosphorylation and insulin sensitivity in mice (Figs. 3 and 4). These results provide strong evidence that reducing Grb2 expression levels is beneficial for insulin-stimulated PI3K signaling and action in vivo.
Our results show that reducing Grb2 expression levels decreased IRS-1 serine phosphorylation in C2C12 cells (Fig. 2, G–J) and in skeletal muscle of mice (Fig. 4, E and F). These results suggest a potential mechanism by which reducing Grb2 levels leads to increased PI3K signaling. There are some data suggesting that p44/42 MAPK negatively regulates PI3K signaling by serine phosphorylation of IRS-1 (6, 12, 20, 21). Several p44/42 MAPK-mediated phosphorylation sites including Ser312 (12), Ser612, and Ser636 (6) have been identified in IRS-1. Thus reducing serine phosphorylation of IRS-1 at these sites could be a mechanism by which CR sensitizes insulin signaling and action. Consistent with this view, the insulin-stimulated p44/42 MAPK phosphorylation in skeletal muscle was notably reduced in CR compared with the AL control mice though the reduction was not statistically significant (Fig. 1, D and F). It is possible that the CR-associated reduction in p44/42 MAPK phosphorylation may reach statistical significance with increased sample size, but another likely explanation for these findings is that additional mechanisms, in addition to or other than reducing p44/42 MAPK activity, may be involved in the insulin-sensitizing effect of CR in mice. Several serine kinases such as the JNK (1, 25), IKK (17), and PKC-θ (18, 39) have been found to negatively regulate insulin-stimulated PI3K signaling by serine phosphorylation of IRS-1/2. Future studies will be needed to determine whether the expression and/or kinase activity of these kinases are downregulated in Grb2-deficient cells or mice.
In summary, our results show that CR reduces the expression levels of Grb2 in skeletal muscle, which potentiates insulin-stimulated Akt phosphorylation. We have also found that downregulation of Grb2 increases insulin-induced glucose uptake in C2C12 muscle cells and insulin sensitivity in mice. Our results show that CR or Grb2 downregulation reduces IRS-1 serine phosphorylation and increases IRS-1 tyrosine phosphorylation, suggesting a potential mechanism underlying increased insulin sensitivity in the CR or Grb2+/− mice. Since activation of PI3K signaling is essential for regulation of glucose metabolism, these findings suggest a potential mechanism by which CR increases insulin sensitivity to regulate glucose homeostasis in skeletal muscle and white adipose tissue in mice.
GRANTS
This study was supported by National Institute of Health Grant (R21-AG-26085).
Acknowledgments
We thank Dr. Tony Pawson for the Grb2+/− mice and Ms. Lixin Wang for carrying out some initial experiments relevant to this research.
REFERENCES
- 1.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Balage M, Grizard J, Manin M. Effect of calorie restriction on skeletal muscle and liver insulin binding in growing rat. Horm Metab Res 22: 207–214, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest 101: 1353–1361, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodkin NL, Ortmeyer HK, Hansen BC. Long-term dietary restriction in older-aged rhesus monkeys: effects on insulin resistance. J Gerontol A Biol Sci Med Sci 50: B142–B147, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52: 1319–1325, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bouzakri K, Zierath JR. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem 282: 7783–7789, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cartee GD, Dean DJ. Glucose transport with brief dietary restriction: heterogenous responses in muscles. Am J Physiol Endocrinol Metab 266: E946–E952, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Cartee GD, Kietzke EW, Briggs-Tung C. Adaptation of muscle glucose transport with caloric restriction in adult, middle-aged, and old rats. Am J Physiol Regul Integr Comp Physiol 266: R1443–R1447, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Cecchin F, Ittoop O, Sinha MK, Caro JF. Insulin resistance in uremia: insulin receptor kinase activity in liver and muscle from chronic uremic rats. Am J Physiol Endocrinol Metab 254: E394–E401, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Cefalu WT, Wagner JD, Bell-Farrow AD, Edwards IJ, Terry JG, Weindruch R, Kemnitz JW. Influence of caloric restriction on the development of atherosclerosis in nonhuman primates: progress to date. Toxicol Sci 52: 49–55, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95: 793–803, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Corbould A, Zhao H, Mirzoeva S, Aird F, Dunaif A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes 55: 751–759, 2006. [DOI] [PubMed] [Google Scholar]
- 13.D'Alessandris C, Lauro R, Presta I, Sesti G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia 50: 840–849, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev 5: 13–19, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Dean DJ, Cartee GD. Calorie restriction increases insulin-stimulated tyrosine phosphorylation of insulin receptor and insulin receptor substrate-1 in rat skeletal muscle. Acta Physiol Scand 169: 133–139, 2000. [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Binder C, Wahren J, Felig P, Ferrannini E, Faber OK. Sensitivity of insulin secretion to feedback inhibition by hyperinsulinaemia. Acta Endocrinol 98: 81–86, 1981. [DOI] [PubMed] [Google Scholar]
- 17.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277: 48115–48121, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18: 2024–2034, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gazdag AC, Sullivan S, Kemnitz JW, Cartee GD. Effect of long-term caloric restriction on GLUT4, phosphatidylinositol-3 kinase p85 subunit, and insulin receptor substrate-1 protein levels in rhesus monkey skeletal muscle. J Gerontol A Biol Sci Med Sci 55: B44–B46; discussion B47–B48, 2000. [DOI] [PubMed] [Google Scholar]
- 20.He J, Usui I, Ishizuka K, Kanatani Y, Hiratani K, Iwata M, Bukhari A, Haruta T, Sasaoka T, Kobayashi M. Interleukin-1alpha inhibits insulin signaling with phosphorylating insulin receptor substrate-1 on serine residues in 3T3-L1 adipocytes. Mol Endocrinol 20: 114–124, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Izawa Y, Yoshizumi M, Fujita Y, Ali N, Kanematsu Y, Ishizawa K, Tsuchiya K, Obata T, Ebina Y, Tomita S, Tamaki T. ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Exp Cell Res 308: 291–299, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Ann NY Acad Sci 928: 287–295, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Langlais P, Dong LQ, Ramos FJ, Hu D, Li Y, Quon MJ, Liu F. Negative regulation of insulin-stimulated MAP kinase signaling by Grb10. Mol Endocrinol 18: 350–358, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–1393, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem 278: 2896–2902, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418: 344–348, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006. [DOI] [PubMed] [Google Scholar]
- 28.McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54: 1349–1356, 2005. [DOI] [PubMed] [Google Scholar]
- 29.McCurdy CE, Davidson RT, Cartee GD. Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle. Am J Physiol Endocrinol Metab 285: E693–E700, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCurdy CE, Davidson RT, Cartee GD. Calorie restriction increases the ratio of phosphatidylinositol 3-kinase catalytic to regulatory subunits in rat skeletal muscle. Am J Physiol Endocrinol Metab 288: E996–E1001, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann NY Acad Sci 928: 305–315, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Roth GS, Ingram DK, Lane MA. Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc 47: 896–903, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–205, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Walford RL, Mock D, MacCallum T, Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: health, aging, and toxicological perspectives. Toxicol Sci 52: 61–65, 1999. [PubMed] [Google Scholar]
- 35.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci 57: B211–B224, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, Defronzo RA, Liu F. Peripheral disruption of Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol 18: 6497–6505, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wick KR, Werner ED, Langlais P, Ramos FJ, Dong LQ, Shoelson SE, Liu F. Grb10 inhibits insulin-stimulated insulin receptor substrate (IRS)-phosphatidylinositol 3-kinase/Akt signaling pathway by disrupting the association of IRS-1/IRS-2 with the insulin receptor. J Biol Chem 278: 8460–8467, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Yamaza H, Chiba T, Higami Y, Shimokawa I. Lifespan extension by caloric restriction: an aspect of energy metabolism. Microsc Res Tech 59: 325–330, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002. [DOI] [PubMed] [Google Scholar]