Abstract
We used extracorporeal perfusion of the reversibly isolated carotid sinus region to determine the effects of specific carotid body (CB) chemoreceptor inhibition on eupneic ventilation (V̇i) in the resting, awake, intact dog. Four female spayed dogs were studied during wakefulness when CB was perfused with 1) normoxic, normocapnic blood; and 2) hyperoxic (>500 mmHg), hypocapnic (∼20 mmHg) blood to maximally inhibit the CB tonic activity. We found that CB perfusion per se (normoxic-normocapnic) had no effect on V̇i. CB inhibition caused marked reductions in V̇i (−60%, range 49–80%) and inspiratory flow rate (−58%, range 44–87%) 24–41 s following the onset of CB perfusion. Thereafter, a partial compensatory response was observed, and a steady state in V̇i was reached after 50–76 s following the onset of CB perfusion. This steady-state tidal volume-mediated hypoventilation (∼31%) coincided with a significant reduction in mean diaphragm electromyogram (−24%) and increase in mean arterial pressure (+12 mmHg), which persisted for 7–25 min until CB perfusion was stopped, despite a substantial increase in CO2 retention (+9 Torr, arterial Pco2) and systemic respiratory acidosis. We interpret these data to mean that CB chemoreceptors contribute more than one-half to the total eupneic drive to breathe in the normoxic, intact, awake animal. We speculate that this CB contribution consists of both the normal tonic sensory input from the CB chemoreceptors to medullary respiratory controllers, as well as a strong modulatory effect on central chemoreceptor responsiveness to CO2.
Keywords: control of breathing, eupnea, peripheral chemoreception
since the landmark findings of Corneille Heymans and colleagues in the 1930s (16), a number of experimental models have been used in an attempt to determine the role of carotid body (CB) chemoreflexes in the regulation of breathing. For example, hypoventilation, which averaged 20% below control, has been reported in several animal species following CB denervation (CBD), thereby demonstrating that CB chemoreceptor afferents provide a significant tonic excitatory input to the medullary respiratory neurons during eupnea (3, 13, 18, 26, 31). However, there are major limitations of the CBD model that severely limit the conclusions that can be drawn from these studies, because CBD has also been shown to result in several time-dependent compensatory and adaptive changes that fundamentally alter the respiratory control system. For example, CBD has been shown to cause the following changes: 1) upregulation of aortic chemoreceptors sensitivity (2, 3); 2) marked reductions in cytochrome oxidase activity in the medullary rhythm-generating neurons in the pre-Bötzinger complex, suggesting a reduced activity of the central respiratory control system after CBD (22) over and above that resulting from loss of carotid chemoreceptor afferent input. 3) A significant reduction of the ventilatory response to focal acidosis with 80% CO2 at multiple raphe sites was found after CBD in the goat (17). 4) Several studies (2, 20, 26) have shown that, after the initial rise in eupneic arterial Pco2 (PaCO2) following denervation, PaCO2 falls, and the gain of the ventilatory response to CO2 rises gradually over days to weeks.
To our knowledge, the role of CB chemoreceptors in eupneic breathing at rest in the intact, unanesthetized preparation has never been tested. Over the past decade, based on the pioneering work of Busch et al. (5) in the goat, we have developed and refined an isolated and perfused CB preparation in the unanesthetized, intact canine, which reversibly separates the circulation of the CB chemoreceptors from that of the systemic circulation (and, therefore, the cerebral circulation supplying the central chemoreceptors). This preparation allows precise and independent control of the chemical milieu of these regions in an intact physiological preparation. Using this technique, our group showed in intact, unanesthetized dogs, that inhibition of the CB chemoreceptors via perfusion of the reversibly isolated carotid sinus region with either hyperoxic or hypocapnic blood transiently reduced eupneic ventilation to an average of 29% below control (38). However, the true contribution of CB chemoreceptors to eupneic ventilation cannot be determined from this study, because the tonic input from these chemoreceptors may not have been maximally inhibited. Moreover, the ventilatory response beyond ∼2 min of inhibition was not studied, so any further compensation for changes in PaCO2 and H+ in the steady-state was not determined. To overcome these limitations, we combined marked hypocapnia and hyperoxia to maximally and physiologically inhibit the isolated CB chemoreceptors over longer durations. With this approach, combined with an analysis of the time course response, we have quantified the CB chemoreceptors' maximum contribution to the eupneic drive to breathe, as well as the compensatory capability of the central chemoreceptors to respond to systemic hypercapnia in the absence of tonic CB chemoreceptor input.
METHODS
Studies were performed during wakefulness on four unanesthetized, spayed female, mixed-breed dogs (20–25 kg). The dogs were trained to lie quietly in an air-conditioned (19–22°C), sound-attenuated chamber. Throughout all experiments, the dogs' behavior was monitored by an investigator seated within the chamber and also by closed-circuit television. The Animal Care and Use Committee of The University of Wisconsin-Madison approved the surgical and experimental protocols for this study.
Chronic Instrumentation
Our preparation required two surgical procedures performed under general anesthesia and with strict sterile surgical techniques and appropriate postoperative analgesics and antibiotics. In the first procedure, dogs were subjected to ovariectomy/hysterectomy, a chronic indwelling catheter was placed into the abdominal aorta via a branch of the femoral artery, and bipolar electromyogram recording electrodes were installed into the costal diaphragm. In the second procedure, the left CB was denervated, and the right carotid sinus was equipped with a vascular occluder and catheter to permit extracorporeal perfusion of the reversibly isolated carotid sinus CB. A chronic indwelling catheter was also placed in the abdominal vena cava via a branch of the femoral vein. Catheters were tunneled subcutaneously to the cephalad portion of the dog's back where they were exteriorized. Dogs recovered for at least 4 days before study. The instrumentation was protected with a heavy nylon jacket and a padded “Elizabethan” collar modified to allow normal eating and drinking.
Carotid Sinus Perfusion
Dogs lay unrestrained on a bed within the sound-attenuated chamber. The extracorporeal circuit was primed with ∼700 ml of saline, 120 ml of allogenic blood, and 2,500 units of heparin and supplemented with 1,000 U/h. Pco2, Po2, and pH in the perfusion circuit were set by adjustment of the gas concentrations supplying the circuit and by addition of NaHCO3. The carotid sinus region was perfused at flow rates <100 ml/min, which raised the pressure in the sinus region by <10 mmHg above the systemic blood pressure. Before data acquisition, a 30-min period of normal perfusion of the carotid sinus region was used to ensure uniformity between systemic and extracorporeal circuit blood. Intravenous boluses of NaCN (∼20 mg/kg) were used to confirm that 1) the nondenervated CB remained completely functional before CB perfusion; and 2) there was complete isolation of the carotid sinus during perfusion and also complete denervation of the contralateral CB. These techniques have been described in detail in previous publications (8, 37, 38).
Experimental Setup and Measurements
Ventilation was measured using a tight-fitting muzzle mask connected to a heated pneumotachograph (model 3700, Hans Rudolph, Kansas City, MO) that was calibrated before each study with four known flows. Costal diaphragm electromyogram (EMGdi) signals were amplified, band-pass filtered, rectified, and moving-time averaged (BMA-931; MA-821RSP, CWE). End-tidal Po2 (PetO2) and Pco2 (PetCO2) were measured using a mass spectrometer (MGA-1100, Perkin-Elmer, Waltham, MA).
Blood pressure was recorded continuously from the femoral artery.
One-milliliter arterial and perfusion circuit blood samples were analyzed for pH, Po2, and Pco2 on a blood-gas analyzer (model ABL-505, Radiometer, Copenhagen, Denmark). The blood-gas analyzer was validated daily with dog blood tonometered with three different combinations of Po2 and Pco2, covering the range encountered in the experiments. Samples were corrected for both body temperature and systematic errors revealed by tonometry.
Ventilation and blood pressure signals were digitized (128-Hz sampling frequency) and stored on the hard disk of a PC for subsequent analysis. Key signals were also recorded continuously on a polygraph (AstroMed K2G, West Warwick, RI). All ventilatory data were analyzed on a breath-by-breath basis by means of custom analysis software developed in our laboratory.
Experimental Protocol
Each test protocol consisted of a 5-min control period (eupnea), during which perfusion of the CB was endogenous, i.e., systemic arterial blood. Two 1-ml blood samples were collected at ∼3 min for determination of blood gases and pH control values. Then, CB perfusion was abruptly switched to the extracorporeal circuit (<2 s), and the ventilatory and EMGdi responses were recorded for at least 7 min before the carotid sinus was abruptly returned to endogenous perfusion.
The dogs were perfused in random order from the extracorporeal circuit with blood gases and pH concentrations matching a given dog's eupneic values (CB normal), or with hyperoxic (>500 mmHg) and hypocapnic (∼20 mmHg) blood gases (CB inhibited). At least two 1-ml blood samples were collected at ∼3 min and ∼7 min of perfusion for determination of blood gases and pH. During inhibition of CB chemoreceptors, inspired O2 fraction was increased to maintain arterial Po2 at control values.
Speed of Response
We determined the time to the first significant ventilatory response [breath-by-breath minute ventilation (V̇i)]. A V̇i response was considered significant if it was 3 SDs lower than the mean of the preceding normocapnic V̇i. We also quantified 1) the time to V̇i nadir, defined as the mean of the lowest V̇i and its two surrounding breaths; 2) the time required for V̇i to reach a steady state, defined as the first three consecutive breaths within 1 SD of the mean V̇i from the last 30 s of CB inhibition; and 3) the mean ventilatory values at 5 min following the beginning of CB inhibition (averaged over a 30-s period) and from the last 30 s of CB inhibition.
We also determined the time to the first significant response in mean arterial pressure (MAP). A response in MAP was considered significant, if it was 3 SDs higher than the mean of the preceding normocapnic MAP. We also quantified the time required for MAP to reach a steady state, defined as the first three consecutive beat values within 1 SD of the mean MAP from the last 30 s of CB inhibition.
Ventilatory and Arterial Blood Variables
Ventilatory data were averaged for the last 2 min of control and CB perfusion periods to determine the ventilatory response to CB inhibition. Duplicate arterial blood samples were also obtained during this 2-min period.
Statistics
Normality of the data was confirmed using the Shapiro-Wilk test. The time-dependent effect of CB inhibition on ventilation (see Fig. 3) was tested using a one-way repeated-measure ANOVA with Holm-Sidak post hoc test. Significance of the differences in mean steady-state data between control and the response to CB perfusion was determined by means of paired t-tests, with Bonferroni correction for multiple comparisons. Differences were considered significant if P < 0.05.
RESULTS
Effects of Perfusion Per Se
Consistent with our laboratory's previous studies (37, 38), CB perfusion with blood gases and pH matched to normal eupneic systemic arterial values did not significantly affect ventilation or cardiovascular parameters (Tables 1 and 2).
Table 1.
Mean steady-state values for blood gas, ventilatory, and EMGdi variables
| Dog No. | PaCO2, Torr | PaO2, Torr | pHa | [HCO3−], meq/l | V̇i, l/min | Vt, liter | f, breaths/min | Ti, s | Te, s | EMGdi, AU |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | ||||||||||
| 1 | 46.4±2.9 | 92.6±3.2 | 7.30±0.02 | 22.1±1.4 | 3.27±0.59 | 0.23±0.05 | 14.8±4.2 | 1.56±0.26 | 2.94±1.13 | 0.35±0.33 |
| 2 | 36.0±1.3 | 104.1±6.7 | 7.35±0.01 | 19.4±0.2 | 5.05±0.40 | 0.31±0.03 | 16.5±0.5 | 1.24±0.13 | 2.43±0.08 | 0.58±0.07 |
| 3 | 48.4±2.7 | 87.0±5.3 | 7.33±0.02 | 24.8±1.7 | 5.33±0.79 | 0.42±0.19 | 16.0±6.5 | 2.42±1.55 | 1.90±0.50 | |
| 4 | 41.1±1.0 | 99.6±9.8 | 7.34±0.02 | 21.4±0.6 | 3.85±0.46 | 0.26±0.02 | 14.2±1.9 | 1.55±0.09 | 2.79±0.49 | 0.46±0.28 |
| Mean±SD | 43.0±5.6 | 95.8±7.6 | 7.33±0.02 | 21.9±2.2 | 4.38±0.98 | 0.31±0.08 | 15.4±1.1 | 1.69±0.51 | 2.52±0.46 | 0.46±0.12 |
| CB perfused normoxic-normocapnic | ||||||||||
| 1 | 43.9±0.1 | 97.3±1.9 | 7.29±0.03 | 20.4±1.3 | 3.90±0.50 | 0.26±0.03 | 15.1±3.6 | 1.60±0.07 | 2.60±0.96 | 0.34±0.06 |
| 2 | 38.8±1.8 | 94.7±0.7 | 7.33±0.03 | 19.9±0.3 | 4.56±0.18 | 0.28±0.02 | 16.5±1.9 | 1.23±0.18 | 2.46±0.24 | 0.54±0.01 |
| 3 | 48.5±1.9 | 91.4±2.7 | 7.31±0.01 | 23.6±1.5 | 5.13±1.34 | 0.53±0.16 | 9.7±0.3 | 3.70±1.60 | 2.55±1.38 | |
| 4 | 40.7±0.8 | 101.0±4.8 | 7.33±0.02 | 20.6±0.4 | 3.78±0.76 | 0.27±0.02 | 13.3±1.8 | 1.69±0.05 | 3.38±0.81 | 0.47±0.25 |
| Mean±SD | 43.0±4.2 | 96.1±4.1 | 7.32±0.02 | 21.1±1.7 | 4.34±0.63 | 0.34±0.13 | 13.7±2.9 | 2.06±1.11 | 2.75±0.43 | 0.45±0.10 |
| CB perfused hyperoxic-hypocapnic | ||||||||||
| 1 | 60.4±3.2 | 91.2±4.7 | 7.24±0.02 | 24.4±0.41 | 1.70±0.56 | 0.18±0.03 | 10.1±3.9 | 1.64±0.27 | 5.36±2.58 | 0.23±0.03 |
| 2 | 45.5±3.2 | 100.4±5.0 | 7.28±0.02 | 20.5±24.4 | 3.77±1.70 | 0.27±0.03 | 14.1±3.9 | 1.38±0.27 | 2.90±2.58 | 0.47±0.07 |
| 3 | 57.1±2.2 | 89.3±5.1 | 7.29±0.02 | 26.1±1.83 | 4.46±0.49 | 0.22±0.08 | 18.4±7.5 | 2.46±2.06 | 1.60±0.39 | |
| 4 | 47.7±1.2 | 97.5±3.4 | 7.27±0.02 | 20.4±2.10 | 2.52±0.73 | 0.26±0.02 | 11.6±3.5 | 2.00±0.67 | 3.47±0.96 | 0.37±0.17 |
| Mean±SD | 52.7±7.2*† | 94.6±5.2 | 7.27±0.02*† | 22.9±2.9 | 3.11±1.24*† | 0.26±0.04*† | 13.6±3.6 | 1.87±0.47 | 3.33±1.56 | 0.36±0.12*† |
Values are means ± SD. CB, carotid body; PaCO2, arterial Pco2; PaO2, arterial Po2; pHa, arterial pH; [HCO3−], HCO3− concentration; V̇i, ventilation; Vt, tidal volume; f, breathing frequency; Ti, inspiratory time; Te, expiratory time; EMGdi, moving-time-averaged electromyogram of the costal diaphragm; AU, arbitrary units. Blood gases during CB perfusion are from the last (steady-state) blood sample. Note that 1) control refers to the condition following the second surgery (denervation of one CB; see methods); 2) for CB, perfused hyperoxic-hypocapnic inspired O2 fraction was increased to maintain PaO2 at control values.
Significant difference from control values, P < 0.05.
Significant difference from CB normal values, P < 0.05.
Table 2.
Mean steady-state values for cardiovascular variables
| Dog No. | MAP, mmHg | HR, beats/min |
|---|---|---|
| Control | ||
| 1 | 101.6±6.8 | 78.7±113 |
| 2 | 92.7±3.6 | 77.0±11.6 |
| 3 | 105.9±8.3 | 67.3±3.4 |
| 4 | 98.4±5.7 | 58.3±4.8 |
| Mean±SD | 99.7±5.6 | 70.3±9.5 |
| CB perfused normoxic-normocapnic | ||
| 1 | 102.8±2.9 | 68.1±9.0 |
| 2 | 99.8±7.8 | 78.1±10.7 |
| 3 | 103.9±3.6 | 65.1±2.3 |
| 4 | 90.0±9.6 | 57.8±4.2 |
| Mean±SD | 99.1±6.3 | 67.3±8.4 |
| CB perfused hyperoxic-hypocapnic | ||
| 1 | 117.7±7.7 | 84.3±10.5 |
| 2 | 108.5±7.0 | 78.4±11.9 |
| 3 | 111.0±10.4 | 69.9±7.3 |
| 4 | 108.6±1.2 | 53.4±0.7 |
| Mean±SD | 111.5±9.2*† | 71.5±13.4 |
Values are means ± SD. MAP, mean arterial pressure; HR, heart rate.
Significant difference from control values, P < 0.05.
Significant difference from CB normal values, P < 0.05.
Response to CB Inhibition: Original Record
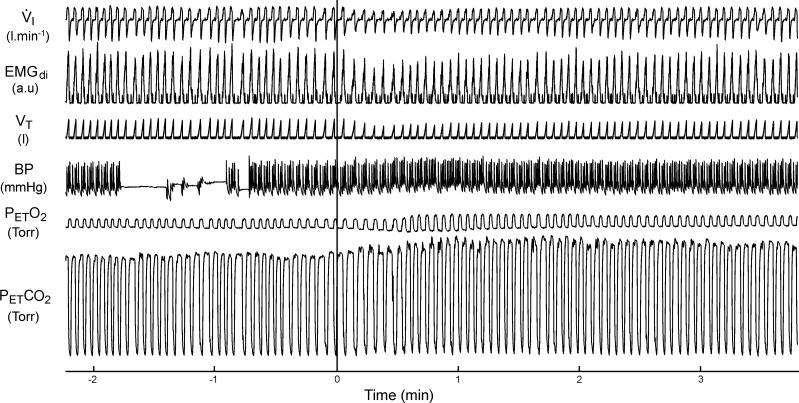
Figure 1 is a segment of an original polygraph record showing the ventilatory, EMGdi, MAP, PetO2, and PetCO2 responses over the first 4 min of perfusion of the isolated CB with hyperoxic and hypocapnic gases in a representative dog (dog 2). Note the rapid reduction in tidal volume (Vt) and the amplitude of EMGdi and the development of hypoventilation (i.e., elevated PetCO2) with the onset of CB perfusion. Also note that, as CB inhibition was maintained, the Vt and EMGdi amplitude tended to increase slowly over time; however, a substantial hypoventilation remained throughout the 4-min period of CB perfusion.
Fig. 1.
Polygraph record of a representative trial of carotid body (CB) inhibition (dog 2). Perfusion begins at time 0 (solid vertical line). V̇i, ventilation; EMGdi, moving-time-averaged electromyogram of the costal diaphragm; Vt, tidal volume; BP, blood pressure; PetCO2, end-tidal Pco2; PetO2, end-tidal Po2; au, arbitrary units. Interruption in the BP trace is due to blood sampling. Note that the immediate hypoventilation and PetCO2 increase with CB inhibition persists for the duration of the trial.
Steady-State Ventilatory Response to CB Inhibition
We performed a total of 27 CB inhibition experiments in four dogs. The mean values for blood gases and ventilatory variables obtained during control and steady-state periods of CB inhibition are summarized in Table 1.
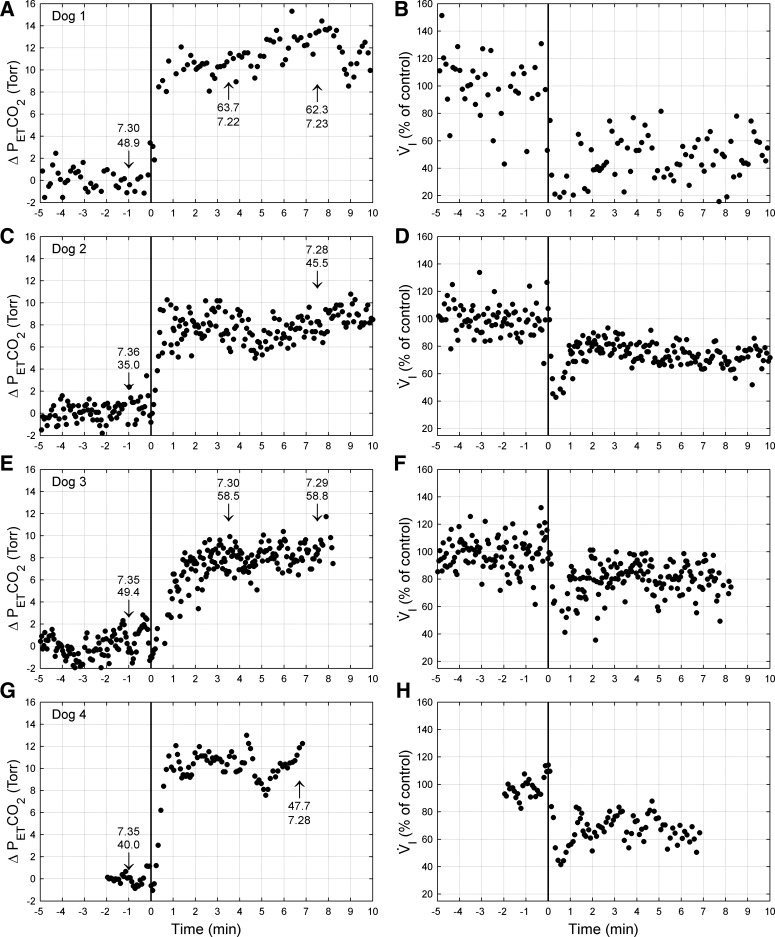
Representative breath-by-breath time course of response of V̇i and PetCO2 are shown in Fig. 2 for each animal. In all four animals, V̇i fell abruptly, and PetCO2 rose with the onset of CB inhibition; shortly thereafter, V̇i rose slightly and reached a steady-state plateau over the remaining several minutes of each trial. In the steady state, PaCO2 (superimposed on the figure) rose to 7–14 Torr greater than control and was accompanied by a significant respiratory acidosis. EMGdi fell 24% (range 19–34%), mean inspiratory flow rate (Vt/Ti, where Ti is inspiratory time) was reduced by 30% (range 22–49%), and V̇i decreased 31% (range 16–48%) below control. This reduction in steady-state V̇i was due almost entirely to a reduced Vt, with only one of the four animals showing a significant decrease in breathing frequency as well as in Vt (see Table 1).
Fig. 2.
PetCO2 (left) and V̇i (right) responses from one representative trial of CB inhibition from each dog: dog 1 (A and B), dog 2 (C and D), dog 3 (E and F), and dog 4 (G and H). Data are breath by breath and normalized to control (endogenous CB perfusion; i.e., CB not inhibited). CB inhibition commenced at time 0 (vertical line). Numbers at arrow indicate arterial Pco2 (Torr) and pH measured at that time. Note that the hypoventilation is maintained throughout CB inhibition, despite marked CO2 retention and arterial acidosis. Δ, Change.
Time Course and Magnitude of the Ventilatory Depression via CB Inhibition
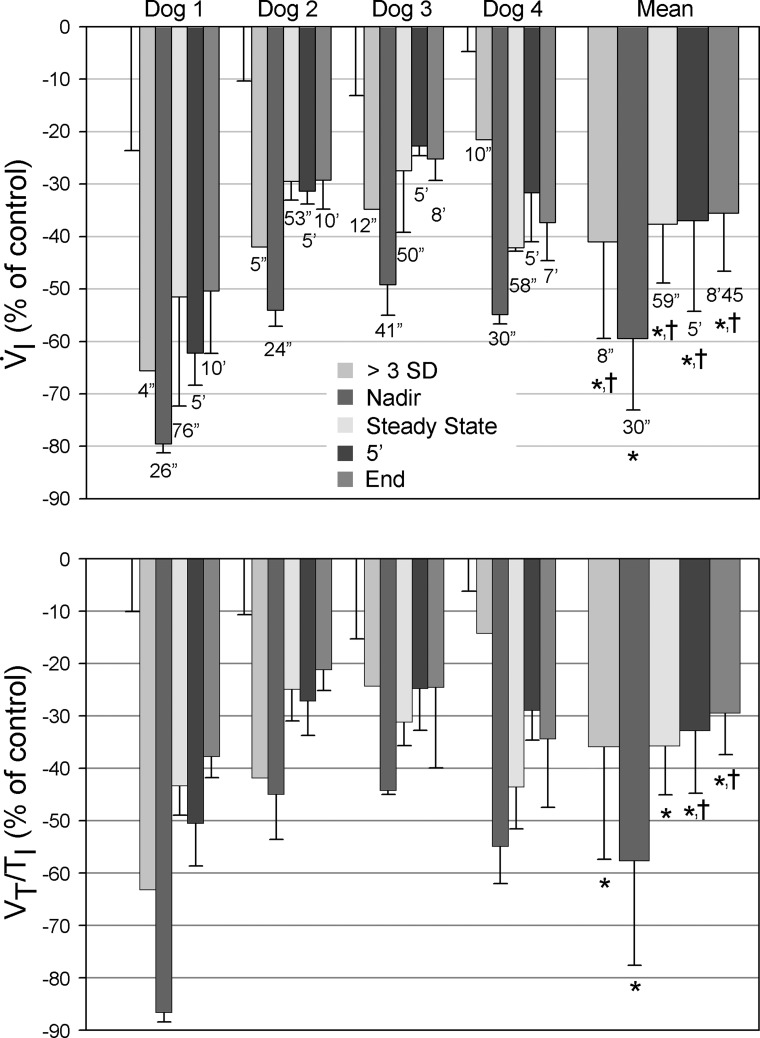
Figure 3 divides the time course of the ventilatory response with CB inhibition into five segments.
Fig. 3.
Bar graph of the time course of V̇i (top) and inspiratory flow rate [ratio of tidal volume to inspiratory time (Vt/Ti), bottom] during CB inhibition. Data are normalized to control (%control, where control is normal, endogenous CB perfusion; i.e., CB not inhibited) and presented as means ± SD. Control is arbitrarily set to zero to more clearly indicate the direction of change. For each dog, the first error bar (without associated bar) indicates the SD of the control values. >3 SD, the first V̇i response > 3 SDs below the control mean; Nadir, the mean of the lowest V̇i and its two surrounding breaths; Steady State, the mean values of the first 3 consecutive breaths within 1 SD of the mean V̇i from the last 30 s of the experiment (>7 min of perfusion); 5′, the mean ventilatory values of the last 30 s of the 5th min of CB inhibition; End, the mean ventilatory values from the last 30 s of each experiment, regardless of duration. Note that the nadir ventilatory response to CB inhibition averaged 60% below control, and the maintained steady-state response was 38% below control. Significant difference from *control values and †nadir values, P < 0.05.
Time to first ventilatory depression.
The time to the first detectable breath with a reduction in V̇i and Vt/Ti to more than 3 SDs below the control mean averaged 8 s (range, 4–12 s) from the onset of CB hyperoxic hypocapnia.
Nadir of the ventilatory response.
Thirty seconds (range 24–41 s) following the onset of CB inhibition, V̇i and Vt/Ti were reduced to an average of 60% (range 49–80%) and 58% (range 44–87%) of control, respectively. These nadir values were averaged over three breaths (13–18 s), during which time the reduced V̇i and Vt/Ti remained unchanged. Thereafter, V̇i and Vt/Ti began to increase systematically, presumably representing a compensatory response to the initial hypoventilation and systemic CO2 retention.
Compensatory response to steady state.
From their nadir, both V̇i and Vt/Ti rose over the subsequent ∼30 s to reach a new steady state, with values that averaged 31% (range 16–48%) and 30% (range 22–49%) less than control, respectively (see Fig. 3 and Table 1).
Individual mean values for V̇i and Vt/Ti are shown after 5 min of continued CB inhibition in each animal, as well as for the average maximum duration of CB inhibition, which varied from 7 to 10 min across the four animals. The constancy of these values in all animals in all trials between 1 and 10 min of CB inhibition demonstrates that the compensatory increases of V̇i and Vt/Ti were essentially complete within 30 s following the nadir of hypoventilation achieved via CB inhibition.
Off-transient.
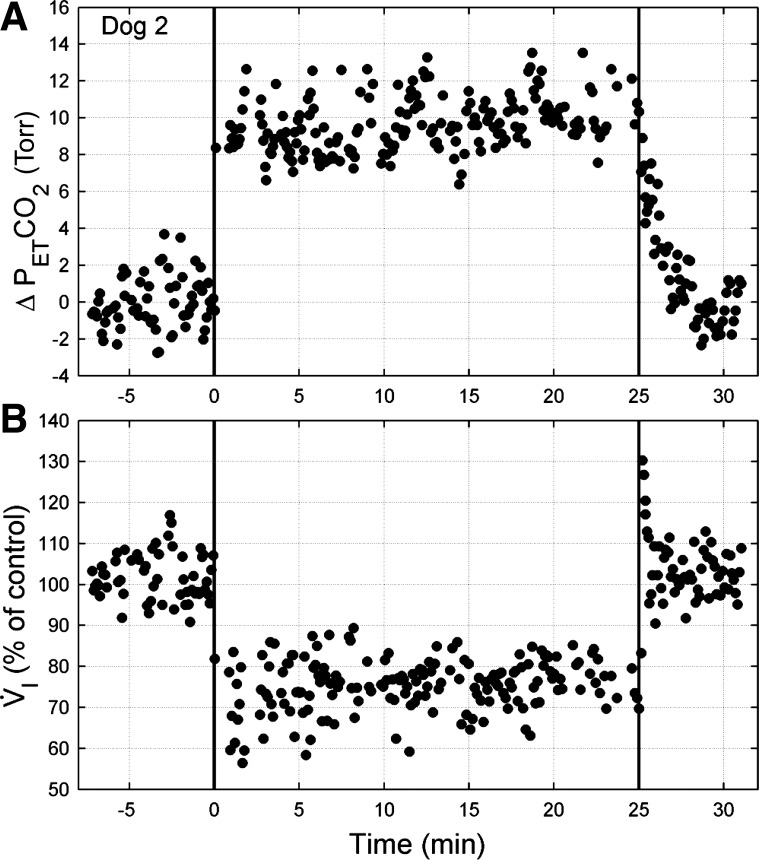
Due to normal behavioral patterns (stretching, licking, etc.), technically acceptable ventilatory and cardiovascular data following the abrupt cessation of CB inhibition were not available in all dogs. In two dogs (dogs 1 and 3) in which acceptable off-transient data were available, cessation of CB inhibition was accompanied by a brief V̇i overshoot, followed by a return to control values within ∼27 s (range 24–29 s; Fig. 4). Note, in this example, that the CB inhibition-induced hypoventilation was maintained throughout 25 min of CB inhibition.
Fig. 4.
Example of PetCO2 (A) and V̇i (B) responses to prolonged (25 min) CB inhibition in dog 2 (same dog shown in Fig. 1). Data are breath by breath and normalized to control (endogenous CB perfusion; i.e., CB not inhibited). Mean PetCO2 and V̇i during control were 43.1 Torr and 5.51 l/min, respectively. CB inhibition commenced at time 0 (first vertical line) and stopped after 25 min of CB inhibition (second vertical line). Note that the hypoventilation is maintained throughout CB inhibition, despite marked CO2 retention, and, after a brief overshoot, it returned to control values within 30 s upon a return to endogenous CB perfusion.
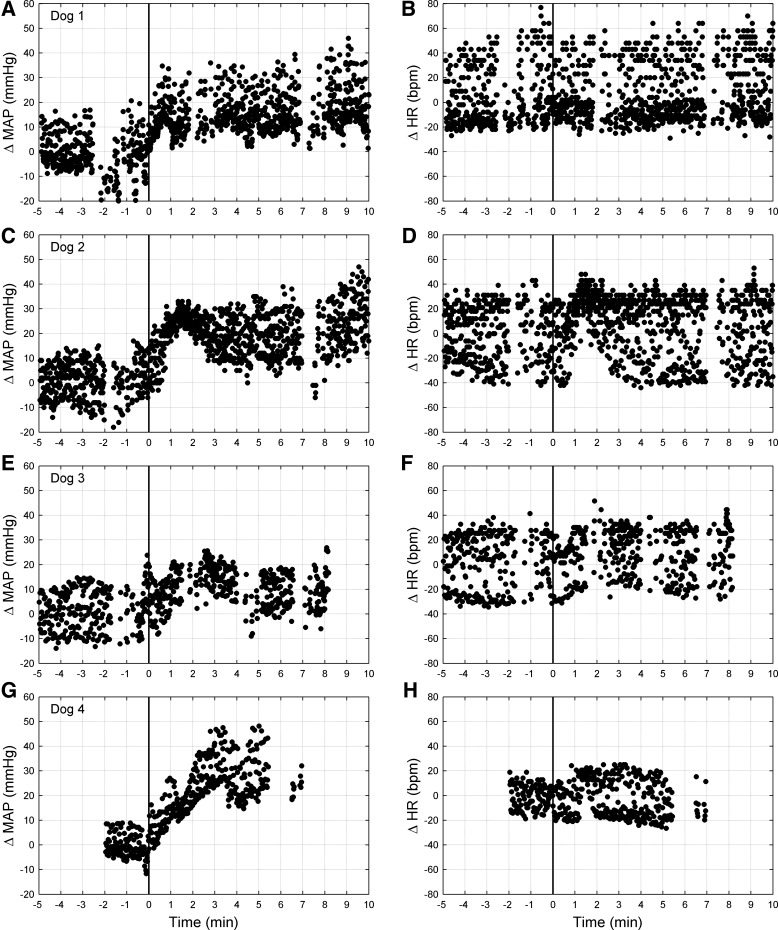
Cardiovascular Response to CB Inhibition
MAP and heart rate changes in response to CB inhibition are shown in Fig. 5 and Table 2. MAP began to rise significantly above 3 SD of control (13.7, range 9–18 mmHg) ∼32 s on average (range 22–45 s) after the onset of CB inhibition. A steady-state response, with MAP increased by 12 mmHg (range 5–17 mmHg) greater than control, was achieved at ∼52 s (range 32–92 s) following the onset of CB inhibition and remained elevated throughout the duration of CB inhibition. Heart rate did not change systematically from control over the time course of CB inhibition. In the two dogs (dogs 1 and 3) in which acceptable off-transient data were available, cessation of CB inhibition was accompanied by a return of MAP to control values within ∼30 s (range 26–34 s).
Fig. 5.
Mean arterial pressure (MAP; left) and heart rate (HR; right) responses from one representative trial of CB inhibition from each dog: dog 1 (A and B), dog 2 (C and D), dog 3 (E and F), and dog 4 (G and H). Data are beat by beat and normalized to control (endogenous CB perfusion; i.e., CB not inhibited). CB inhibition commenced at time 0 (solid line). Discontinuities in the data are due to blood sampling, which interrupted pressure measurement. Note the persistent increase in MAP during CB inhibition. bpm, Beats per minute.
DISCUSSION
Our study shows that physiological inhibition of the carotid chemoreceptor via hyperoxic-hypocapnic perfusion in intact, unanesthetized dogs significantly and markedly inhibits eupneic EMGdi, Vt, and V̇i. The maximal ventilatory depression that was observed consistently within 30 s of the onset of isolated CB inhibition suggests that more than one-half of the eupneic drive to breathe in the normoxic, intact animal is attributable to sensory tonic input from the carotid chemoreceptor. Longer periods of isolated CB inhibition were accompanied by marked hypoventilation and systemic respiratory acidosis for which there was significant but incomplete ventilatory compensation. This limited compensatory response was attributed to a secondary inhibitory effect on medullary chemoresponsiveness (to brain hypercapnia) caused by the absence of CB sensory input.
Significance/Limitations of the Unanesthetized, Intact, Carotid Sinus-Perfused Model
We believe that the present study, utilizing the intact, unanesthetized, carotid sinus-perfused dog model to isolate the CB chemoreceptors from the systemic circulation and central chemoreceptors is an important advance over the CBD model. Indeed, this model, with intact but reversibly isolated CB, preserves the tonic CB sensory input, thereby avoiding 1) any potential compensatory changes in the central respiratory controller or in central and/or non-CB peripheral chemoreceptor sensitivities resulting from CBD (2, 20, 22, 26); and 2) any changes in baseline ventilation or acid-base status, as is commonly observed following bilateral CBD (14, 31) (also see Introduction). Moreover, our study provides unique data in the unanesthetized dog, in which both chemoreflexes, pulmonary mechanoreflexes, and baroreflexes were not obtunded, as is known to occur with anesthesia (10, 15, 29). Finally, the isolated, blood-perfused CB preparation permits reversible physiological inhibition of the CB chemoreceptor, thereby allowing analysis of the time course of the cardiorespiratory response to sustained inhibition of carotid chemoreceptor sensory input in the absence, as well as in the presence, of secondary changes in systemic arterial blood gases (see below).
Our method of CB perfusion does require unilateral CBD on the nonperfused side. The potential effects on the central ventilatory control system following denervation are thus a concern. There is evidence using this preparation in both the goat (5) and the dog (8, 38) showing that unilateral CBD has no consistent effects on ventilation during control, air-breathing conditions, or on the ventilatory response to hypoxia or CO2. In contrast, Pan et al. (26) showed in the goat that unilateral CBD resulted in a transient (i.e., over 7 days) but significant hypoventilation during room-air breathing, yet the ventilatory response to intravenous injections of sodium cyanide was not affected. We also found high PaCO2 values (48.5 ± 1.9 Torr) in one dog (dog 3) during eupneic control in the present study, even though inhibition of the remaining CB in this dog elicited a further marked increase in PaCO2 (+8.7 Torr) in the steady state, which was similar to the increase observed in the remaining three animals (+14, +9.5, and +6.6 Torr), which showed no significant alteration in eupneic PaCO2 following unilateral CBD. Taken together, these findings suggest that animals with one intact CB have a ventilatory control system that responds similarly to that of the intact animal.
To produce retrograde flow through the carotid sinus region sufficient to isolate the CB from systemic blood, perfusion pressure was slightly higher (∼5–10 mmHg) than systemic arterial pressure. However, data from the present study using normoxic and normocapnic control perfusions, as well as a previous study using increases in blood pressure limited to the carotid sinus in the awake dog (33), showed that pressure increases of this magnitude had no significant ventilatory or cardiovascular effects. Consequently, we do not think that the imposed slight increase in carotid sinus pressure is a significant limitation to this study.
Significant sex differences in peripheral and central chemosensitivities could be a potential limitation to our use of spayed female dogs in the present study. However, our interpretation of the human literature is that the preponderance of evidence, when scaled correctly for body mass and/or vital capacity, supports a lack of sex difference in ventilatory responses to hypoxia or hypercapnia (19, 28, 41, 45). In studies that do find a difference, women tend to have a lower apneic threshold for CO2 (i.e., a wider CO2 reserve) (46) or an increased ventilatory response to hypoxia (1) relative to men, although one paper reports an increased peripheral response to CO2 in men vs. women based on single-breath CO2 tests (35). The limited animal literature (again, when scaled correctly) is generally in agreement with that of the human literature in that there are either no differences in the ventilatory responses to hypoxia and hypercapnia (34, 42) or slightly increased ventilatory responses to hypoxia in women (23, 42). It is important to recall that, in the present study, using spayed female dogs, ovarian hormone levels would be low and cycling absent, so our dogs would be most analogous to postmenopausal women. Even if modest non-hormone-mediated sex differences in peripheral and central chemosensitivities do exist, we think it unlikely that there would be qualitative effects on our results. Large differences would, of course, be a greater concern, but, to our knowledge, there is no evidence for such differences.
Carotid Chemoreceptor Contributions to Eupneic Drive to Breathe in Normoxia
Our estimates of the carotid chemoreceptor contribution to eupneic drive to breathe are at least about twofold greater than previously reported. Prior estimates of this contribution include the following: 1) the ∼10% reduction in Vt and V̇i achieved transiently in humans via a few breaths of a hyperoxic inspirate (7, 11, 12); 2) the ∼28% reduction in V̇i achieved transiently in unanesthetized dogs during 100% O2 breathing (6); and 3) the ∼20% reduction in V̇i several days after chemoreceptor denervation in dogs, goats, or ponies (3, 14, 26, 31). Previously, we used extracorporal perfusion of the isolated CB to inhibit the chemoreceptor with either hyperoxic or hypocapnic blood (38). With either of these perfusates, we observed a maximum ∼29% reduction in V̇i after ∼30 s of perfusion. In the present study, our time course analysis revealed again a maximum ventilatory depression ∼30 s following the onset of CB inhibition, but the magnitude was much greater, i.e., ∼60% (range 49–80%) below normocapnic control conditions. It is important to emphasize that this nadir of the ventilatory response did not occur for only a single breath, but was sustained over several breaths and was reproducible upon repeat trials of CB inhibition in the same animal. We propose that the greater ventilatory depression obtained in the present study is due to the combined effects of hyperoxia and hypocapnia, which likely provided a greater inhibition of carotid sinus nerve activity than hyperoxia or hypocapnia alone. Furthermore, in contrast to the reported response to CBD, the response to acute physiological inhibition of the intact CB in the present study was likely influenced little, if at all, by upregulation of other sources of peripheral chemoreception or compensatory alterations in the sensitivity of medullary respiratory control mechanisms (see also Significance/Limitations of the Unanesthetized, Intact, Carotid Sinus-Perfused Model above).
Accordingly, we propose that the present data support the idea that a contribution of the normoxic carotid chemoreceptor contributes more than one-half of the total drive to eupnea in normoxia. Given that the nadir of ventilation was not reached until after ∼30 s of CB inhibition, it is likely that at least some limited level of brain acidosis coincided with and, therefore, limited the reduction of respiratory motor output achievable via CB inhibition per se (also see discussion below, regarding compensation). So, if anything, we may have underestimated the contribution of the CB chemoreceptors.
Modulatory Effects of Carotid Chemoreceptor on the Response to Systemic Hypercapnia
After the initial 30 s of marked ventilatory inhibition in response to CB inhibition, EMGdi, Vt/Ti, and V̇i rose again and plateaued over the remaining several minutes of CB perfusion at an average of 24–31% below control values (see Figs. 2 and 3). The timing of this secondary rise in V̇i coincides with the ventilatory response time of ∼28–30 s for central chemoreceptors observed following a step increase in inspired CO2 fraction in the awake dog, whose intact CB was perfused with normoxic, normocapnic blood (37). The level of CO2 retention presently achieved in the steady state also approximates that obtained 1 day to many years in humans after CB resection in patients with CB tumor (9, 43) or chronic obstructive pulmonary disease (44), in the chronically CB denervated dog (31), pony (2), goat (26), rabbit (4), or rat (25).
It is somewhat surprising that these steady-state levels of systemic CO2 retention and brain acidosis do not yield greater compensatory ventilatory responses. Studies in intact animals using ventricular-cisternal perfusion of brain extracellular fluid (27, 36) or of inhaled CO2 with the isolated CB maintained normocapnic (37), claim that the great majority of the ventilatory response to systemic hypercapnia is attributable to the central chemoreceptors. One explanation for the limited responsiveness of the central chemoreceptors observed in the present study may be that central chemoreceptor responsiveness to brain CO2 is critically dependent on the level of afferent input reaching chemosensitive neurons, including that from carotid chemoreceptors, via pathways through the nucleus of the tractus solitarius. In support of this postulate, Takakura et al. (40) showed that CB stimulation via cyanide or hypoxia activated CO2-sensitive neurons in the retrotrapezoid nucleus in anesthetized rats, and that these responses were prevented via CBD. Furthermore, Hodges et al. (17) observed that CBD caused a significant depression of the ventilatory response in awake goats to focal acidosis in the raphe nucleus. A more direct test of this proposed dependence of central chemoreceptor responsiveness on CB chemoreceptor input might be accomplished in our intact, awake preparation by superimposing progressive, systemic hypercapnia on a normal vs. maximally inhibited CB.
CB Chemoreceptor Inhibition, Systemic Hypercapnia, and Blood Pressure
Numerous studies have reported that normoxic hypercapnia elicits an increase in MAP via sympathoexcitation in both healthy humans (30, 39) and intact dogs (21, 32), which is likely due to CO2 stimulating both the CB and central chemoreceptors. Our findings during inhibition of the tonic input from the CB suggest, however, that a signal of central origin is the main cause of this CO2-induced increase in MAP. This agrees with findings of Oikawa et al. (24), who demonstrated that, in conscious rats at rest, normoxic hypercapnia induced significant increases in MAP and renal sympathetic nerve activity, and that this response was not attenuated by CBD (+7 mmHg in both conditions).
Although the hypercapnia-induced sympathoexcitation should result in a positive cardiac chronotropic response, we observed no change in heart rate during CB inhibition. We postulate that a baroreflex-induced vagal stimulation, in response to MAP increase, counteracted the sympathoexcitation. This hypothesis agrees with previous findings in intact rats (24), which showed a vagally induced bradycardia with normoxic hypercapnia, whereas intravenous administration of atropine resulted in an increase in heart rate.
Conclusions
In conclusion, our findings in an intact and unanesthetized preparation showed that CB chemoreceptors provide a major excitatory input to medullary respiratory neurons, thereby contributing more than one-half of the drive to eupnea in normoxia. Furthermore, we speculate that the contribution of CB input to eupneic breathing is not limited to a direct modulation of the ventilatory controller. This tonic input may also exert a strong modulatory effect on central CO2/H+ chemoreceptors and, therefore, the ventilatory response to systemic hypercapnia/acidosis. There would be major implications for our understanding of the chemical control of breathing if future studies confirm this speculation. 1) Central and peripheral chemoreceptors do not act independently of one another. 2) It follows that interaction of central and peripheral chemosensory inputs to the ventilatory control system would not be simply additive but would likely be more complex than currently appreciated. 3) If, as suggested by Guyenet and colleagues (15a, 40), the chemosensitive neurons of the RTN also possess integrating properties for a variety of sensory inputs (e.g., pulmonary stretch receptors, baroreceptors, central locomotor areas), then there is potential for complex interactions between chemosensory and other nonchemosensory reflexes.
GRANTS
This material is based on work supported, in part, by grants to C. A. Smith and J. A. Dempsey from the National Heart, Lung, and Blood Institute of the National Institutes of Health. G. M. Blain was supported by a postdoctoral fellowship from the American Heart Association.
REFERENCES
- 1.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol 60: 1894–1899, 1986. [DOI] [PubMed] [Google Scholar]
- 2.Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol 49: 964–970, 1980. [DOI] [PubMed] [Google Scholar]
- 3.Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA, Rasmussen B. Hypoventilation in ponies after carotid body denervation. J Appl Physiol 40: 184–190, 1976. [DOI] [PubMed] [Google Scholar]
- 4.Bouverot P, Candas V, Libert JP. Role of the arterial chemoreceptors in ventilatory adaptation to hypoxia of awake dogs and rabbits. Respir Physiol 17: 209–219, 1973. [DOI] [PubMed] [Google Scholar]
- 5.Busch MA, Bisgard GE, Mesina JE, Forster HV. The effects of unilateral carotid body excision on ventilatory control in goats. Respir Physiol 54: 353–361, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Cao KY, Berthon-Jones M, Sullivan CE, Zwillich CW. Ventilatory response to oxygen after eucapnic hypoxia in conscious dogs. J Appl Physiol 74: 1916–1920, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Comroe JHJ, Schmidt CF. The part played by reflexes from the carotid body in the chemical regulation of respiration in the dog. Am J Physiol 121: 75–97, 1938. [Google Scholar]
- 8.Curran AK, Rodman JR, Eastwood PR, Henderson KS, Dempsey JA, Smith CA. Ventilatory responses to specific CNS hypoxia in sleeping dogs. J Appl Physiol 88: 1840–1852, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med 4: e239, 2007. [DOI] [PMC free article] [PubMed]
- 10.Dahan A, van den Elsen MJ, Berkenbosch A, DeGoede J, Olievier IC, Burm AG, van Kleef JW. Influence of a subanesthetic concentration of halothane on the ventilatory response to step changes into and out of sustained isocapnic hypoxia in healthy volunteers. Anesthesiology 81: 850–859, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Dejours P Control of respiration by arterial chemoreceptors. Ann NY Acad Sci 109: 682–695, 1963. [DOI] [PubMed] [Google Scholar]
- 12.Dripps R, Comroe JHJ. The effect of the inhalation of high and low oxygen concentration on respiration, pulse rate, ballistocardiogram and arterial oxygen satiration (oximeter) of normal individuals. J Physiol 149: 277–291, 1947. [DOI] [PubMed] [Google Scholar]
- 13.Forster HV, Bisgard GE, Klein JP. Effect of peripheral chemoreceptor denervation on acclimatization of goats during hypoxia. J Appl Physiol 50: 392–398, 1981. [DOI] [PubMed] [Google Scholar]
- 14.Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119: 199–208, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Fregosi RF, Dempsey JA. Anesthetic effects on [H+]a and muscle metabolites at rest and following exercise. Respir Physiol 65: 85–98, 1986. [DOI] [PubMed] [Google Scholar]
- 15a.Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heymans C, Bouckaert JJ. Sinus caroticus and respiratory reflexes. I. Cerebral blood flow and respiration. Adrenaline apnoea. J Physiol 69: 254–266, 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol 98: 1234–1242, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Honda Y, Myojo S, Hasegawa S, Hasegawa T, Severinghaus JW. Decreased exercise hyperpnea in patients with bilateral carotid chemoreceptor resection. J Appl Physiol 46: 908–912, 1979. [DOI] [PubMed] [Google Scholar]
- 19.Jordan AS, Catcheside PG, Orr RS, O'Donoghue FJ, Saunders NA, McEvoy RD. Ventilatory decline after hypoxia and hypercapnia is not different between healthy young men and women. J Appl Physiol 88: 3–9, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Klein JP, Forster HV, Bisgard GE, Kaminski RP, Pan LG, Hamilton LH. Ventilatory response to inspired CO2 in normal and carotid body-denervated ponies. J Appl Physiol 52: 1614–1622, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Kollai M, Koizumi K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. J Auton Nerv Syst 1: 33–52, 1979. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Kim J, Cinotte J, Homolka P, Wong-Riley MT. Carotid body denervation effect on cytochrome oxidase activity in pre-Botzinger complex of developing rats. J Appl Physiol 94: 1115–1121, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: gender differences. Respir Physiol 106: 21–34, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y. Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo- and baroreceptors. Auton Neurosci 117: 105–114, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Olson EB, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. J Appl Physiol 64: 666–671, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol 85: 1299–1306, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Pappenheimer JR, Fencl V, Heisey SR, Held D. Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol 208: 436–450, 1965. [DOI] [PubMed] [Google Scholar]
- 28.Patrick JM, Howard A. The influence of age, sex, body size and lung size on the control and pattern of breathing during CO2 inhalation in Caucasians. Respir Physiol 16: 337–350, 1972. [DOI] [PubMed] [Google Scholar]
- 29.Phillipson EA, Kozar LF, Murphy E. Respiratory load compensation in awake and sleeping dogs. J Appl Physiol 40: 895–902, 1976. [DOI] [PubMed] [Google Scholar]
- 30.Richardson DW, Wasserman AJ, Patterson JL Jr. General and regional circulatory responses to change in blood pH and carbon dioxide tension. J Clin Invest 40: 31–43, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol 91: 328–335, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Rose CE, Althaus JA, Kaiser DL, Miller ED, Carey RM. Acute hypoxemia and hypercapnia: increase in plasma catecholamines in conscious dogs. Am J Physiol Heart Circ Physiol 245: H924–H929, 1983. [DOI] [PubMed] [Google Scholar]
- 33.Saupe KW, Smith CA, Henderson KS, Dempsey JA. Respiratory and cardiovascular responses to increased and decreased carotid sinus pressure in sleeping dogs. J Appl Physiol 78: 1688–1698, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Schlenker EH, Goldman M. Aspartic acid administered neonatally affects ventilation of male and female rats differently. J Appl Physiol 61: 780–784, 1986. [DOI] [PubMed] [Google Scholar]
- 35.Sebert P, Barthelemy L, Mialon P. CO2 chemoreflex drive of ventilation in man: effects of hyperoxia and sex differences. Respiration 57: 264–267, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Smith CA, Jameson LC, Mitchell GS, Musch TI, Dempsey JA. Central-peripheral chemoreceptor interaction in awake cerebrospinal fluid-perfused goats. J Appl Physiol 56: 1541–1549, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol 100: 13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Smith CA, Saupe KW, Henderson KS, Dempsey JA. Ventilatory effects of specific carotid body hypocapnia in dogs during wakefulness and sleep. J Appl Physiol 79: 689–699, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67: 2101–2106, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarbichi AG, Rowley JA, Shkoukani MA, Mahadevan K, Badr MS. Lack of gender difference in ventilatory chemoresponsiveness and post-hypoxic ventilatory decline. Respir Physiol Neurobiol 137: 41–50, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Influences of gender and sex hormones on hypoxic ventilatory response in cats. J Appl Physiol 71: 1746–1751, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Timmers HJ, Karemaker JM, Wieling W, Marres HA, Folgering HT, Lenders JW. Baroreflex and chemoreflex function after bilateral carotid body tumor resection. J Hypertens 21: 591–599, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Whipp BJ, Ward SA. Physiologic changes following bilateral carotid-body resection in patients with chronic obstructive pulmonary disease. Chest 101: 656–661, 1992. [DOI] [PubMed] [Google Scholar]
- 45.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol 54: 874–879, 1983. [DOI] [PubMed] [Google Scholar]
- 46.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol 89: 192–199, 2000. [DOI] [PubMed] [Google Scholar]