Abstract
Contraction-stimulated glucose transport by skeletal muscle appears to be caused by the cumulative effects of multiple inputs [potentially including AMP-activated protein kinase (AMPK), Ca2+ flux, and force production], making it challenging to isolate the roles of these putative regulatory factors. To distinguish the effects of force production from the direct consequences of Ca2+ flux, the predominantly type II rat epitrochlearis muscle was incubated without (vehicle) or with N-benzyl-p-toluenesulfonamide (BTS), a highly specific myosin II ATPase inhibitor that prevents force production by electrically stimulated (ES) type II fibers without altering cytosolic Ca2+. In ES muscles, BTS vs. vehicle had an 84% reduction in force production and a 57% decrement in contraction-stimulated 3-O-methylglucose transport (3MGT). BTS did not alter the ES increase in phosphorylation of CaMKII (indicative of cytosolic Ca2+) or the amount of glycogen depletion. ES caused significant reductions in ATP (48%) and phosphocreatine (67%) concentrations for vehicle-treated muscles. For BTS-treated muscles, ES did not reduce ATP and caused only a 42% decrease in phosphocreatine. There was an ES increase in phosphorylation of AMPK, acetyl-CoA carboxylase (an AMPK substrate), and TBC1D1 for vehicle-treated muscles but not for BTS-treated muscles. These results point toward an essential role for tension-related events, including AMPK activation, in the 57% contraction-stimulated increase in 3MGT that was inhibited by BTS and further suggest a possible role for TBC1D1 phosphorylation. Non-tension-related events (e.g., increased cytosolic Ca2+ rather than increased AMPK and TBC1D1 phosphorylation) are implicated in the contraction-stimulated increase in 3MGT that persisted in the presence of BTS.
Keywords: N-benzyl-p-toluenesulfonamide, Akt substrate of 160 kDa, contraction, exercise
skeletal muscle contraction triggers increased insulin-independent glucose transport by inducing translocation of the GLUT4 glucose transporter to the cell surface (16, 23, 40), but the precise mechanisms for this effect remain incompletely understood. Using isolated frog skeletal muscle, Holloszy and Narahara (29, 30) provided the initial evidence that increased cytosolic Ca2+ is crucial for contraction-stimulated glucose transport. Subsequent research using rodent muscle supports an important role for increased Ca2+ in activating a portion, but likely not all of the contraction-stimulated increase in glucose transport (7, 57, 60). A number of studies in mammalian muscle have provided evidence that energy metabolism, especially stimulation of AMP-activated protein kinase (AMPK) but potentially other aspects of metabolism, also is important for a portion of contraction-stimulated glucose uptake (15, 27, 34). The current consensus is that glucose transport is increased with contraction as the result of the cumulative effects of multiple inputs, with Ca2+ flux and AMPK activation widely believed to be major factors (19, 20, 28, 33, 42, 57). There also is some evidence for a small role of tension-related processes, possibly independent of altered Ca2+ flux or energy metabolism, in the increased glucose transport induced by contractile activity in mammalian skeletal muscle (31, 32, 45).
Isolating the independent contributions of increased Ca2+, metabolism, and tension on contraction-stimulated glucose transport is challenging because of the complex interactions among these factors. Increased cytosolic Ca2+ triggers both metabolism (via sarcoplasmic reticulum-associated ATPase) and tension development, which further activates metabolism. Elevated metabolism, in turn, can modify Ca2+ flux and tension via the feedback effects of metabolites (inorganic phosphate, protons, and others) (1). One approach has been to incubate isolated muscles with concentrations of caffeine or W7, which cause an increase in cytosolic Ca2+ concentration that is below the threshold for tension development (57, 59, 60). Muscles incubated with caffeine or W7 and dantrolene (to block increased cytosolic Ca2+) do not have increased glucose transport, supporting the idea that elevated cytosolic Ca2+ increases glucose transport. This approach has been very informative, but it does not replicate the much larger and dynamic flux in cytosolic Ca2+ that is typical of physiological contraction. It would be extremely useful to be able to induce the normal, contraction-associated flux of cytosolic Ca2+ without the secondary consequences of activating actinomyosin interaction and tension development.
In this context, the small molecule N-benzyl-p-toluenesulfonamide (BTS) is a valuable new tool for elucidating the mechanisms of contraction-stimulated glucose transport. BTS is an inhibitor of the myosin II ATPase required for force generation in type II (fast twitch) muscle fibers (11, 52). Importantly, BTS is highly specific and does not alter the cytosolic Ca2+ flux in electrically stimulated skeletal muscle (11, 13, 52). Therefore, BTS allows the normal contraction-associated Ca2+ dynamics together with a marked reduction in tension.
We used BTS to probe contraction effects on the rat epitrochlearis, a predominantly type II (∼85%) skeletal muscle (44) that has been instrumental in elucidating the mechanisms that regulate glucose transport (9, 12, 24, 26, 28, 38, 46, 57, 58, 60). Epitrochlearis muscles, electrically stimulated with a protocol that markedly activates tension development, AMPK, and glucose transport (6, 50), were used to determine the effect of BTS on contraction-mediated phosphorylation of two Rab GTPase-activating proteins, Akt substrate of 160 kDa (AS160; also known as TBC1D4) and TBC1D1. AS160 and TBC1D1, which become phosphorylated in response to contractile activity, have been implicated as potential modulators of glucose transport via AMPK and/or Ca2+/calmodulin-related mechanisms (6, 10, 21, 36, 37, 54).
We hypothesized that the BTS-associated decrement in force production of electrically stimulated rat epitrochlearis muscles would be accompanied by 1) unchanged phosphorylation of CaMKII, an indicator of elevated cytosolic Ca2+, 2) a smaller decline in ATP and phosphocreatine (PCr) concentrations, leading to attenuated activation of AMPK and phospho-Akt substrate (PAS)-TBC1D1, and 3) reduced contraction-stimulated glucose transport. We also evaluated the effects of BTS on glycogen levels, which have been suggested to modulate glucose transport (15, 27, 35). In addition, we determined the effects of BTS on contraction-stimulated phosphorylation of Akt and its substrate, glycogen synthase kinase-3 (GSK3). Although increased Akt phosphorylation is not essential for contraction-stimulated glucose transport (21, 49), it has been implicated in some of the adaptations found with chronic contractile activity, e.g., muscle hypertrophy (18, 43).
METHODS
Materials.
BTS and 3-O-([3H]methyl)-d-glucose (3-[3H]MG) were purchased from Sigma-Aldrich (St. Louis, MO). [14C]mannitol was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) was purchased from Calbiochem (San Diego, CA). Anti-phospho-Thr308 Akt (pThr308Akt; no. 9275), anti-phospho-Ser473Akt (pSer473Akt; no. 9271), anti-phospho-Thr172 AMPK (pAMPK; no. 2531), anti-phospho-Ser21/9 glycogen synthase kinase-3α/β (pGSK3; no. 9331), anti-phospho-Ser79 acetyl-CoA carboxylase (pACC; no. 3661), anti-phospho-CaMKII (pCaMKII; no. 3361), anti-phospho-(Ser/Thr) Akt substrate (anti-PAS; no. 9611), and anti-rabbit IgG-horseradish peroxidase (HRP) conjugate (no. 7074) antibodies were purchased from Cell Signaling Technology (Danvers, MA). TBC1D1 polyclonal antibody was provided by Dr. Jianxin Xie (Cell Signaling Technology). Protein G-agarose (no. 16-626) and anti-AS160 (no. 07-741) were purchased from Millipore (Billerica, MA). Reagents and apparatus for SDS-PAGE and immunoblotting were obtained from Bio-Rad (Richmond, CA). SuperSignal chemiluminescence kits (West Dura extended duration substrate), BCA total protein assay kits, and T-PER tissue protein extraction reagent were purchased from Pierce Biotechnology (Rockford, IL). All other reagents were obtained from Sigma-Aldrich or Fisher Scientific (Pittsburgh, PA).
Animal treatment.
Male Wistar rats (∼130–160 g; Harlan, Indianapolis, IN) were maintained on a 12:12-h light-dark cycle and provided with rodent chow (Lab Diet; PMI Nutritional International, Brentwood, MO) and water ad libitum. The animals were fasted at 1700 the night before the experimental day and were only given access to water thereafter. Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (∼60 mg/kg wt) on the following day between 1000 and 1300. Upon loss of pedal reflexes, both epitrochlearis muscles were rapidly dissected out and treated as described below. Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals.
Epitrochlearis muscle treatment.
One epitrochlearis muscle from each animal was incubated in a glass vial containing Krebs-Henseleit buffer (KHB) supplemented with 8 mM glucose (buffer 1) and 50 μM BTS (dissolved in vehicle: 0.05% DMSO) (41). The contralateral muscle was incubated in buffer 1 and 0.05% DMSO (vehicle). In experiments where 3-O-methylglucose (3-MG) transport was assessed, buffer 1 was supplemented with 2 mM mannitol. Vials containing the muscles were incubated for 120 min in a water bath (35°C) with continuous gassing (95% O2-5% CO2) and gentle shaking.
After 120 min of incubation, muscles were mounted in a water-jacketed glass container connected to a temperature-controlled bath containing the same medium as in the preceding incubation step (buffer 1 ± BTS) warmed to 35°C. With the use of stainless steel clips, the distal end of the muscle was attached to a glass rod, and the proximal end was attached to a force transducer (Radnoti, Litchfield, CT) with a resting tension of 0.4 g as previously described (17). Resting muscles were mounted in the same apparatus at tension of 0.4 g for 5 min but were not stimulated to contract. After 5 min of contraction [pulse duration of 0.1 ms, pulse rate of 100 pulses/s, train duration of 10 s, and train rate of 2 min−1 (6)] or resting treatment, some muscles were immediately blotted on filter paper dampened with KHB, rapidly trimmed of connective tissue, freeze-clamped, and then stored at −80°C until subsequent homogenization and analysis. Other muscles to be used for measurement of 3-MG transport were transferred to glass vials containing rinsing medium (KHB supplemented with 2 mM pyruvate and 6 mM mannitol, buffer 2) at 30°C for 10 min before the final incubation step with 3-MG.
For AICAR experiments, muscles were initially incubated for 80 min in buffer 1 with 50 μM BTS or with vehicle followed by 40 min of incubation in the same medium supplemented with or without 2 mM AICAR. Muscles were then either freeze-clamped immediately or incubated for 10 min in buffer 2 before being incubated with 3-MG.
Incubation of epitrochlearis muscles with 3-MG.
After the 10-min incubation in buffer 2, the epitrochlearis muscles were transferred to glass vials containing KHB with 8 mM 3-MG (including 3-[3H]MG at a final specific activity of 0.25 mCi/mmol) and 2 mM mannitol (including [14C]mannitol at a final specific activity of 0.1 mCi/mmol) at 30°C for 10 min. The epitrochlearis muscles were then rapidly blotted on filter paper dampened with KHB, trimmed, freeze-clamped, and stored at −80°C until further processing.
Epitrochlearis muscle force production.
Muscle tension data were captured using the MP100 system and AcqKnowledge software from BIOPAC Systems (Santa Barbara, CA). The peak developed tension was determined by subtracting the baseline tension from the maximum tension. The area under the curve for force production was determined by calculating the area under the curve for all of the tetani and subtracting the baseline tension. Force production for each muscle was normalized relative to wet muscle mass.
Muscle homogenization.
Frozen muscles used for 3-MG transport measurement or immunoprecipitation and/or immunoblotting were homogenized in 1 ml of ice-cold homogenization buffer using glass tissue grinding tubes (Kontes, Vineland, NJ) as described previously (22). Homogenates were subsequently rotated at 4°C for 1 h before being centrifuged (15,000 g for 15 min at 4°C). A portion of the resultant supernatant was used to determine protein concentration with the bicinchoninic acid assay (53). Aliquots of the supernatant from some muscles were used for scintillation counting, and 3-MG transport was determined as previously described (8).
Glycogen, ATP, and PCr measurement.
Muscles used for metabolite analysis were extracted in 100 μl of 3 M perchloric acid for 5 min and then homogenized using glass tissue grinding tubes. After homogenization, 900 μl of H2O were added to the homogenate to yield a 0.3 M perchloric acid muscle extract. An aliquot (400 μl) of the extract was removed and stored at −80°C for subsequent glycogen analysis. Before ATP and PCr analysis, muscle extract was neutralized with KIK (2 N KOH, 0.4 M imidazole, and 0.4 M KCl). ATP and PCr concentrations were determined fluorometrically as previously described (39, 47). Glycogen concentration was determined using the amyloglucosidase method (47).
Immunoprecipitation.
Frozen muscles to be immunoprecipitated by anti-PAS or anti-TBC1D1 were homogenized in T-PER-supplemented homogenization buffer (2 mM Na3VO4, 2 mM EDTA, 2 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/ml leupeptin in T-PER). Homogenized muscles (300–500 μg of protein) were mixed with protein G-agarose beads for 30 min, and the resulting supernatant was immunoprecipitated with 1.5 μg of anti-PAS or anti-TBC1D1 at 4°C. After gentle rotation overnight, the immunoprecipitation mix was centrifuged at 4,000 g and the supernatant was aspirated. After washing (4 times with 500 μl of phosphate-buffered saline), the protein bound to the beads was eluted with 2× Laemmli sample buffer and boiled before being loaded on a polyacrylamide gel.
Immunoblotting.
After SDS-PAGE, proteins were electrophoretically transferred to nitrocellulose. Blots were then rinsed briefly with Tris-buffered saline (TBS; 0.14 M NaCl and 0.02 M Tris base, pH 7.6) and then blocked with 5% nonfat dry milk in TBS with 0.1% Tween (TBST) for 1 h at room temperature. The blots were then washed three times for 5 min at room temperature with TBST and subsequently incubated with primary antibody (1:1,000 in TBST with 5% bovine serum albumin) overnight at 4°C. Nitrocellulose membranes were incubated with the appropriate primary antibody for detection of PAS-AS160 (anti-AS160) and PAS-TBC1D1 (anti-PAS). Blots were again washed three times for 5 min with TBST, incubated with the secondary antibody (goat anti-rabbit IgG HRP conjugate; 1:20,000 in TBST with 5% milk) for 1 h at room temperature, washed three times for 5 min with TBST, washed twice for 5 min in TBS, and visualized with enhanced chemiluminescence. Protein bands were quantified by densitometry (Alpha Innotech, San Leandro, CA). The mean values for resting muscles incubated without BTS on each blot were normalized to equal 1.0, and then all samples on the blot were expressed relative to this normalized control value.
Statistical analysis.
SigmaStat version 2.0 (San Rafael, CA) was used for statistical analyses. Data are means ± SE. A P value ≤0.05 was considered statistically significant. A paired t-test was used to determine significant differences between paired, electrically stimulated muscles for peak tension and the area under the curve for tension developed. Two-way ANOVA was used to identify significant main effects (contraction and BTS; AICAR and BTS) and interactions (contraction × BTS; AICAR × BTS) for glucose transport, protein phosphorylation, and metabolite concentrations. The source of significant variance was detected with a Student-Newman-Keuls post hoc test. The estimated contraction- and AICAR-stimulated differences (estimated-Δ-contraction or estimated-Δ-AICAR) in glucose transport were calculated by subtracting the mean of the basal transport rates of each treatment (vehicle or BTS) from their respective contraction- or AICAR-stimulated glucose transport values. A t-test was used to determine significant differences between treatments for estimated-Δ-contraction and estimated-Δ-AICAR glucose transport.
RESULTS
For basal muscles (no contraction or AICAR treatment), there were no statistically significant differences between BTS and vehicle groups for any of the measurements made (tension, 3-MG transport, metabolite concentrations, or phosphorylated proteins).
Developed tension.
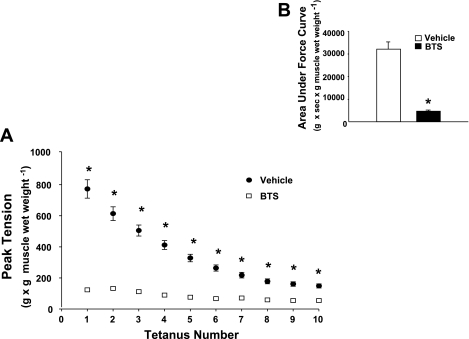
Treatment with BTS compared with vehicle controls resulted in an 83% reduction (P < 0.0001) in peak developed tension by electrically stimulated muscles (Fig. 1A). BTS vs. vehicle treatment also resulted in a similar 84% reduction (P < 0.0001) in the area under the force production curve (Fig. 1B).
Fig. 1.
Tension development. Paired epitrochlearis muscles, incubated with 50 μM N-benzyl-p-toluenesulfonamide (BTS) or vehicle, were electrically stimulated to contract (pulse duration of 0.1 ms, pulse rate of 100 pulses/s, train duration of 10 s, train rate of 2 min−1 for 5 min). A: peak developed tension determined for each tetanus was normalized to the muscle wet weight (g). B: area under the force production curve summed for all 10 tetani was calculated by subtracting the baseline tension from total area under the curve. Values are means ± SE (n = 44). *P < 0.0001, BTS vs. vehicle.
3-MG transport.
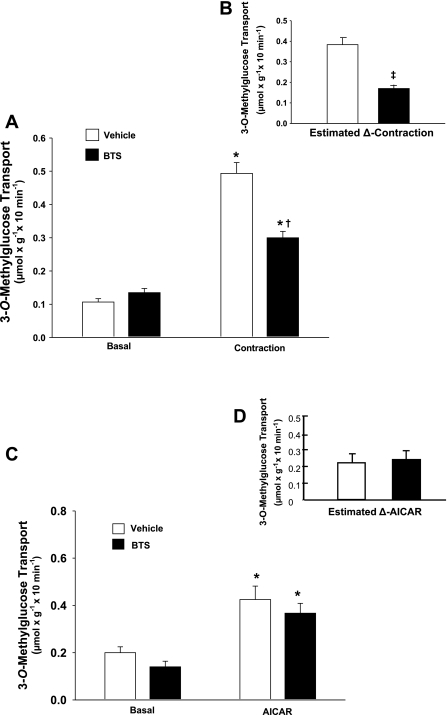
Contracting vs. resting muscles had an ∼4.5-fold increase (P < 0.001) in glucose transport in the vehicle group and an ∼2.5-fold increase (P < 0.001) in the BTS group (Fig. 2A). BTS vs. vehicle treatment significantly (P < 0.001) inhibited glucose transport within the contracting muscles by 39% (Fig. 2A) and yielded a 57% reduction (P < 0.001) in the estimated contraction-induced increase in glucose transport (estimated-Δ-contraction; Fig. 2B).
Fig. 2.
Rate of 3-O-methylglucose (3-MG) transport with or without contraction and with or without 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR). Paired epitrochlearis muscles were incubated with 50 μM BTS or vehicle. A: muscles were mounted at resting tension (basal) or were electrically stimulated (contraction) before measurement of 3-MG transport. *P < 0.001, basal vs. contraction within the BTS or vehicle treatment groups. †P < 0.001, BTS vs. vehicle with contraction. B: estimated contraction-stimulated increase (estimated-Δ-contraction) was calculated by subtracting the mean value for basal 3-MG transport rate of each treatment from the respective contraction-stimulated value. ‡P < 0.001, estimated-Δ-contraction for BTS vs. vehicle. C: muscles were incubated in the absence (basal) or presence of 2 mM AICAR before 3-MG transport measurement. *P < 0.001, basal vs. AICAR within the BTS or vehicle treatment groups. D: estimated AICAR-stimulated increase (estimated-Δ-AICAR) was calculated by subtracting the mean value for basal 3-MG transport rate of each treatment from the respective AICAR-stimulated value. Values are means ± SE (n = 10–17).
To confirm that BTS had no direct inhibitory effects on glucose transport, we incubated epitrochlearis muscles in AICAR with or without 50 μM BTS. AICAR-treated vs. basal muscles had significantly (P < 0.001) elevated glucose transport rates regardless of the presence of BTS (Fig. 2C). The AICAR-induced increase in glucose transport (estimated-Δ-AICAR) was not significantly different in BTS- vs. vehicle-treated muscles (Fig. 2D).
ATP, PCr, and glycogen concentrations.
In the absence of BTS, contraction vs. basal muscles had a significant (P < 0.001) 48% reduction in ATP concentration (Table 1). In BTS-treated muscles, ATP concentration was not different for the contraction vs. basal groups. With or without BTS, PCr concentration decreased significantly (P < 0.001) with contraction vs. respective basal controls, but within the contraction groups, the BTS- vs. vehicle-treated muscles had higher concentrations of PCr (P < 0.05). The contraction vs. basal groups had lower (P < 0.001) glycogen concentrations, with or without BTS, and there was no evidence that BTS vs. vehicle groups differed with regard to glycogen concentration after contraction.
Table 1.
ATP, PCr, and glycogen concentrations in rat epitrochlearis muscles
| Group |
Vehicle |
BTS
|
||
|---|---|---|---|---|
| Basal | Contraction | Basal | Contraction | |
| ATP, μmol/g | 5.54±0.40 | 2.90±0.32* | 4.88±0.32 | 4.68±0.33 |
| PCr, μmol/g | 16.30±1.46 | 5.38±0.62* | 16.74±0.76 | 9.76±0.83*† |
| Glycogen, μmol/g | 19.76±1.59 | 10.1±1.41* | 18.10±0.89 | 10.48±0.96* |
Values are means ±SE (n = 4–5). Paired epitrochlearis muscles were incubated with 50 μM N-benzyl-p-toluenesulfonamide (BTS) or vehicle. Muscles were mounted at resting tension (basal) or electrically stimulated (contraction) before being freeze-clamped and processed for metabolite analyses. PCr, phosphocreatine.
P < 0.001, basal vs. contraction within the BTS or vehicle treatment group.
P < 0.05, BTS vs. vehicle with contraction.
Phosphorylation of CaMKII, AMPK, ACC, Akt, and GSK3.
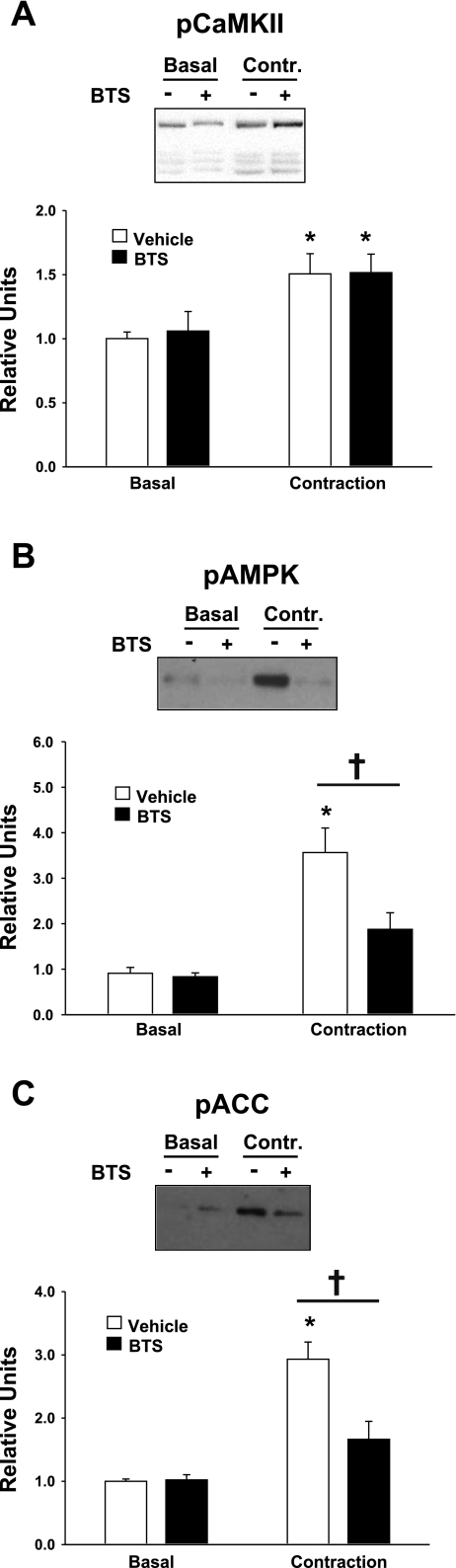
As expected, contraction vs. basal muscles had significantly (P < 0.05) greater pCaMKII in both the vehicle (P < 0.001) and BTS (P < 0.05) groups (Fig. 3A). There was no evidence for a difference in pCaMKII between BTS- and vehicle-treated muscles that were stimulated to contract.
Fig. 3.
Phosphorylation (p) of CaMKII, AMP-activated protein kinase (AMPK), and acetyl-CoA carboxylase (ACC). Paired epitrochlearis muscles were incubated with 50 μM BTS or vehicle. Muscles were mounted at resting tension (basal) or electrically stimulated (contraction) before being freeze-clamped and processed for measurement of pCaMKII (A), pAMPK (B), and pACC (C). A: *P < 0.05, basal vs. contraction within the vehicle treatment group. B and C: *P < 0.001, basal vs. contraction within the vehicle treatment group. †P < 0.01, BTS vs. vehicle with contraction. Values are means ± SE (n = 5–11).
In the absence of BTS, contraction vs. basal muscles had a significant (P < 0.001) increase in pAMPK, but there was only a nonsignificant (P = 0.149) trend for increased pAMPK in contraction vs. basal muscles from the BTS group (Fig. 3B). Furthermore, BTS- vs. vehicle-treated muscles had significantly (P < 0.01) reduced pAMPK within the contraction groups. In the absence of BTS, contraction vs. basal muscles had a significant (P < 0.001) increase in phosphorylation of ACC (an AMPK substrate), whereas in the BTS group, there was a nonsignificant (P = 0.114) trend for an increase pACC in contraction vs. basal muscles (Fig. 3C). Within the contraction groups, BTS vs. vehicle treatment had significantly reduced pACC (P < 0.001). AICAR significantly increased pAMPK (P < 0.05) and pACC (P < 0.01) in both the vehicle- and BTS-treated muscles, and BTS had no affect on these AICAR effects (data not shown).
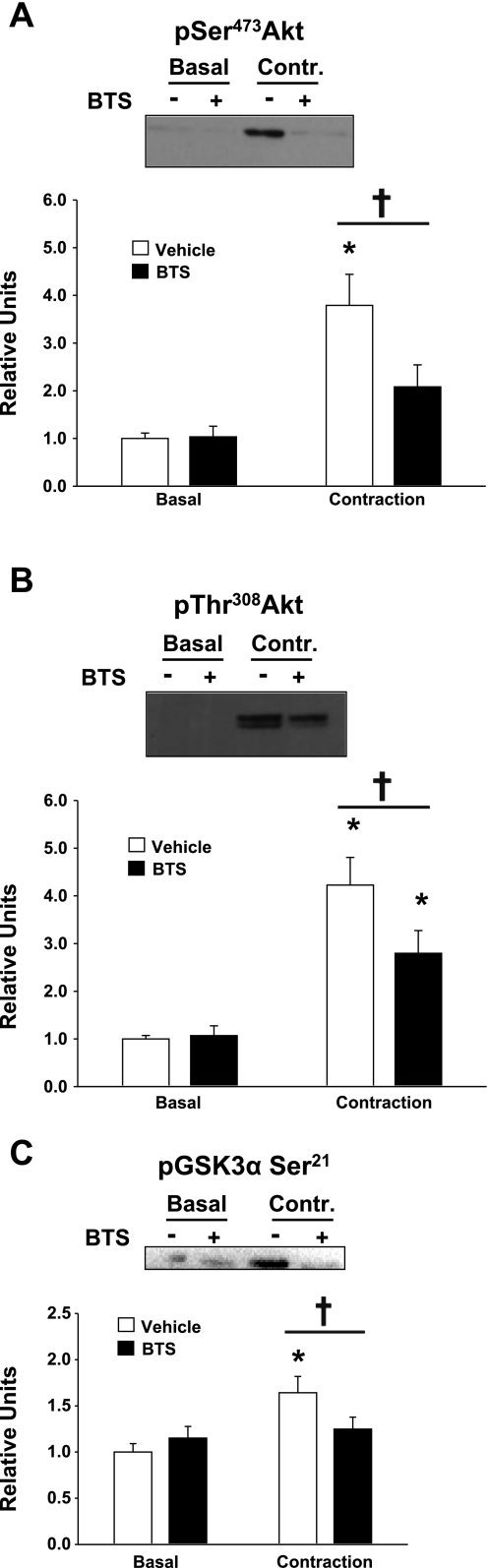
In the absence of BTS, contraction vs. basal muscles showed a significant (P < 0.001) increase in pSer473Akt. In the presence of BTS, contraction vs. basal muscles tended (P = 0.119) to have greater values for pSer473Akt (Fig. 4A). BTS reduced (P < 0.01) the pSer473Akt values in muscles stimulated to contract. For pThr308Akt, there was a significantly (P < 0.001) higher value for contraction vs. basal muscles without BTS and also a significant (P < 0.01) increase in the contraction vs. basal muscles in the presence of BTS (Fig. 4B). BTS- vs. vehicle-treated muscles that were stimulated to contract had significantly (P < 0.05) lower values for pThr308Akt.
Fig. 4.
Phosphorylation of Ser473Akt, Thr308Akt, and Ser21 glycogen synthase kinase-3α (GSK3α Ser21). Paired epitrochlearis muscles were incubated with 50 μM BTS or vehicle. Muscles were mounted at resting tension (basal) or electrically stimulated (contraction) before being freeze-clamped and processed for measurement of pSer473Akt (A), pThr308Akt (B), and pGSK3α Ser21 (C). A: *P < 0.001, basal vs. contraction within the vehicle treatment group. †P < 0.01, BTS vs. vehicle with contraction. B and C: *P < 0.01, basal vs. contraction within the vehicle and BTS treatment groups. †P < 0.05, BTS vs. vehicle with contraction. Values are means ± SE (n = 9–19).
In the absence of BTS, contraction vs. basal muscles had a significant (P < 0.01) increase in pGSK3α Ser21, and this effect was absent in muscles electrically stimulated in BTS compared with basal controls in BTS (Fig. 4C). Muscles stimulated to contract without BTS compared with muscles stimulated in the presence of BTS had significantly (P < 0.05) greater pGSK3α Ser21. There were no significant effects of contraction or BTS on pGSK3β Ser9 (data not shown).
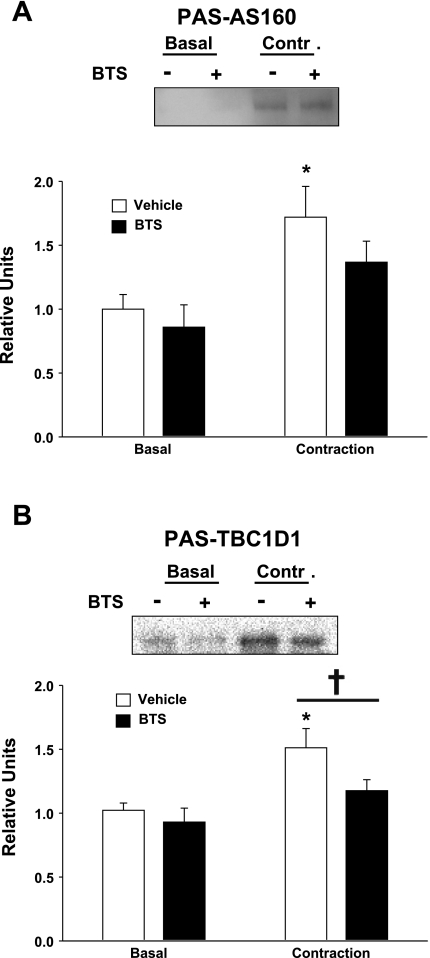
Phosphorylation of AS160 and TBC1D1.
PAS-AS160 was significantly (P < 0.01) increased in muscles stimulated to contract without BTS compared with basal muscles without BTS (Fig. 5A). There was a statistically nonsignificant (P = 0.053) trend for contraction plus BTS to be greater than basal plus BTS. There also was a nonsignificant (P = 0.167) trend for PAS-AS160 to be lower for contraction with BTS compared with contraction without BTS. In the absence of BTS, contraction vs. basal muscles had a significant (P < 0.005) increase in PAS-TBC1D1, and this effect of contraction was eliminated by the presence of BTS (Fig. 5B). Contraction in the presence of BTS compared with contraction without BTS significantly (P < 0.05) reduced the increase in PAS-TBC1D1.
Fig. 5.
Phosphorylation of Akt substrate of 160 kDa (AS160) and TBC1D1. Paired epitrochlearis muscles were incubated with 50 μM BTS or vehicle. Muscles were mounted at resting tension (basal) or electrically stimulated (contraction) before being freeze-clamped and processed for phosphorylation of AS160 (PAS-AS160; A) or TBC1D1 (PAS-TBC1D1; B). A: for PAS-AS160, samples were immunoprecipitated with anti-PAS and then immunoblotted with anti-AS160. *P < 0.01, basal vs. contraction within the vehicle treatment group. B: for PAS-TBC1D1, samples were immunoprecipitated with anti-TBC1D1 and then immunoblotted with anti-PAS. *P < 0.005, basal vs. contraction within the vehicle treatment group. †P < 0.05, BTS vs. vehicle with contraction. Values are means ± SE (n = 11–14).
DISCUSSION
The most important new findings of this study were that BTS induced an 84% decrement in force production in electrically stimulated rat epitrochlearis muscle, leading to a 57% decrease in contraction-stimulated glucose transport despite an unaltered increase in pCaMKII and an unaffected decrease in glycogen concentration. In addition, BTS markedly attenuated the contraction-stimulated depletion of ATP and PCr levels concomitant with substantially diminished activation of AMPK and elimination of the contraction-stimulated increase in PAS-TBC1D1. Together, these findings implicate an essential role for tension-related events, likely including activation of AMPK, in a substantial portion of the contraction-stimulated increase in glucose transport of the predominantly fast-twitch rat epitrochlearis muscle. The data also revealed a tension-related and potentially AMPK-dependent mechanism for the increase in PAS-TBC1D1. The results are consistent with the possibility that increased PAS-TBC1D1 may participate in this portion of contraction-stimulated glucose transport. The 43% of contraction-stimulated glucose transport that was not inhibited by BTS was not closely coupled to the amount of force produced, AMPK activation, or PAS-TBC1D1. Rather, it was apparently triggered by other contraction-associated events such as the increased cytosolic Ca2+.
BTS is a highly specific and potent inhibitor of type II fast-twitch skeletal muscle myosin ATPase and tension development (11, 13, 52). The magnitude of the reduction in tension developed by muscles electrically stimulated in the presence of BTS (84%) corresponded very closely with the proportion of type II fast-twitch fibers (85%) in the rat epitrochlearis muscle (46). The residual tension in BTS-treated muscles (16% of vehicle-treated, electrically stimulated controls) was presumably attributable to the ∼15% type I slow-twitch fibers that are not susceptible to BTS-induced inhibition of force production (11).
In contrast to the marked BTS-induced decline in tension development, the increase in pCaMKII of electrically stimulated muscles was unaltered by the presence of BTS. This finding is expected, because BTS does not alter the flux of Ca2+ in electrically stimulated skeletal muscle (11, 13, 52). The ability of BTS to inhibit force production without altering Ca2+ dynamics allows the direct role of the usual contraction-associated increase in cytosolic Ca2+ to be assessed separately from its secondary consequences, including force production and the associated metabolic consequences.
Most of the published evidence indicates that tension development accounts for the largest portion of the energetic demands of rodent skeletal muscle contraction, with the remainder of energy expenditure used for the sarcolemmal Na+-K+ pump and the sarcoplasmic reticulum Ca2+ pump (2, 3, 55). The BTS-induced decline in tension development in electrically stimulated muscles was accompanied by striking changes in the concentrations of high-energy phosphates. In the absence of BTS, ATP decreased by 48% in electrically stimulated muscles compared with nonstimulated controls, whereas in the presence of BTS, ATP concentration did not decline with electrical stimulation. In addition, the magnitude of the contraction-stimulated decrement in PCr was 28% lower in BTS-treated muscles compared with vehicle-treated controls. The effect of BTS on high-energy phosphates in electrically stimulated muscle is consistent with a substantial BTS-associated decrease in tension-related energy expenditure.
The higher concentrations of ATP and PCr in muscles stimulated to contract with BTS compared with those in muscles without BTS would be predicted to favor the observed decrease in pAMPK with BTS treatment in electrically stimulated muscle. Lower AMPK activation would, in turn, be expected to account for the BTS-associated reduction in phosphorylation of its substrate, ACC, in electrically stimulated muscle. In contrast, BTS did not attenuate the AICAR-induced increases in pAMPK, pACC, and glucose transport. Because incubation in AICAR does not cause force production, these results strongly suggest that the effects of BTS in electrically stimulated muscles were secondary to the BTS-mediated reduction in tension development, i.e., BTS does not appear to directly affect AMPK, ACC, or glucose transport.
Sandstrom et al. (51) recently reported that in vitro BTS treatment resulted in nearly complete inhibition (∼95%) of tension developed by electrically stimulated extensor digitorum longus (EDL) muscles of mice. Surprisingly, they also found that BTS did not reduce the amount of ATP depletion or AMPK activation of electrically stimulated muscles. In an earlier study, they estimated the ATP turnover in electrically stimulated mouse EDL muscle, with and without BTS, and suggested that only ∼20% of ATP consumption during contraction without BTS was attributable to actinomyosin ATPase (61). Subsequently, Barclay et al. (2) determined energy turnover based on measurement of heat production by mouse EDL fiber bundles stimulated using the same contraction protocol with and without BTS. In contrast to the results of Zhang et al. (61), they estimated that actinomyosin ATPase accounted for approximately two-thirds of the energy turnover, with the balance attributable to ion pumping. Regardless of the explanation for the differing results in mouse EDL, the striking reduction in high-energy phosphate depletion in rat epitrochlearis muscles that were electrically stimulated with BTS compared with that in muscles without BTS indicates that actinomyosin ATPase was likely largely responsible for the energy demand of contraction in the epitrochlearis muscles incubated without BTS.
Sandstrom et al. (51) reported that in mouse EDL incubated with BTS compared with EDL without BTS, there was only an ∼5% reduction in the increase in glucose transport with electrical stimulation. Because they found that AMPK activation was unaffected by BTS, and BTS does not alter Ca2+ dynamics (11, 13, 52), the small reduction in glucose transport they observed is consistent with the idea that increased cytosolic Ca2+ and AMPK activation are the major triggers for contraction-stimulated glucose transport. They also evaluated electrically stimulated EDL muscles of rats. The estimated contraction-stimulated increase in glucose uptake above basal values tended to be ∼33% lower for BTS-treated muscle compared with vehicle-treated controls, but they did not report ATP or AMPK values for rat skeletal muscle.
Among the muscles that have been studied with BTS, mouse EDL had the greatest reduction in tension (95%) with the least reduction in glucose transport (∼5%) (51), rat epitrochlearis had an intermediate reduction in tension (84%) and the greatest reduction in glucose transport (57%), and rat EDL had the least reduction in tension (72%) and an intermediate reduction in glucose transport (∼33%) (51). Taking all of these results together, the BTS-induced percent change in force consistently exceeds the percent change in glucose transport, but there does not appear to be a consistent relationship between the magnitude of BTS-induced inhibition of force production and the relative decline in contraction-stimulated glucose transport. These results argue against a large, direct effect of force production (independent of the associated increase in metabolism) on the increased glucose transport with muscle contraction.
The contraction-stimulated increase in PAS-TBC1D1 in skeletal muscle (21, 22, 54) can be eliminated by incubating muscles with the AMPK inhibitor compound C during electrical stimulation (21). Because compound C did not alter tension development, the BTS-induced decline in PAS-TBC1D1 of electrically stimulated muscles seems likely to be attributable to the marked decline in AMPK activation, rather than being a direct effect of reduced force production. The inhibition of contraction-stimulated PAS-TBC1D1 by compound C was accompanied by a 62% reduction in contraction-stimulated glucose transport, which is similar to the 57% decline in contraction-stimulated glucose transport in muscles stimulated in the presence of BTS. We previously found that continuous electrical stimulation of rat epitrochlearis resulted in an increase in glucose transport that reached a plateau between 20 and 60 min of stimulation. The contraction-stimulated increase in PAS-TBC1D1 that was evident at 20 min of stimulation was completely reversed by 60 min of contraction (22). Thus PAS-TBC1D1 may participate in triggering the increase in glucose transport, but it is apparently not required for the long-term maintenance of glucose transport with sustained contraction.
BTS substantially reduced Akt phosphorylation in electrically stimulated muscles. This effect presumably accounted for the lack of a significant electrically stimulated increase in PAS-AS160 in muscles treated with BTS, given that previous research has demonstrated that incubation with wortmannin, which inhibits the contraction stimulation of Akt, eliminates the contraction-stimulated increase in PAS-AS160 of rat skeletal muscle (6, 21). Because wortmannin does not reduce the contraction stimulation of glucose transport, it seems unlikely that the decrements in Akt phosphorylation or PAS-AS160 were important for the BTS-induced reduction in contraction-stimulated glucose transport. Eliminating the contraction-stimulated increase in Akt phosphorylation with wortmannin also did not reduce the increase in PAS-TBC1D1. Although Akt is not implicated in contraction-stimulated glucose transport, contraction-stimulated activation of Akt has other important physiological functions, including regulation of muscle growth (18, 43). The event that triggers the increased Akt phosphorylation with contraction has not been identified, but there is evidence that it may be related to force production. The BTS-associated decrements in pSer473Akt, pThr308Akt, and pGSK3α Ser21 of electrically stimulated muscles are consistent with this idea.
In contrast to high-energy phosphates, the decline in muscle glycogen concentration in electrically stimulated muscles was unaltered by the presence of BTS. Because BTS does not affect the Ca2+ flux, it seems likely that in both the vehicle- and BTS-treated conditions, the increase in cytosolic Ca2+ led to allosteric activation of phosphorylase kinase, which, in turn, phosphorylated and activated glycogen phosphorylase (4, 5), with the end result being similar levels of glycogenolysis. The similar glycogen concentration after contractile activity with BTS compared with that without BTS demonstrates that the amount of glycogenolysis is not necessarily closely coupled to force production.
The similar amount of glycogen breakdown induced by electrical stimulation occurred even though inhibition of type II myosin ATPase by BTS reduced the muscle's ATP demand. Lower ATP demand, in turn, favored the higher ATP and PCr levels in electrically stimulated muscles in the BTS group. Conversely, the greater contraction-stimulated glucose transport in vehicle- compared with BTS-treated muscles provided substrate that could potentially be metabolized to help meet the greater ATP demand of the vehicle group. Although it has been hypothesized that glycogen concentration can modulate glucose transport rate (15, 27, 35), the large BTS-mediated reduction in contraction-stimulated glucose transport was not caused by differences in glycogen concentration. Ultimately, the available energy in each group would also depend on the portion of carbohydrate that was fully oxidized and the amount of intramuscular triglyceride that was oxidized.
The AMPK β-subunit includes a glycogen binding domain (48), and there is evidence for a complex relationship between muscle glycogen concentration and AMPK activation (33). Some studies have shown a negative correlation between muscle glycogen concentration and AMPK activation (14, 56). Despite similar decrements in glycogen concentration with electrical stimulation in the BTS- compared with vehicle-treated group, only the vehicle-treated group had significantly increased pAMPK and pACC. These results demonstrate that the relationship between AMPK activation and glycogen concentration can be uncoupled. AMPK activation in the vehicle-treated group was likely secondary to the substantial contraction-associated reduction in ATP concentration in this group.
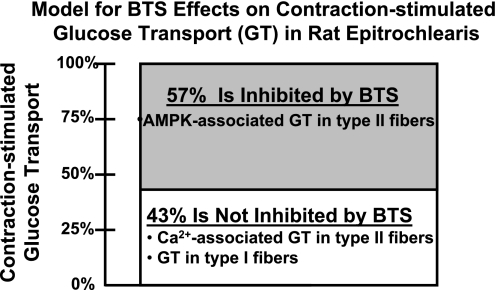
In conclusion, BTS reduced the estimated contraction-stimulated increase in glucose transport by 57%, and this reduction coincided with a BTS-induced decrease in pAMPK and pACC in electrically stimulated muscles. These results, together with the BTS-related decline in PAS-TBC1D1 of electrically stimulated muscles and our earlier findings with the AMPK inhibitor compound C, suggest that TBC1D1 may be downstream of AMPK and upstream of glucose transport. Further research is necessary to establish whether there are causal relationships in this putative AMPK-TBC1D1-glucose transport pathway. The 43% of contraction-stimulated glucose transport that was not inhibited by BTS was similar to the 50% that was previously estimated for cytosolic Ca2+-mediated glucose transport by contracting rat epitrochlearis muscle (57). The contraction-stimulated glucose transport not inhibited by BTS would be expected to include glucose transport by the type I fibers, because they are not susceptible to BTS-induced reductions in force production (11). Earlier research has suggested that Ca2+-related mechanisms, and not increased AMPK activation (42, 57), account for most of the contraction-stimulated increase in glucose transport in type I fibers (57). Regardless of fiber type, BTS would presumably not interfere with mechanisms for increased glucose transport that depend on increased cytosolic Ca2+, and several Ca2+-associated processes, including activation of CaMKII (57) and Ca2+-calmodulin binding to AS160 (36), have been implicated in the contraction-stimulated increase in glucose transport of muscles that are composed predominantly of type II fibers. Our working hypothesis is that in electrically stimulated rat epitrochlearis muscles, the BTS-inhibitable portion of glucose transport (57%) occurred in type II fibers secondary to tension-associated ATPase cycling, leading to depletion of high-energy phosphates, which in turn caused activation of AMPK, and the portion of contraction-stimulated glucose transport that was not inhibited by BTS (43%) was attributable to Ca2+-dependent mechanisms in both type I and type II fibers (Fig. 6).
Fig. 6.
A model for the effects of myosin II ATPase inhibitor BTS on contraction-stimulated glucose transport (GT) in rat epitrochlearis muscle. This model depicts our interpretation of the results of the current study in the context of previously published research on the mechanisms for contraction-stimulated GT in skeletal muscle and the effects of BTS on skeletal muscle function. BTS selectively inhibits myosin II ATPase, which in turn favors attenuated reduction in ATP concentration and less activation of AMPK in electrically stimulated type II fibers. Because BTS does not inhibit myosin I ATPase, it is not expected to alter ATP concentration or AMPK activation in type I fibers. BTS does not alter Ca2+ flux of electrically stimulated muscles, regardless of fiber type. Accordingly, we hypothesize that the 57% reduction in contraction-stimulated GT for BTS- vs. vehicle-treated muscles was attributable to reduced AMPK-associated GT in type II fibers. We further hypothesize that the 43% of contraction-stimulated GT that was not inhibited by BTS was attributable to the summed effects of Ca2+-associated GT in type II fibers and GT in type I fibers.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-071771 (to G. D. Cartee).
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Barclay CJ, Lichtwark GA, Curtin NA. The energetic cost of activation in mouse fast-twitch muscle is the same whether measured using reduced filament overlap or N-benzyl-p-toluenesulphonamide. Acta Physiol(Oxf) 193: 381–391, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Barclay CJ, Woledge RC, Curtin NA. Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell Motil 28: 259–274, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barford D, Johnson LN. The allosteric transition of glycogen phosphorylase. Nature 340: 609–616, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci 4: D618–D641, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Canto C, Chibalin AV, Barnes BR, Glund S, Suarez E, Ryder JW, Palacin M, Zierath JR, Zorzano A, Guma A. Neuregulins mediate calcium-induced glucose transport during muscle contraction. J Biol Chem 281: 21690–21697, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol Endocrinol Metab 258: E390–E393, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol 4: 83–88, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Constable SH, Favier RJ, Cartee GD, Young DA, Holloszy JO. Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. J Appl Physiol 64: 2329–2332, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Dentel JN, Blanchard SG, Ankrapp DP, McCabe LR, Wiseman RW. Inhibition of cross-bridge formation has no effect on contraction-associated phosphorylation of p38 MAPK in mouse skeletal muscle. Am J Physiol Cell Physiol 288: C824–C830, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes 49: 1281–1287, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Derave W, Lund S, Holman GD, Wojtaszewski J, Pedersen O, Richter EA. Contraction-stimulated muscle glucose transport and GLUT-4 surface content are dependent on glycogen content. Am J Physiol Endocrinol Metab 277: E1103–E1110, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Douen AG, Ramlal T, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter.” Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem 265: 13427–13430, 1990. [PubMed] [Google Scholar]
- 17.Dumke CL, Kim J, Arias EB, Cartee GD. Role of kallikrein-kininogen system in insulin-stimulated glucose transport after muscle contractions. J Appl Physiol 92: 657–664, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 103: 378–387, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 280: 39033–39041, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291: E867–E877, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Funai K, Cartee GD. Inhibition of contraction-stimulated AMPK inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes. In press. [DOI] [PMC free article] [PubMed]
- 22.Funai K, Cartee GD. Contraction-stimulated glucose transport in rat skeletal muscle is sustained despite reversal of increased PAS-phosphorylation of AS160 and TBC1D1. J Appl Physiol 105: 1788–1795, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Ren J, Gulve EA, Holloszy JO. Additive effect of contractions and insulin on GLUT-4 translocation into the sarcolemma. J Appl Physiol 77: 1597–1601, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Hansen P, Gulve E, Gao J, Schluter J, Mueckler M, Holloszy J. Kinetics of 2-deoxyglucose transport in skeletal muscle: effects of insulin and contractions. Am J Physiol Cell Physiol 268: C30–C35, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49: 527–531, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369–1373, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Hespel P, Richter EA. Glucose uptake and transport in contracting, perfused rat muscle with different pre-contraction glycogen concentrations. J Physiol 427: 347–359, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holloszy JO A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab 284: E453–E467, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Holloszy JO, Narahara HT. Enhanced permeability to sugar associated with muscle contraction. Studies of the role of Ca++. J Gen Physiol 50: 551–562, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holloszy JO, Narahara HT. Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem 240: 3493–3500, 1965. [PubMed] [Google Scholar]
- 31.Ihlemann J, Ploug T, Hellsten Y, Galbo H. Effect of tension on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol Endocrinol Metab 277: E208–E214, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y, Obara K, Ikeda R, Ishii M, Tanabe Y, Ishikawa T, Nakayama K. Passive stretching produces Akt- and MAPK-dependent augmentations of GLUT4 translocation and glucose uptake in skeletal muscles of mice. Pflügers Arch 451: 803–813, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Jorgensen SB, Rose AJ. How is AMPK activity regulated in skeletal muscles during exercise? Front Biosci 13: 5589–5604, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Katz A Modulation of glucose transport in skeletal muscle by reactive oxygen species. J Appl Physiol 102: 1671–1676, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Kawanaka K, Tabata I, Tanaka A, Higuchi M. Effects of high-intensity intermittent swimming on glucose transport in rat epitrochlearis muscle. J Appl Physiol 84: 1852–1857, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes 56: 2854–2862, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Lee AD, Hansen PA, Holloszy JO. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett 361: 51–54, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic, 1972, p. 291.
- 40.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci USA 92: 5817–5821, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macdonald WA, Pedersen TH, Clausen T, Nielsen OB. N-Benzyl-p-toluene sulphonamide allows the recording of trains of intracellular action potentials from nerve-stimulated intact fast-twitch skeletal muscle of the rat. Exp Physiol 90: 815–825, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Nader GA Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37: 1985–1996, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Nesher R, Karl IE, Kaiser KE, Kipnis DM. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am J Physiol Endocrinol Metab 239: E454–E460, 1980. [DOI] [PubMed] [Google Scholar]
- 45.Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol Cell Physiol 249: C226–C232, 1985. [DOI] [PubMed] [Google Scholar]
- 46.Nesher R, Karl IE, Kipnis DM. Epitrochlearis muscle. II. Metabolic effects of contraction and catecholamines. Am J Physiol Endocrinol Metab 239: E461–E467, 1980. [DOI] [PubMed] [Google Scholar]
- 47.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60: 405–412, 1974. [DOI] [PubMed] [Google Scholar]
- 48.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol 13: 867–871, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab 291: E1031–E1037, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277: 11910–11917, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Sandstrom ME, Zhang SJ, Westerblad H, Katz A. Mechanical load plays little role in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol 579: 527–534, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw MA, Ostap EM, Goldman YE. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry 42: 6128–6135, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985. [DOI] [PubMed] [Google Scholar]
- 54.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wendt IR, Gibbs CL. Energy production of rat extensor digitorum longus muscle. Am J Physiol 224: 1081–1086, 1973. [DOI] [PubMed] [Google Scholar]
- 56.Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51: 284–292, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 53: 330–335, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem 270: 2107–2111, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Youn JH, Gulve EA, Henriksen EJ, Holloszy JO. Interactions between effects of W-7, insulin, and hypoxia on glucose transport in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 267: R888–R894, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Youn JH, Gulve EA, Holloszy JO. Calcium stimulates glucose transport in skeletal muscle by a pathway independent of contraction. Am J Physiol Cell Physiol 260: C555–C561, 1991. [DOI] [PubMed] [Google Scholar]
- 61.Zhang SJ, Andersson DC, Sandstrom ME, Westerblad H, Katz A. Cross bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol 291: C147–C154, 2006. [DOI] [PubMed] [Google Scholar]