Abstract
Ingestion of an essential amino acid-carbohydrate (EAA + CHO) solution following resistance exercise enhances muscle protein synthesis during postexercise recovery. It is unclear whether EAA + CHO ingestion before resistance exercise can improve direct measures of postexercise muscle protein synthesis (fractional synthetic rate; FSR). We hypothesized that EAA + CHO ingestion before a bout of resistance exercise would prevent the exercise-induced decrease in muscle FSR and would result in an enhanced rate of muscle FSR during postexercise recovery. We studied 22 young healthy subjects before, during, and for 2 h following a bout of high-intensity leg resistance exercise. The fasting control group (n = 11) did not ingest nutrients, and the EAA + CHO group (n = 11) ingested a solution of EAA + CHO 1 h before beginning the exercise bout. Stable isotopic methods were used in combination with muscle biopsies to determine FSR. Immunoblotting procedures were utilized to assess cell signaling proteins associated with the regulation of FSR. We found that muscle FSR increased in the EAA + CHO group immediately following EAA + CHO ingestion (P < 0.05), returned to basal values during exercise, and remained unchanged at 1 h postexercise. Muscle FSR decreased in the fasting group during exercise and increased at 1 h postexercise (P < 0.05). However, the 2 h postexercise FSR increased by ∼50% in both groups with no differences between groups (P > 0.05). Eukaryotic elongation factor 2 phosphorylation was reduced in both groups at 2 h postexercise (EAA + CHO: 39 ± 7%; fasting: 47 ± 9%; P < 0.05). We conclude that EAA + CHO ingestion before resistance exercise does not enhance postexercise FSR compared with exercise without nutrients.
Keywords: essential amino acids, resistance exercise, human muscle protein synthesis, protein metabolism, leucine
an acute bout of resistance exercise stimulates muscle protein synthesis within 1–4 h postexercise (3, 8, 9) that can remain elevated for 24–48 h (8, 20, 22). Short-term resistance exercise training of 1–12 wk also increases basal rates of muscle protein synthesis (15, 17, 34, 35, 38).
Anabolic nutrients, primarily leucine and other essential amino acids (EAAs), also stimulate muscle protein synthesis in humans, whether given intravenously or ingested (24, 26, 27, 33). The addition of carbohydrate (CHO) to an EAA solution augments insulin secretion in humans (23, 32). Insulin stimulation of human muscle protein synthesis is dependent on the availability of amino acids (2, 12, 25) and is most likely permissive in nature since the increase in muscle protein synthesis is less than that seen with amino acid ingestion. We have recently shown, in human subjects, that a leucine-enriched EAA and CHO ingestion potently and rapidly increases muscle protein synthesis in association with a substantial activation of the mammalian target of rapamycin (mTOR) signaling pathway (13).
The provision of EAAs with or without CHO following resistance exercise increases the rate of muscle protein synthesis (4, 5, 10, 21, 23, 28). In addition, recent work has also shown that the ingestion of whole proteins such as whey or casein following resistance exercise augments muscle protein synthesis following an acute bout of resistance exercise (11, 18, 19, 30, 31, 36) or when ingested chronically following 3 mo of resistance exercise training (14). On the other hand, it is unclear whether the provision of anabolic nutrients before resistance exercise can enhance postexercise muscle protein synthesis. For example, it has been reported that the ingestion of EAAs and CHO immediately before an acute bout of resistance exercise produced a larger increase in indirect measures of muscle protein synthesis (i.e., leg net amino acid balance and rate of disappearance) compared with when the nutrients were ingested immediately postexercise (29). However, when 20 g of whey protein (rather than purified amino acids) was ingested, there was no difference in leg amino acid uptake between ingesting the protein immediately before exercise and ingestion 1 h postexercise (31). We have recently shown that human muscle protein synthesis is decreased during an acute bout of resistance exercise; however, during postexercise recovery the rate of muscle protein synthesis is increased in association with the activation of mTOR signaling (9). It is not known whether EAA and CHO ingestion before resistance exercise would overcome the reduction in direct measures of muscle protein synthesis (i.e., mixed muscle fractional protein synthetic rate; FSR) by activating signaling proteins linked to the regulation of translation initiation and elongation such as Akt/protein kinase B (PKB), tuberous sclerosis complex 2 (TSC2), mTOR, ribosomal S6 kinase 1 (S6K1), 4E-binding protein 1 (4E-BP1), and eukaryotic elongation factor 2 (eEF2). Exercising in the fed state may also prevent the resistance exercise-induced increase in AMP-activated protein kinase (AMPKα2), a negative regulator of mTOR signaling. Although signaling and muscle protein synthesis alterations during early postexercise recovery may not be optimal markers of measurable muscle growth during training, it does provide necessary data for determining how acute resistance exercise and/or nutrient ingestion can activate cell signaling pathways that promote muscle protein synthesis.
Therefore, we hypothesized that a leucine-enriched EAA acid and CHO (EAA + CHO) ingestion before a bout of high-intensity resistance exercise would prevent the exercise-induced decrease in muscle protein synthesis and would stimulate mTOR signaling and enhance muscle protein synthesis during the early postexercise recovery period.
SUBJECTS AND METHODS
Subjects.
We studied two groups of 22 young healthy subjects. In the first group we recruited 11 young subjects (6 male and 5 female) who consumed a solution of EAA + CHO 1 h before performing a bout of heavy leg resistance exercise (EAA + CHO group). A second group of 11 additional subjects (7 men and 4 women) performed the exact same exercise bout but without consuming nutrients (fasting control group). Subjects for both groups were recruited into the study concurrently. Data from male and female subjects in each group were combined because we did not detect significant sex differences in our variables of interest. The postexercise signaling and muscle protein synthesis data from the fasting control group have been previously published and are not included in results (9). We have included previously published fasting data in all tables and figures to provide clear comparisons between groups (9). All subjects were healthy and physically active but were not currently engaged in a resistance or endurance exercise training program. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch. Screenings of subjects were performed with clinical history, physical exam, and laboratory tests, including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, pregnancy test, thyroid-stimulating hormone (TSH), lipid profile, urinalysis, drug screening, and ECG. All women were not taking oral contraceptives and were studied during their follicular phase. There were no differences in subject characteristics between groups (P > 0.05), and subject characteristics are summarized in Table 1.
Table 1.
Subject characteristics
| Fasting | EAA + CHO | |
|---|---|---|
| n | 11 (M = 7, F = 4) | 11 (M = 6, F = 5) |
| Age, yr | 27±2 | 25±1 |
| Height, m | 1.68±0.03 | 1.70±0.01 |
| Weight, kg | 71.3±4.5 | 69.4±2 |
| Body mass index, kg/m2 | 25.3±1.3 | 23.9±0.8 |
| Lean body mass, kg | 52.5±3.4 | 51.0±1.9 |
| Body fat, % | 23.4±2.4 | 23.3±2.4 |
| Leg lean mass, kg | 8.8±0.6 | 8.4±0.4 |
Values are mean ± SE. Fasting data are from Ref. 9. EAA + CHO, essential amino acids plus carbohydrates; M, men; F, women. There were no significant differences between groups.
Study design.
On two separate occasions (>5 days apart) and more than 5 days before conducting the study, each subject was tested for muscle strength by measuring their 1-repetition maximum (1RM) on a leg extension machine (Cybex-VR2, Medway, MA) located in the General Clinical Research Center (GCRC) Exercise Laboratory. The higher of the two 1RM values obtained was used to determine the starting weight (70% of 1RM) for the resistance exercise portion of our study. On the second visit a dual-energy X-ray absorptiometry (DXA) scan (Hologic QDR 4500W, Bedford, MA) was also performed to measure body composition and lean mass, and a pregnancy test was repeated in the female subjects.
Each subject was admitted to the GCRC of the University of Texas Medical Branch the day before the exercise study. The subjects were then fed a standard dinner, and a snack was given at 2200. The subjects were studied following an overnight fast under basal conditions and refrained from exercise for 24 h before study participation. The morning of the study, polyethylene catheters were inserted into a forearm vein for tracer infusion, in the contralateral hand vein, which was heated for arterialized blood sampling, and in the femoral artery and vein (retrograde placement) of the leg for blood sampling. The femoral lines were placed in the same leg from which muscle biopsies were obtained. The arterial catheter was also used for the infusion of indocyanine green (ICG, Akorn, Buffalo Grove, IL) to determine blood flow.
After drawing a background blood sample, a primed continuous infusion of l-[ring-2H5]phenylalanine (Cambridge Isotope Laboratories, Andover, MA) was begun (time = 0 h at 0800) and maintained at a constant rate until the end of the experiment (Fig. 1). The priming dose for the labeled phenylalanine was 2 μmol/kg, and the infusion rate was 0.05 μmol·kg−1·min−1. All subjects were studied at the exact same time (i.e., between 0800 and 1400).
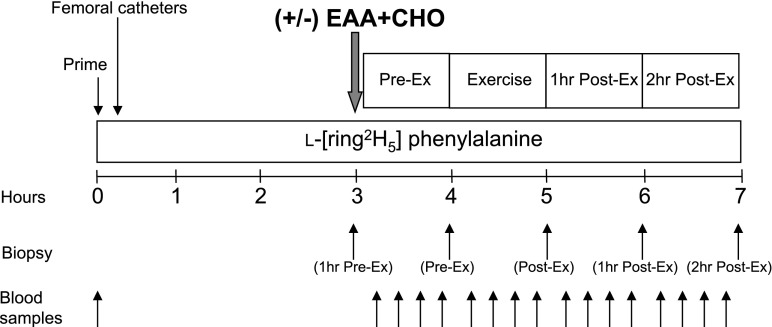
Fig. 1.
Study design. Blood and muscle sampling are indicated by arrows. A detailed description of the study design is provided in the text. Study design was identical for the fasting control group and the leucine-enriched essential amino acid and carbohydrate (EAA + CHO) group except for the ingestion of nutrients (+/−).
Subjects were studied during four time periods: preexercise (Pre-Ex), exercise (Ex), first hour postexercise (1 h Post-Ex), and second hour postexercise (2 h Post-Ex). During the Pre-Ex period, subjects assigned to the EAA + CHO group ingested a nutrient solution, whereas subjects in the fasting group did not receive nutrients although water was provided ad libitum. Marking the beginning of the Pre-Ex period, and 2 h following the initiation of the tracer infusions, the first muscle biopsy was obtained from the lateral portion of the vastus lateralis of the leg with the biopsy site between 15 and 25 cm from the midpatella. The biopsy was performed using a 5-mm Bergström biopsy needle, under sterile procedure and local anesthesia (1% lidocaine). Once harvested the muscle tissue was immediately blotted and frozen in liquid nitrogen (within seconds) and stored at −80°C until analysis. Immediately after the first biopsy, the EAA + CHO group ingested the nutrient solution, and a continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and maintained for 50 min for both groups. Ten minutes after ICG infusion was started, blood samples were drawn four times, at 10-min intervals, from the femoral vein and the arterialized hand vein to measure ICG concentration (Fig. 1). In addition to the blood obtained for ICG measurement, blood samples were taken from the femoral artery and vein and from the arterialized hand vein to measure blood glucose and amino acid concentrations. At the end of baseline, a second biopsy was obtained; however, the biopsy needle was inclined at a different angle so that the second biopsy was taken ∼2 in. (∼5 cm) from the first.
Following the second biopsy, each subject was transported to the exercise lab within the GCRC. The subjects performed 10 sets of 10 repetitions of leg extension exercises on a Cybex leg extension machine (Cybex International, Medway, MA) set to 70% of their 1RM. All subjects started out at 70% of 1RM, but for a few subjects the weight was slightly reduced (60–65% of 1 RM) to achieve 10 repetitions per set. The rest period between sets was 3 min, except during blood collection, which required a few more minutes. As during the basal period, ICG was continually infused during exercise to accurately measure leg blood flow. Blood samples were again drawn for blood pH, glucose, lactate, and amino acid concentrations immediately after the 3rd, 6th, 8th, and 10th sets. Following the last blood collection, subjects performed one final set of 10 repetitions, and a third muscle biopsy was obtained within seconds of completing the last muscle contraction. The subjects were then transported back to the special procedures room of the GCRC for the duration of the study.
During the 1 h Post-Ex period, ICG was again infused continuously (as during the Pre-Ex and Ex periods) to measure leg blood flow, and blood was drawn for the measurement of blood gas, electrolytes, and glucose and lactate concentrations. Samples were obtained every 10 min (as during the Pre-Ex and Ex periods). At the end of the first hour postexercise, a fourth muscle biopsy was obtained through a new incision site ∼5 cm from the first incision.
During the 2 h Post-Ex period (2nd hour postexercise), blood samples were collected in the same manner as during the previous periods. At the end of the second hour postexercise, a final muscle biopsy was collected as described above from the second incision; however, the biopsy needle was inclined at a different angle again so that the biopsy was taken ∼5 cm apart from the prior biopsy.
Composition of the EAA + CHO solution.
The nutrient solution contained leucine-enriched EAAs and CHO (sucrose) as described in our previous study (13). The solution contained EAAs in the following proportions: histidine (8%), isoleucine (8%), leucine (35%), lysine (12%), methionine (3%), phenylalanine (14%), threonine (8%), and valine (10%). To minimize the potential tracer dilution with the addition of the amino acids, phenylalanine tracer was added to the solution at 6.5% of phenylalanine content. Lean mass as determined by DXA was used to calculate the proportion of each EAA [0.35 g/kg of fat-free mass (FFM)] added to the nutrient solution. Similarly, CHO (sucrose) was added at 0.5 g/kg FFM to each nutrient solution. All ingredients (EAA and CHO) were dissolved in a noncaloric, caffeine-free, flavored beverage to increase palatability. The rationale for using this amount of nutrients was to provide a maximal stimulus for muscle protein synthesis via large increases in amino acid availability in combination with an increase in circulating insulin concentrations.
Blood flow, insulin, and glucose uptake.
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at wavelength λ = 805 nm (36). Plasma glucose concentration was measured using an automated glucose and lactate analyzer (YSI, Yellow Springs, OH). Plasma insulin concentrations were determined by ELISA (Linco Research, St. Charles, MO). Leg glucose utilization was calculated as net glucose uptake across the leg:
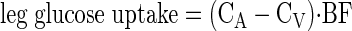 |
where CA and CV are the blood glucose concentrations in the femoral artery and vein, respectively, and BF is leg blood flow. Data are expressed as micromoles of glucose per minute per kilogram of FFM of the leg (μmol·min−1·kg leg FFM−1).
Phenylalanine net balance.
Phenylalanine net balance across the leg was calculated as:
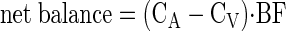 |
where CA and CV are the blood phenylalanine concentrations in the femoral artery and vein, respectively, and BF is leg blood flow. Data are expressed as hourly averages for each period (nmol·min−1·100 ml leg volume−1).
Muscle FSR.
Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as previously described (37). Blood and muscle intracellular free concentration of phenylalanine and leucine and the intracellular free enrichment of phenylalanine were determined by gas chromatography-mass spectrometry (GCMS, 6890 Plus GC, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) using appropriate internal standards (37). Mixed muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (37), using the external standard curve approach (6). We calculated the fractional synthetic rate of mixed muscle proteins (FSR) by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate:
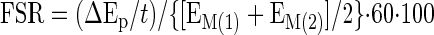 |
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) + EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
Immunoblotting and enzyme activity.
We have published the procedures for SDS-PAGE and immunoblotting in detail elsewhere (9). In short, the amount of total protein loaded per lane was identical, done in duplicate and separated by SDS-PAGE. For mTOR, Akt/PKB, TSC2, S6K1, and eEF2 separation, 7.5% gels were used, whereas for 4E-BP1, separation was accomplished with 15% gels. Following SDS-PAGE, proteins were transferred to polyvinylidene diflouride membranes (PVDF) (Hybond-P, Amersham Biosciences, Piscataway, NJ) at 50 V for 1 h. Once transferred, PVDF membranes were placed in blocking buffer [5% nonfat dry milk (NFDM) in TBST (Tris-buffered saline and 0.1% Tween-20)] for 1 h. Blots were then serially washed two times in deionized water and two more times in TBST and incubated with primary antibody in 5% NFDM in TBST (except 4E-BP1 and TSC2, which were incubated in 5% BSA) overnight at 4°C with constant agitation. The next morning, the blots were washed in TBST twice and incubated with secondary antibody for 1 h in 5% NFDM in TBST at room temperature with constant agitation. After secondary incubation, the blots were washed for 15 min and then serially washed (3 × 5 min) with TBST. Blots were then incubated for 5 min with enhanced chemiluminescence reagent (ECL plus Western Blotting Detection System, Amersham Biosciences, Piscataway, NJ) to detect horseradish peroxidase activity. Optical density measurements were obtained with a CCD camera mounted in a ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA). Once the appropriate image was captured, densitometric analysis was performed using Quantity One 1-D analysis software (version 4.5.2) (Bio-Rad). We found that protein abundance did not change over the short time frame of the study; therefore, all data were expressed as the change in phosphorylation in arbitrary units as we have previously described (9, 10, 13).
AMPKα2 activity was measured as previously described (9). Activity is expressed in picomoles of phosphate incorporated per milligram of muscle protein subjected to immunoprecipitation per minute (pmol·min−1·mg of protein−1).
Antibodies.
The primary antibodies used were all purchased from Cell Signaling (Beverly, MA): phospho-mTOR (Ser2448; 1:1,000); phospho-p70 S6K1 (Thr389; 1:500); phospho-Akt/PKB (Ser473; 1:500); phospho-tuberin/TSC2 (Thr1462; 1:500); phospho-4E-BP1 (Thr37/46; 1:500); and phospho-eEF2 (Thr56; 1:1,000). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2,000).
Statistical analysis.
All values are expressed as means ± SE. Primary outcomes were muscle protein synthesis, phenylalanine net balance, and blood metabolite and amino acid concentrations. Secondary outcomes include intracellular signaling proteins and enzyme activity. Comparisons between EAA + CHO and fasting were performed using ANOVA with repeated measures, the effects being group and time (Pre-Ex, Ex, 1 h Post-Ex, and 2 h Post-Ex), using JMP statistical software version 4.0.5 (SAS Institute). Post hoc testing was performed using t-test as appropriate. Significance was set at P < 0.05.
RESULTS
Blood flow, insulin, and glucose uptake.
Leg blood flow, insulin, and glucose uptake results are shown in Table 2. Leg blood flow was significantly higher after nutritional intake in the EAA + CHO group compared with the fasting group in the Pre-Ex period (baseline; P < 0.05). Leg blood flow increased significantly during exercise in both groups compared with the basal value (P < 0.05) and returned to preexercise baseline values at 1 h and 2 h postexercise (P > 0.05). Insulin concentration significantly increased following EAA + CHO ingestion during the Pre-Ex period (P < 0.05) and remained higher than basal values during the exercise period (P < 0.05). Insulin concentration returned to basal values during recovery in the EAA + CHO group, which was not different from the fasting group (P > 0.05). Glucose uptake across the leg was significantly higher after nutritional intake in the EAA + CHO group compared with fasting group in the Pre-Ex period (P < 0.05). Glucose uptake remained higher during exercise and 1 h postexercise in the EAA + CHO group compared with the fasting group preexercise baseline values (P < 0.05). In the fasting group, leg glucose uptake increased only during the exercise period (P < 0.05).
Table 2.
Leg blood flow, glucose uptake, and blood insulin
| Pre-Ex | Ex | 1 h Post-Ex | 2 h Post-Ex | |
|---|---|---|---|---|
| Leg blood flow, ml·100 ml leg−1·min−1 | ||||
| EAA+CHO | 5.2±0.7 | 13.4±2.0* | 5.8±0.8 | 5.8±0.7 |
| Fasting | 3.5±0.4 | 13.1±1.5* | 5.0±0.6 | 3.9±0.5 |
| Insulin, μU/ml | ||||
| EAA+CHO | 20.5±2.8† | 12.3±2.8* | 7.4±2.0 | 4.6±1.0 |
| Fasting | 4.9±0.8 | 7.6±0.8 | 4.6±0.6 | 3.6±0.6 |
| Glucose uptake, μmol·min−1·100 g LM−1 | ||||
| EAA+CHO | 16.4±4.4† | 31.7±7.2* | 16.3±2.5† | 13.6±2.4 |
| Fasting | 3.6±0.8 | 25.8±3.4* | 7.3±1.9 | 8.9±2.0 |
Values are mean ± SE. Measurements are hourly averages for each time period as shown in Fig. 1: the hour before beginning exercise (Pre-Ex), during exercise (Ex), and during postexercise recovery (1 h Post-Ex and 2 h Post-Ex). LM, lean mass.
P < 0.05 vs. Pre-Ex.
P < 0.05 vs. fasting at respective time period.
Muscle intracellular leucine and phenylalanine concentrations.
Muscle intracellular leucine and phenylalanine concentrations are shown in Table 3. There was no difference in muscle leucine or phenylalanine concentration between groups at baseline. Muscle leucine concentration increased significantly in response to nutritional intake and remained significantly higher immediately following exercise in the EAA + CHO group compared with the fasting group (P < 0.05). Muscle leucine concentrations decreased slightly during postexercise recovery in the fasting group (P < 0.05). Similarly, muscle phenylalanine concentration increased significantly following EAA + CHO ingestion compared with the fasting group (P < 0.05). Muscle phenylalanine concentration was also higher immediately postexercise in the EAA + CHO group (P < 0.05) and gradually declined to baseline levels by 2 h postexercise. Phenylalanine concentration was unchanged throughout the study except for a slight decrease at 2 h postexercise in the fasting group (P < 0.05).
Table 3.
Muscle intracellular amino acid concentrations
| 1 h Pre-Ex | Pre-Ex | Post-Ex | 1 h Post-Ex | 2 h Post-Ex | |
|---|---|---|---|---|---|
| Leucine, μmol/l | |||||
| EAA+CHO | 161±15 | 601±120*† | 430±53*† | 334±32*† | 195±15* |
| Fasting | 204±12 | 165±15 | 239±28 | 149±18* | 132±11* |
| Phenylalanine, μmol/l | |||||
| EAA+CHO | 67±3 | 140±15*† | 149±10*† | 118±12* | 77±2* |
| Fasting | 77±6 | 70±4 | 86±5 | 72±5 | 64±3* |
Values are means ± SE. Muscle amino acid concentrations taken at baseline (1 h Pre-Ex), before exercise but following nutrient ingestion in the EAA+CHO group (Pre-Ex), immediately following exercise (Post-Ex), and during postexercise recovery (1 h Post-Ex and 2 h Post-Ex).
P < 0.05 vs. 1 h Pre-Ex.
P < 0.05 vs. fasting at respective study period.
Muscle protein synthesis and phenylalanine net balance across the leg.
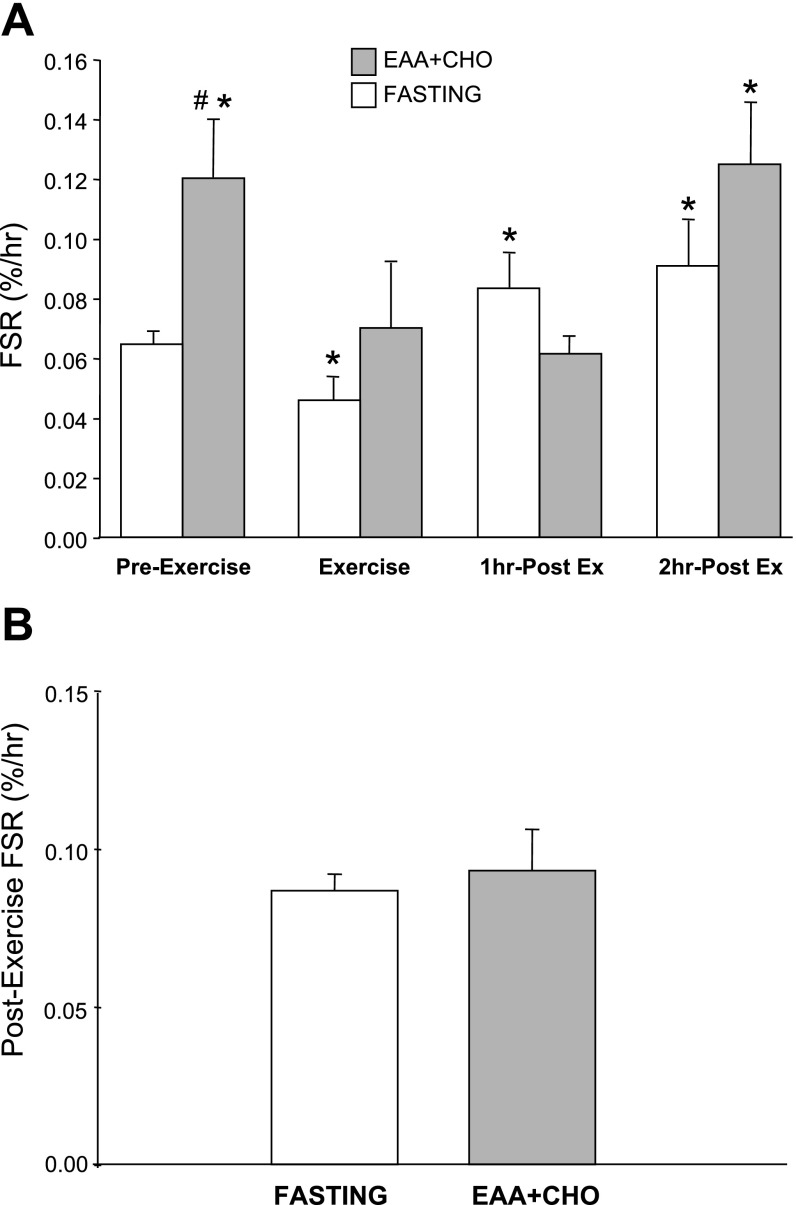
The phenylalanine tracer was added to the nutrient solution to minimize changes in enrichment following nutrient ingestion. In fact, blood and intracellular phenylalanine enrichment was stable throughout the experiment (i.e., enrichments were not different between study periods) in the EAA + CHO group and the fasting group (P > 0.05; data not shown). Mixed muscle protein FSR increased significantly in response to the EAA + CHO ingestion compared with the fasting group basal value (P < 0.05; Fig. 2A). The mixed muscle FSR in the EAA + CHO group returned to basal values during exercise (P > 0.05), remained unchanged during the 1 h postexercise period (P > 0.05), and increased significantly at 2 h postexercise (P < 0.05) compared with basal FSR. The FSR time course for the fasting group has been previously published (9), and the data show that FSR decreased during exercise (P < 0.05); however, FSR increased (compared with basal FSR) during the 1 h and 2 h postexercise periods (P < 0.05; Fig. 2A).
Fig. 2.
A: muscle protein fractional synthetic rate (FSR) time course in both the fasting control group and the EAA + CHO group. FSR was determined during a preexercise period (Pre-Ex), during exercise (Ex), 1 h postexercise (1 h Post-Ex), and 2 h postexercise (2 h Post-Ex). Nutrients were ingested immediately following the first muscle biopsy in the EAA + CHO group during the Pre-Ex period. B: FSR in both the fasting control and EAA + CHO groups over the entire 2 h postexercise recovery period (i.e., rate of incorporation calculated from the biopsy collected immediately postexercise to the biopsy collected at 2 h postexercise). Fasting control group data have been previously published (9). Values are means ± SE. *P < 0.05 vs. fasting Pre-Ex. # P < 0.05 vs. fasting at respective time point.
We also determined the muscle FSR over the entire 2-h postexercise recovery period (i.e., the rate of phenylalanine incorporation from the biopsy collected immediately after exercise to the 2 h postexercise biopsy). We found that there was no difference (P = 0.36) in the entire postexercise muscle FSR between the fasting group (exercise without nutrients) and the EAA + CHO group (nutrient ingestion before exercise), indicating no significant anabolic advantage for ingesting an EAA + CHO solution before exercise on postexercise muscle protein synthesis (Fig. 2B). In addition, we calculated the total muscle FSR during both exercise and recovery [i.e., the rate of tracer incorporation from the Pre-Ex biopsy to the 2 h postexercise biopsy. We found that there was no difference (P = 0.20) between groups (0.089 ± 0.01 vs. 0.073 ± 0.005%/h) for EAA + CHO and fasting groups, respectively].
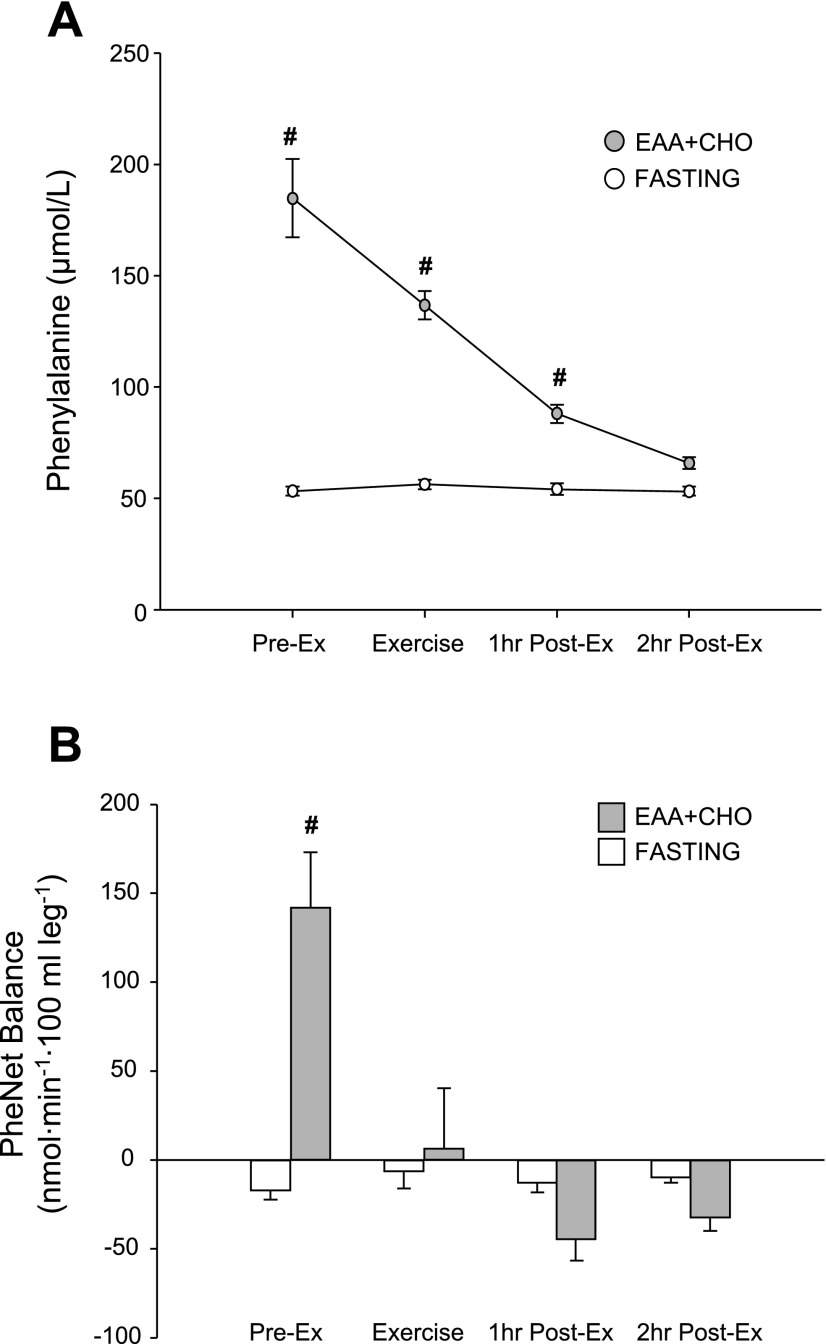
Blood phenylalanine concentration did not change throughout the entire study in the fasting group (P > 0.05; Fig. 3A). In the EAA + CHO group, blood phenylalanine concentration was significantly higher than in the fasting group during the preexercise, exercise, and 1 h postexercise periods (P < 0.05; Fig. 3A). During the first period following ingestion of EAA + CHO, the phenylalanine net balance across the leg significantly increased and became positive in the EAA + CHO group (P < 0.05; Fig. 3B). Postexercise phenylalanine net balance in the EAA + CHO group gradually decreased and became negative during 1–2 h of recovery. The fasting group phenylalanine net balance tended to become less negative during postexercise recovery, but this change did not reach significance (P > 0.05).
Fig. 3.
Time course of blood phenylalanine concentration (A) and net balance (B) across the leg in the preexercise state (Pre-Ex), during exercise (Ex), 1 h postexercise (1 h Post-Ex), and 2 h postexercise (2 h Post-Ex) for both the fasting control and EAA + CHO groups. Data shown are presented as the hourly average. Pre-Ex data for the fasting group are basal data without nutrients, whereas Pre-Ex data for the EAA + CHO are the hourly average following nutrient ingestion. Values are means ± SE. # P < 0.05 vs. fasting at respective time point.
Upstream regulators of mTOR signaling.
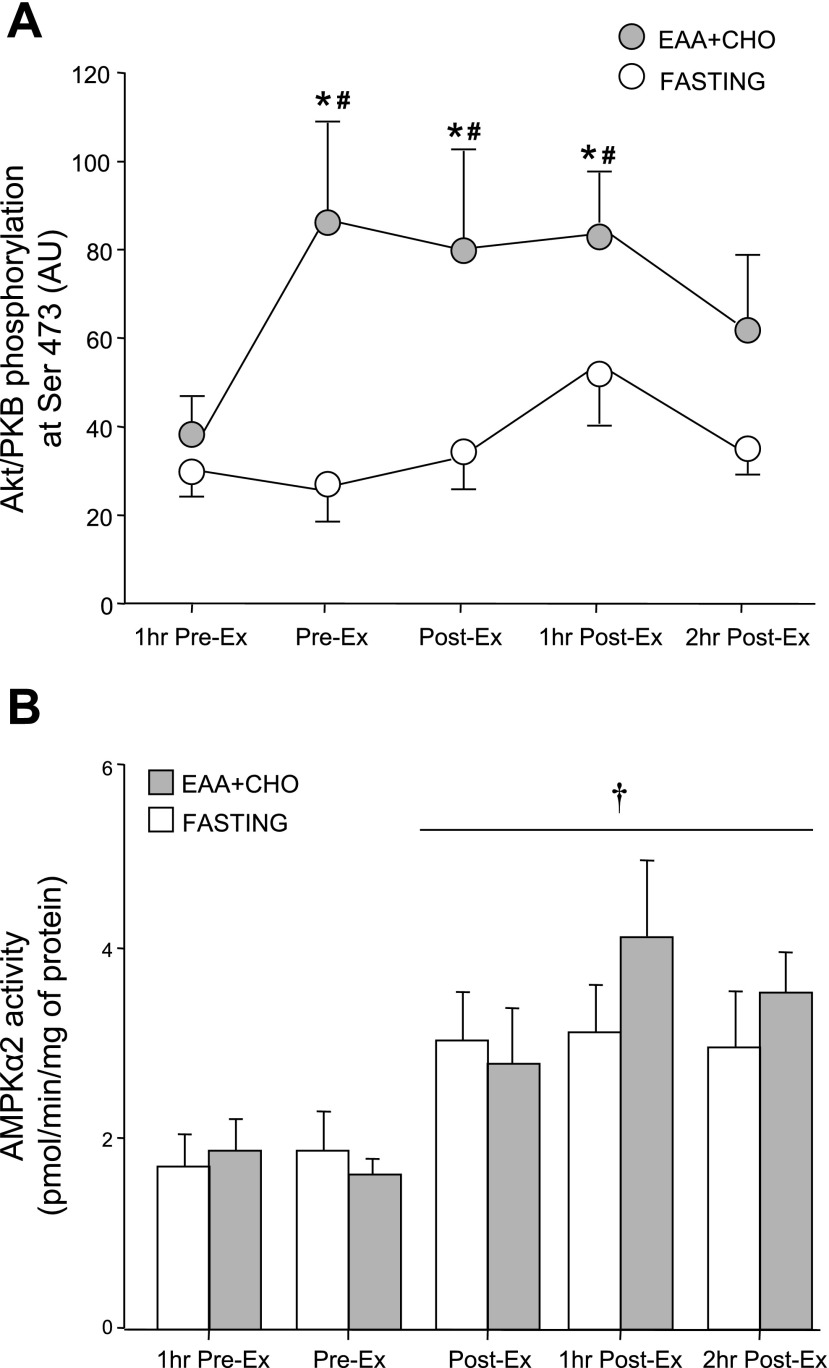
The phosphorylation of Akt/PKB at Ser473 increased significantly in response to the EAA + CHO ingestion before exercise and remained elevated during exercise and 1 h postexercise (P < 0.05; Fig. 4A). Akt/PKB phosphorylation returned to basal levels at 2 h postexercise. Akt/PKB phosphorylation increased only at the end of the first hour postexercise in the fasting group (9).
Fig. 4.
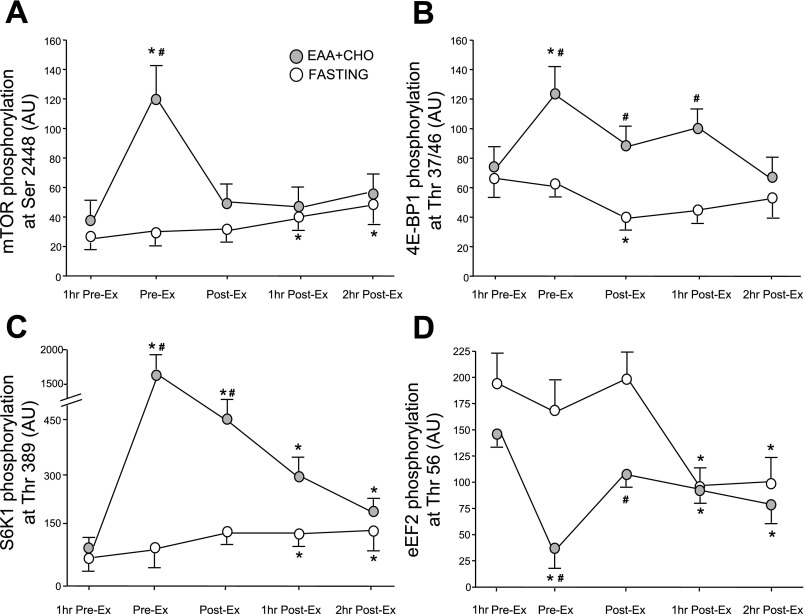
Upstream regulators of the mammalian target of rapamycin (mTOR) signaling pathway in the EAA + CHO and fasting control group 1 h before exercise (1 h Pre-Ex), immediately before beginning exercise (Pre-Ex), immediately following exercise (Post-Ex), 1 h postexercise (1 h Post-Ex), and 2 h postexercise (2 h Post-Ex) . Nutrients were ingested immediately following the first muscle biopsy (1 h Pre-Ex) in the EAA + CHO group. A: Akt/protein kinase B (PKB) phosphorylation at Ser473. B: AMP-activated protein kinase (AMPKα2) activity. The time course data for the fasting control group have been previously published (9). Values are means ± SE. *P < 0.05 vs. 1 h Pre-Ex. # P < 0.05 vs. fasting at respective time point. †P < 0.05 for time effect. There were no differences between groups for the AMPK data.
The phosphorylation of TSC2 at Thr1462 did not change throughout the entire study period in either group (P > 0.05; data not shown) although there was a slight decrease at the end of the first hour postexercise in the fasting group (9).
Muscle AMPKα2 activity tended to decrease following nutrient ingestion in the EAA + CHO group (P > 0.05; Fig. 4B). There was a significant time effect for AMPKα2 activity to be increased postexercise in both groups (P < 0.05; Fig. 4B). There were no differences between groups at any time point (P > 0.05). AMPKα2 activity in the fasting group has been previously published (9).
mTOR and downstream regulators of mTOR signaling and translation elongation.
The phosphorylation of mTOR at Ser2448 increased significantly in response to the EAA + CHO ingestion before exercise (P < 0.05; Fig. 5A). mTOR phosphorylation returned to 1 h Pre-Ex (basal) levels during exercise (P > 0.05) and was not significantly increased at 1 h and 2 h postexercise in the EAA + CHO group compared with baseline or the fasting group (P > 0.05). In comparison, the fasting group mTOR phosphorylation was significantly increased at 1 and 2 h postexercise (P < 0.05) (9).
Fig. 5.
Phosphorylation status of downstream components of mTOR signaling pathway in the EAA + CHO and fasting control group 1 h before exercise (1 h Pre-Ex), immediately before beginning exercise (Pre-Ex), immediately following exercise (Post-Ex), 1 h postexercise (1 h Post-Ex), and 2 h postexercise (2 h Post-Ex). mTOR phosphorylation at Ser2448 (A), 4E-binding protein 1 (4E-BP1) phosphorylation at Thr37/46 (B), S6 kinase 1 (S6K1) phosphorylation at Thr389 (C), and eukaryotic elongation factor 2 (eEF2) phosphorylation at Thr56 (D) are shown. The time course phosphorylation data for the fasting group have been previously published (9). Values are means ± SE. *P < 0.05 vs. 1 h Pre-Ex. #P < 0.05 vs. fasting at respective time point.
Phosphorylation of 4E-BP1 at Thr37/46 increased significantly in response to the EAA + CHO ingestion before exercise (P < 0.05; Fig. 5B) and returned to 1 h Pre-Ex basal levels during exercise (P > 0.05). 4E-BP1 phosphorylation at 1 h and 2 h postexercise also did not differ from 1 h Pre-Ex basal values; however, phosphorylation was higher than the fasting group immediately and 1 h postexercise (P < 0.05). The fasting group 4E-BP1 phosphorylation decreased during exercise (P < 0.05) and was not different from baseline during postexercise recovery (9).
Phosphorylation of S6K1 at Thr389 increased significantly in response to the EAA + CHO ingestion before exercise (P < 0.05; Fig. 5C) and remained higher immediately postexercise and 1 h and 2 h postexercise (P < 0.05). In comparison, the fasting group showed a significant increase in S6K1 phosphorylation at the end of the 2 h postexercise period (9); however, the phosphorylation of S6K1 was higher in the EAA + CHO group compared with the fasting group at Pre-Ex and Post-Ex (P < 0.05).
Phosphorylation of eEF2 at Thr56 decreased significantly in response to EAA + CHO ingestion (P < 0.05; Fig. 5D) and increased to baseline levels during exercise (P > 0.05). eEF2 phosphorylation was significantly reduced (compared with baseline) at the end of the 1 and 2 h postexercise periods (P < 0.05). eEF2 phosphorylation was significantly higher in the fasting group compared with the EAA + CHO group Post-Ex; however, phosphorylation was reduced to a similar extent during postexercise recovery in the fasting group (9).
DISCUSSION
The primary finding from our study was that muscle protein synthesis increased to a similar extent postexercise in both the EAA + CHO (fed) group and control (fasted) group. Thus, performing a bout of resistance exercise in the fed state did not further increase muscle protein synthesis during postexercise recovery compared with the fasted state. However, the postexercise time course for FSR was different between the two groups in that during exercise the FSR did not decrease below basal values in the EAA + CHO group, and the postexercise increase in FSR was delayed compared with the fasting control group. On the other hand, our recent work has shown that providing EAA + CHO following a bout of resistance exercise increases the rate of muscle protein synthesis to a greater extent than when resistance exercise is performed without nutrient ingestion (10). Therefore, our data support the hypothesis that EAA + CHO ingestion following resistance exercise is more effective at enhancing muscle protein synthesis during postexercise recovery.
Our recent data (10) are in agreement with a large number of studies in which nutrient ingestion (i.e., amino acids, protein, or EAA + CHO) following a bout of resistance exercise has been shown to increase muscle protein synthesis in human subjects (4, 5, 10–11, 18–19, 21, 28, 30–31, 36). In addition, recent work has shown that when subjects ingested fat-free milk (i.e., protein + CHO) following each resistance exercise bout (during 3 mo of training), the increase in muscle mass was greater than in either an isoenergetic soy protein group or an isoenergetic CHO control group (14). In contrast to these studies, one study has shown that EAA + CHO ingestion immediately before an acute bout of resistance exercise produced a larger increase in indirect measures of muscle protein synthesis (i.e., leg net amino acid balance and rate of disappearance) compared with when the nutrients were ingested immediately postexercise (29). We cannot completely explain the discrepancies between that study and our present study, but it should be noted that several differences in study design exist. For example, in the prior study (29) the authors used a smaller dose of EAA + CHO than we did, and they provided the nutrients immediately before exercise, whereas we administered the nutrients 1 h before beginning exercise. In addition, it is difficult to determine if muscle protein synthesis was actually increased in the aforementioned study (29) because it was not measured directly. In addition, the ingestion of whey protein immediately before exercise (31) also resulted in similar increases in nondirect measures of muscle protein synthesis and anabolism. Therefore, we cannot rule out the possibility that intact protein ingestion before exercise may have produced different results than what we have reported in the present study. Recently, it has been shown that protein and CHO ingestion “during” a 2-h whole body resistance exercise workout resulted in an increase in leg muscle FSR (although this difference was not significant when the muscle intracellular enrichment was used to calculate FSR) (1). There are a couple of reasons for the discrepancy between that study and the present study. First, the authors (1) studied subjects in the fed state, whereas our subjects were fasted before they consumed a bolus of EAA + CHO 1 h before exercise. Second, we measured FSR during ∼1 h of leg muscle contractions, whereas the subjects in the Beelen et al. (1) study underwent an intermittent exercise routine (i.e., upper body exercise was performed during the first hour of exercise and lower body exercises in the last hour). Thus it is possible that consuming protein/amino acids during an intermittent resistance exercise workout may result in a stimulation of FSR. Future work is still required to determine if muscle protein synthesis is increased during exercise or recovery when EAA + CHO are ingested immediately before performing an exercise bout. In any event, it is clear that net muscle anabolism during recovery is not greater when exercise is performed in the fed state because phenylalanine net balance across the leg is similar to subjects who exercised in the fasted state (Fig. 3B).
Our data do not support the view that exercising in the fed state can increase muscle protein synthesis during a bout of resistance exercise. Specifically, our data show that muscle protein synthesis returns to basal values during a bout of resistance exercise performed while in the fed state (Fig. 2). We have previously shown that muscle protein synthesis decreases during a bout of resistance exercise performed while in the fasting state (9). In the present study, muscle protein synthesis decreased during exercise compared with the large increase detected during the hour following nutrient ingestion. However, muscle protein synthesis was not significantly lower than the fasting group basal values, which may indicate that performing a bout of resistance exercise while in the fed state may prevent muscle protein synthesis from decreasing (i.e., below basal FSR values) during exercise as we originally hypothesized. It remains to be determined whether the blunted decrease in FSR during exercise is physiologically relevant because of the relatively short exercise duration. In fact, we were unable to detect a difference between groups in the FSR calculated during both exercise and recovery periods. During postexercise recovery, the reduction or attenuation of muscle protein synthesis during resistance exercise is rapidly reversed and the rate of protein synthesis is increased within 1 h postexercise (9). In the present study the postexercise time course for muscle protein synthesis in the EAA + CHO group was slightly altered, in that protein synthesis did not increase during the first hour of recovery but an increase was detected at 2 h postexercise. Contrary to our original hypothesis, performing a bout of resistance exercise while in the fed state produces a similar increase in postexercise muscle protein synthesis compared with when exercise is performed in the fasted state (Fig. 2B).
The most likely explanation for no increase in muscle protein synthesis “during” resistance exercise is that the priority of the muscle cell is to provide ATP to support muscle contraction. Thus the anabolic and ATP-consuming process of protein synthesis is given less priority in favor of stimulating catabolic ATP-producing processes such as glycolysis. Our data show that exercising in the fed state may prevent an actual decrease in protein synthesis during exercise; however, it is clear that muscle protein synthesis is not stimulated during exercise. We have previously identified potential cellular mechanisms responsible for reducing muscle protein synthesis during exercise, which include the large increase in muscle acidosis induced by the resistance exercise bout or an increase in cellular energetic stress (9). AMPK, a cellular energy sensor, is increased immediately and 1 h following a bout of resistance exercise in the fasted state (9). In the present study we show that AMPK increased to a similar extent immediately following exercise and during recovery in both groups (Fig. 4B). Therefore, feeding before exercise did not prevent AMPK activation during exercise. It has also been shown that acidosis can reduce the rate of skeletal muscle protein synthesis in both animals and humans (7). In any event, our data clearly show that the local environment within the muscle during exercise in the fed state is not conducive to promoting muscle protein synthesis.
There was an interesting difference in the FSR response between our two groups. In the fasting group, muscle FSR decreased during exercise; however, FSR rebounded quickly and was significantly increased at both 1 and 2 h postexercise. On the other hand, as previously mentioned, in the EAA + CHO group, muscle FSR did not decrease as much during the resistance exercise bout, indicating that the nutrient ingestion before exercise may have prevented the decrease in FSR during exercise. The postexercise increase in FSR was also delayed in the EAA + CHO such that FSR did not increase until 2 h postexercise. The mechanism(s) responsible for the delay in FSR activation in the EAA + CHO group cannot be directly determined in the present study. However, it is possible that the large activation of Akt/mTOR signaling and muscle protein synthesis by the abundant supply of amino acids and insulin immediately following the ingestion of EAA + CHO (before exercise) may have resulted in a partial refractory period for muscle protein synthesis after exercise. In any event, the increase in muscle FSR during recovery was also linked to an elevated muscle protein breakdown rate in both groups since phenylalanine net balance across the leg was negative during postexercise recovery. This would indicate that nutrient ingestion before exercise did not prevent the normal increase in muscle protein breakdown following the resistance exercise bout and provides more support for the hypothesis that EAA + CHO ingestion before exercise does not enhance muscle anabolism during postexercise recovery.
Muscle protein synthesis is stimulated in the recovery period following a bout of resistance exercise (3, 9, 15, 22, 38). We have recently shown that activation of the muscle Akt/mTOR signaling pathway is associated with the increase in muscle protein synthesis following an acute bout of resistance exercise (9–10). In particular, mTOR and S6K1 phosphorylation are increased and eEF2 phosphorylation is decreased during the first 1–2 h postexercise, indicating that both translation initiation and elongation are activated. In the present study, we detected a large activation of the Akt/mTOR-associated signaling proteins 1 h following the ingestion of the EAA + CHO as previously reported (13). Specifically, we found that Akt phosphorylation was increased following the ingestion of the EAA + CHO solution and remained elevated during exercise and at 1 h postexercise. However, phosphorylation of mTOR, S6K1, 4E-BP1, and eEF2 during postexercise recovery were not different between the fasting and EAA + CHO groups (Fig. 5). In fact, eEF2 phosphorylation was significantly reduced at 1 and 2 h postexercise in association with the increased rate of protein synthesis in the fasting group; however, the reduced eEF2 phosphorylation at 1 h postexercise in the EAA + CHO group did not correspond with an increase in muscle protein synthesis. In our previous work we have clearly shown a direct relationship between eEF2 dephosphorylation and an increase in muscle protein synthesis following resistance exercise and/or nutrient ingestion (9, 10, 13), indicating an important role for elongation in the control of muscle FSR. The disconnect between eEF2 phosphorylation and muscle FSR at 1 h postexercise in the EAA + CHO group may be due to the fact that we collected the biopsy at hourly intervals whereas the FSR is a calculated hourly average. It is likely that at 1 h postexercise elongation was activated in the EAA + CHO group, but this was not reflected in the hourly FSR immediately following exercise. Finally, our study was focused on the early postexercise recovery response in signaling and FSR, and we acknowledge the possibility that long-term activation of mTOR signaling and FSR such as at 6, 24, or 48 h postexercise may be better indicators of muscle growth over time.
In summary, we show that performing a bout of resistance exercise in the fed state prevented the normal decrease in FSR during exercise and delayed the normal increase in FSR postexercise. However, the eventual increase in FSR in the fed group did not further increase muscle protein synthesis during postexercise recovery compared with performing resistance exercise in the fasted state. In contrast, our recent findings indicated an enhanced stimulation of muscle protein synthesis when EAA + CHO are ingested 1 h following a bout of resistance exercise (10). We conclude that EAA + CHO ingestion postexercise appears to be more effective (than ingestion preexercise) at inducing further increases in the rate of human skeletal muscle protein synthesis during early postexercise recovery.
GRANTS
This research was supported by Grant R01-AR-049877 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases, Grant S10-RR-16650 from the Shared Instrumentation Grant Program, Grant-P30-AG024832 from the National Institute on Aging, and Grant M01-RR-00073 from the General Clinical Research Branch, National Center for Research Resources.
Acknowledgments
We thank the nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch and Dr. Jerson Cadenas for help with the conduct of the clinical portion of this study. We also thank Ming Zheng, Jessica Lee, Shelley Medina, and Sarah Toombs Smith for technical assistance.
REFERENCES
- 1.Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, van Loon LJ. Protein co-ingestion stimulates muscle protein synthesis during resistance type exercise. Am J Physiol Endocrinol Metab 295: E70–E77, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term Insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab 289: E999–E1006, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab 283: E648–E657, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002-009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 6: 421–424, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Caso G, Garlick PJ. Control of muscle protein kinetics by acid-base balance. Curr Opin Clin Nutr Metab Care 8: 73–76, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 73: 1383–1388, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc 38: 667–674, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 291: E745–E754, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 582: 813–823, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice male weightlifters. Am J Clin Nutr 86: 373–381, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 278: E620–E626, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971. [DOI] [PubMed] [Google Scholar]
- 17.Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568: 283–290, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab 288: E645–E653, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Koopman R, Beelen M, Stellingwerff T, Pennings B, Saris WH, Kies AK, Kuipers H, van Loon LJ. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 293: E833–E842, 2007. [DOI] [PubMed] [Google Scholar]
- 20.MacDougal JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 20: 480–486, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc 35: 449–455, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown following resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88: 386–392, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar affects on muscle protein synthesis in the elderly. J Nutr Health Aging 6: 358–362, 2002. [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab 275: E73–E78, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Svanberg E, Moller-Loswick AC, Matthews DE, Korner U, Andersson M, Lundholm K. Effects of amino acids on synthesis and degradation of skeletal muscle proteins in humans. Am J Physiol Endocrinol Metab 271: E718–E724, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab 276: E628–E634, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall B, Petrini B, Wolfe RR. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab 281: E197–E206, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 36: 2073–2081, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 292: E71–E76, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85: 4481–4490, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welle S, Bhatt K, Thornton CA. Stimulation of myofibrillar synthesis by exercise is mediated by more efficient translation of mRNA. J Appl Physiol 86: 1220–1225, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old subjects after three months of resistance training. Am J Physiol Endocrinol Metab 268: E422–E427, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 85: 1031–1040, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research. Principles and Practice of Kinetic Analysis (2nd ed.). Hobokon, NJ: Wiley-Liss, 2005.
- 38.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab 265: E210–E214, 1993. [DOI] [PubMed] [Google Scholar]