Abstract
Mesolimbic dopamine (DA) circuits mediate a wide range of goal-oriented behavioral processes, and DA strongly influences appetitive and consummatory aspects of male sexual behavior. In both birds and mammals, mesolimbic projections arise primarily from the ventral tegmental area (VTA), with a smaller contribution from the midbrain central gray (CG). Despite the well known importance of the VTA cell group for incentive motivation functions, relationships of VTA subpopulations to specific aspects of social phenotype remain wholly undescribed. We now show that in male zebra finches (Estrildidae: Taeniopygia guttata), Fos activity within a subpopulation of tyrosine hydroxylase-immunoreactive (TH-ir; presumably dopaminergic) neurons in the caudal VTA is significantly correlated with courtship singing and coupled to gonadal state. In addition, the number of TH-ir neurons in this caudal subpopulation dichotomously differentiates courting from non-courting male phenotypes, and evolves in relation to sociality (flocking vs. territorial) across several related finch species. Combined, these findings for the VTA suggest that divergent social phenotypes may arise due to the differential assignment of “incentive value” to conspecific stimuli. TH-ir neurons of the CG (a population of unknown function in mammals) exhibit properties that are even more selectively and tightly coupled to the expression of courtship phenotypes (and appetitive courtship singing), both in terms of TH-ir cell number, which correlates significantly with constitutive levels of courtship motivation, and with TH-Fos colocalization, which increases in direct proportion to the phasic expression of song. We propose that these neurons may be core components of social communication circuits across diverse vertebrate taxa.
Keywords: song, evolution, periaqueductal gray, vocalization
Affiliation behaviors such as courtship, pair bonding, grouping, and parental care can vary dramatically across individuals and species. However, despite the fact that we now know a good deal about the neurobiology of affiliation behaviors (1, 2), we still know very little about the phenotypic variations in neural mechanisms that underlie phenotypic differences in behavior. In fact, nonapeptide systems (the vasopressin- and oxytocin-like peptides) arguably provide the only examples from tetrapod vertebrates in which neural mechanisms have been systematically studied in relation to species-specific social structure (e.g., mating system and sociality) and individual differences in affiliation (3, 4).
Among the many other neurochemical systems that regulate motivational and behavioral states, mesolimbic dopamine (DA) circuits are perhaps the strongest candidates as generators of phenotypic diversity. DA influences numerous affiliation behaviors such as pair bonding (5), sexual communication (6–8), and copulation (9–11). Of particular interest are DA cells in the ventral tegmental area (VTA), which are well known to regulate incentive and reward-related processes (12, 13). These cells project onto multiple areas of the basal (“limbic”) forebrain that are essential to the regulation of social behavior, such as the nucleus accumbens, extended amygdala, septum, and hypothalamus (14, 15). Similar connections are observed for the DA neurons of the midbrain central gray (CG) (14), and although the functions of the CG neurons are less well understood, they are the focus of growing attention among avian neurobiologists, given that they project directly on forebrain regions that regulate song (as do DA neurons of the VTA) (16, 17). DA cells in both the CG and VTA show increased Fos expression (a proxy marker of neural activity) after sexual interactions in birds (18, 19), and similar data are available for the VTA in rodents (20).
In the present experiments, we investigate the relevance of midbrain DA populations to 2 dimensions of phenotypic diversity: individual differences in courtship motivation in male zebra finches (Estrildidae: Taeniopygia guttata), and species differences in sociality (as defined by modal species-typical group size). In both cases, we explore the functional properties of DA cells by quantifying their Fos responses to social stimuli (Fig. 1A; Fig. S1), and by examining the relationships between behavioral phenotype and the number of DA neurons in the VTA and CG. Last, in the zebra finch experiment, we also quantify the Fos responses of DA neurons to a nonsocial reinforcer. The combined assessment of courtship, sociality, and nonsocial reinforcement allows us to determine the functional specificity of DA cell groups much more fully than by exploring a single dimensions of behavior, and in the end, the pattern of results should tell us whether DA phenotypes may relate to affiliation in a behavior-specific manner (as we conclude for the CG) or in a manner that suggests mechanistic interrelationships between multiple aspects of social phenotype (as we show for the caudal VTA).
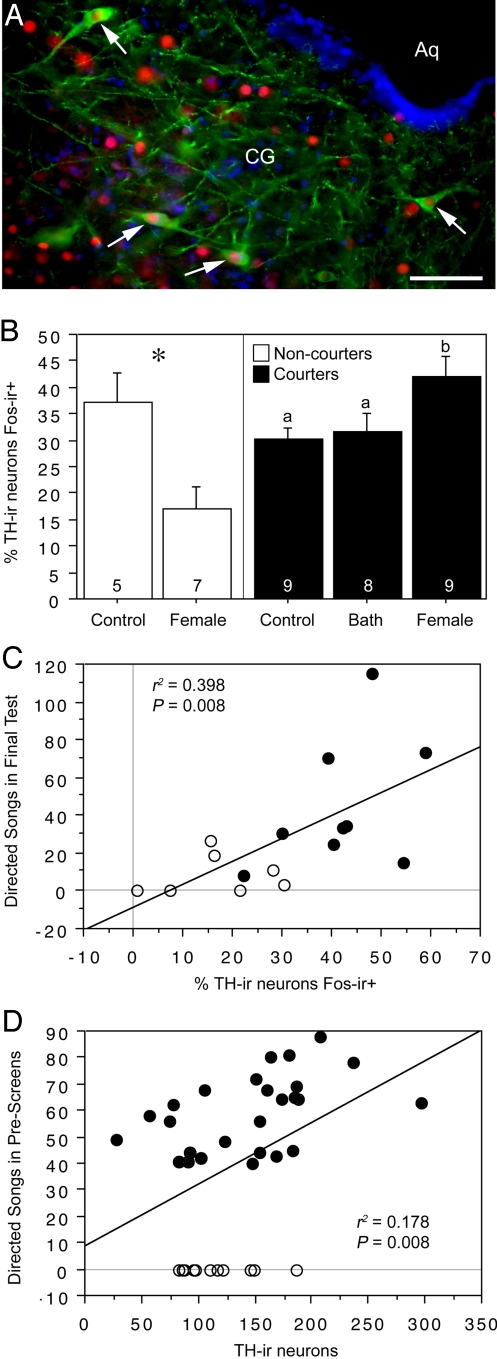
Fig. 1.
TH-Fos colocalization and TH-ir cell number in the CG of male zebra finches that reliably court (courters; black fills) or fail to court (non-courters; no fills). (A) Representative double-labeling for TH (Alexa Fluor 488; green) and Fos (Alexa Fluor 594; red) in the CG of a normal (courter) male zebra finch after exposure to a female. DAPI nuclear stain is shown as blue. (Scale bar, 100 μm.) Arrows indicate neurons double-labeled for TH and Fos. (B) Percentage of TH-ir neurons that express Fos after exposure to control conditions, a positive nonsocial stimulus (water bath; courters only), or a female. Data are shown as means ± SEM. *, P = 0.01, unpaired t test. Different letters above the error bars denote significant differences between courter groups (Fisher's PLSD P < 0.05 after significant ANOVA). Group n's are indicated at the base of each bar. (C) Correlation between TH-Fos colocalization in the CG and the number of directed songs sung in the final test by the 16 subjects exposed to females. (D) Correlation between TH-ir cell number in CG (summed across 4 sections) and the number of directed songs sung in prescreenings. Separate analysis of courters yields an r2 of 0.228 (P = 0.01). Total n = 38.
Results
Courters and Noncourters.
To examine the relationships between DA and courtship phenotypes, we screened 127 male zebra finches for courtship behavior (directed singing to a female) on 2 separate occasions. Twelve males did not sing on either occasion, and are hence designated as “non-courters.” For the test before killing and perfusion for tissue fixation, these non-courter males were exposed to a female (n = 7) or control conditions (n = 5). Three groups of reliable “courters” (n = 9 each) were created that were matched based on the number of directed songs given during screenings. Males in these 3 groups were exposed to a female, a water bath, or control conditions. We maintained all subjects on sipper tubes for 30 days before the final test, ensuring that subjects would be highly motivated to bathe when offered bath water (zebra finches are an arid-adapted species and bathe whenever open water is available). In the bath group, 8 of the 9 males bathed during the final test. The remaining male was excluded from the study. Four of the non-courters that were exposed to females sang a modest amount to the female stimulus in this final test (perhaps stimulated by the long overnight isolation in the testing room), although non-courters exhibited only ≈19% of the song number exhibited by courters (8.6 ± 10.8 vs. 44.9 ± 34.3 songs, respectively; P = 0.01, unpaired t test). Importantly, song number in the final test (for the 16 males exposed to females) was strongly predicted by their behavior in prescreenings, which were conducted a month before final testing (r2 = 0.427, P = 0.006).
CG.
Labeling was analyzed at 4 rostrocaudal levels distributed throughout the length of the CG. No significant differences were observed across levels; therefore, data are shown pooled. Relative to controls, courters that were exposed to females showed a significant increase in the percentage of tyrosine-hydroxylase-immunoreactive (TH-ir) neurons in the CG that expressed Fos-ir nuclei (henceforth referred to as “TH-Fos colocalization”). In contrast, non-courters exposed to females exhibited a significantly lower level of TH-Fos colocalization than did controls (Fig. 1B; note that 90 min was allowed between the beginning of the test and perfusion). For males that were exposed to females (courters and non-courters combined), TH-Fos colocalization correlated significantly with the number of songs given in the final test before perfusion (Fig. 1C). The anatomy of the DA cell group in the CG likewise reflected phenotypic variation in courtship, but in a graded manner. Thus, the number of TH-ir neurons correlated strongly with the number of songs that subjects sang during the behavioral prescreenings (Fig. 1D; note that this measure is inclusive of all 38 subjects, regardless of group assignments), but there was only a weak trend for non-courters to differ from courters in TH-ir cell number (28.3 ± 2.3 vs. 36.3 ± 2.9 cells per section, respectively; P = 0.09, unpaired t test). Fos-ir cell counts in the medial CG (corresponding to the region of TH-ir neurons) were very similar across groups, although Fos-ir cell counts tended to correlate with song number in the males exposed to females (r2 = 0.231, P = 0.059).
VTA.
The rostral and caudal VTA are morphologically distinct, and yielded very different patterns of results. As in mammals (21), TH-ir cells in the caudomedial VTA are substantially smaller than are cells in the rostral and caudolateral VTA. However, caudal subpopulations are not completely segregated; thus, the parvocellular TH-ir neurons that predominate medially tend to intersperse with larger cells laterally (Fig. S1). We here analyzed labeling at rostral and caudal levels comparable with those shown in Fig. S1 A and C, respectively.
TH-Fos colocalization in the rostral VTA did not differ across courter groups, but was lower in non-courter males exposed to females relative to non-courter controls (Fig. S2A). For the subjects that were exposed to females, song number was significantly correlated with the number of TH-negative (potentially GABAergic) (20, 22) cells that expressed Fos (Fig. S2B). Despite this positive correlation, Fos-ir cell counts in the rostral VTA were not elevated in the courter group exposed to females (relative to the control group), and were significantly lower than in the courter group given a water bath (Fig. S2C). TH-ir cell number did not differ significantly between courters and non-courters (114.0 ± 11.3 vs. 146 ± 13.4 TH-ir neurons per section, respectively; P = 0.13, unpaired t test), and the total number of TH-ir neurons in the rostral VTA did not correlate with the number of songs exhibited in prescreenings (r2 = 0.060, P = 0.13).
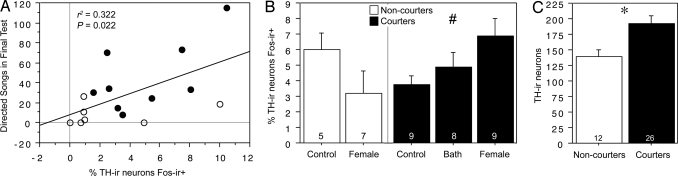
In a reversal of the rostral VTA, the total number of Fos-ir cells in the caudal VTA did not correlate with directed singing in males that were exposed to females (r2 = 0.091, P = 0.26), but rather song was significantly correlated with the percentage of TH-ir neurons that expressed Fos (Fig. 2A). TH-Fos colocalization showed near-significant differences across courter groups, with the greatest colocalization in courters that were exposed to females (Fig. 2B). Although we strongly expected that results would differ between medial and lateral subpopulations of the caudal VTA, based on differences in cellular morphology, we found that the results for these 2 areas were comparable. Courters exhibited significantly more TH-ir neurons in the caudal VTA than did non-courters (Fig. 2C). This difference between courters and non-courters was strongly dichotomous and not reflective of a graded relationship between TH-ir cell number and song, because a virtually flat (even slightly negative) correlation was observed between TH-ir cell number and the number of songs given by courter males in prescreenings (r2 = −0.103, P = 0.11).
Fig. 2.
TH-Fos colocalization and TH-ir cell number in the caudal VTA reflect singing and differentiate courters (black fills) and non-courters (no fills). (A) Correlation between TH-Fos colocalization in the caudal VTA and the number of directed songs sung in the final test by the 16 male zebra finches exposed to females. (B) Percentage of TH-ir neurons that express Fos after exposure to control conditions, a positive nonsocial stimulus (water bath; courters only), or a female. Data are shown as means ± SEM. #, ANOVA P = 0.053. (C) TH-ir cell number in the caudal VTA is significantly lower in non-courters than in courters. Data are shown as means ± SEM. *, P = 0.01, unpaired t test. Group n's are indicated at the base of each bar.
Correlations with Gonadal State.
Although gonadosomatic index (GSI; gonad/body weight × 100) was not correlated with any variables in the CG or rostral VTA, it was significantly correlated with TH-ir cell number in the caudal VTA (Fig. S3A), and with the percentage of those neurons that expressed Fos in subjects that were exposed to females (Fig. S3B). Non-courters exhibited significantly lower GSI values than courters (138.4 ± 12.0 vs. 191.7 ± 12.4 TH-ir neurons per section, respectively; P = 0.01, unpaired t test), producing a significant positive correlation between GSI and the number of songs exhibited in prescreenings (r2 = 0.107, P = 0.05). However, this latter correlation is not observed if the analysis is restricted to courters, and even tends to reverse direction (r2 = −0.133, P = 0.07).
Species Differences in Sociality.
The findings reported above suggest that differences in courtship phenotype relate to multiple aspects of DA anatomy and function. Those linkages between appetitive sexual behavior and DA may be specific to the context of social communication, sexual interactions, or may reflect broader relationships between DA and affiliation phenotypes. To address this question, we quantified TH-Fos colocalization and TH-ir cell number in 5 species of estrildid finches (2 territorial, 1 modestly gregarious, and 2 highly gregarious; closely matched for other aspects of behavior and ecology) after a 90-min exposure to a same-sex conspecific (separated by a wire barrier) or control conditions. The protocol for testing minimizes the expression of overt social responses, although 5 of 34 experimental subjects (including both sexes and 3 species) vocalized extensively during the 10-min observation period. These vocalizations included various species-specific call types. Subject behavior was qualitatively categorized (calm, aroused with little or no vocalization, or highly vocal) by a single observer. Quantification of all calls was not feasible. No sex differences were observed for any of the dependent measures, and therefore, sexes are pooled in the following analyses.
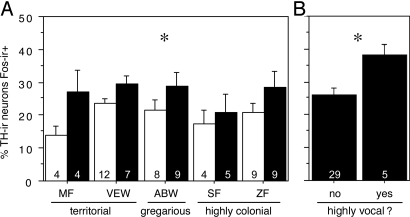
Exposure to a same-sex conspecific increased TH-Fos colocalization modestly (although significantly) within the CG, with no species differences (Fig. 3A), and a similar increase was observed for Fos-ir cells per section (69.8 ± 2.8 control vs. 93.5 ± 6.8 conspecific; main effect of condition F(57,4,1) = 12.693, P = 0.0007). These findings indicate that the TH-ir neurons of the CG are not sensitive to the valence of stimuli, given that comparable responses were observed in territorial and gregarious birds. However, the 5 subjects that were highly vocal exhibited signficantly greater TH-Fos colocalization in the CG than did the remaining 29 subjects (Fig. 3B), and this difference was remarkably specific to the TH-ir neurons (90.6 ± 9.9 Fos-ir cells per section in highly vocal subjects vs. 89.41 ± 4.8 in others; P = 0.92, unpaired t test). No significant effects were obtained for TH-Fos colocalization or Fos response within the rostral or caudal VTA, although <1% of TH-ir neurons expressed Fos-ir nuclei, even after same-sex exposure (see Discussion).
Fig. 3.
TH-Fos colocalization in the CG reflects social vocalization, but not species differences in sociality. (A) Percentage of TH-ir neurons in the CG that express Fos after exposure to a same-sex conspecific (through a wire barrier; solid bars) or control conditions (open bars) in 5 species of estrildid finches. Data are shown as means ± SEM, with sexes pooled. *, ANOVA main effect of condition, P = 0.0057. Group n's are indicated at the base of each bar. ABW, Angolan blue waxbill; MF, melba finch; SF, spice finch; VEW, violet-eared waxbill; ZF, zebra finch. (B) TH-Fos colocalization is signifcantly greater in subjects that were observed to be actively calling during the exposure to a same-sex conspecific (*, P = 0.033, unpaired t test).
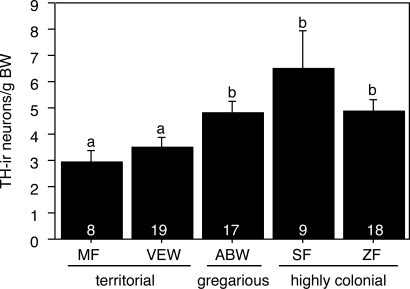
The number of TH-ir neurons in the caudal VTA showed a close correspondence to sociality (Fig. 4). Thus, the 2 territorial species exhibited significantly lower numbers of TH-ir cells (expressed as cells per gram of body mass to correct for species differences in body size) than did the 3 gregarious species. A similar pattern of results was obtained in an analysis of cell packing (number of TH-ir neurons/100 μm2; main effect of species F(58,4) = 5.622, P = 0.0007). TH-ir cell number in the CG and rostral VTA did not differ across species.
Fig. 4.
TH-ir cell number in the caudal VTA (corrected for differences in body size) reflects sociality in 5 species of estrildid finches. Data are shown as means ± SEM, sexes pooled. Different letters above the error bars denote significant differences between species (Fisher's PLSD P < 0.05 after significant ANOVA). Group n's are indicated at the base of each bar.
Discussion
Mesolimbic DA circuits are fundamental to the organization of the vertebrate brain (15, 23, 24), and have long been the focus of intensive study, given that they are essential for the generation of reward prediction, incentive motivation, and a myriad of appetitive behaviors (13, 25–27). It seems likely, then, that naturally occurring variation in affiliation behaviors such as social grouping (e.g., flocking) and appetitive sexual behavior (e.g., courtship singing) may be reflected in the anatomy and functional properties of midbrain DA populations, but evidence for such links has been surprisingly absent. We now demonstrate that 2 separate groups of DA neurons do indeed reflect social phenotypes, but in distinctly different ways. Thus, the DA neurons of the CG are tightly coupled to courtship phenotype (and perhaps social communication behavior more broadly), whereas DA neurons of the caudal VTA are linked to affiliation in a much broader sense. The social responses of the VTA are even further intriguing, in that song-related activity is restricted to TH-negative neurons rostrally, and confined to TH-positive neurons caudally.
Immediate early genes influence numerous cellular functions, and thus, interpretations of immediate gene activity are often difficult, particularly when the activity cannot be directly correlated with a quantifiable behavioral or physiological output. However, we here observed very close relationships between TH-Fos colocalization and courtship singing, and also between TH cell number and constitutive courtship motivation, as established through behavioral prescreenings. Considering that TH-ir neurons in the CG and VTA project directly to areas involved in singing and sexual motivation (16, 17), and that DA promotes singing (8, 28), our interpretations for courting males are fairly straightforward; the more DA neurons that a male exhibits in the CG and caudal VTA, and the greater their Fos activity, the greater the male's behavioral output. This interpretation is likewise consistent with the fact that non-courters express low levels of colocalization after exposure to female.
Overall, non-courters exhibit an abnormal neural profile that is characterized by a higher level of TH-Fos colocalization in controls than in subjects exposed to females. Given that the final test began 90 min before killing, these differences may reflect a socially-induced decrease in colocalization (Fos protein decays in 2 phases, with half-lives of 45 and 90–120 min, respectively) (29). An alternative interpretation is that non-courter controls exhibited stress-induced neural activation due to overnight isolation in the test chambers, and that exposure to females provided a social buffer to that response. Regardless of the cause, non-courters appear to exhibit numerous neural abnormalities that are as striking as their behavioral deficits. For example, non-courters also fail to exhibit socially-induced Fos expression within vasotocin neurons of the extended amygdala (30), despite the fact this induction is an extremely robust phenomenon in normal zebra finches (and is observed regardless of the subject's housing condition or reproductive status) (30, 31). Thus, based on the combined data for DA and VT, we hypothesize that non-courters exhibit elevated stress reactivity or anxiety, which serves to dysregulate neural affiliation systems and decrease the probability of courtship.
DA Neurons of the CG Are Strongly Coupled to Song and Reflect Courtship Phenotypes.
In contrast to the VTA, the DA neurons of the CG appear to be tightly coupled to vocalization, and show no species differences that would suggest a valence sensitivity or broader involvement in general affiliation. Thus, these neurons are equally responsive to same-sex stimuli in territorial and gregarious species, and cell numbers do not differ across species.
As in the caudal VTA, TH-Fos colocalization in the CG is positively correlated with the phasic expression of song (i.e., in the final test), but more uniquely, the number of TH-ir neurons in the CG population is significantly correlated with phenotypic variation in directed singing, as assessed in 2 behavioral prescreenings with females. Given that a month was allowed between screening and tissue collection, this latter relationship appears to be constitutive. However, despite these strong links to song, the species comparisons suggest a somewhat more general role for the CG neurons in social communication, because calling birds showed greater TH-Fos colocalization in the CG than did quiet birds. Similarly, male zebra finches that sing undirected songs exhibit higher levels of TH/egr-1 colocalization than do silent males (32), although in contrast to our present Fos results, TH/egr-1 colocalization does not correlate with song number.
Although the present data alone cannot demonstrate the DA neurons of the CG are “specialized” for communication, their tight coupling to song, heightened activity in callers, and modest response to same-sex conspecifics suggests that these neurons may be fairly specifically involved in social communication. The fact that these neurons discriminate heterospecific from conspecific stimuli (19) is certainly consistent with that view. CG DA neurons are anatomically well situated to integrate motivation-related information from the basal forebrain with the regulation of song by the telencephalic song system (33), and hence these neurons may be particularly important for appropriate context-dependent expression of song. However, because CG DA neurons are common to virtually all tetrapods, including non-songbirds (14, 18), it seems likely their relevance to song is evolutionarily derived from non-song communicative functions. Comparative data from diverse taxa are therefore necessary to fully elucidate the conserved functions of these neurons.
Findings in several vertebrate classes demonstrate that communication functions of the CG are strongly conserved (2), although the specific role of CG DA neurons has been addressed only in songbirds. In taxa ranging from fish to primates, the CG is vocally active and provides an anatomical link between social behavior circuits of the basal forebrain (e.g., amygdala, hypothalamus, and preoptic area) and vocal-motor systems that subserve social communication (34, 35). Indeed, extensive work in primates demonstrates that the CG (periaqueductal gray) is an essential component of vocal control (35).
DA Neurons of the Caudal VTA Reflect Social Motivation and Differentiate Affiliation Phenotypes.
Recent findings demonstrate that DA signals derived from the VTA-substantia nigra pars compacta complex are essential for the context-dependent modulation of song in the zebra finch (6, 36), and various other findings likewise show that DA is important for the regulation of song (8, 28, 37). However, previous experiments have found that singing is correlated with immediate early gene expression only within nondopaminergic neurons of the VTA (6, 32), although those studies appear to have focused predominantly on the rostral portion of the VTA. VTA neurons show electrophysiological responses to female stimuli and in relation to singing, but again, at least a substantial portion of the sampled neurons appears to be nondopaminergic interneurons (38). We here confirm that song-related Fos expression in the rostral VTA is limited to TH-negative neurons, but we additionally show that this biased expression is reversed at the caudal end of the VTA. Thus, singing is associated with increased Fos expression in TH-positive neurons of the caudal VTA, but not with Fos expression in TH-negative interneurons.
In mammals, the caudal VTA contains a predominance of small cells medially that intersperse with large cells laterally, and these different cell types exhibit distinct electrophysiological and projection profiles (21). Electrophysiological properties of VTA DA neurons in songbirds are closely matched to those in mammals (22), and we here describe a pattern of cellular morphology in the caudal VTA that is likewise highly similar to mammals. Given the presence of distinct cellular types in the caudal VTA that may have different projection targets, we expected that courtship singing might correlate predominantly with Fos expression in one cell type or the other, but this prediction was not confirmed. Rather, singing is correlated with TH-Fos colocalization throughout the caudal VTA, indicating that functional variations within the VTA occur primarily along the rostrocaudal axis. Rostral-caudal distinctions in function are known for the mammalian VTA, as well (39), and thus, further study may reveal them to be fairly ubiquitous across taxa.
In addition to the positive correlation between singing and TH-Fos colocalization, the DA neurons of the caudal VTA also exhibit Fos responses to female stimuli that are positively correlated with GSI, indicating that these neurons influence song in a manner that is coordinated with other aspects of reproductive physiology. This finding likely reflects direct hormonal regulation, because the VTA expresses both androgen and estrogen receptors (40), and manipulations of sex steroids influence the number of TH-ir neurons in the VTA and TH-ir fiber density in telencephalic song regions (41, 42).
Overall, these findings demonstrate a strong link between caudal VTA DA and courtship singing, particularly in light of the fact that the gross anatomy of this population (in terms of cell number) distinguishes male sexual phenotypes, with non-courters exhibiting significantly fewer TH-ir neurons than courters. This link receives further support from findings in male starlings (Sturnis vulgaris), in which TH immunoreactivity in the VTA correlates with singing in a breeding context, but not in a nonbreeding context (36). These observations suggest the interesting possibility that DA neurons of the caudal VTA may be selectively involved in sexual behavior, and consistent with this idea, we were unable to detect significant effects of same-sex interactions on TH-Fos colocalization in the VTA. However, TH-ir neurons in the VTA express Fos at very low levels (e.g., after copulation, only ≈5% of TH-ir neurons express Fos) (19, 20); thus, colocalization alone may be a poor marker for functional specificity. Indeed, we here observed large species differences in TH-ir cell numbers that show close correspondence to sociality, suggesting that DA neurons of the caudal VTA are not selectively involved in appetitive singing or sexual behaviors per se, but much more broadly involved in social motivation.
Conclusions
DA systems arising from the midbrain are essential for the expression of appetitive behaviors, but it has remained unclear whether variations in affiliation phenotypes are associated with discrete, identifiable aspects of DA anatomy and function. We now demonstrate previously undescribed links between DA neurons and song, and show that multiple DA cell groups reflect social phenotype. The DA subpopulation of the caudal VTA exhibits functional and anatomical features that reflect singing, courtship phenotype, and species-typical levels of sociality, a suite of characteristics that is virtually unique and that places this subpopulation on a very short list of cell groups that show close coupling to naturally-occurring diversity in affiliation (30, 31). In a striking contrast, the DA neurons of the CG show a more restrictive relationship to vocal communication. The number of these neurons is tightly coupled to constitutive, individual differences in courtship, and their phasic Fos activity is directly reflective of the phasic expression of song. To our knowledge, such direct structure-activity-behavior relationships are unknown for any other cell group that regulates the motivational components of social communication behavior, providing a strong impetus for further research on this relatively little-studied DA population.
Methods
Animals.
For the courtship experiment, 127 male zebra finches (T. guttata) were screened for courtship behavior, and 38 were subsequently used for the current study. These males were removed from long-term mixed-sex housing ≈5 weeks before killing. For the species comparisons, we used birds that were in reproductive condition and housed in nesting cages. Subjects were removed from nesting cages 2 days before killing. Tissue for all species, except zebra finches, was collected in conjunction with an earlier experiment (31). In total, we here present species comparison data for 18 zebra finches (9 males, 9 females), 9 spice finches (Lonchura punctulata; 4 males, 5 females), 17 Angolan blue waxbills (Uraeginthus angolensis; 9 males, 8 females), 19 violet-eared waxbills (U. granatina; 10 males, 9 females), and 8 melba finches (Pytilia melba; males only). Birds were collected and housed as previously described (31) and in compliance with all federal and institutional regulations.
Behavioral Testing.
For the first experiment described here, male zebra finches were screened twice for courtship singing to a female, and were then assigned to groups as described in Results. Subjects were acclimated to testing rooms for 1 h per day for 3 days, after which they remained in the rooms overnight. The following morning, subjects were exposed to a test stimulus (a female or a water bath) for 15 min or a control manipulation (all procedures without placement of a stimulus into the subject's cage). Observations were conducted from a curtained blind. Subjects were perfused 90 min after the start of the test. Methods for the species comparison experiment were very similar, with the exception that subjects were exposed to same-sex stimuli through a wire barrier for a period of 90 min. Methods for this latter experiment are described in full elsewhere (31).
Immunocytochemistry and Data Analysis.
Tissue was immunofluorescently labeled by a standard protocol (19) using a sheep anti-TH antibody (Novus Biologicals), rabbit anti-Fos antibody (Santa Cruz Biotechnology), and secondary antibodies conjugated to Alexa Fluor 488 and 594 (Invitrogen), respectively. Monochrome photomicrographs were shot for each fluorophore at 10× and quantification was subsequently conducted from layered monochrome images using Adobe Photoshop 7 (Adobe Systems) and Image J (National Institutes of Health). Data were analyzed using simple regressions or ANOVA followed by Fisher's PLSD, with log transformations as necessary to normalize data.
Supplementary Material
Acknowledgments.
We thank Olga Gorobet, Christine Kemp, Da Lim Ki, Sara Schrock, and Yiwie Wang for assistance with immunocytochemistry and/or cell counts, and Marcy Kingsbury for comments on the manuscript. This work was supported by the National Institute of Mental Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811821106/DCSupplemental.
References
- 1.Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 2.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 4.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragona BJ, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 6.Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riters LV, Olesen KM, Auger CJ. Evidence that female endocrine state influences catecholamine responses to male courtship song in European starlings. Gen Comp Endocrinol. 2007;154:137–149. doi: 10.1016/j.ygcen.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Rauceo S, et al. Dopaminergic modulation of reproductive behavior and activity in male zebra finches. Behav Brain Res. 2008;187:133–139. doi: 10.1016/j.bbr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- 10.Cornil CA, Dejace C, Ball GF, Balthazart J. Dopamine modulates male sexual behavior in Japanese quail in part via actions on noradrenergic receptors. Behav Brain Res. 2005;163:42–57. doi: 10.1016/j.bbr.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- 15.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 17.Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- 18.Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 19.Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- 21.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- 23.Reiner A, Karle EJ, Anderson KD, Medina L. In: Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Smeets WJ, Reiner A, editors. Cambridge: Cambridge Univ Press; 1994. pp. 135–181. [Google Scholar]
- 24.Reiner A, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 26.Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuber GD, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiol Behav. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 30.Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Physiol Behav. 2008;93:870–876. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: Possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- 34.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 35.Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- 39.Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A. Dissociation between rewarding and psychomotor effects of opiates: Differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci. 2008;28:8406–8416. doi: 10.1523/JNEUROSCI.1958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- 41.Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003;121:801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- 42.LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.