Abstract
Advanced sarcopenia is prevalent among octogenarian women; yet little is known about myocellular quality and plasticity in this cohort. The aim of this investigation was to examine single muscle fiber contractile function and whole muscle characteristics before and after 12 wk of high-intensity progressive resistance training (PRT) in very old (85 ± 1 yr) women (OW, n = 6). Young women [YW (21 ± 2 yr old), n = 9] were included as a control group. Whole muscle strength [1 repetition maximum (RM)] and size (CT scans) were assessed before and after PRT. Functional experiments (size, peak force, velocity, and power) were performed on vastus lateralis myosin heavy chain (MHC) I and IIa muscle fibers before and after PRT. With PRT, 1-RM strength increased (P < 0.05) in YW (36%) and OW (26%). Thigh muscle cross-sectional area increased (5%) in YW (P < 0.05), but thigh muscle did not hypertrophy in OW. Before PRT, there were no differences in single-fiber parameters between YW and OW. With PRT, MHC IIa fiber size (28%), peak force (31%), and power (28%) improved, but no changes were observed in MHC I fibers, in YW (P < 0.05). There were no improvements in MHC I or IIa single-fiber function in OW. These data show that the myocellular functional profile in OW is similar to that in YW but that OW have a blunted hypertrophic response to PRT at the whole muscle and myocellular level. The limited myocellular plasticity in OW with PRT contrasts with that in YW and previous PRT studies in elderly women only a decade younger. These data suggest that attempts to greatly enhance skeletal muscle mass and function should begin before 80 yr of age.
Keywords: skeletal muscle, single-fiber physiology, aging, exercise
skeletal muscle atrophy is a well-known characteristic of aged individuals and is associated with a reduction in whole muscle strength and function (9). Progressive resistance training (PRT) has been implemented successfully in the elderly population to partially reverse age-related atrophy, resulting in whole muscle size and strength increases (12, 14, 26, 36, 40, 46, 47, 51–53). Our laboratory previously reported substantial improvements in whole muscle size and strength after PRT, with myocellular improvements primarily in the slow myosin heavy chain (MHC) I muscle fibers, in 74-yr-old men (53) and women (51). Although resistance training has been effective in the elderly population, the data remain equivocal: some reports suggest that elderly individuals have a diminished capacity to adapt to PRT (29, 45). We recently reported that 82-yr-old men demonstrate limited skeletal muscle plasticity, on a myocellular and whole muscle level, in response to high-intensity PRT (48). There is also evidence to suggest that the rate of skeletal muscle mass (34) and strength loss (1, 8) is accelerated in octogenarian individuals and aged rodents (19). The accelerated loss of skeletal muscle mass and strength, combined with the reduced plasticity, suggests that alterations in muscle biology may occur with advancing age (>80 yr), thus warranting further investigation.
Women represent ∼70% of the age group that reaches the age of 80 yr and beyond (21). There is very limited information on skeletal muscle plasticity and myocellular quality in octogenarian women. Furthermore, women are more vulnerable to a loss of whole muscle function with age, because women, in particular, are susceptible to MHC IIa atrophy (29, 50). The fast MHC IIa muscle fibers are important: they are five to six times more powerful than MHC I muscle fibers (5, 50), and these fibers are relied on during fast movements such as to help prevent a fall. Collectively, octogenarian women represent a large, rapidly expanding, and important group of our elderly population, and further research is needed in this cohort.
Therefore, the objective of this investigation was to investigate whole muscle and single muscle fiber contractile properties before and after PRT in 85-yr-old women. For a direct comparison with baseline and training response, we included a control group of young women. At the onset of this multiyear investigation, we hypothesized that octogenarian individuals would have a response to PRT similar to that of the 74-yr-old men (53) and women (51) we previously studied, that is, an increase in whole muscle size and strength and myocellular adaptations targeted to the slow MHC I muscle fibers. We recently reported limited myocellular plasticity in 82-yr-old men (who were part of this multiyear investigation) after 12 wk of PRT. Because identical methods and instrumentation were used in the present study and in our previous study on 74-yr-old women (51), we were able to make a novel comparison of skeletal muscle plasticity at the myocellular level in response to high-intensity PRT among 21-, 74-, and 85-yr-old female populations.
METHODS
Subjects.
Nine young (21 ± 2 yr, 164 ± 2 cm, 65 ± 4 kg) and six older (85 ± 1 yr, 158 ± 1 cm, 67 ± 3 kg) women were recruited from the local community. The criteria for subject qualification included nonexercising, nonobese, nonsmoking, normotensive, healthy women. The older women were not engaged in and had no past history of hormone replacement therapy. Before participating in resistance training, the older women underwent a physical examination, which included medical history, blood and urine samples for general health markers, and resting and exercising electrocardiogram and blood pressure. Before engaging in the experimental protocol, the subjects were informed of all procedures and risks associated with the protocol, and written informed consent was obtained from each participant. The Institutional Review Board of Ball State University and Ball Memorial Hospital approved the experimental design before initiation of the study.
Experimental design.
After an initial familiarization period (2–3 sessions), the women completed 12 wk of high-intensity PRT for the knee extensors, with 3 training sessions per week for a total of 36 sessions. Each training session consisted of 3 sets of 10 repetitions at 70–75% of one-repetition maximum (1-RM). Whole thigh muscle size and strength (1-RM) were assessed before and after the training period. Skeletal muscle biopsies were obtained from the vastus lateralis before and after the training period, and MHC I and IIa muscle fiber size and contractile properties were analyzed. All subjects were instructed to continue an ad libitum diet throughout the study.
Whole muscle strength.
Bilateral isotonic 1-RM strength of the knee extensor muscles was assessed before, every 2 wk during, and after the training program for which a seated knee extension device (Cybex Eagle, Medway, MA) was used. The 1-RM was determined by an incremental increase of the amount of weight on the device with each successful lift until the subject was unable to maintain proper form and/or fully extend her legs at a given weight. The 1-RM trials were designed such that each individual would reach 1-RM within two to three attempts. The subjects rested for 2 min after each 1-RM attempt. After it appeared that the subject had reached her 1-RM, an additional attempt was performed at a heavier load to ensure that 1-RM had been reached. The last weight successfully lifted was determined to be the 1-RM.
Whole muscle size.
To measure muscle CSA of the right thigh before and after PRT, we used CT on a helical scanner (CTI helical scanner, General Electric, Milwaukee, WI), as described previously (52). Briefly, subjects were in a supine position for ≥30 min before the scan (3). A scout scan was performed to locate the anatomic midpoint of the femur, which was used as the point of measurement for pre- and posttraining CT measurements. The CSA of the thigh minus the area of bone and subcutaneous fat was determined using computerized planimetry (NIH Image Program, version 1.61, National Institutes of Health, Bethesda, MD).
High-intensity PRT program.
Subjects performed 12 wk of a supervised high-intensity PRT program known to effectively strengthen the quadriceps muscles in young (56) and elderly (6, 14, 31, 48, 51, 53) individuals. Subjects performed bilateral isotonic leg extensions on 3 nonconsecutive days per week (36 total sessions). Subjects performed three sets of 10 repetitions (3 × 10) at 70–75% of their 1-RM, with 2 min of rest between sets. Each repetition consisted of a concentric and an eccentric phase; each phase lasted ∼2–3 s. The program was progressive, since 1-RM was assessed every 2 wk, and the weight was adjusted accordingly to maintain an intensity of 70–75%. All 15 women completed all 36 training sessions for 100% compliance to the training program.
Muscle biopsy.
Muscle biopsies (4) were obtained from the vastus lateralis of each subject before and 72 h after the training period. Each muscle sample was processed into longitudinal sections, and a portion was placed in cold skinning solution (see below) and stored at −20°C for single muscle fiber physiology experiments.
Skinning, relaxing, and activating solutions.
The skinning solution contained 125 mM K propionate, 2.0 mM EGTA, 4.0 mM ATP, 1.0 mM MgCl2, 20.0 mM imidazole (pH 7.0), and 50% (vol/vol) glycerol. The relaxing and activating solutions contained (mM) 7.0 EGTA, 20.0 imidazole, 14.5 creatine phosphate, 1.0 free Mg2+, 4.0 free MgATP, and KCl and KOH to produce an ionic strength of 180 mM and pH 7.0; pCa of relaxing and activating solutions was 9.0 and 4.5, respectively.
Single muscle fiber contractile function.
Individual muscle fibers were analyzed for diameter, peak force (Po), maximal unloaded shortening velocity (Vo), and force-power characteristics. Detailed descriptions and illustrations of these procedures have been previously published by our laboratory (50, 53). After a single muscle fiber experiment, each single fiber was analyzed for MHC composition (see below). All single muscle fiber physiology experiments were completed within 4 wk of the muscle biopsy.
Single muscle fiber physiology experiments.
For each experiment, a 2- to 3-mm muscle fiber segment was isolated from a muscle bundle and transferred to an experimental chamber filled with pCa 9.0 solution. Each end of the fiber was securely fastened between a force transducer (model 400A, Cambridge Technology) and a direct-current torque motor (model 308B, Cambridge Technology) as described by Moss (37). The apparatus was mounted on a microscope (model BH-2, Olympus, Tokyo, Japan), so that the fiber could be viewed (×800) during an experiment. An eyepiece micrometer was used to adjust sarcomeres along the isolated muscle segment length to 2.5 μm, and the fiber length was determined. All single muscle fiber experiments were performed at 15°C.
Unamplified force and length signals were sent to a digital oscilloscope (model 310, Nicolet, Madison, WI), which enabled us to monitor muscle fiber performance throughout data collection. Analog force and position signals were amplified (Dual Differential Amplifier 300-DIF2, Positron Development, Ingelwood, CA), converted to digital signals (National Instruments), and transferred to a computer (Gateway, Irvine, CA) for analysis using customized software. Servo-motor arm and isotonic force clamps were controlled using a computer-interfaced force-position controller (model 300-FC1, Positron Development).
single-fiber diameter.
Fiber diameter was determined from a captured computer image (Sony CCD-IRIS, DXC-107A) obtained while the fiber was briefly (<3 s) suspended in air. Fiber width (diameter) was determined at three points along the segment length (2.5-μm-long sarcomeres) of the captured image using NIH public domain software (Scion Image, release Beta 4.0.2, for Windows). CSA of the muscle fibers was calculated on the basis of the diameter data and with the assumption that the muscle fiber has a circular shape. Previous research has shown that muscle fiber shape is not altered after resistance training in older individuals (22), thus supporting the notion that the CSA calculations could be performed before and after the training program under the assumption that muscle fiber shape did not change.
single-fiber Po.
Resting force was monitored, and then the fiber was maximally activated in pCa 4.5 solution. Po was determined in each fiber by computer subtraction of the baseline force from the peak force in the pCa 4.5 solution.
single-fiber Vo.
Fiber Vo was measured by the slack test technique as described by Edman (11). Four different activation and length steps [150, 200, 250, and 300 μm, each ≤15% of fiber length (FL)] were used for each fiber, with the slack distance plotted as a function of the duration of unloaded shortening. Fiber Vo (FL/s) was calculated by dividing the slope of the fitted line by the fiber segment length, and the data were normalized to a sarcomere length of 2.5 μm.
single-fiber power.
Submaximal isotonic load clamps were performed on each fiber for determination of force-velocity parameters and power. Each fiber segment was fully activated in pCa 4.5 solution and then subjected to a series of isotonic load steps. Force and shortening velocity data points derived from the isotonic contractions were fit using the hyperbolic Hill equation (24). Fiber peak power was calculated from the fitted force-velocity parameters (Po, Vmax, and a/Po, where a is a force constant and Vmax is the y-intercept). Absolute power (μN·FL·s−1) was defined as the product of force (μN) and shortening velocity (FL/s). Normalized power (W/l) was defined as the product of force and shortening velocity normalized to cell size.
MHC isoform identification.
SDS-PAGE analysis, as described elsewhere (57), was used to determine the MHC isoform of each isolated single muscle fiber. Briefly, each single muscle fiber was solubilized in 80 μl of 1% SDS sample buffer [1% SDS, 6 mg/ml EDTA, 0.06 M Tris (pH 6.8), 2 mg/ml bromphenol blue, 15% glycerol, and 5% β-mercaptoethanol] and subjected to SDS-PAGE and then silver staining. The MHC isoforms were identified according to their migration distances.
Statistical analyses.
The data were tested for normality using the Shapiro-Wilk test. Since several single muscle fiber parameters (size, peak power, Po/CSA, and Vmax) were not normally distributed, nonparametric statistical analyses were performed. Baseline comparisons of whole muscle strength and size and each single muscle fiber parameter were conducted between groups using a nonparametric version of an independent t-test, the Mann-Whitney test. Effects of the training program on whole muscle strength and size and each single muscle fiber parameter (size, Po, Po/CSA, Vo, Vmax, peak power, and normalized power) were evaluated using the nonparametric version of a paired t-test, Wilcoxon's signed-rank test. Bonferroni's multiple comparison adjustment was applied for Wilcoxon's signed-rank tests to reduce the risk of type I error. The nonparametric tests chosen have a power efficiency of ∼95% for small sample sizes compared with equivalent parametric t-tests (44). For each single muscle fiber parameter within a subject, a mean for each fiber type (MHC I and IIa) was determined. The mean value was used to represent all fibers of that type within the given individual. The hybrid fibers (i.e., MHC I/IIa and IIa/IIx) constituted a reasonable proportion of fibers studied, but when partitioned into the individual subtypes, there were insufficient data for age and pre- vs. posttraining comparisons. MHC IIx fibers were also excluded from the analysis, because these fibers rarely occurred. Significance was accepted at P < 0.05. Values are means ± SE.
RESULTS
Thigh muscle size and strength.
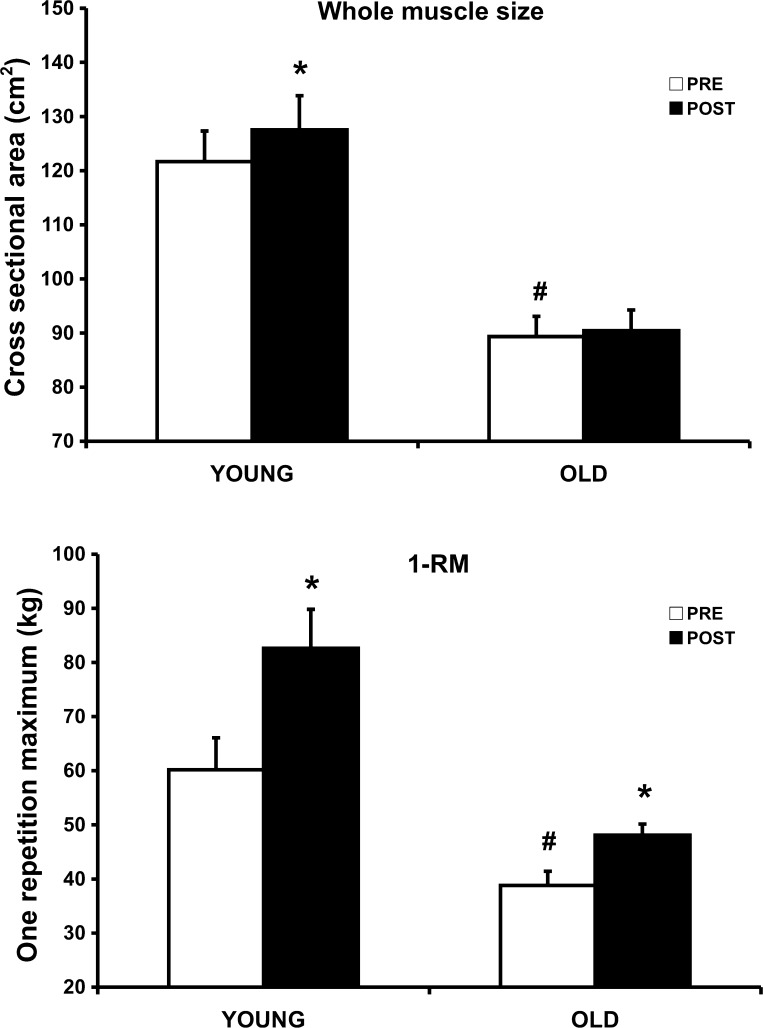
At the beginning of the study, thigh muscle CSA was 23% smaller (P < 0.05) in OW than YW, and OW were weaker (36%) in 1-RM strength (36%) than YW (Fig. 1). With PRT, 1-RM strength increased (P < 0.05) in YW (22 kg, 36%) and OW (10 kg, 26%). In YW, thigh muscle CSA also increased (P < 0.05) by 6 cm2 (5%) after PRT. There was no change in thigh muscle CSA after PRT in OW.
Fig. 1.
Whole thigh muscle adaptations to 12 wk of high-intensity progressive resistance training in young (21-yr-old) and older (85-yr-old) women. Top: thigh cross-sectional area (CSA) before (Pre) and after (Post) 12 wk of training. Bottom: 1-repetition maximum (1-RM) strength of knee extensors before and after 12 wk of training. *P < 0.05 vs. Pre. #P < 0.05 vs. young at Pre.
Single muscle fiber size and Po.
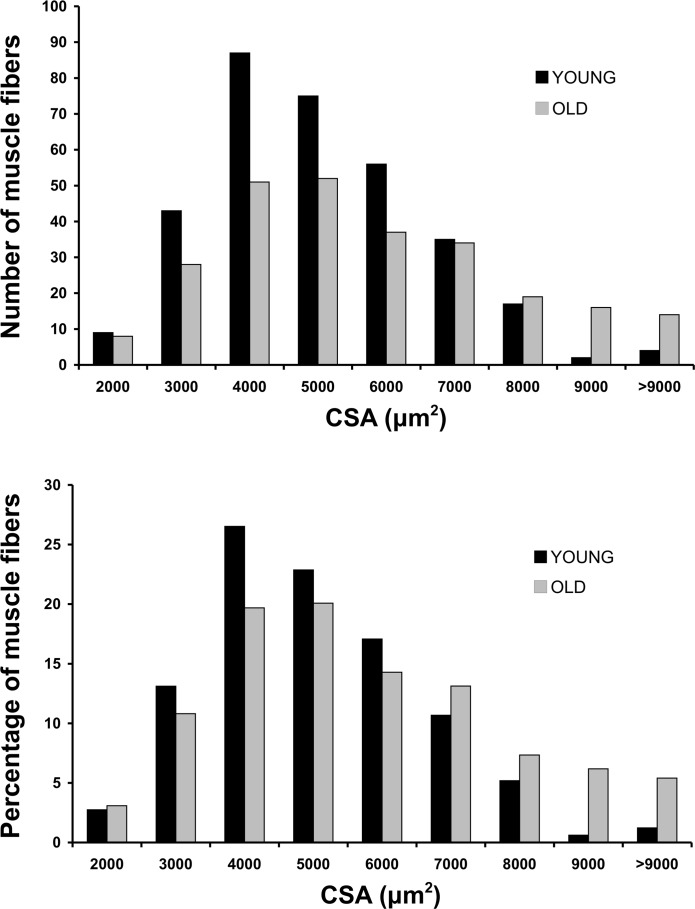
A total of 372 MHC I and 213 MHC IIa single-fiber experiments were completed as part of this investigation. At baseline, there were no differences between YW and OW in MHC I or IIa muscle fiber CSA (Table 1); in OW, however, MHC I muscle fibers were larger (P < 0.05) than MHC IIa muscle fibers. A CSA frequency histogram of the muscle fibers sampled among the YW and OW can be seen in Fig. 2. There were also no differences between YW and OW in MHC I and IIa peak force or peak force normalized to cell size at baseline. As a result of PRT, YW increased (P < 0.05) MHC IIa muscle fiber CSA (28%) and peak force (31%), whereas there was no change in these variables for MHC I muscle fibers. In OW, there were no changes in MHC I or IIa muscle fiber CSA or peak force after PRT.
Table 1.
CSA, Po, and Po/CSA of vastus lateralis MHC I and IIa single muscle fibers before and after high-intensity PRT in young and older women
| Fiber Type | Young Women |
Older Women | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| CSA μm2 | ||||
| MHC I | 4,459±382 | 4,235±308 | 5,383±654† | 5,223±538 |
| MHC IIa | 3,915±427 | 5,000±458* | 4,164±508 | 4,694±551 |
| Po, mN | ||||
| MHC I | 0.51±0.04 | 0.54±0.05 | 0.57±0.06 | 0.59±0.06 |
| MHC IIa | 0.61±0.05 | 0.80±0.07* | 0.69±0.07 | 0.75±0.09 |
| Po/CSA, kN/m2 | ||||
| MHC I | 115.7±7.7 | 126.9±6.2 | 108.3±3.9 | 110.8±2.9 |
| MHC IIa | 164.2±12.2 | 162.4±7.1 | 160.8±4.1 | 158.6±1.4 |
Values are means ± SE. Young women were 21 yr old; older women were 85 yr old. PRT, progressive resistance training; Po, peak isometric force; Po/CSA, peak isometric force per fiber cross-sectional area (CSA); MHC, myosin heavy chain.
P < 0.05 vs. Pre.
P < 0.05 vs. MHC IIa in older women.
Fig. 2.
Frequency histograms of muscle fiber CSA among sampled vastus lateralis muscle fibers in young and older women. Top: absolute number of fibers studied (328 and 257 for young and older, respectively) for each given muscle fiber CSA. Bottom: percentage of muscle fibers studied for each given muscle fiber CSA.
Single muscle fiber shortening velocity.
The contractile velocity of the muscle fibers was assessed using the slack test procedure (Vo) and the force-velocity procedure (Vmax). Both of these measurements provided a measure of shortening velocity, and data are shown in Table 2. At baseline, shortening velocity for MHC I or IIa muscle fibers did not differ between YW and OW. PRT had no impact on MHC I or IIa muscle fiber shortening velocity in either age group.
Table 2.
Vo and Vmax of vastus lateralis MHC I and IIa single muscle fibers before and after high-intensity PRT in young and older women
| Fiber Type | Young Women |
Older Women | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Vo, FL/s | ||||
| MHC I | 1.22±0.06 | 1.23±0.06 | 1.35±0.09 | 1.38±0.08 |
| MHC IIa | 3.46±0.15 | 3.64±0.14 | 3.35±0.12 | 3.35±0.21 |
| Vmax, FL/s | ||||
| MHC I | 0.90±0.04 | 0.94±0.06 | 0.92±0.05 | 0.86±0.04 |
| MHC IIa | 3.13±0.12 | 3.19±0.14 | 3.00±0.10 | 3.18±0.21 |
Values are means ± SE. Vo, maximal unloaded shortening velocity; FL, fiber length.
Single muscle fiber power.
Absolute peak power and peak power normalized for muscle cell size of the MHC I and IIa muscle fibers are shown in Table 3. At baseline, MHC I or IIa muscle fiber peak power or normalized power did not differ between YW and OW. With PRT, peak power increased (28%) in MHC IIa muscle fibers (P < 0.05), whereas MHC I peak power did not change, in YW. There was no change in peak or normalized power for either fiber type in OW after PRT.
Table 3.
Peak power and peak power normalized to cell size of vastus lateralis MHC I and IIa single muscle fibers before and after high-intensity PRT in young and older women
| Fiber Type | Young Women |
Older Women | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Peak power, μN·Fl/s | ||||
| MHC I | 8.8±0.5 | 10.0±0.8 | 11.2±1.5 | 12.4±1.6 |
| MHC IIa | 45.5±4.3 | 58.3±5.1* | 56.2±6.5 | 63.9±10.3 |
| Normalized power, W/l | ||||
| MHC I | 2.0±0.2 | 2.2±0.2 | 2.1±0.1 | 2.4±0.2 |
| MHC IIa | 12.2±0.9 | 12.0±0.8 | 13.1±0.3 | 13.3±0.5 |
Values are means ± SE.
P < 0.05 vs. Pre.
DISCUSSION
The novel aspect of this investigation was the examination of whole muscle and single muscle fiber characteristics and plasticity of independent-living octogenarian women. Young women were included as a comparison but also provide the first data set in the literature on myocellular adaptations to high-intensity resistance training in this population. The main findings from this investigation are as follows: 1) among the large pool of single muscle fibers sampled, MHC I and IIa muscle fiber size and quality were similar in young and 85-yr-old women, and 2) skeletal muscle plasticity was limited, at the whole muscle and myocellular level, in response to a high-intensity resistance training program in 85-yr-old women.
Baseline comparisons.
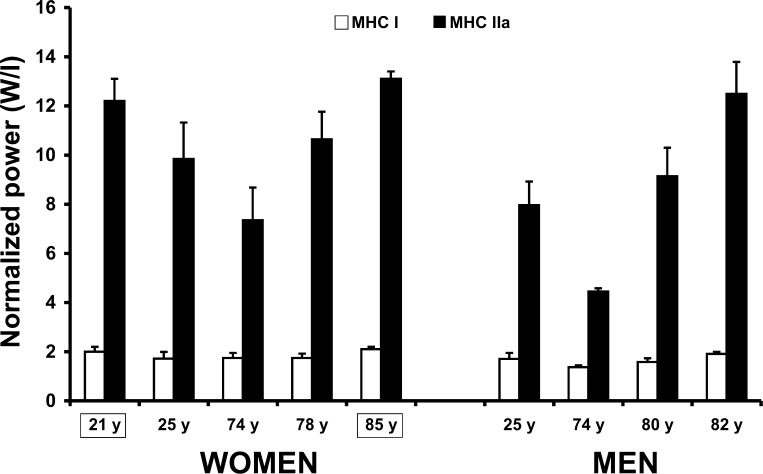
The 85-yr-old women entered the study with sarcopenia, as evidenced by 23% smaller thigh muscle size and 36% less knee extensor strength than the young women. However, on a myocellular level, there were no differences between the young women and the 85-yr-old women in any of the single muscle fiber parameters (CSA, Po, Po/CSA, Vo, peak power, and normalized power), suggesting that myocellular size and quality were preserved in the 85-yr-old women. A decrease in myocellular quality with age was initially reported in the literature (7, 16, 30, 32, 38). However, recent investigations with more extensive single muscle fiber yields and stricter controls on physical activity levels suggest that single muscle fiber quality is preserved with age (13, 15, 28, 48, 50).
Several reports in the scientific literature support the idea that myocellular quality is preserved with age. 1) A review of the human single-fiber literature (see Table 5 in Ref. 48) cites 14 studies (representing 20 different groups of subjects) of normalized muscle fiber power among young and older adults, athletes, and spinal cord-injured (SCI) patients. From these studies, an interesting pattern, displaying some of the highest normalized power values among slow- and fast-twitch muscle fibers, emerged among 82-yr-old men and SCI patients. 2) The MHC I and IIa normalized power observed in the 85-yr-old women from the present study concurs with our recent data in 82-yr-old men (48) and is on the upper end of the spectrum of our laboratory's observations of the vastus lateralis muscle of inactive individuals (Fig. 3). 3) The 85-yr-old women entered the present study with 6% smaller thigh muscle size than our previously studied 74-yr-old women (51) but with identical 1-RM strength, which would suggest a compensation by the intrinsic properties of the muscle fibers, nervous system, or both. Our data support compensation by the intrinsic properties of the muscle and, when combined with the extensive human literature in this area, strongly support the idea that advanced skeletal muscle atrophy, as experienced by octogenarian individuals and SCI patients, appears to be characterized by surviving muscle fibers that are relatively large and of good quality (15, 48) to compensate for the large loss of whole muscle mass and function. The relatively large muscle fibers remaining are consistent with the idea of compensatory muscle fiber hypertrophy, which has been reported in humans (1, 15) and animals (23). The apparent disconnect between skeletal muscle atrophy and average- to large-sized muscle fibers also points to a loss of motor units. The age-related loss of motor units and, thus, muscle fibers is well supported by previous research among elderly individuals (10, 35, 49).
Fig. 3.
Normalized power comparison of vastus lateralis myosin heavy chain (MHC) I and IIa muscle fibers among inactive young (50) and older (48, 50, 51, 53) individuals. Data from 21- and 85-yr-old women are from the present study.
It is noteworthy that none of the 85-yr-old women's MHC IIa muscle fibers failed during the physiological experiments in the present investigation. We previously reported that in vitro study of MHC IIa muscle fibers of women in their 70s has been difficult, because the fibers have a stringy and atrophic appearance, and muscle fiber failure frequently occurs (∼50%) on exposure to a series of maximal contractions (50, 51). The appearance of the skeletal muscle fibers under the microscope in the present study was markedly different between the young and 85-yr-old women (see supplemental Fig. 4 in the online version of this article), but the appearance did not negatively affect the integrity of the muscle fibers during our isolated contraction experiments. Collectively, these findings suggest that women in their 70s may be undergoing an accelerated atrophy process (i.e., loss of muscle fibers), where many muscle fibers are in a transition phase of elimination and have a high failure rate (50, 51). Conversely, the surviving muscle fibers of the 85-yr-old women most likely represent a more robust pool of fibers that have survived exogenous and endogenous stressors that may cause necrosis or apoptosis of weaker muscle cells and/or loss of entire motor units. Although the CSA frequency histograms (Fig. 2) suggest that we studied muscle fibers with a wide range in size (from 2,000 to >9,000 μm2), it is possible that some very small and fragile muscle fibers escaped the isolation procedure. The heterogeneity of fiber size in the present study is consistent with previous reports on aging human (33) and rodent (23) skeletal muscle. Further study is needed to support the idea that only the most robust fibers remain in very old healthy independent-living individuals.
Whole muscle adaptations to high-intensity PRT.
As expected, in the young women, thigh muscle size and strength increased with 12 wk of high-intensity PRT, confirming the efficacy of our PRT protocol. In the 85-yr-old women, 1-RM strength increased by ∼10 kg (26%), but the thigh muscle did not hypertrophy. These findings are considerably different from findings in the young women in the present study and the 74-yr-old women in our previous study (51). Interestingly, thigh muscle of young and 74-yr-old women hypertrophied to a similar degree and 1-RM strength increased by ∼22 kg. The lack of thigh muscle hypertrophy in the 85-yr-old women is noteworthy and supports the theory that the ability to hypertrophy with a training program diminishes with advancing age (>80 yr) (45, 48).
The aging literature shows a wide range in strength increases with resistance training (from 7% to 174%), but the range can likely be explained by different training protocols and subject profiles. Resistance training studies in subjects exclusively over 80 yr of age are limited but show an average ∼23-kg increase of 1-RM bilateral strength, which corresponded to 41% (48), 134% (31), and 174% (12) strength increase. Our recent study in 82-yr-old men (48) is the most pertinent comparison for >80 yr of age: their strength increased by 41%, and hypertrophy was minimal. The strength gains in the older men (41%) and women (26%) were most likely of neural origin, given the blunted whole muscle growth and single muscle fiber findings. Furthermore, the apparent differences in strength gains may point to a potential difference in neural plasticity between very old men and women.
Myocellular adaptations to high-intensity PRT.
The physiology data collected on single muscle fibers from the 85-yr-old women concur with the modest whole muscle adaptations observed in these older women: no myocellular hypertrophy and no functional improvements in MHC I or IIa muscle fibers after high-intensity PRT. These findings are in agreement with our most recent findings in age-matched older men (48). It is noteworthy that there were modest, but nonsignificant, improvements in MHC IIa size and function in the 85-yr-old women. A heterogeneous response (3 of 6 women improved size and function of MHC IIa muscle fibers) and a relatively small sample size among the older women may have contributed to the lack of significance at the cell level. Other than the fact that the women we studied were very old, the heterogeneous response may also be related to genetic, dietary, or other environmental factors that are outside the scope of the present investigation. The lack of a robust change in myocellular function is unique, and as Slivka et al. (48) reported, the literature supports the theory that most (22 of 25 studies) interventions (i.e., sports training, unloading paradigms, and resistance training) result in functional alterations at the myocellular level in humans.
In contrast, the myocellular adaptations were more homogeneous among the young women (8 of 9 women adapted similarly) than the older women. The single muscle fiber improvements were targeted to the MHC IIa muscle fibers, inasmuch as these fibers hypertrophied and peak force and peak power increased as a result of the high-intensity PRT program. Only three other studies on single muscle fiber functional adaptations with short-term (54) or long-term (39, 43) resistance training in young adults have been published. Two of these studies reported adaptations (increased size, peak force, and peak power) in MHC I and IIa fibers, which is in slight contrast to our findings. The differences in results may, in part, be explained by the differences in training volume and/or sex. The present study employed 90 contractions per week, which is considerably less than the multiple leg exercises (>250 contractions per week) in the short-term study (54) and years (>7 yr) of training in the long-term study (43).
Our laboratory has myocellular data from three female age groups performing an identical 12 wk high-intensity PRT program. The myocellular adaptations differ across the age span: young women display a homogeneous adaptation in the fast MHC IIa fibers, 74-yr-old women adapt homogeneously in the slow MHC I fibers, and the 85-yr-old women do not adapt robustly in either fiber type (Table 4). The present data provide additional insight into skeletal muscle plasticity with age and suggest that 85-yr-old women have limited myocellular plasticity with 12 wk of PRT, which likely contributes to their modest whole muscle adaptations with PRT.
Table 4.
Comparison of vastus lateralis MHC I and IIa single muscle fiber size and contractile function changes with 12 wk of high-intensity PRT in 21-, 74-, and 85-yr-old women
| 21-Year-Old Women |
74-Year-Old Women | 85-Year-Old Women | ||||
|---|---|---|---|---|---|---|
| MHC I | MHC IIa | MHC I | MHC IIa | MHC I | MHC IIa | |
| CSA, μm2 | ⇆ | ↑28% | ↑55% | ⇆ | ⇆ | ⇆ |
| Po, mN | ⇆ | ↑31% | ↑32% | ↑14% | ⇆ | ⇆ |
| Vo, FL/s | ⇆ | ⇆ | ⇆ | ⇆ | ⇆ | ⇆ |
| Power, μN·FL−1·s−1 | ⇆ | ↑28% | ↑51% | ↑25% | ⇆ | ⇆ |
Values are percentages. Data for 74-yr-old women are from Ref. 51. ↑, P < 0.05, pre- to posttraining. ⇆, No change pre- to posttraining.
Collectively, the literature supports the idea that the positive adaptations (increase in force and power) that occur on a myocellular level with resistance training in young (43, 54) and older (51, 53) individuals is primarily a result of increased muscle fiber size (i.e., quantity). In the absence of muscle fiber hypertrophy, the functional properties (i.e., quality) of the muscle fibers do not improve with PRT, as was the case in the 85-yr-old women. The lack of muscle fiber hypertrophy with PRT in these 85-yr-old women is a novel finding, and, at this point, we can only speculate as to what molecular events may contribute to this outcome. The biology of skeletal muscle hypertrophy is complex, and aged skeletal muscle has been reported to show alterations in a number of cellular events related to growth, such as cell signaling (17, 18, 55), gene expression (20, 27, 41, 42), protein expression (2), and satellite cell count (25). Pertinent to the present data set is our recent report of a similar myogenic gene expression induction (muscle homogenate) in response to the first training bout in the PRT program in the young and 85-yr-old women in the present study (41). Concomitantly, the 85-yr-old women showed evidence of an exaggerated proteolytic gene expression induction after the first training bout (42). It is possible that increased levels of protein degradation after each training session, among other cellular events, influenced the blunted hypertrophy process of the 85-yr-old women.
In summary, these are the first physiological data from single muscle fibers of octogenarian women, and this is the first study to investigate the effects of high-intensity PRT on myocellular contractile properties in young and octogenarian women. The results from this investigation emphasize that myocellular size and quality were not different between young and 85-yr-old women, but skeletal muscle plasticity was limited in response to high-intensity PRT in 85-yr-old women. Although the results of this investigation are interesting and novel, more research is necessary in this unique age group to substantiate these initial findings. We suggest that our collective findings of limited skeletal muscle plasticity among octogenarian women and men (48) are likely related to age, since we previously resistance trained 74-yr-old men (53) and women (51) (identical protocol) with robust adaptations on a myocellular and whole muscle level. On the basis of our findings, we propose that older individuals should engage in some resistance training once they reach the age of 60 and take advantage of their ability to gain muscle mass during the 7th and 8th decades of life. Once independent-living individuals reach the age of 80 yr and beyond, it appears to be more difficult to produce hypertrophy with a high-intensity resistance training program. Future research should focus on the attenuation of whole skeletal muscle atrophy and achievement of hypertrophy of remaining muscle fibers in the rapidly expanding cohort of independent-living octogenarian individuals.
GRANTS
This investigation was supported by National Institute on Aging Grant AG-18409 (to S. Trappe).
Supplementary Material
REFERENCES
- 1.Aniansson A, Grimby G, Hedberg M. Compensatory muscle fiber hypertrophy in elderly men. J Appl Physiol 73: 812–816, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol 97: 1329–1337, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom J Muscle electrolytes in man. Scand J Clin Lab Invest 68: 1–110, 1962. [Google Scholar]
- 5.Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol 9: 87–95, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol 69: 1725–1733, 1990. [DOI] [PubMed] [Google Scholar]
- 7.D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danneskiold-Samsoe B, Kofod V, Munter J, Grimby G, Schnohr P, Jensen G. Muscle strength and functional capacity in 78- to 81-year-old men and women. Eur J Appl Physiol Occup Physiol 52: 310–314, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Doherty TJ Aging and sarcopenia. J Appl Physiol 95: 1717–1727, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit. Can J Appl Physiol 18: 331–358, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Edman KA The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990. [PubMed] [Google Scholar]
- 13.Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 28: 601–608, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64: 1038–1044, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol 105: 637–642, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTOR signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 290: R1080–R1086, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Haddad F, Adams GR. Aging sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100: 1188–203, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hagen JL, Krause DJ, Baker DJ, Fu MH, Tarnopolsky MA, Hepple RT. Skeletal muscle aging in F344BN F1-hybrid rats. I. Mitochondrial dysfunction contributes to the age-associated reduction in V̇o2 max. J Gerontol A Biol Sci Med Sci 59: 1099–1110, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547: 247–254, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Sengupta M, Velkoff VA, DeBarros KA. 65+ in the United States: 2005. Washington, DC: US Census Bureau, 2005.
- 22.Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflügers Arch 433: 238–244, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Hepple RT, Ross KD, Rempfer AB. Fiber atrophy and hypertrophy in skeletal muscles of late middle-aged Fischer 344 × Brown Norway F1-hybrid rats. J Gerontol A Biol Sci Med Sci 59: 108–117, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hill A The heat of shortening and the dynamic constants of muscle. Proc R Soc B 126: 136–195, 1938. [Google Scholar]
- 25.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29: 120–127, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kalapotharakos VI, Michalopoulou M, Godolias G, Tokmakidis SP, Malliou PV, Gourgoulis V. The effects of high- and moderate-resistance training on muscle function in the elderly. J Aging Phys Act 12: 131–143, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol 99: 2149–2158, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol 101: 906–917, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil 80: 447–455, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports 17: 422–430, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272: C638–C649, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat 174: 239–249, 1991. [PMC free article] [PubMed] [Google Scholar]
- 34.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 35.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Morse CI, Thom JM, Mian OS, Muirhead A, Birch KM, Narici MV. Muscle strength, volume and activation following 12-month resistance training in 70-year-old males. Eur J Appl Physiol 95: 197–204, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Moss RL Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol 292: 177–192, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62: 375–381, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Pansarasa O, Rinaldi C, Parente V, Miotti D, Capodaglio P, Bottinelli R. Resistance training of long duration modulates force and unloaded shortening velocity of single muscle fibres of young women. J Electromyogr Kinesiol. In press. [DOI] [PubMed]
- 40.Ramsbottom R, Ambler A, Potter J, Jordan B, Nevill A, Williams C. The effect of 6 months training on leg power, balance, and functional mobility of independently living adults over 70 years old. J Aging Phys Act 12: 497–510, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101: 53–59, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407–1412, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Shoepe TC, Stelzer JE, Garner DP, Widrick JJ. Functional adaptability of muscle fibers to long-term resistance exercise. Med Sci Sports Exerc 35: 944–951, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill, 1988, p. 87–137.
- 45.Singh MA, Ding W, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab 277: E135–E143, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Sipila S, Elorinne M, Alen M, Suominen H, Kovanen V. Effects of strength and endurance training on muscle fibre characteristics in elderly women. Clin Physiol 17: 459–474, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Skelton DA, Young A, Greig CA, Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc 43: 1081–1087, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219, 1977. [DOI] [PubMed] [Google Scholar]
- 50.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci 57: B138–B143, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol 89: 143–152, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Widrick JJ, Stelzer JE, Shoepe TC, Garner DP. Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283: R408–R416, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547: 977–987, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol 91: 1955–1961, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol 88: 627–633, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.