Abstract
Glucose, fat, and glucagon availability are increased in diabetes. The normal response of the liver to chronic increases in glucose availability is to adapt to become a marked consumer of glucose. Yet this fails to occur in diabetes. The aim was to determine whether increased glucagon and lipid interact to impair the adaptation to increased glucose availability. Chronically catheterized well controlled depancreatized conscious dogs (n = 21) received 3 days of continuous parenteral nutrition (TPN) that was either high in glucose [C; 75% nonprotein calories (NPC)] or in lipid (HL; 75% NPC) in the presence or absence of a low dose (one-third basal) chronic intraportal infusion of glucagon (GN; 0.25 ng·kg−1·min−1). During the 3 days of TPN, all groups received the same insulin algorithm; the total amount of glucose infused (GIR) was varied to maintain isoglycemia (∼120 mg/dl). On day 3 of TPN, hepatic metabolism was assessed. Glucose and insulin levels were similar in all groups. GIR was decreased in HL and C + GN (∼30%) and was further decreased in HL + GN (55%). Net hepatic glucose uptake was decreased ∼15% in C + GN, and HL and was decreased ∼50% in HL + GN. Lipid alone or combined with glucagon decreased glucose uptake by peripheral tissues. Despite impairing whole body glucose utilization, HL did not limit whole body energy disposal. In contrast, glucagon suppressed whole body energy disposal irrespective of the diet composition. In summary, failure to appropriately suppress glucagon secretion adds to the dietary fat-induced impairment in both hepatic and peripheral glucose disposal. In addition, unlike increasing the percentage of calories as fat, inappropriate glucagon secretion in the absence of compensatory hyperinsulinemia limits whole body nutrient disposition.
Keywords: glucose, intralipid
the liver has a unique adaptive response to continuous nutrient availability as occurs during total parenteral nutrition (TPN). The liver transitions from a site of modest glucose uptake and storage to an organ that efficiently converts glucose to lactate (9, 10). This adaptation shifts the role of the liver from a site of glycogen synthesis to a major site of lactate production. As a consequence of this shift in hepatic metabolic flux, the normal acute regulators of liver glucose uptake and disposition no longer exert the same degree of control in the adapted setting.
Glucagon plays an important role in the adaptive response of the liver. Glucagon concentrations fall markedly during TPN. Although a very potent regulator of hepatic glucose production, glucagon is thought to regulate hepatic glucose uptake only weakly in the acute setting (23). However, our recent work suggests that chronic reversal of the fall in basal glucagon during TPN markedly attenuates adaptive response of the liver (11). The potency of this effect was surprising. However, this is consistent with the gluconeogenic effect of glucagon, which is amplified in the chronic setting (26). Thus very small changes in glucagon have profound long-term effects on hepatic glucose metabolism.
Our recent work suggests that chronic prevention of the fall in glucagon during TPN impairs peripheral glucose uptake as well (11). Although in humans (7) acute increases in glucagon above basal can inhibit glucose uptake by peripheral tissues, suppression of glucagon below basal is not thought to have significant peripheral effects (12). The chronic effects of glucagon suppression have not been examined. The impact of chronic defects in α-cell function on glucose metabolism is important since insulin-resistant states are characterized by a relative excess of glucagon.
Nonesterified fatty acids (NEFA) are modulators of hepatic glucose metabolism, but their impact on the adaptive response to the liver to TPN is unknown. NEFA enhance gluconeogenesis and inhibit glycolysis both in vitro and in vivo (22, 25). Increases in NEFA do not always stimulate glucose production (6, 33) because of reciprocal effects on gluconeogenesis and glycogenolysis (14, 17). However, in the setting of relative insulin deficiency and nicotinic acid-induced suppression of lipolysis, acute restoration of NEFA can impair liver glucose uptake (28). NEFA concentrations decrease during chronic TPN. Since NEFA are potent regulators of glycolysis and gluconeogenesis (32), the chronic fall in NEFA may amplify the hepatic adaptation to TPN.
NEFA can also impair peripheral glucose uptake. Suppression of NEFA improves insulin action in hepatic and peripheral tissues (1–3, 21). It is unknown whether the chronic failure of glucagon to fall will amplify any NEFA-mediated impairment in peripheral glucose uptake.
NEFA concentrations are elevated in type 2 diabetes and, like glucagon, fail to suppress appropriately in response to insulin (16, 34). When combined with the increased nutrient availability in individuals with diabetes, the failure of liver glucose production to suppress in response to insulin delivery may be limited by the combined failure of glucagon and NEFA to suppress. We wished to determine whether a failure of NEFA to suppress below basal can aggravate the hepatic and peripheral effects of glucagon in a setting where insulin secretion and hyperglycemia are controlled. Our data suggest that very subtle increases in glucagon impair whole body nutrient disposal by targeting glucose utilization in both hepatic and peripheral tissues. In contrast, although increased lipid availability limits glucose disposal and adds to any glucagon-mediated inhibition, it does not limit total calorie disposal.
METHODS
Animal preparation.
Twenty-one male and female nonpregnant mongrel dogs were fed standard Kal-Kan meat (Vernon, CA) and Purina Lab Canine Diet no. 5006 (Purina Mills, St. Louis, MO) once daily and had free access to water. The composition of the diet based on dry weight was 52% carbohydrate, 31% protein, 11% fat, and 6% fiber. Pancrease was added to the diet to facilitate digestion after the pancreas was removed. Dogs were housed in a facility that met American Association for the Accreditation of Laboratory Animal Care International guidelines. All dogs were depancreatized and treated with regular (Eli Lilly and Co., Indianapolis, IN; 9.7 ± 0.3 U) and NPH (15.1 ± 0.2 U) insulin daily until the animal was placed on total parenteral nutrition (TPN). The Vanderbilt University Medical Center Animal Care Committee approved the protocols. The health of the animals was determined before surgery and before TPN administration as having a good appetite (i.e., consumed at least three-fourths of the daily ration), normal stools, hematocrit of >35%, and leukocyte count of <18,000 mm−3.
Experimental preparation.
A laparotomy was performed using sterile techniques under general anesthesia (15 mg/kg thiopental sodium iv for induction and 1.0% isoflurane as an inhalant during surgery) on healthy dogs. During the laparotomy, the pancreas was removed. Blood sampling catheters [0.04-in. inner diameter (ID)] were positioned in the portal and left common hepatic veins. Silastic catheters (0.04-in. ID) were placed in the splenic vein for hormone (insulin and/or glucagon) infusion. Two catheters (0.04-in. ID), one for infusion of nutrients and the other for sampling to monitor the blood glucose level, were placed in the inferior vena cava (IVC). The free ends of the catheters placed in the splenic vein and IVC were exteriorized and tunneled subcutaneously behind the left clavicle. Flow probes (Transonic Systems, Ithaca, NY) were positioned about the portal vein, hepatic artery, and right external iliac artery. After an incision in the right inguinal region, a sampling catheter (0.04-in. ID) was placed in the right common iliac vein, and the tip was positioned distal to its confluence with the IVC; another catheter was advanced from the left external iliac artery to the abdominal aorta.
All catheters were filled with 0.9% NaCl (saline) containing heparin (200 U/ml). The free ends of the catheters and flow probes were placed in subcutaneous pockets. The dogs received penicillin G (500,000 U iv) in 1 liter of saline to minimize the possibility of infection. Flunixamine (0.1 mg/kg; Fort Dodge Laboratory, Fort Dodge, IA) was injected intramuscularly immediately after wound closure for acute pain relief. Dogs also received penicillin G (600,000 U im) for 3 days after surgery.
Nutritional support.
After allowing ≥14 days for recovery from surgery, the free ends of the IVC and splenic vein catheters were exteriorized from their subcutaneous pocket behind the left clavicle under local anesthesia (2% lidocaine; Abbott, North Chicago, IL). Parenteral nutrients (TPN) were infused into one of the IVC catheters with an ambulatory infusion pump (Dakmed, Buffalo, NY); the other IVC catheter was used for daily blood sampling. Insulin and/or glucagon were infused into catheter in the splenic vein using an infusion pump (Walkmed-350; McKinley, Lakewood, CO). Dogs wore a jacket (Alice King Chatham, Los Angeles, CA) with two large pockets for the TPN bag and pumps.
The dogs received TPN as the sole exogenous caloric source for 3 days. The TPN was designed to be isocaloric, based on predicted resting energy expenditure (30). The composition of the TPN included glucose, lipids, amino acids, saline (2.9 ml·kg−1·min−1), potassium phosphates (90 mg·kg−1·day−1), and a multivitamin supplement (MVI-12; Astra USA, Westborough, MA). In the low-lipid group (C), glucose (50% dextrose, Abbott) made up 75% of the nonprotein calories, and a fat emulsion (20% Intralipid; Baxter Healthcare, Deerfield, IL) met the remaining 25% of the energy requirements. In the high-lipid group (HL), glucose comprised 25% of nonprotein calories, and intralipid contributed the remaining 75% of the nonprotein calories. Heparin was included in the HL groups (0.5 mU·kg−1·min−1). In all groups, Travasol (10%; Baxter; Deerfield, IL) was infused to supply basal nitrogen requirements (∼12 g protein/day), calculated with the formula 1.5 × body wt0.67 (in kg). TPN was prepared under sterile conditions.
Experimental design.
Animals were assigned to one of four groups receiving 1) low-lipid TPN (C; n = 5); 2) high-lipid TPN (HL; n = 5); 3) C and one-third of basal glucagon (C + GN; 0.25 ng·kg−1· min−1; n = 5); 4) HL TPN and glucagon (HL + GN; 0.25 ng·kg−1·min−1; n = 6). In all groups during the first 33 h of TPN, the insulin infusion rate was decreased in a stepwise manner from 1.7 (0–6 h) to 1.0 (6–16 h) to 0.8 (16–33 h) to 0.4 mU·kg−1·min−1 (33–72 h). Insulin was given into the portal vein by a catheter in the splenic vein. After 33 h, the insulin infusion rate was held constant at 0.4 mU·kg−1·min−1 for the duration of the study. The glucose infusion rate (GIR) was varied to maintain isoglycemia (∼120 mg/dl) for the duration of the study. The glucose levels were monitored four times a day. TPN was changed twice daily. The glucose content of the TPN was varied. This allowed a variable amount of glucose to be administered, while not altering the infusion rate of the other TPN components. After 72 h of TPN, hepatic metabolism was assessed.
Experimental protocol.
Hepatic substrate balance was assessed 72 h after initiation of the TPN. On the morning of the study, the free ends of all catheters were exteriorized under local anesthesia, their contents were aspirated, and they were flushed with saline. The free ends of the flow probes were also exteriorized and connected to a flow meter (Transonic Systems). The dog was placed in a Pavlov harness for the duration of the study. The study consisted of two periods: a tracer equilibration (0–120 min), a sampling period (120–240 min). The TPN and hormones were continuously infused for the duration of the study. At least 120 min before blood samples were taken to assess organ substrate balance, a primed (44 μCi) constant infusion (0.4 μCi/min) of HPLC purified [3-3H]glucose (PerkinElmer, Waltham, MA) was given into the inferior vena cava and continued for the duration of the study.
Blood pressure and heart rate (Micro-Med, Louisville, KY) were assessed. Blood samples in the artery, portal vein, hepatic vein, and iliac vein were taken every 30 min during the 120-min experimental period. At the end of the study, the animals were euthanized with an overdose of pentobarbital sodium (Veterinary Lab, Lenexa, KS). Tissue samples from each of the seven liver lobes and a muscle (adductor magnus et brevis) were freeze-damped with wal-lenburg clamps precooled in liquid nitrogen. Samples were stored at −70°C until analysis. The remaining liver was removed rapidly and weighed.
Sample processing.
Blood samples were placed in chilled tubes containing potassium EDTA (15 mg). The collection and immediate processing of blood samples have been described previously (9). Blood samples were centrifuged at 3,000 rpm for 10 min. For the glucagon assay, 1 ml of plasma was added to 50 μl of Trasylol (500 kallikrein inhibitor units; Miles, Kankakee, IL). Plasma was deproteinized with Ba(OH)2 and ZnSO4 to assess plasma [3H] glucose specific activity (SA). The remaining plasma was stored at −70°C for later analyses.
Analysis.
Immunoreactive insulin and glucagon were assayed using a radioimmunoassay [Millipore/Linco Research; intra-assay coefficient of variation (CV) of insulin and glucagon was 11 and 10%, respectively], and cortisol was assayed with Diagnostic Products (Los Angeles, CA) RIA Kit (CV 12%).
Analysis of lactate, alanine, beta hydroxybutyrate, and glycerol in blood was performed in a 96-well plate using a modification of the method of Lloyd et al. (24). The plasma concentration of nonesterified fatty acids (NEFA) was determined spectrophotometrically (Wako Chemicals, Richmond, VA).
Plasma lipids were extracted using the method of Folch-Lees (18). The extracts were filtered, and lipids were recovered in the chloroform phase. Individual lipid classes were separated by thin-layer chromatography using Silica Gel 60 A plates developed in petroleum ether, ethyl ether, acetic acid (80:20:1) and visualized by rhodamine 6G. Triglycerides and NEFAs were scraped from the plates and methylated using BF3 /methanol as described by Morrison and Smith (29). The methylated fatty acids were extracted and analyzed by gas chromatography. Gas chromatographic analyses were carried out on an HP 5890 gas chromatograph equipped with flame ionization detectors, an HP 3365 Chemstation, and a capillary column (SP2380, 0.25 mm × 30 m, 0.25-μm film; Supelco, Bellefonte, PA). Helium was used as a carrier gas. The oven temperature was programmed from 160 to 230°C at 4°C/min. Fatty acid methyl esters were identified by comparing the retention times to those of known standards. Inclusion of lipid standards with odd chain fatty acids permitted quantification of the amount of lipid in the sample.
Calculations.
Hepatic substrate load (load in) was calculated as As × HABF + PVs × PVBF, where As and PVs represent the blood or plasma substrate concentrations in the iliac artery and portal vein, and HABF and PVBF represent blood flow in the hepatic artery and portal vein, respectively. Similarly, the substrate load leaving the liver (load out) was the product of HVs × HBF, in which HVs and HBF represent the hepatic vein substrate concentration and total hepatic blood (HABF + PVBF) or plasma flow [blood flow × (1 − hematocrit)]. Net hepatic substrate uptake was calculated as the difference between load in and load out. Net hepatic substrate fractional extraction was calculated as the ratio of net hepatic substrate uptake and load in.
These equations were used to calculate net hepatic glucose, lactate, alanine, glycerol, beta-hydroxybutyrate, and NEFA balances. Plasma glucose was converted to blood glucose by a correction factor of 0.73. Unidirectional hepatic glucose uptake (HGU) was calculated as the ratio of hepatic [3H]glucose uptake and the corresponding [3H]glucose inflowing glucose-specific activity. In cases where the liver was a producer of substrate (i.e., negative uptake), these data were presented as positive values and denoted as net output. The liver can simultaneously produce and consume glucose. Hepatic glucose production (HGP) was calculated as the difference between unidirectional HGU and net HGU (NHGU). Net hindlimb glucose uptake was calculated with the formula (Ag − Vg) × ABF, where Ag and Vg represent glucose concentrations in the iliac artery and iliac vein and ABF represents blood flow in the iliac artery. Plasma flow was calculated by multiplying blood flow by (1 − hematocrit ratio). Net nonhepatic glucose uptake was calculated as the difference between exogenous GIR and NHGU.
Tissue analysis.
Hepatic glycogen content was determined using the enzymatic method of Chan and Exton (8). Tissue glucokinase (GK) and glucose-6-phosphatase (G-6-Pase) activities were analyzed on the quadrate lobe with the methods described by Barzilai and Rossetti (4). Total GK activity was calculated as the difference between activities at 100 and 0.5 mM glucose. G-6-Pase was measured at 10 mM G-6-P. Glycogen synthase and phosphorylase activity were assessed as described by Golden et al. (20). Protein content was assessed with the Biuret method.
Statistics.
All data are expressed as means ± SE. Statistical analysis was performed using ANOVA or Student's unpaired t-test. All data are reported as the averages of the five points of the sampling period. P ≤ 0.05 was regarded as significant.
RESULTS
Prestudy data.
During the first 33 h of TPN, the exogenous insulin infusion rate was initially 1.7 mU·kg−1·min−1 but was then gradually diminished to plateau at 0.4 mU·kg−1·min−1. The insulin infusion rate after 33 h was then held constant at 0.4 mU·kg−1·min−1 for the duration of the study. The glucose infusion rate in the TPN was adjusted to maintain isoglycemia (120 mg/dl). Interestingly, for the first 33 h of TPN, the period where the insulin infusion was relatively high (1.0–0.8 mU·kg−1·min−1), the GIR was only lower in animals receiving additional lipid (9.4 ± 1.3, 6.9 ± 0.9, 9.1 ± 0.4, 6.4 ± 1.2 mg·kg−1·min−1, C, HL, C + GN, and HL + GN, respectively). After 33 h of TPN when the insulin infusion rate was 0.4 mU·kg−1·min−1, the GIR was lower relative to C when either glucagon or lipid was infused (9.2 ± 0.2, 6.3 ± 0.2, and 6.7 ± 0.6 mg·kg−1·min−1, C, HL, C + GN, respectively; P < 0.05, C vs. both HL and C + GN). The GIR was further decreased in HL + GN (4.1 ± 1.1 mg·kg−1·min−1; P < 0.05 vs. C, HL, and C + GN).
As expected, the intralipid infusion was elevated in HL and HL + GN (3.1 ± 0.1 and 3.1 ± 0.1 mg·kg−1·min−1, respectively) compared with C and C + GN (P < 0.05; 1.2 ± 0.02 and 1.2 ± 0.06 mg·kg−1·min−1, respectively). Since intralipid also contains unesterifed glycerol, the glycerol infusion rate was also higher in the HL infusion groups (3.9 ± 0.1 and 3.4 ± 0.5 vs. 1.4 ± 0.03 and 1.5 ± 0.1 μmol·kg−1·min−1, HL and HL + GN vs. C and C + GN, respectively; P < 0.05). The caloric equivalent of the combined carbohydrate and intralipid infusion was 0.045 ± 0.002, 0.053 ± 0.001, 0.035 ± .0025, and 0.046 ± 0.004 kcal·kg−1·min−1 in C, HL, C + GN, and HL + GN, respectively. Glucagon infusion significantly decreased total caloric intake by 22 and 13% in the presence of the high-carbohydrate and HL diet, respectively.
Hemodynamics and hormones.
Blood pressure, heart rate, and organ blood flow were not different between four groups during the study period. In C, HL, C + GN, and HL + GN, insulin and cortisol concentrations were similar. As expected, the arterial plasma glucagon concentration increased when glucagon was infused (Table 1).
Table 1.
Body and liver weights, basal hemodynamic parameters, and hormones in C, HL, C + GN, and HL + GN groups receiving TPN for 3 days before a study
| C | HL | C + GN | HL + GN | |
|---|---|---|---|---|
| n = 5 | n = 5 | n = 5 | n = 6 | |
| Body weight, kg | 21±1 | 20±1 | 21±1 | 21±1 |
| Liver weight, g/kg body wt | 49±3 | 48±3 | 36±3* | 45±3 |
| Mean arterial pressure, mmHg | 104±3 | 97±7 | 112±8 | 101±3 |
| Heart rate, beats/min | 101±14 | 94±7 | 93±12 | 93±11 |
| Hepatic arterial blood flow, ml·kg−1·min−1 | 4.8±0.7 | 4.1±0.4 | 5.2±0.4 | 4.3±0.3 |
| Portal vein blood flow, ml·kg−1·min−1 | 22.8±2.5 | 21.2±1.0 | 27.1±3.3 | 26.9±1.1 |
| Iliac arterial blood flow, ml·kg−1·min−1 | 8.2±1.1 | 7.3±0.7 | 6.4±0.7 | 7.0±1.0 |
| Arterial plasma insulin, μU/ml | 6.9±0.8 | 6.8±0.3 | 4.8±0.9 | 6.6±0.4 |
| Glucagon (artery), pg/ml | 15±2 | 20±6 | 24±1* | 25±4† |
| Cortisol, μg/dl | 3.4±0.6 | 3.9±0.7 | 3.3±0.7 | 4.3±1.0 |
Data are means ± SE. TPN, parenteral nutrition; C, control with low lipid content nutrients [25% nonprotein calories (NPC)]; HL, high lipid (75% NPC) in intravenous nutrient delivery (ND); C + GN, low lipid ND + one-third basal glucagon dose; HL + GN, one-third basal glucagon plus higher amount of intralipid in ND.
Significant difference compared with C (P < 0.05).
Significant difference compared with C (P = 0.063).
Hepatic glucose metabolism.
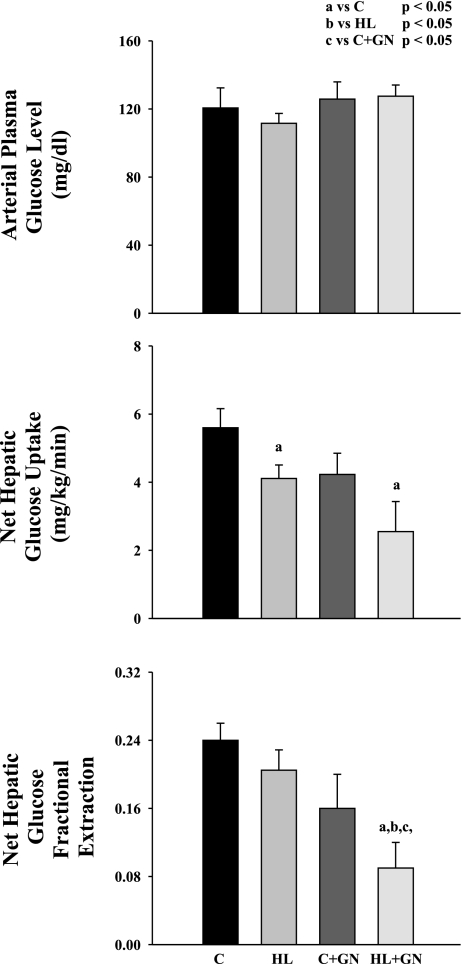
The arterial plasma glucose concentration (Fig. 1) was clamped at similar concentrations in the four groups. In C, NHGU was decreased by ∼15% in HL and C + GN. In HL + GN, NHGU was markedly decreased by ∼50% of C (P < 0.05 vs. C). The decrease in NHGU was due to parallel decreases in net fractional hepatic glucose extraction.
Fig. 1.
Arterial plasma glucose concentration, net hepatic glucose uptake, and net hepatic glucose fractional extraction in chronically catheterized pancreatectomized conscious dogs receiving low-lipid total parenteral nutrition (TPN) [control (C)], high-lipid TPN (HL), low-lipid TPN and one-third of basal glucagon (C + GN), and high-lipid TPN plus one-third of basal glucagon (HL + GN). Data are means ± SE. aSignificant difference compared with C (P < 0.05). bSignificant difference compared with HL (P < 0.05). cSignificant difference compared with HL + GN (P < 0.05).
Unidirectional HGU did not differ among the four groups (Table 2). However, hepatic glucose production was higher in HL + GN compared with C + GN.
Table 2.
Enzyme and substrate analysis of the liver in C, HL, C + GN, and HL + GN groups receiving TPN for 3 days before a study
| C | HL | C + GN | HL + GN | |
|---|---|---|---|---|
| Unidirectional hepatic glucose uptake, mg·kg−1·min−1 | 6.1±0.9 | 4.8±0.4 | 4.6±0.7 | 3.7±0.8 |
| Hepatic glucose production, mg·kg−1·min−1 | 0.3±0.4 | 0.8±0.3 | 0.1±0.2 | 1.0±0.3† |
| Glycogen, mg/g liver | 123±18 | 121±17 | 96±15* | 97±6* |
| Glycogen, g/kg body wt | 6.3±1.2 | 6.1±1.3 | 3.5±0.8* | 4.4±0.5* |
| G-6-Pase, nU/mg protein | 182±51 | 118±27 | 180±55 | 145±26 |
| GK, μU/mg protein | 15.8±3.0 | 12.9±1.7 | 16.6±2.5 | 13.7±1.9 |
| Phosphorylase (%active) | 16±2 | 27±5 | 26±4 | 33±4* |
| Glycogen synthase (%active) | 18±4 | 15±3 | 16±6 | 20±3 |
Data are means ± SE. G-6-Pase, glucose-6-phosphatase; GK, glucokinase.
Significant difference compared with C (P < 0.05).
Significant difference compared with C + GN (P < 0.05).
Hepatic glucose-6-phosphatase and glucokinase activity as well as glycogen synthase and phosphorylase (except in HL + GN) activity ratios were not altered (Table 2). The glycogen contents when expressed on a per gram liver basis were only modestly decreased by glucagon infusion. However, since glucagon infusion also decreased liver mass, liver glycogen content expressed on a body weight basis decreased. This decrease was not amplified by the additional lipid in the TPN (Table 2).
Metabolic substrate kinetics.
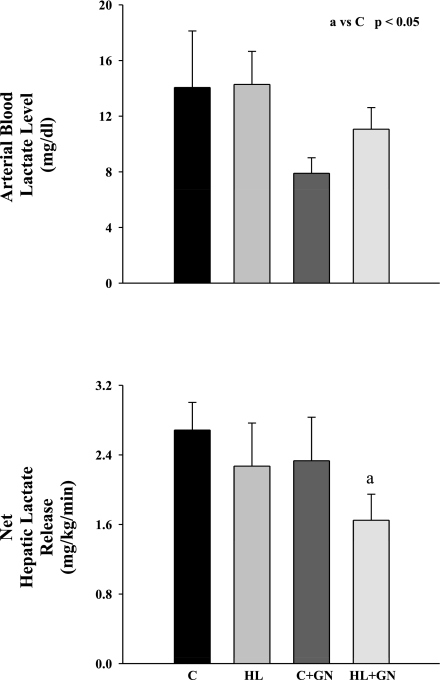
The arterial blood lactate concentration was similar in all four groups (Fig. 2). Net hepatic lactate release was unchanged when lipid or glucagon was infused alone; however, it decreased when HL and glucagon were combined (P < 0.05).
Fig. 2.
Arterial blood lactate concentration and net hepatic lactate release in chronically catheterized pancreatectomized conscious dogs in C, HL, C + GN, and HL + GN. Data are means ± SE. aSignificant difference compared with C (P < 0.05).
The arterial blood alanine and β-hydroxybutyrate concentrations and hepatic balance were unaltered by lipid or glucagon infusion (Table 3). Arterial blood glycerol concentrations were higher in HL and HL + GN. Net hepatic glycerol uptake was only significantly higher in the HL group (Table 3). Arterial plasma NEFA concentration, hepatic NEFA uptake, and net hepatic NEFA fractional extraction increased in HL and HL + GN groups (Table 3). The plasma triglyceride concentration was not altered (504 ± 47, 492 ± 182, 589 ± 119, and 715 ± 200 μg/ml; C, HL, C + GN, and HL + GN, respectively). The fatty acid composition of NEFA and triglyceride is presented in the supplementary data section (Tables S1 and S2, available online at the American Journal of Physiology-Endocrinology and Metabolism website).
Table 3.
Arterial substrate concentration and hepatic substrate uptake and fractional extraction in C, HL, C + GN, and HL + GN groups receiving TPN for 4 days
| C | HL | C + GN | HL + GN | |
|---|---|---|---|---|
| Alanine | ||||
| Concentration | 850±117 | 1108±183 | 648±105 | 944±164 |
| Uptake | 1.0±0.5 | 2.1±0.7 | 1.1±0.5 | 2.0±0.5 |
| FE | 0.05±0.02 | 0.06±0.02 | 0.04±0.02 | 0.08±0.02 |
| Glycerol | ||||
| Concentration | 62±10 | 131±10* | 54±10 | 120±19* |
| Uptake | 1.28±0.24 | 1.99±0.21* | 1.21±0.21 | 1.67±0.41 |
| FE | 0.68±0.02 | 0.66±0.06 | 0.71±0.03 | 0.63±0.04 |
| β-Hydroxybutyrate | ||||
| Concentration | 30±4 | 30±4 | 22±7 | 33±4 |
| Uptake | −0.3±0.1 | −0.7±0.2 | −0.8±0.5 | −0.5±0.1 |
| NEFA | ||||
| Concentration | 246±63 | 852±118* | 279±25 | 781±117* |
| Uptake | 0.27±0.15 | 2.61±0.36* | 0.13±0.40 | 2.56±0.75* |
| FE | 0.04±0.04 | 0.23±0.05* | 0.03±0.05 | 0.18±0.05* |
Data are means ± SE. FE, net fractional hepatic extraction; NEFA, nonesterified fatty acids. Concentrations are in μM; uptake rates are in μmol·kg−1·min−1.
Significant difference compared with C (P < 0.05).
Nonhepatic and hindlimb glucose metabolism.
Net nonhepatic glucose uptake (non-HGU) tended (P = 0.06) to decrease by HL and low glucagon infusion (3.6 ± 0.6, 2.3 ± 0.4, and 2.5 ± 0.3 mg·kg−1·min−1; C, HL, and C + GN, respectively). Non-HGU was significantly decreased further in HL + GN (1.5 ± 0.5 mg·kg−1·min−1). We were unable to detect a statistically significant decrease in net hind limb glucose uptake (0.53 ± 0.22, 0.16 ± 0.01, 0.27 ± 0.03, and 0.20 ± 0.07 mg/min; C, HL, C + GN, and HL + GN, respectively) or fractional extraction (0.09 ± 0.04, 0.03 ± 0.003, 0.05 ± 0.02, and 0.03 ± 0.01; C, HL, C + GN, and HL + GN, respectively) because of the high variance in C.
DISCUSSION
In this study, we examined the chronic interaction between NEFA and glucagon in regulating the metabolic response to chronic nutrient delivery. The chronically catheterized depancreatized canine model allowed us to independently control the concentration of glucagon and fatty acids as well as insulin and glucose. When animals were placed on a high-lipid diet (75 vs. 25% of NPC) to increase NEFA availability, hepatic and peripheral glucose uptakes were impaired. A similar response was observed when very low doses of glucagon were infused in animals adapted to a high-carbohydrate diet. When the high-lipid diet was combined with glucagon infusion, the inhibition was additive. Although both lipid and glucagon impaired whole body glucose utilization, only glucagon limited the ability to meet whole body energy requirements without inducing hyperglycemia. Thus subtle α-cell dysfunction when combined with inappropriate fatty acid availability significantly impairs both hepatic and peripheral glucose metabolism.
The shift from the high-carbohydrate to the high-fat diet impaired whole body glucose disposal (∼30%) by decreasing both hepatic and peripheral (i.e., nonhepatic) glucose uptake. The shift to a high-fat TPN increased NEFA from rather low concentrations (∼200 μM) to concentrations seen in animals on a chow diet (∼900 μM). Most strikingly, whole body glucose disposal was impaired from the onset of TPN in the face of initially elevated insulin concentrations, and this impairment persisted for the duration of the study (72 h). Increases in NEFA do not always impair whole body glucose disposal. In dogs, a 2-h increase in NEFA of the range observed in this study (100–900 μM) also blunted liver, but not peripheral, glucose uptake in the presence of hyperglycemia and basal insulin concentrations (28). Nevertheless, the current findings agree with studies where NEFA were elevated for longer durations (5). A common theme for individuals placed on isocaloric diets where fat content is increased is that insulin action may diminish, but the primary effect is impairment in whole body glucose oxidation (13, 31). In the normal feasting-fasting cycle, glycogen deposition plays a central role in control of glucose tolerance. However, during continuous TPN, after the adaptive phase oxidation is the primary metabolic fate. Since glucose oxidation is sensitively controlled by lipid availability, this may explain why a persistent inhibition of glucose disposal was observed. The decrease in hepatic glucose uptake was not due to an increase in glucose-6-phosphatase activity. In fact, prior work suggests that acyl-COA inhibits this enzyme (19). It is possible that the increase in saturated long-chain fatty acids was not high enough (see Table S1 available online at the American Journal of Physiology-Endocrinology and Metabolism website) to induce a change in that process. Not surprisingly, liver triglyceride content was unaffected, since it was not a hypercaloric diet.
Hepatic glycogen deposition during TPN was not impaired by a high-fat diet. When humans are placed on high-fat, low-carbohydrate diets, nonoxidative glucose disposal increases (13, 31), which most likely reflects enhanced glycogen deposition in muscle. In the present study, after 72 h of TPN, NHGU was decreased while lactate release was unaltered, implying that glucose oxidation by the liver was diminished (28). This would allow a greater fraction of NHGU to be diverted to glycogen, thus preserving glycogen deposition in the face of a decrease in NHGU. Increased NEFA did not alter hepatic glycogen synthase or phosphorylase activities. The increased availability of NEFA increased net hepatic NEFA uptake to support hepatic energy demand. A comparable fall in peripheral glucose uptake was also noted, and this was accompanied by an increase in NEFA uptake by muscle. In contrast to fractional extraction of NEFA by the liver, which increased to over 20% with increased NEFA availability, hindlimb NEFA extraction was low (7% in C) and only increased to 11% in HL.
A very-low-dose infusion of glucagon impaired whole body glucose uptake. This is consistent with our prior work using higher doses of glucagon (11) and observations in the glucagon receptor knockout mice (15). In contrast to the impact of increased NEFA availability, the impairment in whole body glucose disposal was not evident until after 33 h of TPN when the insulin concentration was at its nadir. However, what was surprising was that the very low dose of glucagon impaired hepatic glycogen deposition by nearly 45%. Glycogen deposition occurs during the adaptive phase (0–30 h) to TPN. Yet whole body glucose uptake was not decreased until after 33 h of TPN, and other variables that are regulated by glucagon such as fractional extraction of alanine by the liver and BOHB release were unaffected by the low dose of glucagon. One possibility is that glucose during the first 33 h was diverted from glycogen deposition to other metabolic fates (oxidation, lipogenesis), but after 33 h, when insulin was stabilized at a lower concentration, the inhibitory effect of glucagon on these fates was manifest. Alternatively, glycogen deposition did occur at a normal rate during the first 33 h, and when insulin was decreased the stored glycogen was mobilized. The later possibility is the most likely explanation, since our prior work demonstrate in the already adapted setting replacement with basal glucagon can potently mobilize the stored glycogen (11). Irrespective of the mechanism, the impact of α-cell dysfunction is most evident after the adaptive phase is complete. Thus, in individuals with type 2 diabetes and significant impairment in pancreatic insulin secretion, associated defects in α-cell function amplify any underlying alteration in whole body glucose homeostasis.
When glucagon and elevated NEFA were combined, the effects on glucose disposal were additive rather than synergistic. This implies they may be modifying whole body glucose metabolism by differing mechanisms. Interestingly, acute increases in NEFA actually impair glucagon-stimulated glycogenolysis while not altering gluconeogenesis (17). This would suggest the chronic and acute interaction of NEFA and glucagon is very different. In a setting where the liver is a producer of glucose, increases in NEFA sensitize the liver to increases in gluconeogenic precursor supply (27). In the present study the decrease in hepatic glucose uptake was in part due to a stimulation of hepatic glucose production and inhibition of hepatic glucose entry. The molecular target for this inhibition is unclear since the activation of glycogen phosphorylase was modest and glucokinase and glucose-6-phosphatase activity were unaltered. The glucagon-mediated decrease in hepatic glycogen was not amplified by NEFA.
Although both NEFA and glucagon induce insulin resistance, only glucagon limits whole body calorie disposal. When the animals were either placed on a HL TPN infusion (∼75% of their NPC were derived from intralipid) or given a low-dose glucagon infusion with a high-carbohydrate TPN (75% NPC from glucose), whole body glucose requirements decreased by ∼30% compared with animals on a high-carbohydrate TPN. In the case of HL, the impairment in whole body glucose uptake was appropriate, since total caloric requirements were met by the corresponding rise in lipid availability. In contrast, glucagon infusion impaired whole body glucose disposal in the absence of a change in lipid availability. To meet the total energy requirements, either with additional lipid or glucose, would necessitate an increase in insulin requirements to maintain normoglycemia. A number of studies suggest high-fat diets cause insulin resistance and result in elevated insulin concentrations. However, in the majority of studies, total caloric intake is increased above metabolic demand when the high-fat diet is instituted. In the present study, caloric intake was at or below metabolic demand. As a consequence, fat-induced insulin resistance was appropriate, and the body shifted to fat oxidation. Glucagon infusion replacing the deficit in caloric intake would likely require additional insulin even if the additional calories were supplied as fat (11). This is exemplified by the fact that, when fat and glucagon were combined, as is seen in the HL + GN group, total glucose requirements fell even further. Thus, if glucagon fails to suppress, to prevent hyperglycemia, insulin availability must increase to sustain whole body glucose disposal and to supply the calories required to meet energy demands.
Diabetes is characterized by marked alteration in both fatty acid availability and α-cell dysfunction. Our data suggest that both increased NEFA and glucagon impair hepatic and peripheral glucose utilization and that they do so in an additive manner. Increased NEFA supply appropriately inhibits oxidative carbohydrate disposal. Thus NEFA, when not given in excess of caloric needs, place no additional demand on the beta cell to maintain normoglycemia. In contrast, defects in α-cell secretion specifically target carbohydrate disposal. To meet energy requirements in the setting of α-cell, dysfunction increases the demand on the beta cell to maintain normoglycemia.
GRANTS
This work was supported by the American Diabetes Association, and the National Institutes of Health (DK-43748; private investigator, O. McGuinness), Clinical Nutrition Research Unit (DK-26657), Digestive Disease Research Center (DK-058404), VMMPC (DK-59637), and the VDRTC (DK-20593).
Supplementary Material
Acknowledgments
We are grateful for the expert technical assistance of Wanda Snead, Patrick Donahue, Greg Poffenberger, Eric Allen, Bakula Trivedi, and Angie Penaloza in the Vanderbilt Diabetes Training and Research Center (VDRTC)/Vanderbilt Mouse Metabolic Phenotyping Center (VMMPC) hormone core laboratory and Carla Harris in the VMMPC lipid core. We will miss the dedication and expertise of D. Brooks Lacy, who passed away on July 18, 2008.
REFERENCES
- 1.Ahren B Reducing plasma free fatty acids by acipimox improves glucose tolerance in high-fat fed mice. Acta Physiol Scand 171: 161–167, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj M, Medina-Navarro R, Suraamornkul S, Meyer C, DeFronzo RA, Mandarino LJ. Paradoxical changes in muscle gene expression in insulin-resistant subjects after sustained reduction in plasma free fatty acid concentration. Diabetes 56: 743–752, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty acyl-CoAs and insulin action in Type 2 diabetic patients. Diabetes 54: 3148–3153, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Barzilai N, Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem 268: 25019–25025, 1993. [PubMed] [Google Scholar]
- 5.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44: 1239–1242, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. Effects of fat on insulin stimulated carbohydrate metabolism in normal men. J Clin Invest 88: 960–966, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson MG, Snead WL, Campbell PJ. Regulation of free fatty acid metabolism by glucagon. J Clin Endocrinol Metab 77: 11–15, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Chan TM, Exton JH. A method for the determination of glycogen content and radioactivity in small quantities of tissues or isolated hepatocytes. Anal Biochem 71: 96–105, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Chen SS, Donmoyer C, Zhang Y, Hande SA, Lacy DB, McGuinness OP. Impact of enteral and parenteral nutrition on hepatic and muscle glucose metabolism. JPEN 24: 255–260, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Chen SS, Torres-Sanchez CJ, Hosein N, Zhang Y, Lacy DB, McGuinness OP. Time course of the hepatic adaptation to TPN: interaction with glycogen depletion. Am J Physiol Endocrinol Metab 288: E163–E170, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Chen SS, Zhang Y, Santomango TS, Williams PE, Lacy DB, McGuinness OP. Glucagon chronically impairs hepatic and muscle glucose disposal. Am J Physiol Endocrinol Metab 292: E928–E935, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cherrington Banting Lecture 1997 AD. Control of glucose uptake and release by the liver in vivo. Diabetes 48: 1198–1214, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Chokkalingam K, Jewell K, Norton L, Littlewood J, van Loon LJC, Mansell P, Macdonald IA, Tsintzas K. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: an important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 92: 284–292, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Chu CA, Sherck SM, Igawa K, Sindelar DK, Neal DW, Emshwiller M, Cherrington AD. Effects of free fatty acids on hepatic glycogenolysis and gluconeogenesis in conscious dogs. Am J Physiol Endocrinol Metab 282: E402–E411, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50: 142–150, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in Type 2 diabetes and therapeutic implications. Endocr Rev 28: 253–283, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Everett-Grueter C, Edgerton DS, Donahue EP, Vaughan S, Chu CA, Sindelar DK, Cherrington AD. The effect of an acute elevation of NEFA concentrations on glucagon-stimulated hepatic glucose output. Am J Physiol Endocrinol Metab 291: E449–E459, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 19.Gilles Mithieux CZ Mechanisms by which fatty-acyl-CoA esters inhibit or activate glucose-6-phosphatase in intact and detergent-treated rat liver microsomes. Eur J Biochem 235: 799–803, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Golden S, Wals PA, Katz J. An improved procedure for the assay of glycogen synthase and phosphorylase in rat liver homogenates. Anal Biochem 77: 436–445, 1977. [DOI] [PubMed] [Google Scholar]
- 21.Gormsen LC, Jessen N, Gjedsted J, Gjedde S, Norrelund H, Lund S, Christiansen JS, Nielsen S, Schmitz O, Moller N. Dose-response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. J Clin Endocrinol Metab 92: 1834–1842, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Groop LC, Ferrannini E. Insulin action and substrate competition. Baillieres Clin Endocrinol Metab 7: 1007–1032, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Holste LC, Connolly CC, Moore MC, Neal DW, Cherrington, AD. Physiological changes in circulating glucagon alter hepatic glucose disposition during portal glucose delivery. Am J Physiol Endocrinol Metab 273: E488–E496, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd B, Burrin J, Smythe P, Alberti KG. Enzymatic fluorometric continuous-flow assays for blood glucose, lactate, pyruvate, alanine, glycerol, and 3-hydroxybutyrate. Clin Chem 24: 1724–1729, 1978. [PubMed] [Google Scholar]
- 25.McGarry JD Glucose-fatty acid interactions in health and disease. Am J Clin Nutr 67: 500s–504s, 1998. [DOI] [PubMed] [Google Scholar]
- 26.McGuinness OP, Burgin K, Moran C, Bracy D, Cherrington AD. Role of glucagon in the metabolic response to stress hormone infusion in the conscious dog. Am J Physiol Endocrinol Metab 266: E438–E447, 1994. [DOI] [PubMed] [Google Scholar]
- 27.McGuinness OP, Ejiofor J, Audoly LP, Schrom N. Regulation of glucose production by NEFA and gluconeogenic precursors during chronic glucagon infusion. Am J Physiol Endocrinol Metab 275: E432–E439, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Moore MC, Satake S, Lautz M, Soleimanpour SA, Neal DW, Smith M, Cherrington AD. Nonesterified fatty acids and hepatic glucose metabolism in the conscious dog. Diabetes 53: 32–40, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 5: 600–608, 1964. [PubMed] [Google Scholar]
- 30.Nutrition Sod. Nutrient Requirements of Dogs. Washington, DC: National Academy Press, 1985.
- 31.Pehleman TL, Peters SJ, Heigenhauser GJF, Spriet LL. Enzymatic regulation of glucose disposal in human skeletal muscle after a high-fat, low-carbohydrate diet. J Appl Physiol 98: 100–107, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes 46: 187–196, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Stingl H, Krssak M, Krebs M, Bischof MG, Nowotny P, Furnsinn C, Shulman GI, Waldhausl W, Roden M. Lipid-dependent control of hepatic glycogen stores in healthy humans. Diabetologia 44: 48–54, 1901. [DOI] [PubMed] [Google Scholar]
- 34.Woerle HJ, Szoke E, Meyer C, Dostou JM, Wittlin SD, Gosmanov NR, Welle SL, Gerich JE. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 290: E67–E77, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.