Abstract
We have previously reported that in ovine fetal pulmonary venous smooth muscle cells (FPVSMC), decreased expression of cGMP-dependent protein kinase (PKG) by hypoxia could explain hypoxia-induced SMC phenotype modulation. In this study, we investigated the role of myocardin, a possible downstream effector of PKG, in SMC phenotype modulation induced by 1 and 24 h of hypoxia. Hypoxia for 1 h induced the phosphorylation of E-26-like protein 1 (Elk-1), indicating a quick activation of Elk-1 after hypoxia. Either hypoxia (1 h) or treatment with DT-3, a PKG inhibitor, increased associations of Elk-1 with myosin heavy chain (MHC) gene and serum response factor (SRF), which was paralleled by a decrease in association of myocardin with MHC gene and SRF. Exposure to hypoxia of FPVSMC for 24 h significantly decreased the promoter activity of multiple SMC marker genes, downregulated protein and mRNA expression of myocardin, and upregulated mRNA expression of Elk-1, but had no significant effects on the phosphorylation of Elk-1. Inhibition of myocardin by siRNA transfection downregulated the expression of SMC marker proteins, while overexpression of myocardin prevented the hypoxia-induced decrease in expression of SMC marker proteins. Inhibition of PKG by siRNA transfection downregulated the expression of myocardin, but upregulated that of Elk-1. Overexpression of PKG prevented hypoxia-induced effects on protein expression of myocardin and Elk-1. These data suggest that PKG induces displacement of myocardin from SRF and upregulates myocardin expression, thus activating the SMC genes transcription. The inhibitory effects of hypoxia on PKG may explain hypoxia-induced SMC phenotype modulation by decreasing the effects of PKG on myocardin.
Keywords: Elk-1
the mature fully differentiated vascular smooth muscle cell (SMC) expresses SMC contractile proteins necessary for the unique contractile properties of the SMC. SMC are remarkably plastic and can undergo reversible changes in their phenotype in response to a variety of stimuli (20). During this process, vascular SMC undergo a transition in phenotype from a contractile, differentiated to a proliferative, dedifferentiated state (6, 10), and this reversible transition is defined as phenotype modulation (20). SMC phenotype modulation contributes to the pathogenesis of numerous cardiovascular disorders, including pulmonary hypertension, persistent pulmonary hypertension of the newborn (PPHN), postangioplasty restenosis, and atherosclerosis. PPHN is characterized by elevated pulmonary vascular resistance and pulmonary arterial pressure with increased muscularization of small arteries and thickening or fibrosis of the intima (26). Chronic hypoxia is an important contributing factor to the development of pulmonary hypertension. Chronic hypoxia triggers pulmonary vascular remodeling, which is associated with a modulation of the SMC phenotype. Moreover, this SMC phenotype modulation is an early but key process in chronic hypoxia-induced pulmonary hypertension (42). However, little is known regarding the mechanisms that regulate SMC phenotype modulation in vivo or in vitro (27).
It is known that a variety of signals regulate SMC phenotype, but the transcriptional mechanisms that ultimately regulate SMC differentiation are still largely unknown. Expression of many SMC marker genes has been shown to be dependent on multiple CC(A/T)6GG (CArG) elements and their binding factor, serum response factor (SRF) (16, 23, 34). One of the most important discoveries in the field of SMC differentiation was the identification of myocardin, a coactivator of SRF-dependent transcription exclusively expressed in cardiac and SMC lineages (33, 39). By interacting with SRF, myocardin is able to selectively induce expression of all CArG-dependent SMC marker genes tested to date, including smooth muscle α-actin (αSMA), SM-myosin heavy chain (MHC), SM22α, and calponin (38). E-26-like protein 1 (Elk-1), another principal signal-regulated SRF cofactor, competes with myocardin for interaction with a common docking site on SRF. Cytoplasmic signaling events, which lead to the phosphorylation of Elk-1, allow phosphorylated Elk-1 to displace myocardin from SRF, resulting in downregulation of a subset of SMC marker genes, whereas formation of Elk-1/SRF complex activates expression of genes involved in cell growth. This process is critical in the phenotypic “switch” from a contractile to a proliferative state in SMC (16, 22). In addition, myocardin can induce histone modification by loosening chromatin structure, which also facilitates SMC marker gene expression (11). It is not known how the functions of myocardin are affected by hypoxia.
Lincoln et al. (9) first described a role for cGMP-dependent protein kinase (PKG) in modulation of aortic SMC phenotype and implicated its role in vascular disease (4). We previously reported that hypoxia (24, 48, and 72 h) induced downregulation of PKG in ovine fetal pulmonary venous SMC (FPVSMC) (42). We also reported that short-term hypoxia (0.5 h) only decreased PKG activity, but did not alter PKG protein expression (18). Furthermore, exposure of FPVSMC to hypoxia (24, 48, and 72 h) or transfection of normoxic cells with PKG-specific small interfering RNA is associated with decreased expression of SMC contractile marker proteins, which indicates that PKG plays a regulatory role in the modulation of SMC phenotype switch to a dedifferentiated state in hypoxia. However, the mechanism by which PKG regulates SMC phenotype modulation at the transcriptional level is not clear. Additionally, it is also not known if PKG regulates SMC phenotypic modulation by regulating the function of myocardin.
In this study, we investigated the roles of myocardin in hypoxia-induced phenotype modulation of SMCs and explored the role played by PKG in regulating the functions of myocardin.
MATERIALS AND METHODS
Cell culture.
Primary ovine FPVSMC were isolated from pulmonary veins of three near-term ovine fetuses (42). All procedures and protocols used in the present study were approved by the Animal Research Committees of Los Angeles Biomedical Research Institute at Harbor-UCLA. All experiments were performed with cells at passages 4 to 6. The media used for cell growth and maintenance was DMEM supplemented with 10% heat-inactivated FBS, 400 ng/ml amphotericin B, and 160 U/ml penicillin and streptomycin (Invitrogen).
Hypoxia treatment.
Subconfluent FPVSMC were subjected to 1 or 24 h of normoxia or hypoxia at 37°C for at least three separate experiments. All studies were performed with growth media containing 0.5% FBS after starvation for 16 h. To induce hypoxia, cells were placed in a special hypoxia chamber (C-chamber) into which a gas mixture of 0% O2, 5% CO2 and balance nitrogen was flowed in at a rate sufficient to keep oxygen concentration within the chamber at ∼2%. The concentration of O2 within the C-chamber was constantly monitored and controlled with an oxygen controller (Model ProOxC, Biospherix) (42). For normoxia experiments, cells were incubated in a humidified incubator with a constant supply of 5% CO2 at 37°C. The Po2 in the cell media during hypoxia was 30–40 Torr and during normoxia was ∼100 Torr.
siRNA transfection.
We used PKG-specific small interfering RNA (siRNA) to knock down PKG expression in FPVSMC as previously reported (42). The effectiveness of exogenously introduced siRNAs targeting PKG was demonstrated by measuring PKG protein expression and kinase activity (42). Additionally, we used the same approach to block myocardin in the current study. For myocardin siRNA, a pool of three target-specific siRNAs designed to knock down human myocardin gene expression was obtained from Santa Cruz Biotechnology (cat. no. SC-43953). A nonsilencing oligonucleotide sequence (nonsilencing siRNA) that does not recognize any known homology to mammalian genes was obtained from Qiagen as a negative control (cat. no. 1022563). FPVSMC were transfected with siRNAs using HiperFect transfection kit (Qiagen) in a 12-well plate format as described before (42).
DNA transfection.
To help FPVSMC overexpress PKG, a plasmid encoding a full-length PKG1α (PKG-GFP) tagged with green fluorescent proteins (GFP) was transfected into FPVSMC. A plasmid encoding a full-length myocardin tagged with FLAG (33) was kindly provided by Dr. Gary K. Owens (Department of Molecular Physiology and Biological Physics, Univ. of Virginia) and transfected into FPVSMC to overexpress myocardin in FPVSMC. Transient transfections were done by using Lipofectamine LTX reagent (Invitrogen) in six-well plates. Transfection protocols were performed following the manufacturer's instructions, and the corresponding vector was also transfected as a negative control. Briefly, cells were seeded at a density of ∼6.3 × 104/cm2 in DMEM with 10% FBS and allowed to attach overnight. Transfection was performed 1 day after seeding using 2.5 μg of DNA, 2.5 μl of PLUS reagent, and 6.25 μl of Lipofectamine LTX per well. One day after transfection, cells were starved overnight and then treated with hypoxia or normoxia for 24 h. To determine the transcriptional activity of αSMA, MHC, and SM22α genes, we used reporter plasmids containing the rat αSMA, rat MHC, or mouse SM22α promoters linked to the luciferase gene in pGL3-basic vector (Promega). These three reporter plasmids were generously provided by Dr. Gary K. Owens (39). pRL-SV40 encoding the Renilla luciferase gene was cotransfected as an internal control reporter. Reporter plasmid DNA was transiently transfected into FPVSMC using Lipofectamine LTX (Invitrogen) in a 96-well plate format. Each well was transfected for 4 h with 100 ng of pGL3-promoter construct encoding a firefly luciferase gene (or the empty pGL3 vector) and cotransfected with 20 ng of pRL-SV40 (Promega) encoding the Renilla luciferase gene. Myocardin-FLAG plasmid DNA or control vectors were also cotransfected into FPVSMC. One day after transfection, cells were starved overnight and then treated with hypoxia or normoxia for another 24 h.
Luciferase assay.
Luciferase activity was measured as chemiluminescence with a Victor 1420 multilabel counter (Perkin-Elmer) by using the Dual-Glo system (Promega). For each experimental condition, data were collected from four wells in the culture plate and repeated five times. Promoter activities were expressed as a ratio of firefly luciferase to Renilla luciferase luminescence in each well.
Western blot analysis.
Total protein from cells was dissolved in cell lysis buffer (Cell Signaling), and protein concentration was determined by using BCA protein assay kit (Pierce). Equal amounts of total protein (2–10 μg) from cells were subjected to SDS-PAGE on 4–12% Bis-Tris gels in 1× MES running buffer using the NuPage minigel system (Invitrogen) at 200 V for 1 h. Proteins were transferred to nitrocellulose membrane for 1 h at 30 V. Membranes were blocked for 1 h at room temperature in Tris-buffered saline (TBS) containing 5% nonfat powdered milk and probed with primary antibody in TBS with 5% nonfat powdered milk at concentrations from 1:2,000 to 1:20,000 overnight according to the manufacturer's suggestions for each antibody. In all cases, a secondary antibody labeled with horseradish peroxidase (GE Lifesciences) was used at concentrations from 1:2,000 to 1:20,000 for 1 h at room temperature, and immunoreactive bands were detected by using SuperSignal West Pico Chemiluminescent Substrate (Pierce) and recorded on photosensitive film. The relative intensities of immunoreactive bands detected by Western blot analysis in cells were quantified by densitometry using UN-SCAN-IT gel version 5.1 (Silk Scientific, UT) and normalized with density of total actin (including α, β, γ forms of actin). The apparent molecular masses of the bands were also compared. The primary antibodies used for this study include: anti-MHC, anti-calponin, anti-αSMA (Sigma-Aldrich); anti-myocardin, anti-ELK1, anti-SRF (Santa Cruz Biotech); anti-phospho-Elk-1 (Cell signaling); anti-PKG, anti-total actin (Calbiochem); and anti-SM22α (Abcam).
Immunoprecipitation.
Total proteins from cells treated with hypoxia (1 h) or normoxia were dissolved in RIPA buffer (Boston Biotech). One microgram of polyclonal anti-myocardin or anti-Elk-1 antibody was prebound with Ultralink immobilized protein A/G (Pierce) by rocking for 1 h in room temperature. The prebound primary antibody and protein A/G agarose complex were then added to each sample (200 μg protein), and rocking continued overnight in 4°C. The agarose beads were washed in TBS three times and boiled in 4× Nupage LDS sample buffer (Invitrogen). Western blot analysis was performed for each immunoprecipitated sample as described above except that the secondary antibody was ImmunoPure Recomb Protein A-Peroxidase conjugated (Pierce, cat. no. 32490) to avoid the detection of heavy chain and light chain of IgG in the samples.
Total mRNA preparation, synthesis of cDNA, and real-time quantitative PCR.
Total RNA was prepared from cells by using RNeasy Plus Mini kit (Qiagen), and cDNA were synthesized by using SuperScript III reverse transcriptase (Invitrogen). Real-time PCR was performed using the ABI Prism 7700 sequence detection system and a kit of qPCR master mix for SYBR Green Reagents (Eurogenetec). All the above procedures were performed by following the respective manufacturer's instructions. The primers used were: calponin, 5′-GGC TGA GGT CAA GAA CAA GC-3′ (forward) and 5′-CCA GTT CTG GGT GGA CTC AT-3′ (reverse); SRF, 5′-TGT TAC CGA GCC AAG CTG-3′ (forward) and 5′-CCC GCT CCA GAC GAC TCT-3′ (reverse); Elk-1, 5′-GGT GGT GAA TTC AAG CTG GT-3′ (forward) and 5′-TTG CGG ATG ATG TTC TTG TC-3′ (reverse); myocardin, 5′-CAG AGA AGG ACC CAG GAA CA-3′ (forward) and 5′-GAC CTC TCT GCA GTG GAA GC-3′ (reverse). Samples (100 ng of cDNA per well) were run in triplicate in 20-μl reactions. The real-time PCR cycling conditions were 95°C for 15 min, followed by 45 cycles of 94°C for 30 s, 60°C for 15 s, and 72°C for 15 s, followed by a dissociation curve. The amount of target gene, normalized to an endogenous reference (GAPDH) and relative to a calibrator (reference RNA, in this study, the normoxia samples), was given by 2−Δ ΔCt calculation.
Chromatin immunoprecipitation.
The chromatin immunoprecipitation (ChIP) assay was performed by using the EZ-Magna ChIP G kit (Upstate, cat. no. 17–409) following the manufacturer's instructions. In brief, cells were grown to 60–70% confluence in 100-mm dishes, starved in growth medium containing 0.5% FBS overnight, and then treated with hypoxia, normoxia, or the peptide PKG inhibitor DT-3 (EMD) at 3 × 10−5 M concentration for 1 h. Upon completion of treatment, proteins were cross-linked to DNA by adding the formaldehyde directly to the culture medium to a final concentration of 1% for 10 min. The unreacted formaldehyde was quenched by incubating with 1× glycine buffer for 5 min. After rinsing twice with ice-cold PBS, the cells were resuspended in 1 ml of PBS with protease inhibitor by scraping the cells from the dishes. After brief centrifugation to pellet cells, cells were resuspended in 0.5 ml of cell lysis buffer and then nuclear lysis buffer. Sonication of cell lysate was performed four times for 8 s each, followed by centrifugation at 4°C for 10 min. Supernatants were collected and diluted 1:10 with dilution buffer. A portion of diluted supernatant (1%) was kept to estimate the amount of DNA present in different samples. It is referred to as “input” sample. Immunoprecipitation was carried out overnight at 4°C by adding the immunoprecipitating antibody and 20-μl fully suspended protein G magnetic beads. The antibodies used were rabbit-polyclonal anti-myocardin and anti-Elk-1 (2 μg for each sample, Santa Cruz Biotechnology). Anti-RNA-polymerase (1 μg) and normal rabbit IgG (1 μg) were also included as the positive and negative controls, respectively. After washing the protein G bead-antibody/chromatin complex, ChIP elusion buffer with proteinase K were added and incubated at 62°C for 2 h. DNA was recovered and purified with DNA spin columns. Both the immunoprecipitated samples and the input samples were processed in the same way. After ChIP assays, we chose real-time quantitative PCR as recommended by the ChIP kit from Upstate to evaluate the interaction of SRF cofactors and MHC promoter since it is a convenient, sensitive, and more reliable method to quantify the DNA in the samples. Real-time PCR was performed using primers of MHC promoter, 5′-GGA GGA TGA GAT CCT GGT CA-3′ (forward) and 5′-GCT CCC TCA GGT TGT GTA GC-3′ (reverse). Relative quantities of MHC promoter of each sample were calculated as described in the real-time PCR section, except the samples were normalized with each corresponding input. Data are expressed as percentage relative to the untreated control.
Statistical analysis.
SigmaStat 3.1 software was used for data analysis. Unpaired t-tests were used to compare differences in density of Western blots, and a probability value under 0.05 was considered significant. If the data did not pass the Normality test, Mann-Whitney Rank Sum tests were used as an alternate, as recommended by the software SigmaStat.
RESULTS
Myocardin is disassociated from SRF after 1 h of hypoxia.
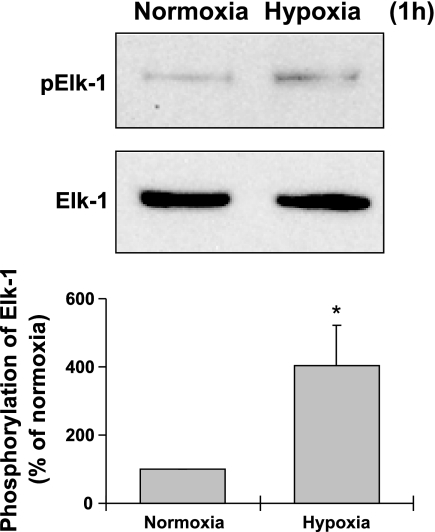
To determine the early effect of hypoxia on myocardin and Elk-1, FPVSMC were treated with hypoxia for 1 h (Fig. 1). Hypoxia for 1 h resulted in a threefold increase in Elk-1 phosphorylation, indicating that hypoxia activates Elk-1 in an early stage. Hypoxia (1 h) had no significant effects on the protein expression of myocardin (data not shown).
Fig. 1.
Effects of hypoxia (1 h) on phosphorylation of Elk-1. Representative Western blots (top) and quantification of Western blots by densitometry (bottom) are shown. Data are expressed as means ± SE of 3 independent experiments. *P < 0.05 compared with the cells exposed to normoxia (expressed as 100%).
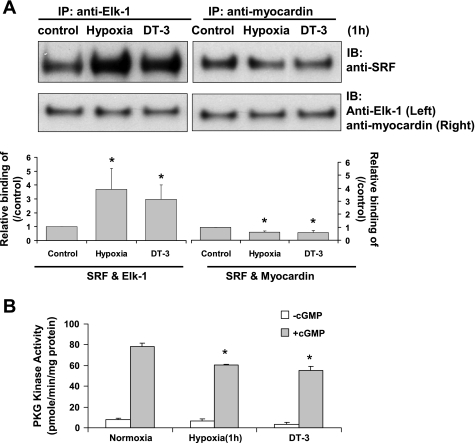
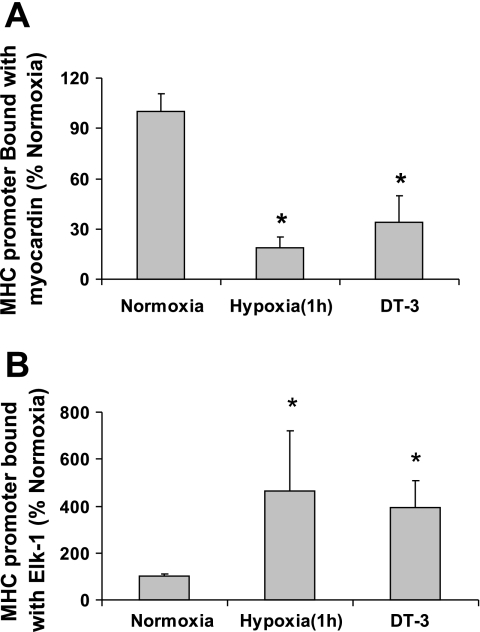
The associations of myocardin or Elk-1 with SRF or MHC promoter after 1 h of hypoxia were also studied. The 1-h hypoxia time period selected as the displacement of myocardin from SRF by Elk-1 has been reported to occur as quickly as 30 min after PDGF treatment in SMCs (40). FPVSMC were treated with hypoxia for 1 h, and cell lysates were immunoprecipitated with anti-myocardin or anti-Elk-1 antibody and then probed with SRF antibody (Fig. 2A). The interactions between SRF cofactors and SMC marker genes within intact chromatin were studied by ChIP assays. Figures 2A and 3 show that hypoxia treatment for 1 h decreased the association of myocardin, but increased the association of Elk-1 with SRF and the promoter of the MHC gene.
Fig. 2.
After hypoxia or DT-3 treatment, less myocardin and more Elk-1 binds to the serum response factor (SRF). A: interactions of Elk-1 and myocardin with SRF in fetal pulmonary venous smooth muscle cells (FPVSMC) after hypoxia or DT-3 treatment. FPVSMC were treated with normoxia, hypoxia, or DT-3 (30 μM) for 1 h, and immunoprecipitations (IP) were performed in cell extracts with anti-myocardin and anti-Elk-1 antibody followed by immunoblot (IB) with anti-SRF antibody. Data are expressed as means ± SE of 3 independent experiments. *P < 0.05 compared with control (expressed as 100%). B: PKG activities at basal conditions and stimulated with cGMP (3 × 106 M) of FPVSMC after hypoxia or DT-3 (30 μM) treatment (1 h). Data are shown as means ± SE; n = 4 for each group. *P < 0.05 compared with normoxia control.
Fig. 3.
After hypoxia or DT-3 treatment, less myocardin and more Elk-1 binds to the MHC promoter in chromatin immunoprecipitation (ChIP) assays. FPVSMC were treated with hypoxia, normoxia, or DT-3 for 1 h. ChIP assays on MHC genes were performed on cross-linked chromatin in the treated FPVSMC with antibodies against myocardin or Elk-1 as described in materials and methods. DNA was analyzed by real-time PCR. A: data from ChIP on Elk-1. B: data from ChIP on myocardin. Data are generated from 3 independent experiments and expressed as means ± SE of 3 independent experiments. *P < 0.05 compared with the cells exposed to normoxia (expressed as 100%).
PKG regulates the displacement of myocardin from SRF after 1 h of hypoxia.
Both hypoxia and treatment with 30 μM DT-3 for 1 h significantly decreased PKG activity in FPVSMC (Fig. 2B). Treatment with DT-3 in FPVSMC resulted in decreased myocardin and increased Elk-1 binding to SRF and MHC gene, which was similar to the effects of hypoxia for 1 h (Figs. 2A and 3).
Expression of myocardin is downregulated after 24 h of hypoxia.
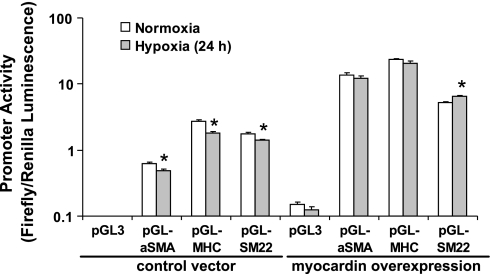
To confirm our hypothesis that hypoxia modulates SMC contractile protein expression at the gene transcriptional level, FPVSMC were transiently transfected with αSMA, SM22α, or MHC promoter reporter plasmid DNA, treated with hypoxia or normoxia for 24 h, and promoter activities were measured. Exposure of FPVSMC to 24 h of hypoxia significantly inhibited the activity of multiple SMC marker gene promoters including αSMA, MHC, and SM22α (Fig. 4).
Fig. 4.
The effect of hypoxia on SMC marker gene promoter activity in the absence and presence of myocardin overexpression. FPVSMC were cotransfected with the indicated SMC gene promoter-firefly luciferase constructs, FLAG-tagged myocardin plasmid DNA or control vector, together with pRL-SV40 as a Renilla luciferase reporter transfection control. Luciferase activities were measured after 24 h of hypoxia. Promoter activity is expressed as a ratio of firefly/Renilla luciferase activity. Data are presented as mean values ± SE of 5 separate experiments, each of which comprised 4 replicates. *Significantly different from respective normoxia controls (P < 0.05).
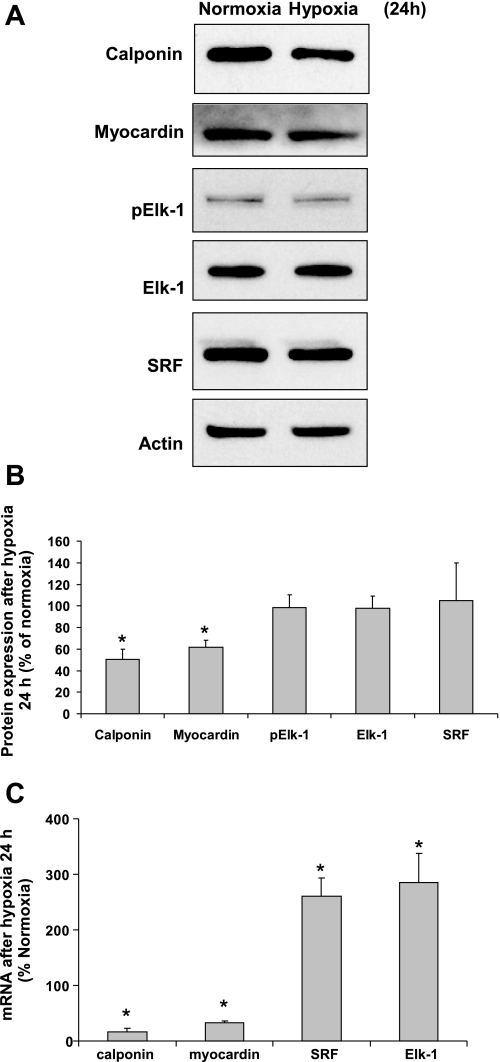
The late effects of hypoxia (hypoxia for 24 h) on the expression of SRF cofactors myocardin and Elk-1 were also investigated. Western blot data showed that 24 h of hypoxia significantly downregulated the protein expression of myocardin and that of calponin, whereas having no significant effects on phospho-Elk-1, Elk-1, and SRF levels (Fig. 5). mRNA expression levels of these factors were also determined by real-time PCR (Fig. 5C). Hypoxia (24 h) downregulated the mRNA expression levels of calponin and myocardin, but upregulated the levels of SRF and Elk-1.
Fig. 5.
Effects of hypoxia (24 h) on protein and mRNA expression of myocardin, Elk-1, and SRF. A and B: hypoxia (24 h) downregulates the expression of myocardin and calponin. A: representative Western blots. B: quantification of Western blots by densitometry. C: effects of hypoxia (24 h) on the mRNA expressions of SRF and its cofactors. Data are expressed as means ± SE of 3 independent experiments. *P < 0.05 compared with the cells exposed to normoxia (expressed as 100%).
Effect of decreased myocardin expression after 24 h of hypoxia.
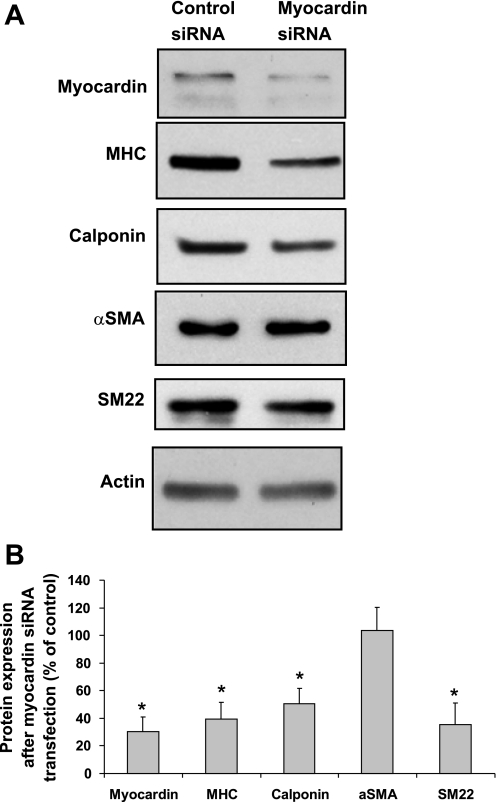
To study the role of myocardin in hypoxia-induced SMC phenotype modulation, myocardin-specific siRNA was transfected into FPVSMC to decrease the expression of endogenous myocardin in cells. The results (Fig. 6) showed that transfection with myocardin siRNA was able to knock down myocardin protein expression to 30% of expression in control siRNA transfected cells. Myocardin-specific siRNA transfection also downregulated SMC marker proteins, MHC, calponin, and SM22, which was similar to the effects of hypoxia treatment in FPVSMC (42).
Fig. 6.
Transfection with myocardin siRNA in FPVSMC results in downregulation of SMC marker proteins including MHC, calponin, SM22, and αSMA. FPVSMC were transfected with myocardin siRNA or control siRNA. A: representative Western blots. B: quantification of Western blots by densitometry. Data are expressed as means ± SE of 4 independent experiments. *P < 0.05 compared with the cells transfected with control siRNA (expressed as 100%).
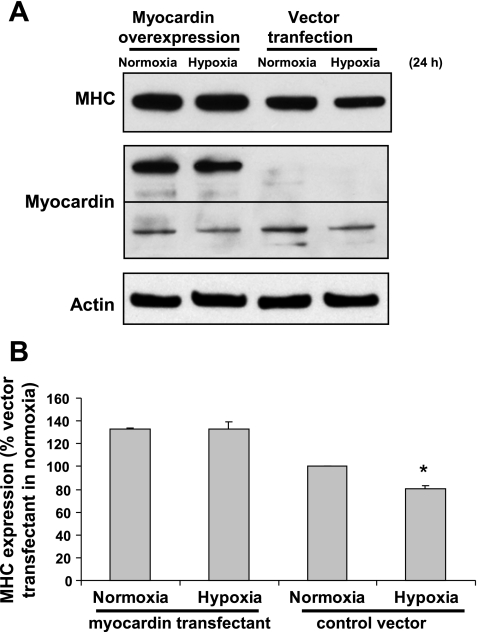
To further elucidate the role of myocardin in SMC phenotype modulation, exogenous myocardin was introduced in FPVSMC by transiently transfecting cells with FLAG-tagged myocardin construct DNA. Western blot analysis using anti-myocardin antibody (Fig. 7) showed that FLAG-tagged myocardin construct had been successfully introduced into FPVSMC as evidenced by the detection of a protein band with higher molecular mass in the myocardin-FLAG-transfected cells, whereas no such band was present in control vector-transfected cells. Hypoxia (24 h) induced downregulation of MHC protein expression in control vector-transfected cells, although it had no significant effect on MHC protein expression in myocardin overexpressing cells. This is in agreement with measurements of SMC marker gene promoter activity in myocardin overexpressing cells, in which hypoxia had no significant effect on the activities of αSMA and MHC gene promoters, and even significantly increased activity of SM22α gene promoter (Fig. 4). The above data indicate that overexpression of myocardin in cells can generally reverse the effect of hypoxia on SMC phenotype modulation.
Fig. 7.
Overexpression of myocardin in FPVSMC reverses hypoxia-induced SMC phenotype modulation. Cells were transiently transfected with FLAG-tagged myocardin construct or control vector. Transfected cells were treated with hypoxia or normoxia for 24 h. A: representative Western blots. B: quantification of Western blots by densitometry. Data are expressed as means ± SE of 3 independent experiments. *P < 0.05 compared with the cells transfected with control vector (expressed as 100%).
PKG regulates the expression of myocardin in cells exposed to 24 h of hypoxia.
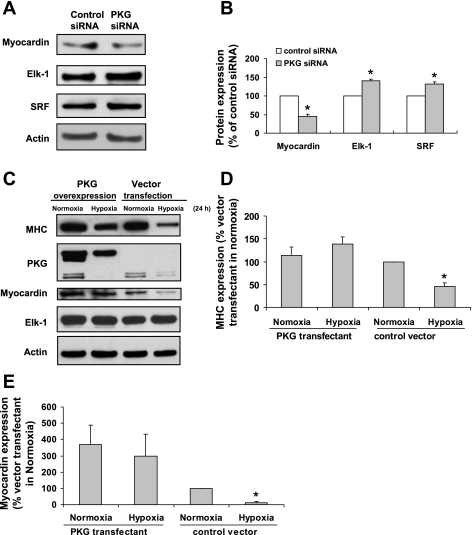
Our published data have demonstrated the role of PKG in hypoxia-induced SMC phenotype modulation (42). A combination of loss- and gain-of-function experiments in cultured SMC were performed (Fig. 8). We inhibited PKG in FPVSMC by PKG-specific siRNA transfection, which significantly downregulated myocardin expression but upregulated SRF and Elk-1 (Fig. 8, A and B). To see if the overexpression of PKG can prevent the downregulation of myocardin and MHC expression in hypoxia, FPVSMC were transiently transfected with a PKG-GFP construct. Western blot analysis (Fig. 8, C–E) showed that GFP-tagged PKG1α construct had been successfully introduced into FPVSMC since a higher molecular mass protein band was detected in the PKG-GFP-transfected cells, whereas no such band was present in control vector-transfected cells. In control vector-transfected cells, hypoxia (24 h) was able to downregulate the protein expression of myocardin and MHC, whereas it had no significant effect on the protein expression of Elk-1. In PKG-transfected cells, the effect of hypoxia on myocardin and MHC was abrogated, indicating that increased expression of PKG was able to maintain FPVSMC in a contractile state even in the presence of hypoxia.
Fig. 8.
The effect of PKG on the expression of myocardin and Elk-1. A and B: inhibition of PKG by PKG siRNA transfection in FPVSMC downregulates myocardin but upregulates SRF and Elk-1. FPVSMC were transfected with PKG siRNA, and the protein expression of myocardin, SRF, and Elk-1 was measured by Western blot. Data are expressed as means ± SE of 4 independent experiments. *P < 0.05 compared with the cells transfected with control siRNA (expressed as 100%). C–E: overexpression of PKG in FPVSMC reverses hypoxia-induced downregulation of myocardin and MHC. FPVSMC were transfected with PKG tagged with GFP plasmid DNA or control vector and treated with hypoxia for 24 h after transfection, and the protein expression of myocardin, SRF, and Elk-1 was measured by Western blot. Blots are representatives from 3 independent experiments. C: representative Western blots. D: densitometric data of MHC. E: densitometric data of myocardin. Data are expressed as means ± SE of 4 independent experiments. *P < 0.05 compared with the cells transfected with control siRNA (expressed as 100%).
DISCUSSION
This is the first study to demonstrate the role of myocardin in hypoxia-induced SMC phenotype modulation. This study also characterizes myocardin as a downstream effector of PKG and its involvement in hypoxia-induced inhibition of SMC contractile marker protein expression in FPVSMC (42).
In this study, we selected MHC, αSMA, calponin, and SM22α as SMC contractile marker proteins. While MHC is expressed exclusively in SMC and considered the “definitive” SMC marker (15), other SMC markers, including αSMA, calponin, and SM22α, are expressed predominantly in SMC during postnatal development, but also transiently in embryonic cardiac and skeletal myocytes (19). The expressions of genes encoding SMC contractile markers are dynamically regulated by a complex, synergistic interplay between SRF accessory factors, the direct interaction of SRF and CArG, and histone modifications within promoter chromatin (12).
The expression of genes encoding SMC markers is dependent on a SRF-dependent transcriptional program (14, 21). SRF is a 508-amino acid protein that is a member of the ancient MADS (MCM1, agamous, deficiens, SRF) box family of transcription factors (29). SRF binds the consensus nucleotide sequence [CC(A/T)6GG], which has been variably designated as a serum response element (SRE) or CArG box, a homodimer or heterodimeric protein complex (17). SRF is a transcription factor required for SMC marker gene expression and smooth muscle differentiation. Paradoxically, SRF also regulates genes involved in cell proliferation, which opposes the SMC differentiation program (1, 14, 30, 32, 37).
SRF regulates different downstream effector genes depending on its association with positive and negative cofactors (28, 31). Recent important publications in this field have demonstrated that two families of SRF cofactors, the ternary complex factors (TCFs) including Elk-1, SAP-1, and NET/SAP-2/Erp, as well as the myocardin-related transcription factors (MRTFs) including myocardin, MRTF-A/megakaryoblastic leukemia 1 (MKL-1)/MAL, and MRTF-B/MKL-2, are regulated by separate signaling pathways and thereby control SRF target genes, i.e., SMC marker genes, differentially (23). Wang et al. (35) showed that when SMC were treated with PDGF, Elk-1 was phosphorylated via a MAP kinase signaling pathway, and that phosphorylated Elk-1 associates better with SRF, which favors the displacement of myocardin. Although Elk-1 is a coactivator of SRF, its activity is substantially weaker than that of myocardin (22). Moreover, Elk-1 and myocardin compete for binding at the same docking site on SRF, and their binding is mutually exclusive such that binding of myocardin with SRF activates SMC marker gene expression while formation of a TCF/SRF complex represses SMC marker gene expression and activates cell growth gene expression. The latter process is critical in the phenotypic switch from a contractile, differentiated to a proliferative, dedifferentiated state in smooth muscle cells (22).
Myocardin not only transactivates SMC marker gene expression by physically interacting with SRF, but also helps modifications in chromatin structure that are required for SMC differentiation marker gene expression (11). The SMC differentiation marker genes are packaged in dynamic chromatin structures that affect access of transcription factors to conserved CArG and other promoter elements. Myocardin recruits the histone 3 lysine 9 (H3K9)-specific demethylase, Jmjd1a, near the CArG-containing region of SMC-specific promoter in chromatin (11). The SRF/myocardin complex may subsequently recruit the histone acetyl transferase (HAT), p300, and increase histone acetylation of the SMC-specific promoters (2, 13). These histone modifications within chromatin result in further loosening of chromatin structure, the formation of the transcription initiation complex, and facilitation of SMC differentiation marker gene expression (11).
It has been reported that PDGF treatment for 30 min in SMCs induces activation of Elk-1 and the displacement of myocardin from SRF by Elk-1 (40). Although our data show no significant activation of Elk-1 after 24 h of hypoxia, phosphorylation of Elk-1 was increased after 1 h of hypoxia, indicating that hypoxia induces Elk-1 activation at an early stage. Such alteration of SRF cofactors may play an important role in modulating cell phenotype after hypoxic exposure. Jin et al. (7) and Preston et al. (24) provided direct evidence that hypoxia activates MAP kinases including Jun-NH2-terminal kinase (JNK), extracellular signal-regulated protein kinase (ERK), and p38 MAP kinase. Their data suggested that hypoxia- induced activation of JNK is an early response to hypoxic stress and that activation of ERK and p38 kinase is associated with hypoxia-induced pulmonary arterial remodeling (7, 24). Therefore, we hypothesize that hypoxia at an early stage activates ERK, which phosphorylates TCFs, such as Elk-1. Phosphorylated Elk-1 is recruited to a subset of SMC marker genes where it can displace myocardin with subsequent downregulation of SMC marker gene expression. In other words, when FPVSMC are subjected to hypoxia, the MAPK-Elk-1-SRF axis is engaged and SRF-Elk-1 complex formation is favored over SRF-myocardin complexes. As a result, growth-related genes are more likely to be activated than SMC marker genes. Conversely, when FPVSMC is subjected to normoxia, the MAPK-Elk-1-SRF axis is not engaged, and SRF-myocardin complexes are favored and induce SMC marker gene expression. Since there is no significant change of the protein expression level of myocardin, it may be less likely that changes of myocardin-induced histone modification occur after 1 h of hypoxia.
We previously reported that PKG plays an important role in hypoxia-induced SMC phenotype modulation (42). Recently, Zhang et al. (40) found that PKG1 phosphorylates a new cysteine-rich LIM-only protein CRP4 that facilitates recruitment of SRF cofactors and aids a ternary nucleoprotein complex formation with SRF and SRF cofactors over the SRE of SM-specific gene promoters, which then increases transcription of SM-specific gene expression. Our hypothesis is that hypoxia for 1 h may inhibit PKG quickly, thus quickly regulating myocardin and Elk-1 binding with SRF. Our present and previous data (18, 42) show that basal PKG activity is high in unstimulated normoxic FPVSMC, which is similar to that reported in PASMC (40). We also show that hypoxia can inhibit PKG activity as early as 1 h. The PKG inhibitor DT-3 had a similar effect as hypoxia, namely that less myocardin and more Elk-1 was bound to SRF. All of these findings suggest that hypoxia in the early stage (1 h of hypoxia) modulates SMC phenotype, most likely by inhibiting PKG activity quickly, displacing myocardin from SRF, thus helping repress SMC marker gene transcription.
Our data show that the exposure of FPVSMC to hypoxia for 24 h significantly decreases MHC, SM22α, and αSMA promoter activity. Hypoxia also significantly downregulates expression of SMC contractile marker protein calponin as we previously reported (42). Both findings support the notion that FPVSMC switches from a differentiated to a dedifferentiated phenotype after exposure to hypoxia for 24 h. Hypoxia for 24 h also downregulates myocardin at both protein and mRNA levels. For SRF and Elk-1, hypoxia for 24 h upregulated the mRNA expression level, but had no significant effects on the protein expression level of both factors. The possible reason for these discrepancies is that the changes in protein expression levels may occur later than the changes in mRNA expression level. Alternatively, changes in posttranscriptional regulation (translational regulation) of these genes and/or the rates of mRNA and protein synthesis/degradation may be different due to an altered cellular environment under hypoxia. A previous study has identified the SRF gene as a target for Elk-1, thereby providing a positive-feedback loop where Elk-1 activation leads to enhanced expression of SRF (8). This may help explain our data that SRF mRNA expression is upregulated after hypoxia for 24 h.
Knockdown of myocardin does not decrease αSMA expression, and overexpression of myocardin even increases hypoxia-induced expression of SM22α promoter activity. This may reflect either different sensitivity to repressor pathways or differential dependence on myocardin for the expression of SMC marker genes, as suggested by Zhou et al. (41).
It is known that myocardin is expressed exclusively in SMC and cardiomyocytes (33) and is extremely efficacious in activating multiple CArG-dependent SMC marker genes, including αSMA, MHC, SM22α, and calponin (3, 5, 36, 39). The promoter selectivity of myocardin depends on the cooperative interaction of multiple CArG elements. Myocardin is unable to activate growth-related genes such as c-fos, which only contain a single CArG element (33). Although the SRF promoter contains two highly conserved CArG elements, the expression of SRF gene was not regulated by myocardin (38). Therefore, it is not surprising that there were no changes in SRF protein expression during phenotype modulation in vitro and in vivo, suggesting that alterations in SRF expression are not the basis for the attenuated SMC differentiation program (3). The ability of SRF to regulate SMC phenotype modulation depends on its binding to cofactors such as myocardin, and the present study shows that more SRF binds with Elk-1 and less SRF binds with myocardin in hypoxia.
Our finding that hypoxia for 24 h downregulates myocardin mRNA and protein expression in FPVSMC is in contradiction to reports by Zhu et al. (43) and Reynolds et al. (25). They showed that hypoxia significantly upregulates myocardin mRNA in rat pulmonary vessels and in porcine pulmonary artery endothelial cells (43) and that hypoxia causes a time-dependent increase in myocardin mRNA expression in the lungs of CAST/eiJ mice (25). This seeming discrepancy is likely due to species differences and/or the tissues or cells studied as well as experimental methods. However, all of these studies provide compelling evidence that downregulation of myocardin is associated with marked decreases in SMC marker gene expression, whereas upregulation of myocardin is associated with marked increases in SMC marker gene expression. Consistent with the results of our studies, Yoshida et al. (38) also reported that angiotensin II-induced expression of αSMA was associated with increased expression of myocardin, and siRNA-induced suppression of myocardin attenuated angiotensin II-induced transcription of this gene. These results indicate that myocardin plays a key role in regulation of SMC differentiation and serves as a point of convergence in mediating effects of environmental cues on expression of SMC marker genes.
Our data show that knockdown of myocardin gene expression in FPVSMC by myocardin siRNA suppresses the expression of MHC, calponin, and SM22α, which is similar to the effect of hypoxia. On the other hand, overexpression of myocardin reverses hypoxia-induced inhibition of the SMC contractile marker gene MHC and αSMA promoter activity. These results substantially demonstrate that myocardin plays a key role in regulation of SMC differentiation in hypoxia in FPVSMC. Consistent with our results, several groups have reported that forced expression of myocardin activates the transcription of multiple genes encoding SMC contractile markers including MHC, αSMA, SM22α, and calponin (3, 5, 36, 39). In addition, knockdown of myocardin gene expression in SMC, by either siRNA or transfection of a dominant-negative myocardin mutant, suppressed transcription of genes encoding SMC contractile markers (5).
Our data show that the phosphorylation of Elk-1 is not significantly changed after hypoxia for 24 h, which suggests that it would be less likely to be involved in the displacement of myocardin from SRF, because phosphorylation of Elk-1 is a prerequisite for this step (36). Our data also show that myocardin is downregulated after hypoxia for 24 h, which may result in less histone modification, thus prohibiting SMC marker gene expression (11).
Our data also show that hypoxia downregulates both PKG and myocardin expression in FPVSMC and that this is associated with the repression of SMC contractile marker genes. Our data show that inhibition of PKG by PKG siRNA transfection in FPVSMC downregulates myocardin but upregulates SRF and Elk-1. These changes are similar to the effects of hypoxia for 24 h. In contrast, overexpression of PKG in FPVSMC reversed hypoxia-induced myocardin downregulation. The above data suggest that PKG is an upstream regulator that regulates the expression of myocardin, thus modulating SMC marker gene transcription. Hypoxia at a later stage (24 h of hypoxia) modulates SMC phenotype, most likely by downregulating myocardin expression via inhibiting PKG, which may result in less myocardin-induced histone modification within chromatin and eventually assists in repression of SMC marker gene transcription.
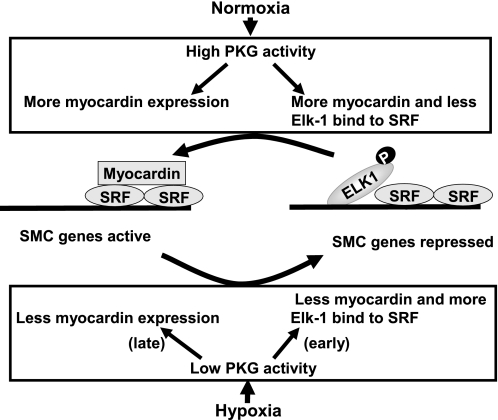
In summary, this study shows that under normoxic conditions, PKG activity is high in FPVSMC, more myocardin binds with SRF, more myocardin protein is expressed, and SMC marker gene transcription is active. However, under hypoxic conditions, at an early stage (1 h of hypoxia), PKG activity is low, Elk-1 is activated, and phosphorylated Elk-1 replaces myocardin at the binding sites with SRF. Under hypoxic conditions at a later stage (24 h of hypoxia), less myocardin protein is expressed. After both early and late hypoxia (1 and 24 h), SMC marker gene transcription is repressed (depicted in Fig. 9). Our data suggest that PKG may help myocardin displace Elk-1 from SRF and also upregulate myocardin expression, thus modulating the transcription of SMC genes. We speculate that the inhibitory effects of hypoxia on PKG, via its effects on myocardin, may be one of the mechanisms involved in hypoxia-induced SMC phenotype modulation.
Fig. 9.
A model to account for the modulation of SMC marker genes by PKG in response to hypoxia.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-75187 and HL-59435 (to J. U. Raj).
REFERENCES
- 1.Browning CL, Culberson DE, Aragon IV, Fillmore RA, Croissant JD, Schwartz RJ, Zimmer WE. The developmentally regulated expression of serum response factor plays a key role in the control of smooth muscle-specific genes. Dev Biol 194: 18–37, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Cao D, Wang Z, Zhang CL, Oh J, Xing W, Li S, Richardson JA, Wang DZ, Olson EN. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol 25: 364–376, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Dey NB, Boerth NJ, Murphy-Ullrich JE, Chang PL, Prince CW, Lincoln TM. Cyclic GMP-dependent protein kinase inhibits osteopontin and thrombospondin production in rat aortic smooth muscle cells. Circ Res 82: 139–146, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 23: 2425–2437, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol 23: 1510–1520, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Jin N, Hatton N, Swartz DR, Xia X, Harrington MA, Larsen SH, Rhoades RA. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am J Respir Cell Mol Biol 23: 593–601, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Kasza A, O'Donnell A, Gascoigne K, Zeef LA, Hayes A, Sharrocks AD. The ETS domain transcription factor Elk-1 regulates the expression of its partner protein, SRF. J Biol Chem 280: 1149–1155, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Lincoln TM, Dey NB, Boerth NJ, Cornwell TL, Soff GA. Nitric oxide–cyclic GMP pathway regulates vascular smooth muscle cell phenotypic modulation: implications in vascular diseases. Acta Physiol Scand 164: 507–515, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11: 356–367, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Lockman K, Taylor JM, Mack CP. The histone demethylase, Jmjd1a, interacts with the myocardin factors to regulate SMC differentiation marker gene expression. Circ Res 101: e115–e123, 2007. [DOI] [PubMed] [Google Scholar]
- 12.McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res 100: 1428–1441, 2007. [DOI] [PubMed] [Google Scholar]
- 13.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 116: 36–48, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miano JM Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 35: 577–593, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res 75: 803–812, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292: C70–C81, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Mohun TJ, Chambers AE, Towers N, Taylor MV. Expression of genes encoding the transcription factor SRF during early development of Xenopus laevis: identification of a CArG box-binding activity as SRF. EMBO J 10: 933–940, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negash S, Gao Y, Zhou W, Liu J, Chinta S, Raj JU. Regulation of cGMP-dependent protein kinase-mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am J Physiol Lung Cell Mol Physiol 293: L1012–L1020, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Owens GK Molecular control of vascular smooth muscle cell differentiation. Acta Physiol Scand 164: 623–635, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Parmacek MS Transcriptional programs regulating vascular smooth muscle cell development and differentiation. Curr Top Dev Biol 51: 69–89, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev 20: 1545–1556, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol 16: 588–596, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Preston IR, Hill NS, Warburton RR, Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 290: L367–L374, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 279: 37124–37132, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 361: 1533–1544, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg 45, Suppl A: A25–A32, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Shore P, Sharrocks AD. The MADS-box family of transcription factors. Eur J Biochem 229: 1–13, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Treisman R Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J 6: 2711–2717, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treisman R The serum response element. Trends Biochem Sci 17: 423–426, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Treisman R Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev 4: 96–101, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Walsh K Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol 9: 2191–2201, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev 14: 558–566, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428: 185–189, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA 100: 7129–7134, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson EJ, Raab K, Browning CA, Hosty TA. Familial hepatic cirrhosis in infants associated with alpha1-antitrypsin SZ phenotype. J Pediatr 85: 159–164, 1974. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida T, Kawai-Kowase K, Owens GK. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler Thromb Vasc Biol 24: 1596–1601, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 92: 856–864, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Zhuang S, Casteel DE, Looney DJ, Boss GR, Pilz RB. A cysteine-rich LIM-only protein mediates regulation of smooth muscle-specific gene expression by cGMP-dependent protein kinase. J Biol Chem 282: 33367–33380, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Hu G, Herring BP. Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1. Mol Cell Biol 25: 9874–9885, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L1459–L1466, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Zhu P, Huang L, Ge X, Yan F, Wu R, Ao Q. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int J Exp Pathol 87: 463–474, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]