Abstract
IL-8 is a key mediator in the pathophysiology of acute lung injury. TNFα stimulates IL-8 production in respiratory epithelial cells by activating both the NF-κB and MAP kinase pathways. The precise mechanism by which these pathways are downregulated to terminate IL-8 production remains unclear. We studied the regulatory role of the serine/threonine phosphatase, PP2A, on the signaling pathways involved in IL-8 production from respiratory epithelial cells. Inhibition of PP2A using okadaic acid or gene knockdown using siRNA resulted in an augmentation of TNFα-induced IL-8 production. We also found that PP2A inhibition resulted in prolonged activation of JNK, p38, and ERK resulting in both increased transcriptional activation of the IL-8 promoter and posttranscriptional stabilization of IL-8 mRNA. Because TNFα had been shown to activate ceramide accumulation, and separate studies had linked ceramide with activation of PP2A, we hypothesized the pathway of TNFα-inducing ceramide to activate PP2A comprised an endogenous regulatory pathway. Inhibition of the immediate sphingomyelinase-dependent pathway as well as the de novo synthesis pathway of ceramide production reduced serine/threonine phosphatase activity and augmented IL-8 production. These data suggest that ceramide plays a role in activating PP2A to terminate ongoing IL-8 production. In summary, our data suggest that in respiratory epithelium, TNFα induces ceramide accumulation, resulting in subsequent activation of PP2A, which targets those kinases responsible for transcriptional activation of IL-8.
Keywords: interleukin-8, airway epithelium, tumor necrosis factor-α, ceramide, phosphatases
one of the key aspects in the pathogenesis of acute lung injury (ALI) and its most severe manifestation, the acute respiratory distress syndrome (ARDS), is the substantial local production of the chemokine CXCL8/IL-8 (IL-8) that serves as one of the principal recruiters of neutrophils from the blood into the lung alveolar space (1, 5, 6, 46). Once localized in the lung parenchyma and alveolar space, neutrophil release of oxygen radicals, proteases, and other toxic moieties are responsible for the subsequent tissue destruction and loss of barrier function leading to pulmonary edema, intrapulmonary shunt, and hypoxemia hallmarks of ALI (1, 5, 6, 46). Importantly, the amount of IL-8 produced has correlated with the severity of lung injury (12, 16, 25, 31, 32), and levels have been reported to be an important prognostic factor for mortality from ARDS (2, 45). From a pathophysiological basis, IL-8's pivotal role in ALI has been elucidated using experimental models of lung injury in which IL-8 neutralization resulted in improvements in lung injury markers and reduced mortality (9, 18, 49). Intriguingly, applying this therapeutic strategy in humans has been hampered by growing concern about creating IL-8 antigen:antibody immune complexes known to potentially exacerbate lung inflammation and injury (19). Thus, there remains a need to identify alternative strategies for downregulating IL-8 gene expression independently of antibody neutralization. To that end, we attempted to gain a better understanding of endogenous counterregulatory pathways of IL-8 gene expression that could be utilized in a therapeutic approach. We hypothesized that elucidating the endogenous mechanism regulating IL-8 expression would enable identification of potential therapeutic targets aimed at attenuating IL-8 production thereby reducing ALI-associated mortality and morbidity.
IL-8 gene activation and protein production is complex but has been well characterized. In the setting of ALI, IL-8 production is dependent on the early inflammatory gene products, notably TNFα, which activates both the NF-κB and MAPK pathways (38). In respiratory epithelial cells, once TNFα binds to its receptor, the NF-κB pathway is activated, resulting in its translocation to the nucleus where it binds and activates the IL-8 promoter (38). Thus, whereas NF-κB binding to the IL-8 promoter is required for IL-8 gene transcription (17, 21, 22), it is not sufficient for maximal production of IL-8, which requires activation of the JNK, ERK, and the p38 mitogen-activated protein kinase (p38) pathways (29). In respiratory epithelial cells, the JNK and ERK arms of the MAPK pathway, along with NF-κB, activate IL-8 transcription. IL-8 mRNA is subsequently stabilized by p38 via posttranscriptional modifications. Thus, the maximal production of IL-8 relies on cooperative interactions between the NF-κB and MAPK signaling pathways.
The signal mechanisms involved in the induction of IL-8 production have been well delineated; in contrast, the downregulation signals have not been well characterized. Both NF-κB and MAPK pathways utilize cascades of kinases to transduce and amplify the cellular signal. These kinases are activated by serial phosphorylation via upstream kinases and notably deactivated through phosphatase-mediated dephosphorylation. Importantly, the protein phosphatase PP2A, a serine/threonine phosphatase, has been shown to regulate both the NF-κB and the MAPK pathways (13, 27, 28, 36, 37, 43, 50), but its role in regulating the production of IL-8 gene expression is unknown. More recently, the sphingomyelin signal transduction pathway has also been shown to be involved in the TNFα induction of IL-8 gene expression (11). TNFα induces sphingomyelinases that convert sphingomyelin to ceramide, the key second messenger in the sphingomyelin pathway (30). Ceramide may be further converted to sphingosine-1-phosphate, which may be active in IL-8 production (11). Interestingly, ceramide is also known to be a potent activator of PP2A (10, 14, 15, 20, 47). To date, the interaction of TNFα, ceramide, and PP2A in lung epithelium and their effects on IL-8 production have not been studied. We hypothesized that in respiratory epithelial cells, induction of ceramide by TNFα activates PP2A, which serves as an endogenous regulator of the MAPK signaling pathways crucial to IL-8 production. Our data show that blocking PP2A activity by pharmacological inhibition, RNA silencing, or diminishing ceramide production, augmented TNFα-induced IL-8 production from A549 respiratory epithelial cells and suggest that PP2A is a key endogenous counterregulator of IL-8 expression.
MATERIALS AND METHODS
Cells and reagents.
The human alveolar epithelial cell line (A549) was purchased from American Type Culture Collection (ATCC; Manassas, VA) and was cultured in Ham's F-12 medium (Invitrogen, Carlsbad, CA) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and fetal calf serum (10%). The human bronchial epithelial cell line (BEAS-2B) was purchased from ATCC and was cultured in Ham's F-12 medium (Invitrogen) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and fetal calf serum (10%). Okadaic acid (OA) was purchased from Biomol International (Plymouth Meeting, PA) and dissolved in DMSO. TNFα was purchased from PeproTech Incorporated (Rocky Hill, NJ). Anti-phospho-p38 (Thr180/Tyr182; Imgenex, San Diego, CA), anti-p38 (Cell Signaling, Danvers, MA), anti-phospho-JNK (Promega, Madison, WI), anti-JNK (Cell Signaling), anti-phospho-ERK (Cell Signaling), anti-ERK (Cell Signaling), anti-IκBα (Cell Signaling), and anti-GAPDH (Abcam, Cambridge, MA) were used per the manufacturers' suggestions.
Cellular model of alveolar epithelial cell production of IL-8.
Our experiments were based on the cell model described by Standiford et al. (41), which provides a well-established model to examine respiratory epithelial cell biological responses. A549 or BEAS-2B cells were plated to a density of 0.8 × 106 cells/well, unless otherwise indicated, in six-well culture plates in medium (Invitrogen) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and fetal calf serum (10%). Cells were allowed to adhere for 4–6 h. The media was then changed to serum-free medium (Invitrogen) supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml), and the cells were incubated overnight (14–16 h). IL-8 production was induced by incubating the cells in serum-free media containing TNFα for the times indicated in the various experiments. Cells pretreated with inhibitors before TNFα stimulation were washed twice with serum-free media following the pretreatment and before TNFα stimulation. Conditions of pretreatment before TNFα treatment are detailed in subsequent methods. Due to the inhibition of the complex cellular processes involved in these experiments, the duration of inhibitor or TNFα exposure as well as the concentration of inhibitors and TNFα were optimized for each experiment to yield maximal IL-8 production while limiting cell death.
IL-8 determination (ELISA).
A549 cells were exposed to the experimental conditions outlined, and media was harvested after treatment. Immunoreactive IL-8 concentrations were determined using a commercially available human IL-8 ELISA kit (BioSource International, Camarillo, CA). All procedures were performed according to the manufacturer's protocol.
Serine/threonine phosphatase assay.
Cells were exposed to the experimental conditions indicated in results. After two washes with 0.9% NaCl, total cellular proteins were extracted in lysis buffer containing 50 mM Tris·HCl, pH 7.5, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 1% Triton X-100, and 0.5% Nonidet P-40 with no phosphatase inhibitors. A non-radioactive, malachite green phosphatase assay kit (Millipore, Billerica, MA) was used to measure total serine/threonine activity. Specific PP2A activity was measured using the PP2A Immunoprecipitation Phosphatase Assay Kit (Millipore). All procedures were performed according to the manufacturer's protocol, and changes in absorbance were measured at 650 nm in a Spectra-MAX 250 (Molecular Devices, Sunnyvale, CA) plate reader.
siRNA transfection.
A549 cells were plated in Ham's F-12 media containing 10% fetal bovine serum without antibiotics to a concentration of ∼0.1 × 106 cells/well. Following overnight incubation (14–16 h), the cells were transfected using Lipofectamine 2000 (Invitrogen) per the manufacturer's protocol with 5 μl of 20 μM stock of control siRNA (cat. no. D-001810-10), human PP2A-siRNA (cat. no. L-003598-01), or human PP1-siRNA (cat. no. L-008685-00) purchased from Dharmacon (Lafayette, CO). Cells were cultured for an additional 48 h and then incubated overnight (14–16 h) in serum-free medium before stimulation with TNFα (4 ng/ml) for 4 h.
Immunoblotting.
Cells were pretreated with OA (1 μM for 15 min) and then stimulated with TNFα (4 ng/ml) or stimulated with TNFα (4 ng/ml) alone for indicated lengths of time. Cells were harvested and lysed using RIPA buffer containing 25 mM Tris·HCl, pH 7.6, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% SDS with 10 μl of Halt Protease Inhibitor Cocktail (Pierce, Rockford, IL) added for each 1 ml of buffer. Samples were separated using SDS-PAGE and transferred to nitrocellulose. Nitrocellulose blots were probed with antibodies against phospho-JNK, JNK, phospho-p38, phospho-ERK, ERK, IκBα, or GAPDH as indicated in results.
Luciferase assay.
Cells were transiently transfected with IL-8-luc constructs using Fugene 6 (Roche, Indianapolis, IN) per the manufacturer's protocol. One day after transfection, cells were either pretreated with 1 μM OA for 15 min and then stimulated with TNFα (4 ng/ml) or stimulated with TNF-α alone. Cells were harvested 4 h following TNFα stimulation in Reporter Lysis Buffer (Promega, Madison, WI). Luciferase activity was measured using a Lucifer Assay System (Promega) and a Berthold Autolumat Plus LB 953 luminometer (Berthold Technologies, Oak Ridge, TN).
Ceramide inhibition.
The effects of acid sphingomyelinase inhibition were tested by incubating cells with 10 μM desipramine (Sigma-Aldrich, St. Louis, MO) for 1 h. The cells were then stimulated with 2 ng/ml TNFα for 4 h. To determine the effects of neutral sphingomyelinase inhibition, A549 cells were incubated with 15 μM GW4869 (Calbiochem, Madison, WI) for 2 h and then stimulated with TNFα (2 ng/ml for 4 h). The effects of de novo ceramide synthesis inhibition were tested by incubating the cells with 35 μM fumonisin B1 (Calbiochem) for 24 h and then stimulating them with TNFα (2 ng/ml for 4 h). Under all conditions, cell media was collected, and IL-8 production was assessed by ELISA.
Ceramide assay.
A549 cells were incubated for 24 h in media containing fumonisin B1 and then were stimulated with TNFα as described above. Following treatment, lipids from the cells were extracted by the method of Van Veldhoven et al. (44), and total cellular ceramide was measured using the technique described by Preiss et al. (34).
IL-8 mRNA stability.
A549 cells were either pretreated with OA (1 μM for 15 min) followed by TNFα stimulation (4 ng/ml) or stimulated with TNFα alone (4 ng/ml). After 2 h of TNFα stimulation, the media was changed to media containing actinomycin D (5 μg/ml, Sigma-Aldrich) to inhibit further transcription. Total RNA was isolated from the cells by use of the RNeasy Mini Kit (Qiagen, Valencia, CA) at the times indicated. Quantitative RT-PCR was performed on the samples using IL-8 TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) following cDNA production using the High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems).
Statistical analysis.
Results are reported as means ± SE. Statistical significance for parametric data was determined using an unpaired t-test for experiments comprising two groups and a one-way ANOVA for experiments comprising three or more groups. Statistical tests were conducted using Sigma Stat 3.0 (Systat, San Jose, CA).
RESULTS
Inhibition of PP2A augments IL-8 production.
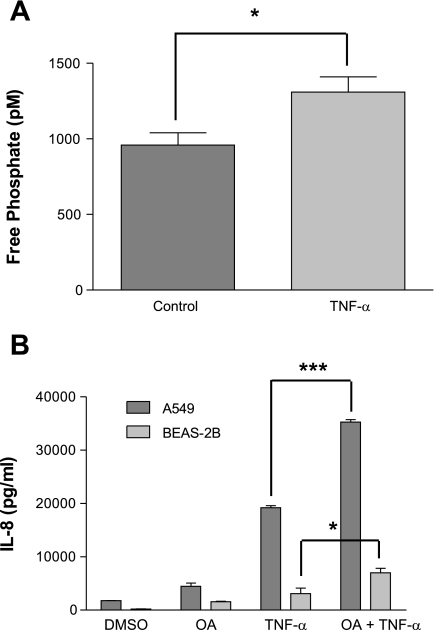
To investigate the effect of TNFα stimulation specifically on PP2A activity, we immunoprecipitated PP2A and detected a 36% increase in PP2A activity (Fig. 1A) following TNFα stimulation in A549 cells. Before determining if PP2A regulated the TNFα induction of IL-8 production, we optimized conditions to inhibit PP2A activity and found that incubating cells in 1 μM OA, a serine/threonine (Ser/Thr) phosphatase inhibitor, for 15 min decreased phosphatase activity by one-third with little effect on cell viability (data not shown). This dose and duration of pretreatment with OA was used in all subsequent experiments. Treatment of both A549 and BEAS-2B cells with TNFα after OA pretreatment nearly doubled the production of IL-8 compared with cells treated with vehicle control and TNFα (0.01% DMSO) (Fig. 1B). These data indicated that the TNFα induction of IL-8 in respiratory epithelial cells is regulated by a Ser/Thr phosphatase.
Fig. 1.
Serine/threonine inhibition augments IL-8 production. A: A549 cells were stimulated with TNFα (4 ng/ml for 4 h), and PP2A phosphatase activity in cell lysates was measured using a malachite green phosphatase assay after PP2A immunoprecipitation. B: A549 and BEAS-2B cells were stimulated with TNFα (4 ng/ml for 4 h) following okadaic acid (OA; 1 μM for 15 min) inhibition, and IL-8 ELISAs were performed on cell media. Data are shown as means ± SE. *P < 0.05; ***P < 0.001.
Using in vitro phosphatase assays and purified proteins, OA shows preferential inhibition of PP2A at lower doses, but may also inhibit PP1, the other major Ser/Thr phosphatase, at higher concentrations. Thus, because the intracellular concentration of OA was unknown in our model, we aimed to determine the contribution of PP1 and PP2A to this IL-8 regulation. To do so, we used siRNA directed at both PP1 and PP2A to confirm that the increase in IL-8 production following OA treatment was specifically due to PP2A inhibition. Knockdown of both PP2A and PP1 was confirmed by Western blot (Fig. 2A). Following knockdown of PP2A, we detected a 3.5-fold increase (P < 0.001) in TNFα-induced IL-8 production (Fig. 2B) compared with cells transfected with scrambled (control) siRNA. Importantly, no change in IL-8 production occurred following knockdown of PP1 (Fig. 2B). These results indicated that regulation of IL-8 production observed with OA inhibition was specific for PP2A.
Fig. 2.
IL-8 augmentation following PP2A knockdown. A549 cells were incubated with siRNA (siRNA = non-target control siRNA; siPP1 = siRNA to PP1; siPP2A = siRNA to PP2A) and then stimulated with TNFα (4 ng/ml for 4 h). A: Western blots probed with antibodies against PP1, PP2A, and GAPDH confirm knockdown of PP2A and PP1. B: IL-8 ELISAs were performed on media collected from A549 cell cultures following gene knockdown (either PP1 or PP2A as indicated) and TNFα (4 ng/ml for 6 h) stimulation. Data are shown as means ± SE. ***P < 0.001. These results are representative of 3 experiments.
PP2A inhibition increases phosphorylation of JNK, p38, and ERK to affect both gene activation and posttranscriptional stabilization of IL-8.
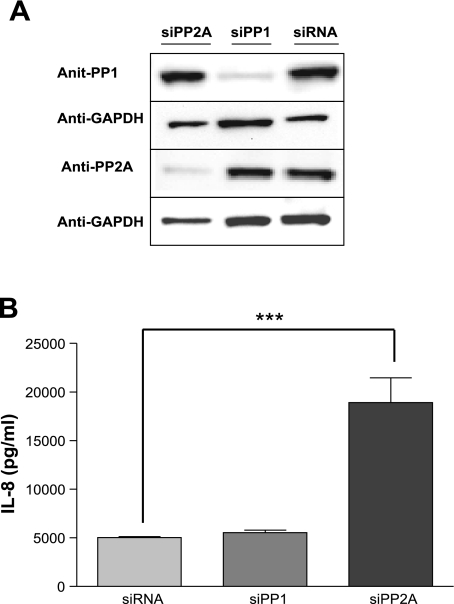
We next sought to determine the mechanism by which PP2A regulated the signaling pathways involved in TNFα-induced IL-8 production. A549 cells were similarly pretreated with OA (1 μM for 15 min) or vehicle control followed by TNFα stimulation (4 ng/ml) for various durations up to 90 min. Cell lysates subjected to Western blot analysis were probed with antibodies to the phosphorylated terminal kinases of the MAPK pathways (JNK, p38, and ERK) as well as the inhibitor protein, IκBα, of the NF-κB pathway. Pretreatment with OA increased phosphorylation of JNK, p38, and ERK compared with cells treated with TNFα and vehicle control (Fig. 3). The phosphorylation and subsequent degradation of IκBα was delayed following inhibition of PP2A (Fig. 3). These data support the involvement of the three arms of the MAPK pathway in the PP2A regulation of IL-8 production. Interestingly, the data also confirm a positive regulatory role of PP2A on the NF-κB pathway as previously reported by Kray et al. (28).
Fig. 3.
Effect of PP2A inhibition on the MAPK and NF-κB signal pathways. A: A549 cells were treated with OA (1 μM for 15 min) followed by TNFα stimulation (4 ng/ml) for the times indicated. Western blots of cell lysates probed with antibodies to the phosphorylated forms of JNK, p38, and ERK indicate prolonged activation of JNK, p38, and ERK following PP2A inhibition. Densitometric comparisons show the percentage of phosphorylated forms to total forms of 1) p38, 2) JNK, 3) ERK p44, and 4) ERK p42 (**P < 0.01, ***P < 0.001). B: Western blots of cell lysates probed with antibodies to IκBα show delayed IκBα degradation following PP2A inhibition. Densitometric data compare IκBα at the various time points. These blots are representative of 3 experiments.
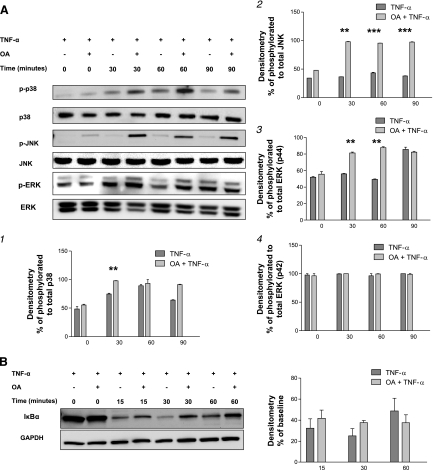
Our data supported a dual regulatory role for PP2A (a negative regulation of MAPK activation and a positive regulation of the NF-κB pathway). We next determined the overall effect of PP2A inhibition on the transactivation of the IL-8 gene using an IL-8-promoter-driven luciferase construct (IL-8-luc). Following transfection of the A549 cells with the IL-8-luc construct, cells were pretreated with OA followed by TNFα stimulation (4 ng/ml, 4 h). Phosphatase inhibition with OA significantly augmented the promoter transactivation (Fig. 4A; P < 0.001) compared with TNFα stimulation alone. This increased promoter activation was consistent with a role for PP2A as an endogenous deactivator of JNK and ERK, thereby reducing the activation of the IL-8 promoter.
Fig. 4.
PP2A regulates IL-8 production in both a transcriptional and posttranscriptional manner. A: A549 cells were transfected with IL-8 promoter luciferase construct followed by phosphatase inhibition with OA (1 μM for 15 min) before TNFα stimulation (4 ng/ml for 4 h) resulted in an increase in IL-8 gene promoter activity. A549 cells were pretreated with OA (1 μM for 15 min) followed by TNFα stimulation (4 ng/ml for 4 h) at which time further transcription was inhibited with actinomycin D (5 μg/ml). B: total RNA was harvested at the times indicated, and quantitative RT-PCR showed an increase in the half-life of IL-8 mRNA following OA pretreatment. Data are shown as means ± SE. ***P < 0.001.
Furthermore, because p38 had been shown to be crucial to posttranscriptional regulation of IL-8 gene expression (23, 24, 29), we investigated the effect of PP2A inhibition on p38 activation by examining the rate of IL-8 mRNA degradation following treatment with OA. In these experiments, inhibition of PP2A significantly prolonged the stability of IL-8 mRNA from 45 min following TNFα stimulation to 75 min when PP2A was inhibited before TNFα treatment (Fig. 4B). These data are consistent with PP2A regulation of p38 with a consequent effect on IL-8 mRNA stabilization. Together, these results suggest that PP2A modulates IL-8 production primarily by regulating the three MAPK pathways affecting both transcriptional and posttranscriptional processes.
Ceramide synthesis inhibition results in increased IL-8 production.
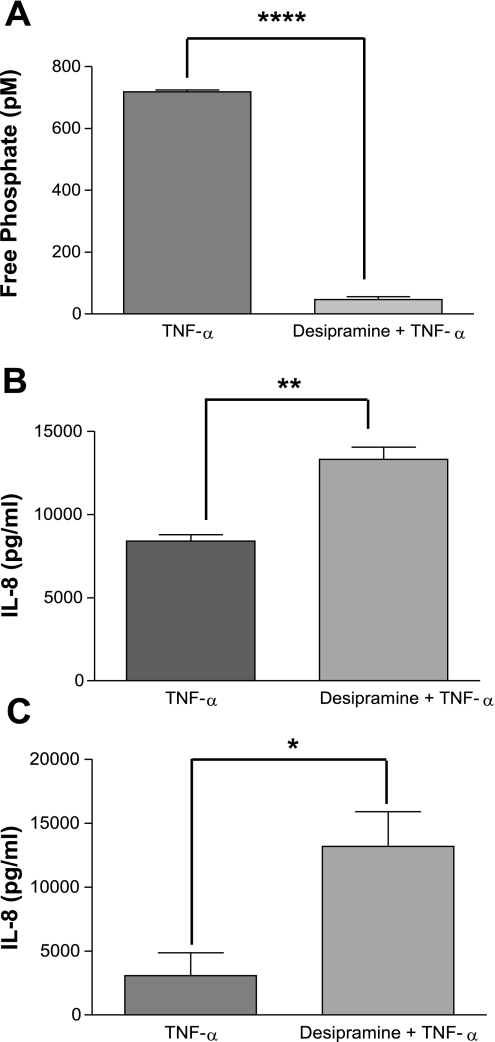
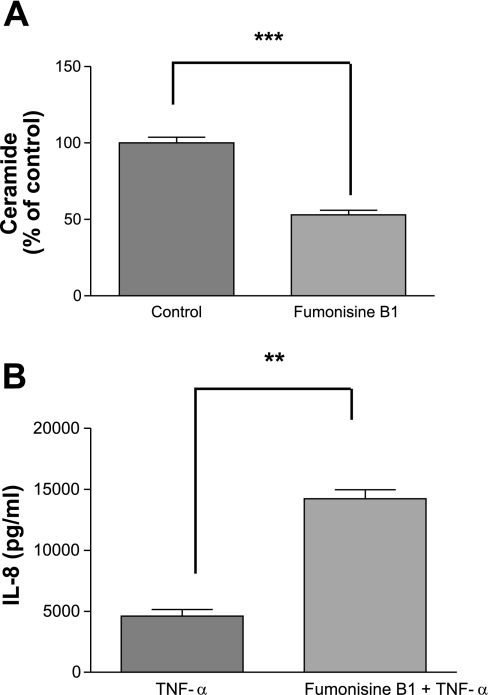
The sphingolipid ceramide is a second messenger produced following TNFα stimulation in a number of cell types (30) and participates in myriad cell functions. Relevant to these current studies, ceramide has been shown to stimulate PP2A activity (10, 14, 15, 20, 47). As TNFα induces both IL-8 and ceramide, we hypothesized that an endogenous regulatory pathway existed whereby TNFα induction of ceramide subsequently activates PP2A, which then downregulates the MAPK pathways. We therefore examined the role of ceramide in the activation of PP2A and the subsequent effect on IL-8 production. Given the complexity of the ceramide metabolic pathways, we approached this by inhibiting various targets within the immediate sphingomyelinase-dependent pathway, as well as the longer de novo synthesis pathway (ceramide synthase). Inhibition of acid sphingomyelinase with desipramine (10 μM for 1 h) before TNFα stimulation decreased the Ser/Thr phosphatase activity to minimal levels (Fig. 5A; P < 0.0001) while augmenting IL-8 production by 1.5-fold in A549 cells (Fig. 5B; P < 0.01) and 4-fold in BEAS-2B cells. In contrast, no significant difference in Ser/Thr phosphatase activity or IL-8 production was detected following neutral sphingomyelinase inhibition using GW4869 (data not shown). To confirm the regulation of IL-8 production was influenced by ceramide levels, we also inhibited the de novo synthesis pathway by inhibiting ceramide synthase with fumonisin B1 (35 μM for 24 h), which resulted in a 53% decrease in basal ceramide levels (Fig. 6A; P < 0.001) and a 3-fold increase in TNFα-induced IL-8 production (Fig. 6B; P < 0.01) in cells pretreated with fumonisin B1 compared with cells pretreated with vehicle before TNFα stimulation. These results establish a strong link between TNFα stimulation and subsequent ceramide accumulation and the activation of PP2A, which serves as an endogenous regulator of the MAPKs to affect IL-8 production.
Fig. 5.
Inhibition of immediate ceramide production augments IL-8 production. A549 cells were pretreated with the sphingomyelinase inhibitor desipramine to alter the TNFα induction of ceramide production. A: following acid sphingomyelinase inhibition with desipramine (10 μM for 1 h) and subsequent TNFα stimulation (2 ng/ml for 4 h), phosphatase activity, as measured by malachite green phosphatase assay, decreased. IL-8 ELISA performed on the cell culture media indicated an augmented production of IL-8 in A549 cells (B) and in BEAS-2B cells (C). Data are shown as means ± SE. ****P < 0.0001, **P < 0.01, *P < 0.05.
Fig. 6.
Inhibition of de novo synthesis of ceramide augments IL-8 production. A549 cells were pretreated with fumonisine B1 (35 μM for 24 h) followed by TNFα stimulation (2 ng/ml for 6 h). Ceramide levels decreased following fumonisine B1 pretreatment (A) while IL-8 ELISA performed on the cell culture media indicated an augmented production of IL-8 (B). Data are shown as means ± SE. ***P < 0.001 significance compared with control cells; **P < 0.01 significance compared with TNFα treatment alone.
DISCUSSION
IL-8 is a key mediator of the pathophysiology of ALI and its most severe clinical manifestation, ARDS. A strong correlation between the severity of ALI and increasing IL-8 levels, both in bronchoalveolar lavage fluid and in blood, has been established in a number of clinical investigations (12, 16, 25, 31, 32). More recently, IL-8 has been purported as a stratification factor in pediatric septic shock (48). Thus, the targeting of IL-8 gene expression has been the focus of a number of studies. Despite this emphasis, little is known about endogenous mechanisms that may downregulate IL-8 gene expression. In respiratory epithelial cells, IL-8 production following TNFα stimulation is known to involve both the NF-κB and the MAPK signaling pathways (17, 21, 22, 29). These two pathways interact to increase IL-8 gene transcription through promoter activation as well as posttranscriptional stabilization of the mRNA to facilitate maximal IL-8 production. We provide data that indicate a role for PP2A in the regulation of these pathways involved in TNFα-induced IL-8 production.
PP2A regulation of IL-8 gene expression is consistent with prior reports of the involvement of Ser/Thr phosphatases in IL-8 regulation in neutrophils, macrophages, and promyelocitic cells (3, 33, 40). Refsnes et al. (35) also detected increases in fluoride-induced IL-8 production in A549 cells following OA inhibition, although the mechanism of this finding was not explored. The nonselective nature of OA used in all of these studies does not distinguish between the contribution of PP1 or PP2A to the regulation of the pathways involved. However, using siRNA knockdown of PP2A, we clearly showed that the changes in IL-8 production following OA inhibition are due to PP2A, as no change in IL-8 production occurred following PP1 knockdown (Fig. 2).
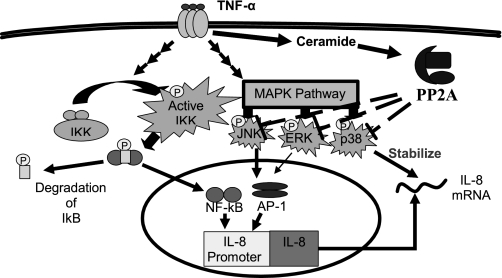
It has also been recognized that sphingolipids are also involved in both TNFα signaling (30) as well as IL-8 gene expression (11). Given that ceramide is both a key component of the sphingolipid pathways induced by TNFα and an established activator of PP2A (formerly cloned as the ceramide-activated protein phosphatase), we hypothesized that these molecules were intrinsically linked in an endogenous counterregulatory pathway targeting IL-8 gene expression. Our results provide evidence that while TNFα stimulates production of IL-8 to mount a necessary host response of neutrophil infiltration, it simultaneously activates acid sphingomyelinase and increases ceramide. In turn, ceramide activates PP2A, which consequently downregulates the MAPK pathways, thereby serving to terminate ongoing production of IL-8 (Fig. 7).
Fig. 7.
PP2A is an endogenous regulator of TNFα induction of IL-8 in alveolar epithelial cells. TNFα activates both the NF-κB and MAPK pathways resulting in NF-κB and AP-1 binding to the IL-8 promoter allowing maximal IL-8 mRNA production. The IL-8 mRNA is stabilized in a posttranslational stabilization by p38 resulting in maximal IL-8 production. TNFα also activates acid sphingomyelinase resulting in the increased production of ceramide. Ceramide activates PP2A, which deactivates the JNK, p38, and ERK MAPK pathways, resulting in the reduction of ongoing IL-8 production.
A broader role for PP2A in regulating the JNK, p38, and ERK pathways is well established. Previous data from our lab showed that the PP2A regulates the JNK pathway (37) by acting on upstream kinases (50). We have confirmed in our cell model that PP2A not only regulates JNK but also contributes to a decrease in transactivation of the IL-8 promoter (Fig. 4A). The regulation of the p38 MAPK pathway by PP2A has also been established (4, 7, 8, 42), and this has been shown to involve stabilization of mRNA by altering tristetraprolin (TTP) binding to the 3′-untranslated regions of unstable transcripts (42). Our findings extend this regulatory role to a critical pathological pathway in ALI, TNFα induction of IL-8 from alveolar epithelial cells. While we show that PP2A regulates p38 to affect IL-8 mRNA stability as reflected by significant changes in the half-life of the IL-8 transcript (Fig. 4B), the role of TTP in this regulation needs to be further elucidated. The involvement of ERK in the TNFα-induced expression of IL-8 in the respiratory epithelium (29) and PP2A's involvement in ERK deactivation (26) have previously been shown. Here we show that PP2A-mediated deactivation of ERK occurs in the lung epithelium and contributes to the regulation of IL-8 production.
In contrast to the MAPK pathways, our data indicated a positive regulatory role for PP2A on NF-κB signaling in respiratory epithelial cells similar to the positive regulatory role for PP2A on the NF-κB pathway reported by Kray et al. (28). Although this positive regulation seems counterproductive for an endogenous system designed to downregulate ongoing IL-8 production, it seems plausible that such a mechanism enables the cell to decrease IL-8 and reduce damage from overproduction but enables the cell to continue to signal through NF-κB, allowing a rapid escalation of IL-8 production in response to a second and/or later stimulus. Further investigations are needed to confirm such a hypothesis.
Having established that PP2A regulated IL-8 production, we wanted to determine the mechanism by which TNFα stimulation resulted in PP2A activation. The sphingolipid second messenger, ceramide, was identified as a prime candidate for a link between TNFα and PP2A activation on the basis of TNFα inducing sphingomyelinases to produce sphingomyelin, which is actively hydrolyzed to ceramide (30). Subsequently, this accumulation of ceramide appears to activate PP2A (10, 15, 20). The presence of this axis in both alveolar and bronchial epithelial cells is supported by our observation that pharmacological modulation aimed at decreasing ceramide accumulation upon TNFα stimulation resulted in a significant augmentation of IL-8 production similar to the degree observed with direct PP2A inhibition (Figs. 5 and 6). From these data, we propose a model (Fig. 7) in which TNFα induces the production of IL-8 by activating both the NF-κB and MAPK pathways. At the same time, TNFα stimulates the conversion of sphingosine to ceramide, which then activates PP2A and then serves as an endogenous regulator of MAPK activation to stop the ongoing production of IL-8.
A limitation to these studies is that the two isolated cell lines used may respond differently in patients with ALI. Confirmatory studies in animal models of ALI will be necessary before designing therapeutic strategies aimed to make use of this endogenous regulatory system. Additionally, the regulation of IL-8 is extremely complex, and although we have shown that ceramide and PP2A are involved in the regulation of IL-8 production, we cannot eliminate the possible involvement of other phosphatases or sphingolipids.
In summary, we have provided evidence that PP2As act as an endogenous regulator of the TNFα-induced IL-8 production by alveolar epithelial cells. We have shown that ceramide provides a second messenger pathway in which TNFα may activate PP2A to prevent the overproduction of IL-8. Future studies are aimed at examining this axis utilizing animal models of ALI in trying to understand whether related chemokine production is regulated in a similar manner in vivo.
GRANTS
This work was supported by National Institutes of Health Grants K12-HD-047349 (T. T. Cornell) and RO1-GM-66839 (T. P. Shanley).
Acknowledgments
We thank Dr. Yong Han for careful reading of and critical review regarding this manuscript and Kyle Mahoney for technical assistance.
REFERENCES
- 1.Abraham E Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Amat M, Barcons M, Mancebo J, Mateo J, Oliver A, Mayoral JF, Fontcuberta J, Vila L. Evolution of leukotriene B4, peptide leukotrienes, and interleukin-8 plasma concentrations in patients at risk of acute respiratory distress syndrome and with acute respiratory distress syndrome: mortality prognostic study. Crit Care Med 28: 57–62, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Antonicelli F, Brown D, Parmentier M, Drost EM, Hirani N, Rahman I, Donaldson K, MacNee W. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant Nacystelyn. Am J Physiol Lung Cell Mol Physiol 286: L1319–L1327, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Avdi NJ, Malcolm KC, Nick JA, Worthen GS. A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH(2)-terminal kinase pathway in human neutrophils. J Biol Chem 277: 40687–40696, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis 116: 589–615, 1977. [DOI] [PubMed] [Google Scholar]
- 6.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 3: 35–56, 1982. [PubMed] [Google Scholar]
- 7.Boudreau RT, Conrad DM, Hoskin DW. Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T leukemia cells is negatively regulated by PP2A-associated p38 mitogen-activated protein kinase. Cell Signal 19: 139–151, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Boudreau RT, Hoskin DW, Lin TJ. Phosphatase inhibition potentiates IL-6 production by mast cells in response to FcepsilonRI-mediated activation: involvement of p38 MAPK. J Leukoc Biol 76: 1075–1081, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Broaddus VC, Boylan AM, Hoeffel JM, Kim KJ, Sadick M, Chuntharapai A, Hebert CA. Neutralization of IL-8 inhibits neutrophil influx in a rabbit model of endotoxin-induced pleurisy. J Immunol 152: 2960–2967, 1994. [PubMed] [Google Scholar]
- 10.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem 274: 20313–20317, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Chandru H, Boggaram V. The role of sphingosine 1-phosphate in the TNF-alpha induction of IL-8 gene expression in lung epithelial cells. Gene 391: 150–160, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun 61: 4553–4559, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388: 548–554, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Dobrowsky RT, Hannun YA. Ceramide-activated protein phosphatase: partial purification and relationship to protein phosphatase 2A. Adv Lipid Res 25: 91–104, 1993. [PubMed] [Google Scholar]
- 15.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem 268: 15523–15530, 1993. [PubMed] [Google Scholar]
- 16.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341: 643–647, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of TNF-alpha-induced NF-kappaB activation and IL-8 release in A549 cells with the proteasome inhibitor MG-132. Am J Respir Cell Mol Biol 19: 259–268, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96: 107–116, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fudala R, Krupa A, Stankowska D, Allen TC, Kurdowska AK. Anti-interleukin-8 autoantibody:interleukin-8 immune complexes in acute lung injury/acute respiratory distress syndrome. Clin Sci 114: 403–412, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Galadari S, Kishikawa K, Kamibayashi C, Mumby MC, Hannun YA. Purification and characterization of ceramide-activated protein phosphatases. Biochemistry 37: 11232–11238, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Hancox RJ, Stevens DA, Adcock IM, Barnes PJ, Taylor DR. Effects of inhaled beta agonist and corticosteroid treatment on nuclear transcription factors in bronchial mucosa in asthma. Thorax 54: 488–492, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med 158: 1585–1592, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Holtmann H, Enninga J, Kalble S, Thiefes A, Dorrie A, Broemer M, Winzen R, Wilhelm A, Ninomiya-Tsuji J, Matsumoto K, Resch K, Kracht M. The MAPK kinase kinase TAK1 plays a central role in coupling the interleukin-1 receptor to both transcriptional and RNA-targeted mechanisms of gene regulation. J Biol Chem 276: 3508–3516, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D, Malinin NL, Cooper JA, Resch K, Kracht M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol 19: 6742–6753, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorens PG, Van Damme J, De Backer W, Bossaert L, De Jongh RF, Herman AG, Rampart M. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine 4: 592–597, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 22: 954–965, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Keyse SM Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12: 186–192, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kray AE, Carter RS, Pennington KN, Gomez RJ, Sanders LE, Llanes JM, Khan WN, Ballard DW, Wadzinski BE. Positive regulation of IkappaB kinase signaling by protein serine/threonine phosphatase 2A. J Biol Chem 280: 35974–35982, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Kartha S, Iasvovskaia S, Tan A, Bhat RK, Manaligod JM, Page K, Brasier AR, Hershenson MB. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am J Physiol Lung Cell Mol Physiol 283: L690–L699, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J 335: 465–480, 1998. [DOI] [PMC free article] [PubMed]
- 31.Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med 24: 1448–1454, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Miller EJ, Cohen AB, Nagao S, Griffith D, Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M, Christophers E, Matthay MA. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis 146: 427–432, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Miskolci V, Castro-Alcaraz S, Nguyen P, Vancura A, Davidson D, Vancurova I. Okadaic acid induces sustained activation of NFkappaB and degradation of the nuclear IkappaBalpha in human neutrophils. Arch Biochem Biophys 417: 44–52, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Preiss JE, Loomis CR, Bell RM, Niedel JE. Quantitative measurement of sn-1,2-diacylglycerols. Methods Enzymol 141: 294–300, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Refsnes M, Thrane EV, Lag M, Thoresen GH, Schwarze PE. Mechanisms in fluoride-induced interleukin-8 synthesis in human lung epithelial cells. Toxicology 167: 145–158, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Reyes JG, Robayna IG, Delgado PS, Gonzalez IH, Aguiar JQ, Rosas FE, Fanjul LF, Galarreta CM. c-Jun is a downstream target for ceramide-activated protein phosphatase in A431 cells. J Biol Chem 271: 21375–21380, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Shanley TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J Immunol 166: 966–972, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Shanley TP, Wong HR. Signal transduction pathways in acute lung injury: NF-κB and AP-1. In: Molecular Biology of Acute Lung Injury, edited by Wong HR and Shanley TP. Boston: Kluwer Academic Publishers, 2001, p. 1–16.
- 40.Sonoda Y, Kasahara T, Yamaguchi Y, Kuno K, Matsushima K, Mukaida N. Stimulation of interleukin-8 production by okadaic acid and vanadate in a human promyelocyte cell line, an HL-60 subline. Possible role of mitogen-activated protein kinase on the okadaic acid-induced NF-kappaB activation. J Biol Chem 272: 15366–15372, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP 3rd, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest 86: 1945–1953, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-alpha mRNA. J Biol Chem 282: 3766–3777, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Sun SC, Maggirwar SB, Harhaj E. Activation of NF-kappa B by phosphatase inhibitors involves the phosphorylation of I kappa B alpha at phosphatase 2A-sensitive sites. J Biol Chem 270: 18347–18351, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Van Veldhoven PP, Bishop WR, Yurivich DA, Bell RM. Ceramide quantitation: evaluation of a mixed micellar assay using E. coli diacylglycerol kinase. Biochem Mol Biol Int 36: 21–30, 1995. [PubMed] [Google Scholar]
- 45.Ware LB Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit Care Med 33: S217–S222, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, Hannun YA. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem 269: 19605–19609, 1994. [PubMed] [Google Scholar]
- 48.Wong HR, Cvijanovich N, Wheeler DS, Bigham MT, Monaco M, Odoms K, Macias WL, Williams MD. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med 178: 276–282, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokoi K, Mukaida N, Harada A, Watanabe Y, Matsushima K. Prevention of endotoxemia-induced acute respiratory distress syndrome-like lung injury in rabbits by a monoclonal antibody to IL-8. Lab Invest 76: 375–384, 1997. [PubMed] [Google Scholar]
- 50.Zhao B, Sun L, Haas M, Denenberg AG, Wong HR, Shanley TP. PP2A regulates upstream members of the c-jun N-terminal kinase mitogen-activated protein kinase signaling pathway. Shock 29: 181–188, 2008. [DOI] [PubMed] [Google Scholar]