Abstract
Cardiomyocyte apoptosis is a critical process in the pathogenesis of ischemic and diabetic cardiomyopathy, but the mechanisms are not fully understood. Thioredoxin-interacting protein (TXNIP) has recently been shown to have deleterious effects in the cardiovascular system and we therefore investigated whether it may also play a role in diabetes-associated cardiomyocyte apoptosis. In fact, TXNIP expression was increased in H9C2 cardiomyocytes incubated at high glucose, and cardiac expression of TXNIP and cleaved caspase-3 were also elevated in vivo in streptozotocin- and obesity-induced diabetic mice. Together, these findings not only suggest that TXNIP is involved in diabetic cardiomyopathy but also that it may represent a novel therapeutic target. Surprisingly, testing putative TXNIP modulators revealed that calcium channel blockers reduce cardiomyocyte TXNIP transcription and protein levels in a dose-dependent manner. Oral administration of verapamil for 3 wk also reduced cardiac TXNIP expression in mice even in the face of severe diabetes, and these reduced TXNIP levels were associated with decreased apoptosis. To determine whether lack of TXNIP can mimic the verapamil-induced decrease in apoptosis, we used TXNIP-deficient HcB-19 mice, harboring a natural nonsense mutation in the TXNIP gene. Interestingly, we found significantly reduced cleaved caspase-3 levels in HcB-19 hearts, suggesting that TXNIP plays a critical role in cardiac apoptosis and that the verapamil effects were mediated by TXNIP reduction. Thus our results suggest that TXNIP reduction is a powerful target to enhance cardiomyocyte survival and that agents such as calcium channel blockers may be useful in trying to achieve this goal and prevent diabetic cardiomyopathy.
Keywords: diabetic cardiomyopathy, cardiomyocyte apoptosis, verapamil
thioredoxin-interacting protein (TXNIP) has recently been shown to have detrimental effects on the cardiovascular system including oxidative stress, endothelial cell inflammation, and cardiomyocyte apoptosis (25, 29, 33). In addition, TXNIP expression was increased in cardiomyocytes in response to myocardial infarction (MI) (31), hypobaric hypoxia (14), and pressure overload (34). In contrast, knockdown of TXNIP resulted in reduced apoptosis and fibrosis and prevented left ventricular remodeling and hypertrophy after MI and pressure overload, suggesting that downregulation of TXNIP may offer a novel therapeutic approach to protect cardiomyocytes (31).
TXNIP is a ubiquitously expressed protein that binds and inhibits thioredoxin and thereby can induce oxidative stress and modulate the cellular redox state (13, 20, 22, 32). In fact, alterations of the thioredoxin system have been implicated in different cardiovascular diseases, suggesting that endogenous regulators such as TXNIP may represent important therapeutic targets especially in disorders associated with oxidative stress (30). However, very little is known about the regulation of TXNIP especially in the heart.
In pancreatic beta cells, we have previously shown that TXNIP expression is strongly induced by glucose and by diabetes and that TXNIP overexpression induces apoptosis (18, 19, 26). In contrast, TXNIP deficiency protected pancreatic beta cells against oxidative stress, glucose toxicity, and apoptosis (4, 6) and even was able to rescue mice from Type 1 and Type 2 diabetes by preserving beta cell mass and function (5).
The present study was therefore aimed at assessing whether diabetes also induces cardiac TXNIP expression and at identifying pharmacological means to reduce TXNIP expression and apoptosis in the heart.
MATERIALS AND METHODS
Cell culture.
H9C2 rat cardiomyocytes (ATCC) were maintained in DMEM modified to contain 4 mM l-glutamine, 4,500 mg/l glucose, 1 mM sodium pyruvate, 1,500 mg/l sodium bicarbonate, 1.8 mM CaCl2, and 0.8 mM MgCl2, pH 7.3 and supplemented with 10% FBS. Cells were incubated for 24 h in the absence or presence of verapamil (MP Biomedicals, Irvine, CA) or diltiazem (Sigma). To assess the role of calcium, cells were incubated in media depleted of calcium by pretreatment with the chelator ethylene glycol tetraacetic acid (EGTA, 500 μM for 1 h) (Sigma).
Animal studies.
All mouse studies were approved by the University of Wisconsin Animal Care and Use Committee and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Wild-type, 6- to 8-wk-old, male C57BL/6/Sv129 F1 mice (Jackson Laboratory) were rendered diabetic by a single intraperitoneal injection of streptozotocin (STZ; 200 mg/kg) and hyperglycemia of >300 mg/dl was confirmed after 48 h. Mice were euthanized 4 days after the injection and their hearts were collected for protein and RNA extraction.
As a model of Type 2 diabetes, leptin-deficient, obese, insulin-resistant, and diabetic BTBRlepob/ob (ob/ob) (Jackson Laboratory) were used and compared with lean control BTBR mice. The ob/ob mice develop severe diabetes ∼6 wk of age and were euthanized at 12 wk for tissue collection (blood glucose >400 mg/dl).
Mice received verapamil in their drinking water (1 mg/ml) for 3 wk, resulting in an average dose of 100 mg·kg−1·day−1 as described previously (7). Control mice were housed under identical conditions without verapamil. The heart rate and blood pressure of control and verapamil-treated mice were assessed as described previously (3).
TXNIP-deficient HcB-19 mice and the control C3H/DiSnA strain were a generous gift of Dr. Aldons J. Lusis, UCLA, and have been described previously (2, 5, 11, 27). In brief, HcB-19 mice represent a recombinant congenic strain that was derived from the C3H/DiSnA strain and was found to harbor a naturally occurring inactivating nonsense mutation in exon 2 at codon 97 of their TXNIP gene, resulting in dramatically reduced, but not absent, TXNIP mRNA and protein levels (2).
Immunoblotting.
Protein extracts were prepared using a lysis buffer containing HEPES (50 mM), Nonidet P-40 (10%), sodium fluoride (100 mM), sodium pyrophosphate (10 mM), EDTA (4 mM), PMSF (1 mM), leupeptin (2 μM), activated sodium orthovanadate (2 mM), and okadaic acid (100 nM) and were separated by 4–20% SDS-PAGE and blotted onto polyvinylidene difluoride membranes. Antibodies used were TXNIP (1:400) (JY2, MBL International), cleaved caspase-3 (1:200) (Cell Signaling), β-actin (1:200) (Abcam), and anti-mouse IgG (1:5,000) (Amersham). Bands were visualized by Lumigen PS-3 detection reagent (Amersham) and quantified by ImageQuant.
Transfection experiments.
H9C2 cells were grown in 12-well plates and transiently transfected with the human TXNIP luciferase reporter construct (0.8 μg/well) (18) and pRL-TK (5 ng/well) using Lipofectamine Plus (Invitrogen) and the transcriptional activity of the TXNIP promoter was assessed after 24 h by Dual Luciferase Assay (Promega) and corrected for transfection efficiency by Renilla luciferase.
Quantitative real-time RT-PCR.
Mouse heart RNA was isolated using TRIzol (Invitrogen), and 1 μg RNA was used for conversion to cDNA by First Strand cDNA Synthesis Kit for RT-PCR (Roche). Real-time RT-PCR was run on a 7000 Sequence Detection System (Applied Biosystems). Primers for COL1A2 were forward 5′-ACGTGCCGGGACTTAAGACTC-3′ and reverse 5′-GTAGTAATCGCTGTT CCACTCTGG-3′. All other primers have been described previously (4, 18).
TUNEL analysis.
Hearts were fixed in 4% formaldehyde and paraffin embedded, and 5-μm sections were prepared. The DeadEnd Fluorometric TUNEL System kit (Promega, Madison, WI) was used to detect apoptotic nuclei according to the manufacturer's instructions, but including a permeabilization step (5 min in a 1% Triton X-100 PBS solution). The Vectashield with DAPI mounting solution (Vector, Burlingame, CA) was used for visualization of nuclei.
Statistical analysis.
To calculate the significance of a difference between two means, we used Student's t-tests. For data sets of more than two groups we utilized one-way ANOVA calculations. A P value of <0.05 was considered statistically significant.
RESULTS
Glucose induces cardiomyocyte TXNIP expression.
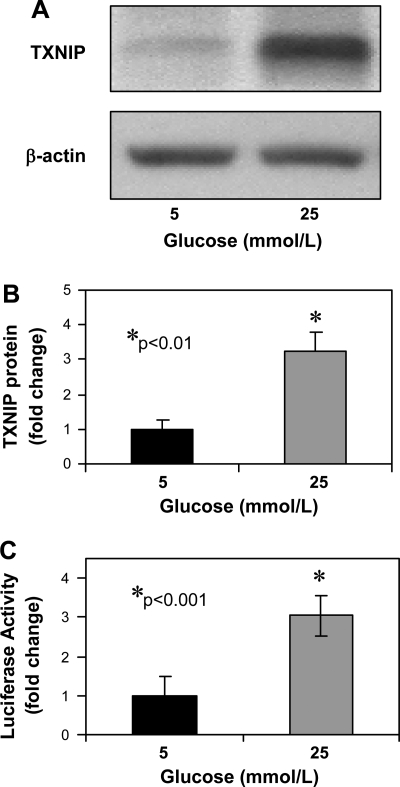
To investigate whether glucose induces TXNIP expression in cardiomyocytes, we incubated H9C2 cardiomyocytes at low (5 mM) and high (25 mM) glucose for 24 h. We found that glucose induces cardiomyocyte TXNIP protein expression (>3-fold, P < 0.01) as measured by immunoblotting (Fig. 1, A and B) and that this induction is mediated by transcriptional activation of the TXNIP promoter as determined by transfection studies (Fig. 1C). This is consistent with our previous human pancreatic islet microarray study where TXNIP was the most dramatically upregulated gene in response to glucose (26) and with the observation that glucose induces beta cell TXNIP expression at the transcriptional level (18).
Fig. 1.
Glucose effects on cardiomyocyte thioredoxin-interacting protein (TXNIP) expression. TXNIP protein levels as assessed by immunoblotting in H9C2 cardiomyocytes incubated at low or high glucose for 24 h. A: representative blot. B: means ± SE of 4 independent experiments. C: glucose effects on TXNIP transcription. H9C2 cells were transfected with the human TXNIP reporter construct and incubated at 5 or 25 mM glucose for 24 h. Bars represent mean fold change ± SE in firefly luciferase activity. Three independent experiments were performed in triplicate.
Cardiac TXNIP expression is elevated in diabetes.
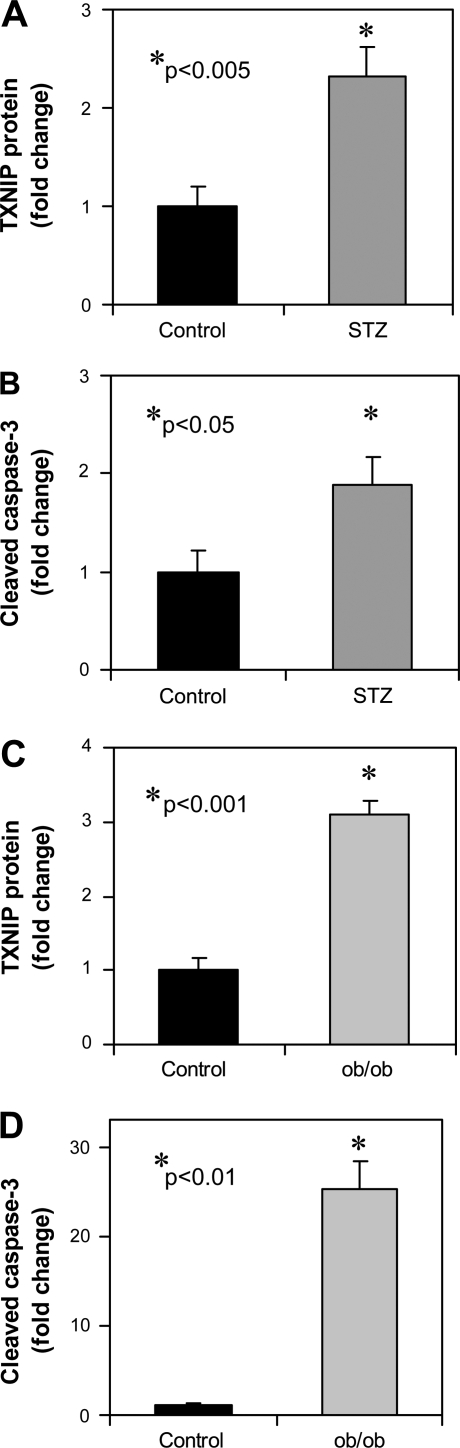
To test whether cardiac TXNIP expression is also elevated in vivo in diabetes, we measured TXNIP in hearts of mice rendered diabetic by STZ. The results of these studies revealed that TXNIP expression is significantly elevated in diabetic animals (Fig. 2A) and this increase in TXNIP expression is accompanied by increased apoptosis as measured by cleaved caspase-3 (Fig. 2B). These effects were even more pronounced in the leptin-deficient, obese, and insulin-resistant ob/ob mice as a model of Type 2 diabetes (Fig. 2, C and D).
Fig. 2.
Effects of diabetes on cardiac TXNIP expression. Wild-type mice were rendered diabetic by a single intraperitoneal injection of streptozotocin (STZ; 200 mg/kg) and euthanized 4 days later. Their hearts were harvested for immunoblotting. Bars represent mean fold change ± SE (n = 6 per group) in TXNIP (A) and cleaved caspase-3 (B) protein levels compared with control mice that received vehicle alone. Obese, insulin-resistant, and diabetic BTBRlepob/ob mice were euthanized at 12 wk of age and their cardiac TXNIP (C) and cleaved caspase-3 (D) expression was compared with age-matched lean control BTBRlep+/+ mice. Bars represent mean fold change ± SE (n = 3 per group).
These findings are in agreement with the hyperglycemia-induced increase in vascular TXNIP expression (25) and the TXNIP-mediated apoptosis previously observed in cardiomyocytes (29, 31) and suggest that TXNIP may be involved in the detrimental effects of diabetes on cardiomyocytes. They further raise the possibility that decreasing TXNIP expression may provide cardioprotection, a notion that is supported by the beneficial effects observed with TXNIP deletion in the context of MI and pressure overload (31, 34). However, to achieve this goal, agents that can lower cardiac TXNIP expression have to be identified.
While studying regulation of TXNIP expression in pancreatic beta cells, we tested different calcium channel blockers in an attempt to block glucose-induced insulin secretion. In the process we noticed that verapamil, and to a lesser degree diltiazem, led to a significant reduction in TXNIP expression in INS-1 beta cells and primary human islets (data not shown). We therefore decided to test whether verapamil could be used to lower cardiac TXNIP expression.
Calcium channel blockers reduce TXNIP expression in cardiomyocytes.
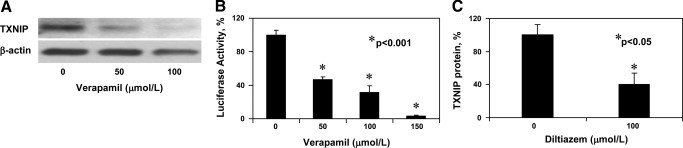
To assess the effects of verapamil on cardiomyocytes, we incubated H9C2 cells at 25 mM glucose and treated them with different concentrations of verapamil. The results of these experiments demonstrated that verapamil reduces TXNIP expression in a dose-dependent manner in cardiomyocytes (>5-fold at 100 μM) (Fig. 3A) and that this reduction is mediated by decreased transcriptional activity of the TXNIP promoter (Fig. 3B).
Fig. 3.
Effects of calcium channel blockers on cardiomyocyte TXNIP expression. H9C2 cardiomyocytes were incubated at 25 mM glucose and treated with the designated doses of verapamil for 24 h. A: dose-dependent decrease in TXNIP protein levels in response to verapamil as measured by immunoblotting. One representative of 2 independent experiments is shown. B: dose-dependent decrease in TXNIP transcription in response to verapamil as assessed by transfection studies using the human TXNIP reporter construct. Bars represent %transcriptional activity of the human TXNIP promoter as measured by luciferase activity. Means ± SE of 3 independent experiments performed in triplicates are shown. C: H9C2 cardiomyocytes were treated with diltiazem for 24 h and TXNIP protein levels assessed by immunoblotting. Means ± SE of 4 independent experiments are shown.
Even though less pronounced, we also observed a reduction in TXNIP expression in response to diltiazem (Fig. 3C) consistent with our prior INS-1 results. This suggests that the TXNIP-lowering effect is not agent specific, but rather a common feature of calcium channel blockers. To further determine whether changes in intracellular calcium can mimic this effect, we incubated H9C2 cardiomyocytes in the presence of the calcium chelator EGTA. We observed a small but significant 35% reduction in TXNIP expression (P = 0.02) in response to EGTA. This modest effect size may be due to the fact that on the basis of the ionic conditions of our culture media, EGTA may reduce but not completely eliminate calcium. In any case, these results suggest that the TXNIP-lowering effects of calcium channel blockers are at least in part mediated by decreased intracellular calcium.
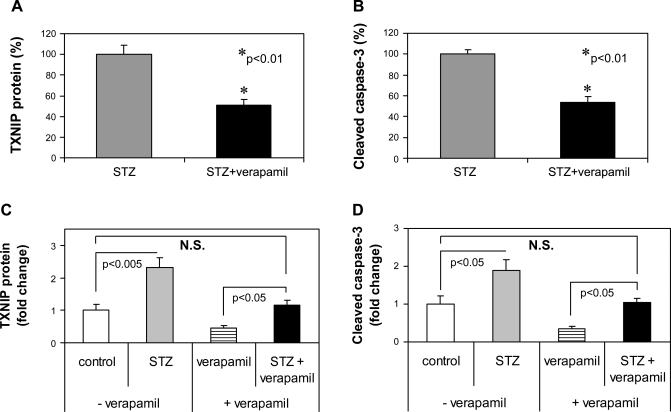
Verapamil administration reduces cardiac expression of TXNIP and cleaved caspase-3 in vivo.
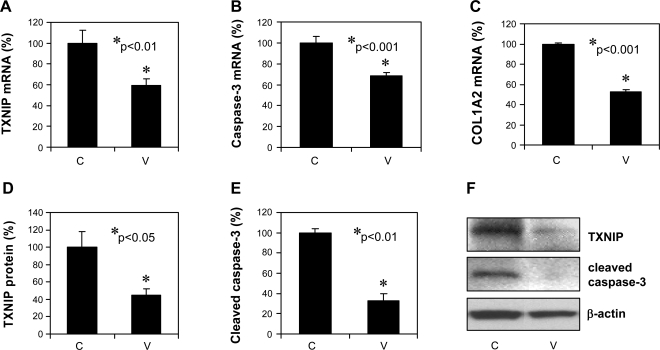
To determine whether verapamil can also reduce cardiac TXNIP in vivo, mice received verapamil in their drinking water for 3 wk; after euthanasia their hearts were analyzed for TXNIP expression as well as for markers of apoptosis and fibrosis at the mRNA and at the protein level. Quantitative real-time RT-PCR revealed a significant decrease in the mRNA expression of TXNIP, caspase-3, and collagen type 1 α2 (P < 0.01) in response to verapamil (Fig. 4, A–C). Immunoblotting further confirmed these findings (Fig. 4, D–F), demonstrating that even a short course of oral verapamil administration can inhibit cardiac TXNIP expression and apoptosis and therefore may be cardioprotective. On the other hand, 3 wk of oral verapamil did not lead to any significant changes in body weight, blood glucose, heart rate, or blood pressure (data not shown).
Fig. 4.
In vivo verapamil effects on the expression of cardiac TXNIP and markers of apoptosis and fibrosis. Mice were treated with or without verapamil (100 mg/kg po) for 3 wk and their hearts were harvested for RNA extraction (n = 4 per group) or for preparation of protein extracts (n = 3). Bars represent changes ± SE in TXNIP (A), caspase-3 (B), and collagen type 1 α2 (COL1A2) (C) mRNA expression as measured by quantitative real-time RT-PCR and corrected for 18S as well as TXNIP protein levels and cleaved caspase-3 levels corrected for β-actin (D–F). C, untreated control; V, verapamil-treated mice.
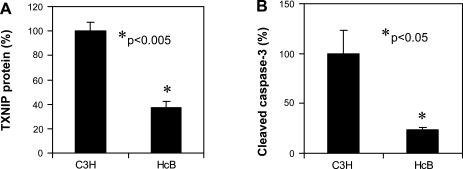
Cleaved caspase-3 expression is reduced in hearts of TXNIP-deficient HcB-19 mice.
Although the observed verapamil-induced reduction in cardiac expression of cleaved caspase-3 was associated with decreased TXNIP expression, these findings do not provide direct proof that the effects are mediated by TXNIP. To address this question, we studied hearts of TXNIP-deficient HcB-19 mice. [HcB-19 mice have a naturally occurring inactivating nonsense mutation in the TXNIP gene, resulting in dramatically reduced, but not totally absent, TXNIP mRNA and protein levels (2).] If TXNIP reduction was responsible for the reduced apoptosis in response to verapamil, TXNIP deficiency should mimic this effect and HcB-19 mice should have lower cardiac expression of cleaved caspase-3. In fact, this is exactly what we observed in our experiments (Fig. 5, A and B). These findings strongly suggest that TXNIP plays a key role in cardiac apoptosis and that genetic or pharmacological reduction of TXNIP expression is capable of promoting cardiomyocyte survival.
Fig. 5.
Effects of TXNIP deficiency on apoptotic factors in the heart. Expression levels of TXNIP (A) and cleaved caspase-3 (B) were assessed by immunoblotting of protein extracts from hearts of 6-mo-old TXNIP-deficient HcB-19 (HcB) and wild-type control (C3H) mice. Bars represent means ± SE; n = 3 animals per group.
Verapamil reduces TXNIP and apoptosis in the diabetic heart.
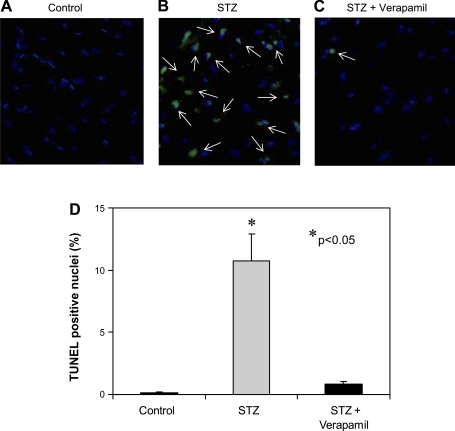
To further test whether verapamil is also capable of reducing proapoptotic TXNIP in the face of diabetes, we again rendered wild-type mice diabetic with STZ and treated them with or without verapamil. Although both groups developed severe diabetes and there was no significant difference in their blood glucose (>400 mg/dl), verapamil-treated mice showed significantly lower levels of TXNIP and cleaved caspase-3 expression in their hearts (Fig. 6, A and B). We also compared the effects of STZ in the absence or presence of verapamil. Interestingly, although STZ-induced diabetes still increased cardiac TXNIP and cleaved caspase-3 expression compared with the very low levels in mice treated with verapamil only, verapamil prevented any increase beyond the levels observed in nondiabetic control mice (Fig. 6, C and D) and thereby led to a complete normalization of these parameters even in the face of severe diabetes. These protective effects of verapamil became even more apparent when we directly assessed apoptosis by TUNEL, demonstrating a >10-fold increase in apoptotic nuclei in diabetic hearts that was almost completely blunted by verapamil (Fig. 7).
Fig. 6.
Verapamil effects on TXNIP expression and apoptosis in the diabetic heart. Wild-type mice were divided into 2 groups (n = 3) and treated with or without verapamil (100 mg/kg po) for a total of 3 wk. Four days prior to euthanasia, mice of both groups were rendered diabetic by STZ injection (200 mg/kg). After euthanasia, their hearts were harvested and assessed for TXNIP (A) and cleaved caspase-3 (B) by immunoblotting; bars represent means ± SE. Comparison of the STZ effects in the presence or absence of verapamil on TXNIP protein (C) and cleaved caspase-3 (D) levels. Bars represent fold change ± SE compared with untreated nondiabetic control mice (open bar).
Fig. 7.
TUNEL analysis of the antiapoptotic effects of verapamil in the heart. Wild-type mice were treated with or without verapamil (100 mg/kg po) for 3 wk and were rendered diabetic by STZ injection (200 mg/kg) or were left untreated for the nondiabetic control group (n = 3 per group). After euthanasia, their hearts were harvested and processed for immunohistochemistry and TUNEL analysis. Representative pictures (×40) of nondiabetic control (A), STZ-diabetic (B), and verapamil-treated STZ-diabetic (C) heart sections are shown. White arrows point at TUNEL-positive apoptotic nuclei. D: quantification of TUNEL-positive cardiomyocytes. Three different mouse hearts and >2,000 nuclei were analyzed per group. Bars represent means ± SE.
DISCUSSION
The results of the present study demonstrate that cardiac TXNIP expression is increased in STZ- and obesity-induced diabetes and associated with increased cardiomyocyte apoptosis. This is consistent with our previous findings in pancreatic beta cells and the role of TXNIP as a proapoptotic factor (18, 29). Furthermore, it suggests that TXNIP may be involved in the pathogenesis of diabetic cardiomyopathy in which cardiomyocyte apoptosis represents a key mechanism (9, 10, 28). Recently, TXNIP has also been implicated in cardiomyocyte damage associated with MI, and inhibition of TXNIP was suggested as a potential cardioprotective approach on the basis of knockdown experiments (31). Moreover, in vivo studies in cardiomyocyte-specific TXNIP knockout mice revealed reduced cardiac hypertrophy in response to pressure overload (34). On the basis of these collective data, TXNIP emerged as an attractive therapeutic target for a variety of cardiovascular disorders, but feasible inhibitors remained elusive.
Our findings now reveal for the first time that calcium channel blockers (and in particular verapamil) can act as potent TXNIP inhibitors and can reduce cardiac TXNIP expression and apoptosis even in the face of severe diabetes. These approved agents (widely used as antihypertensive drugs) thereby may represent a readily available pharmacological tool to achieve the desired TXNIP inhibition and cardioprotection.
Our results indicate that the observed calcium channel blocker effects on TXNIP expression occur at the transcriptional level and indeed calcium has been found to control several transcriptional regulators such as CREB, DREAM, MEF2, NFAT, and NF-κB (16). Interestingly, analysis with the Genomatix software revealed that TXNIP does contain putative binding sites for most of them, and CREB and NF-κB have been shown to be also involved in glucose sensing (8, 12) and diabetic cardiomyopathy (35), suggesting that the calcium channel blocker effects may be mediated by a complicated signaling network. Studies to unravel this network and identify the critical transcription factors are currently ongoing.
Despite some controversy in the past (17), large patient studies have demonstrated that calcium channel blockers are effective in reducing the mortality and morbidity of cardiovascular disease (21, 23) and verapamil has been shown to have beneficial effects especially in diabetic cardiomyopathy (1). Given the increased TXNIP expression and apoptosis we observed in diabetic hearts and the TXNIP-lowering antiapoptotic capacity of verapamil revealed in the present study, this further supports the notion that inhibition of cardiac TXNIP expression (e.g., by verapamil) may help treat and/or prevent diabetic cardiomyopathy. In addition, calcium channel blockers have previously been shown to have protective, anti-oxidant effects in the cardiovascular system (15) and our results suggest that some of these effects might be mediated by TXNIP reduction. In fact, decreased TXNIP expression has also been shown to be part of the antioxidant effects of nitric oxide (24). Moreover, TXNIP deficiency in the HcB-19 mice mimicked the beneficial effects of calcium channel blocker in terms of reduction in apoptotic factors. However, we cannot exclude the possibility that some of the protective effects observed with verapamil are independent of its TXNIP-lowering capacity and are based on the reduction of intracellular calcium levels and/or other factors.
Finally, our findings of decreased apoptosis in TXNIP-deficient hearts underline the importance of TXNIP as a potential target for cardioprotection, be it through calcium channel blockers or by novel agents designed to specifically inhibit cardiac TXNIP expression. Thus, although additional clinical studies are necessary, reducing TXNIP, e.g., by calcium channel blockers, may provide a novel approach to promote cardiomyocyte survival and cardiac function, especially in diabetes.
GRANTS
This work was supported by a grant from the National Heart, Lung and Blood Institute (R21 HL-089205) as well as grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-078752), the American Diabetes Association (7-07-CD-22), and the Juvenile Diabetes Research Foundation (1-2007-790) to A. Shalev.
Acknowledgments
We thank Dr. Timothy Hacker for measuring mouse heart rates and blood pressure. This work was supported with resources and use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, WI.
REFERENCES
- 1.Afzal N, Ganguly PK, Dhalla KS, Pierce GN, Singal PK, Dhalla NS. Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes 37: 936–942, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar JS, Chatterjee A, Castellani LW, Ross DA, Ohmen J, Cavalcoli J, Wu C, Dains KM, Catanese J, Chu M, Sheth SS, Charugundla K, Demant P, West DB, de Jong P, Lusis AJ. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat Genet 30: 110–116, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickson S, Fitzsimons DP, Pereira L, Hacker T, Valdivia H, Moss RL. In vivo left ventricular functional capacity is compromised in cMyBP-C null mice. Am J Physiol Heart Circ Physiol 292: H1747–H1754, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun 346: 1067–1074, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, Lusis AJ, Davis RA, Shalev A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta cell mass and protects against diabetes. FASEB J 22: 3581–3594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta cell apoptosis. Diabetes 57: 938–944, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest 107: R1–R7, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dentin R, Hedrick S, Xie J, Yates J 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319: 1402–1405, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Dyntar D, Sergeev P, Klisic J, Ambuhl P, Schaub MC, Donath MY. High glucose alters cardiomyocyte contacts and inhibits myofibrillar formation. J Clin Endocrinol Metab 91: 1961–1967, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, Salio M, Savino C, Melucci S, Santangelo F, Scanziani E, Masson S, Ghezzi P, Latini R. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol 37: 959–968, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Hui TY, Sheth SS, Diffley JM, Potter DW, Lusis AJ, Attie AD, Davis RA. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem 279: 24387–24393, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Jansson D, Ng AC, Fu A, Depatie C, Al Azzabi M, Screaton RA. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci USA 105: 10161–10166, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 164: 6287–6295, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Karar J, Dolt KS, Mishra MK, Arif E, Javed S, Pasha MA. Expression and functional activity of pro-oxidants and antioxidants in murine heart exposed to acute hypobaric hypoxia. FEBS Lett 581: 4577–4582, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Mak IT, Weglicki WB. Comparative antioxidant activities of propranolol, nifedipine, verapamil, and diltiazem against sarcolemmal membrane lipid peroxidation. Circ Res 66: 1449–1452, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev 88: 421–449, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Messerli FH What, if anything, is controversial about calcium antagonists? Am J Hypertens 9: 177S–181S, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 146: 2397–2405, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Minn AH, Pise-Masison CA, Radonovich M, Brady JN, Wang P, Kendziorski C, Shalev A. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem Biophys Res Commun 336: 770–778, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life 52: 29–33, 2001. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor CM, Carson PE, Miller AB, Pressler ML, Belkin RN, Neuberg GW, Frid DJ, Cropp AB, Anderson S, Wertheimer JH, DeMets DL. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective Randomized Amlodipine Survival Evaluation. Am J Cardiol 82: 881–887, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip: evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem 281: 21884–21891, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW. A calcium antagonist vs. a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 290: 2805–2816, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Schulze PC, Liu H, Choe E, Yoshioka J, Shalev A, Bloch KD, Lee RT. Nitric oxide-dependent suppression of thioredoxin-interacting protein expression enhances thioredoxin activity. Arterioscler Thromb Vasc Biol 26: 2666–2672, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 279: 30369–30374, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 143: 3695–3698, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Sheth SS, Castellani LW, Chari S, Wagg C, Thipphavong CK, Bodnar JS, Tontonoz P, Attie AD, Lopaschuk GD, Lusis AJ. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res 46: 123–134, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 113: 544–554, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, De Keulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem 277: 26496–26500, 2002. [DOI] [PubMed] [Google Scholar]
- 30.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med 84: 997–1003, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Xiang G, Seki T, Schuster MD, Witkowski P, Boyle AJ, See F, Martens TP, Kocher A, Sondermeijer H, Krum H, Itescu S. Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem 280: 39394–39402, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka H, Maehira F, Oshiro M, Asato T, Yanagawa Y, Takei H, Nakashima Y. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun 271: 796–800, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest 115: 733–738, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res 101: 1328–1338, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation 113: 1235–1243, 2006. [DOI] [PubMed] [Google Scholar]