Abstract
The anti-inflammatory actions of endogenous glucocorticoids (GCs) are regulated by the activities of the GC-activating and -inactivating enzymes, 11β-hydroxysteroid dehydrogenase (11β-HSD)-1 and 11β-HSD2, respectively, that catalyze the interconversion of the inert GC, cortisone, and its bioactive derivative, cortisol. Proinflammatory cytokines regulate 11β-HSD1 expression in various cell types and thereby modulate the bioavailability of cortisol to the glucocorticoid receptor (GR). Since endogenous GCs reportedly attenuate the airway asthmatic response to allergen exposure, we investigated whether airway smooth muscle (ASM) exhibits cytokine-induced changes in 11β-HSD1 expression that enable the ASM to regulate its own bioavailability of GC and, accordingly, the protective effect of GR signaling on airway function under proasthmatic conditions. Human ASM cells exposed to the primary proasthmatic T helper type 2 (Th2) cytokine, IL-13, exhibited upregulated expression of 11β-HSD1, an effect that was attributed to activation of the transcription factor, AP-1, coupled to MAPK signaling via the ERK1/2 and JNK pathways. The induction of 11β-HSD1 expression and its oxoreductase activity by IL-13 (also IL-4) served to amplify the conversion of cortisone to cortisol by the cytokine-exposed ASM and, hence, heighten GR-mediated transcriptional activation. Extended studies demonstrated that this amplified 11β-HSD1-dependent GC activation enabled physiologically relevant concentrations of cortisone to exert enhanced protection of ASM tissues from the proasthmatic effects of IL-13 on ASM constrictor and relaxation responsiveness. Collectively, these novel findings identify a Th2 cytokine-driven homeostatic feedback mechanism in ASM that enhances its responsiveness to endogenous GCs by upregulating 11β-HSD1 activity, thereby curtailing the adverse effects of the proasthmatic cytokine on airway function.
Keywords: asthma, T helper type 2 cytokines, mitogen-activated protein kinase activation, activator protein-1, glucocorticoid receptor transactivation
the adrenal cortex releases endogenous glucocorticoids (GCs) that regulate a host of critical physiological functions such as stress and inflammatory responses, cellular growth and differentiation, and a variety of metabolic processes. The actions of GCs are mediated by their binding to the intracellular glucocorticoid receptor (GR), enabling it to become dissociated from its chaperone proteins, hyperphosphorylated, and translocated to the nucleus. Apart from its transactivation function involving stimulation of the glucocorticoid response element (GRE) in GC-responsive genes, the GR can also inhibit gene expression via transrepression, a mechanism that includes direct binding of the GR to proinflammatory transcription factors such as AP-1 and NF-κB, thereby inhibiting their potential for proinflammatory gene expression (3). The latter mechanism is believed to be principally involved in mediating the anti-inflammatory actions of GCs (53).
Downstream of their systemic vascular distribution, the actions of endogenous GCs are influenced by the tissue-specific activities of GC-activating and -inactivating enzymes that regulate the concentration of active GC available to the intracellular GR. The latter prereceptor control of cellular GC bioavailability is attributed to the actions of the 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes that act by interconverting inert and bioactive endogenous GCs (5, 49). Accordingly, 11β-HSD1, which is widely distributed in most tissues, largely acts in vivo as an NADPH-dependent oxoreductase that converts the inactive 11β-oxoglucocorticoid, cortisone, into the bioactive 11β-hydroxyglucocorticoid, cortisol. Conversely, the isozyme, 11β-HSD2, is a NAD+-dependent dehydrogenase that is primarily expressed in mineralocorticoid-responsive tissues (e.g., kidney and colon) where it catalyzes the inactivation of cortisol by converting the latter to cortisone, thereby protecting the mineralocorticoid receptors in these tissues from being occupied by GCs and enabling these receptors to be stimulated by circulating mineralocorticoids (5, 9, 49). Thus the 11β-HSD1 and 11β-HSD2 enzymes serve as key “gate keepers” that regulate the bioavailability of endogenous GCs and mineralocorticoids at their respective tissue-specific receptors.
In recent years, a number of studies have demonstrated that the localized anti-inflammatory effects of GCs are importantly regulated by the surrounding proinflammatory cytokine milieu, which can modulate the expression of 11β-HSD1 and thereby the intracellular concentration of bioactive GC (5, 49). In this context, 11β-HSD1 expression in human monocytes was found to be potently induced by the T helper type 2 (Th2)-type cytokines, IL-4 and IL-13, and this effect was shown to be inhibited by the Th1 cytokine, IFN-γ (48). Comparably, in a variety of other immune and nonimmune cell types, including both vascular and bronchial smooth muscle cells (7), the pleiotropic cytokines, TNFα and IL-1β, were found to also upregulate 11β-HSD1 expression (7, 9, 11, 14, 57), whereas 11β-HSD2 expression was downregulated by these cytokines, implicating reduced GC inactivation (7, 9, 11). Collectively, these previous studies suggest that the localized adverse effects of specific proinflammatory cytokines may be mitigated by their induced upregulation of 11β-HSD1 oxoreductase activity, the latter serving an anti-inflammatory feedback function by facilitating endogenous GC bioavailability at the affected tissue site. In support of this localized feedback mechanism, it was recently reported that, relative to serum, the levels of cortisone detected in synovial fluid samples from patients with rheumatoid arthritis are reduced, a finding that was attributed to enhanced localized GC activation due to upregulated 11β-HSD1 reductase activity primarily exhibited by synovial fibroblasts (26).
In parallel with the well-established therapeutic benefit of exogenously administered GCs in the treatment of asthma, it has been demonstrated that serum levels of endogenous GCs are increased in asthmatic individuals following allergen challenge and that their release plays an important role in attenuating the bronchoconstrictor response to allergen exposure (6, 15, 39). Similarly, studies on animal models of allergic asthma have reported that endogenously produced GCs serve to suppress both the pulmonary eosinophilic infiltration and airway constrictor hyperresponsiveness elicited by allergen exposure (13, 16, 36, 40, 45).
In view of the above findings together with those demonstrating upregulated 11β-HSD1 expression in different cell types exposed to proinflammatory cytokines (7, 9, 11, 14, 57), the present study was undertaken to determine whether airway smooth muscle (ASM) exhibits cytokine-induced changes in 11β-HSD1 expression that modulate its generation of bioactive GC and thereby the effects of downstream signaling via the GR on airway function under proasthmatic conditions. The results provide new evidence demonstrating that: 1) the principal proasthmatic Th2-type cytokine, IL-13, elicits upregulated expression of 11β-HSD1 in ASM that is MAPK-dependent and mediated by activation of the transcription factor, AP-1; and 2) the latter effect of IL-13 (also IL-4) is associated with enhanced 11β-HSD1 oxoreductase activity in ASM, which facilitates its ability to convert physiologically relevant concentrations of inert cortisone into bioactive cortisol and thereby suppress the opposing proasthmatic effects of IL-13 on ASM constrictor and relaxation responsiveness. Collectively, these findings identify that ASM exhibits a Th2 cytokine-induced homeostatic mechanism that amplifies the prereceptor bioavailability and consequent actions of endogenous GCs, thereby serving to curtail the proasthmatic impact of the cytokine on airway function.
MATERIALS AND METHODS
Reagents.
All chemicals used were purchased from Sigma (St. Louis, MO) unless otherwise indicated. The human ASM cells and smooth muscle basal medium (SMBM) were obtained from BioWhittaker (Walkersville, MD), Ham's F-12 media was purchased from Mediatech (Herndon, VA), and 10% FBS was from HyClone Laboratories (Logan, UT). The antibodies used for Western blotting were purchased from Cell Signaling Technology (Boston, MA), and the enhanced chemiluminescence (ECL) reagents were obtained from Amersham (Little Chalfont, United Kingdom). The nuclear protein extraction kit (NucBuster) was obtained from Novagen (San Diego, CA), and the EMSA kits were from Panomics (Redwood City, CA). The NF-κB inhibitors, BAY 11-7082 and SN50, were obtained from Alexis Biochemicals (San Diego, CA). The AP-1 inhibitor, SR 11302, was obtained from Tocris Bioscience (Ellisville, MO), and nordihydroguaiaretic acid (NDGA), the MEK-ERK1/2 inhibitor, U0126, the p38 MAPK inhibitor, SB-202190, and the JNK inhibitor, SP-600125, were purchased from Calbiochem (La Jolla, CA). The anti-IL-4Rα receptor blocking MAb were obtained from R&D Systems (Minneapolis, MN), and human recombinant IL-13 and IL-4 were purchased from PeproTech (Rocky Hill, NJ).
Animals.
Twenty-one adult New Zealand White rabbits were used in this study, which was approved by the Biosafety and Animal Research Committee of the Joseph Stokes Research Institute at Children's Hospital of Philadelphia. The animals had no signs of respiratory disease for several weeks before the study, and their care and use were in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council.
Culture and treatment of ASM cells.
Human ASM cells were grown in SMBM supplemented with 10% FBS and 1% of penicillin-streptomycin solution and maintained throughout in a humidified incubator containing 5% CO2 in air at 37°C. Cells were used within passages 3-5 for all experiments. The experimental protocols involved growing the cells to ∼85% confluence in the above medium. Thereafter, in separate experiments, the cells were starved in unsupplemented Ham's F-12 media for 24 h, treated with different concentrations and for varying durations with IL-13 or IL-4 in the absence and presence of inhibitors directed against specific transcription factors or MAPKs, and then examined for induced changes in 11β-HSD mRNA expression, transcription factor activation, and 11β-HSD1 oxoreductase activity, as described.
Detection of 11β-HSD transcripts.
Total RNA was extracted from the cultured ASM cells using the TRIzol method (Invitrogen; Carlsbad, CA), and cDNAs were isolated by RT-PCR using the SuperScript First Strand Synthesis System kit from Invitrogen with the following oligonucleotide primer sets (Integrated DNA Technologies): for 11β-HSD1, 5′-TGCTCATTCTCAACCACATCAC-3′ (forward) and 5′-ACAGAACAGTCCCAAAATCCC-3′ (reverse); for 11β-HSD2, 5′-GGCTGTGACTCTGGTTTTG-3′ (forward) and 5′-AACTGCCCATGCAAGTGCTC-3′ (reverse); and for β-actin, 5′-GAGAAGAGCTACGAGCTGCCTGAC-3′ (forward) and 5′-CGGAGTACTTGCGCTCAGGAGGAG-3′ (reverse). The reaction volume was 20 μl, and cycling conditions used were 30 cycles of 15-s denaturation at 95°C followed by 30-s annealing at 60°C and elongation at 72°C for 30 s. Ex Tag (TaKaRa Biotechnology) was used as DNA polymerase. Expressed bands were confirmed by sequencing, and their intensities were analyzed with respect to the densitometric ratio of 11β-HSD1 or 11β-HSD2 to the β-actin intensity detected in the same sample. Quantitative PCR determinations were conducted using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol, wherein fluorescence signals generated by binding of SYBR Green I dye to double-stranded (ds) DNA were recorded at the elongation phase of each cycle, and normalized fluorescence at the threshold cycle (half-maximum intensity) was used to compare the mRNA content in each sample. A typical reaction volume used was 30 μl, and cycling conditions were 40 cycles of 30 s. Denaturation at 95°C was followed by 1-min annealing and elongation at 60°C. To verify binding to specific dsDNA, melting curve analysis was conducted involving initial denaturation of the samples at 95°C and then cooling to 63°C followed by slow heating to 95°C over 20 min. The specificity of the amplified PCR product was determined based on the melting temperature established during optimization of the gene-specific primer set. The mRNA levels in the samples were determined using a standard curve based on the relationship between serial dilutions of known amounts of specific template and the corresponding cycle threshold (Ct) values. The absolute levels of mRNA were normalized to those of β-actin in the same samples.
EMSA of transcription factor binding activity.
Gel shift assays were performed as previously described (42). Briefly, following serum deprivation for 24 h, confluent cultures of ASM cells were treated for varying durations with IL-13, as indicated. Thereafter, the cells were harvested, and nuclear extracts were prepared using a protein extraction kit (Novagen). In separate preparations, the nuclear extracts (5 μg) were incubated with a biotin-tagged oligodeoxynucleotide probe containing the DNA consensus binding site for either NF-κB, CCAAT/enhancer binding protein-α (C/EBP-α), or AP-1, according to the protocol described in the EMSA kits by the manufacturer. The extracts were electrophoresed using a 5% polyacrylamide gel at 4°C and transferred to Biodyne B nylon membrane (Pall, East Hills, NY). The blots were developed using the detection protocol provided in the EMSA kits and visualized by ECL.
siRNA knockdown of C/EBP-α.
ASM cells were seeded into six-well plates, and, at ∼40% confluency, the medium was replaced with the reduced serum-containing medium, Opti-MEM (Invitrogen). The cells were then transfected twice during a 24-h interval with either each of three small interfering RNA (siRNA) duplexes targeted against C/EBP-α (Applied Biosystems; ID numbers 114406, 114407, and 146390), a pool of these siRNAs, or a nontargeted control (scrambled; scRNA) siRNA duplex, using Oligofectamine (Invitrogen) as the transfection agent. The siRNAs were applied to each well at a final concentration of 100 nM for each siRNA preparation. As previously described (28), this double-transfection approach was associated with a high transfection efficiency and, as detected by Western blot analysis, markedly inhibited C/EBP-α expression by the targeted siRNA duplexes, with maximal inhibition detected at 72 h following siRNA transfection (see results).
Kinetic analysis of 11β-HSD1 oxoreductase activity.
Confluent cultures of human ASM cells were treated with either vehicle alone or IL-13 (50 ng/ml) for 24 h and then lysed with 1% Triton X-100 in assay buffer containing 100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 250 mM sucrose, and 20 mM Tris·HCl. Based on preliminary studies designed to determine appropriate protein concentration and the linear time reaction velocity of oxoreductase activity, the enzyme assays were performed using 50 μg of sample protein incubated for 1 h at 37°C in 600 μl of assay buffer containing 500 μM NADPH and varying concentrations of cortisone in the range of 50–5,000 nM. Immediately following incubation, the samples were placed in a boiling water bath for 5 min. 11β-HSD1 oxoreductase activity was then assessed by measuring cortisol production using the Cortisol ELISA kit from R&D Systems as per the manufacturer's protocol, and enzyme activity was expressed in units of picomoles of cortisol produced per milligram of protein per minute. Kinetic analysis of the data was subsequently performed to derive the apparent Michaelis-Menten constant (Km) and maximal velocity (Vmax) values based on nonlinear regression using the Michaelis-Menten equation as well as sigmoidal analysis of the log dose-response relationships, and the latter analysis was also used to derive the Hill coefficients. The enzyme kinetic parameters were determined using the Prism 4 computer program by GraphPad Software (San Diego, CA).
GRE-driven transactivation.
GR-dependent transcriptional activation was assayed using the Pathway Profiling System from Clontech (Mountain View, CA). After being grown to ∼85% confluence, ASM cells were transfected using calcium phosphate with a vector containing a promoter-reporter construct, pGRE-SEAP, that is comprised of three GRE sequences upstream from a TATA box and a reporter gene that encodes a secretable form of alkaline phosphatase (SEAP). Cells were also transfected with pTAL-SEAP, an identical plasmid but without the GRE promoter, serving as a negative control, as well as a plasmid expressing β-galactosidase (pCMV-SPORT-β-gal) serving as an internal control. After 18 h of transfection, the medium was changed to fresh serum-free Ham's F-12 media for 8 h. The cells were then treated for 24 h with IL-13 (50 ng/ml) in the absence and presence of 1 μM cortisone or dexamethasone (DEX) under different experimental conditions, as described. Thereafter, the conditioned culture medium was harvested and assayed for SEAP activity using the Great EscAPe SEAP Chemiluminescence Kit from Clontech according to the manufacturer's protocol. The measured levels of AP activity were then normalized to β-galactosidase activity to correct for any variations caused by differences in transfection efficiency between the cell culture wells.
Preparation and treatment of rabbit ASM tissues.
Following initial sedation and subsequent general anesthesia with intramuscular injections of xylazine (10 mg/kg) and ketamine (50 mg/kg), respectively, rabbits were killed with an intravenously administered overdose of pentobarbital (125 mg/kg). Briefly, as described previously (25), the tracheae were removed via open thoracotomy, the loose connective tissue and epithelium were scraped and removed, and the tracheae were divided into ring segments of 6–8 mm in length. The airway segments were then placed in modified Krebs-Ringer solution containing indomethacin (10 μM), and each alternate ring was incubated for 24 h at room temperature with either vehicle alone (control) or IL-13, each in the absence and presence of either 0.1 μM cortisone or 1 μM DEX, with and without carbenoxolone (CBX; 1 μM). In separate experiments, both control and IL-13-exposed ASM tissues were coexposed to conditioned medium from cultured human ASM cells that were incubated for 24 h with 500 nM cortisone in the absence or presence of IL-13 (50 ng/ml). All the tissues studied were continuously aerated with a gas mixture containing 95% O2 -5% CO2 throughout the incubation phase.
Pharmacodynamic studies of ASM constrictor and relaxant responsiveness.
Following incubation, the tissues were placed in organ baths containing modified Krebs-Ringer solution aerated with 5% CO2 in oxygen (pH 7.35–7.40), and the tissues were attached to force transducers from which isometric tension was continuously displayed on a multichannel recorder, as previously described (25). Cholinergic contractility was then assessed in the ASM segments by cumulative administration of ACh in final bath concentrations ranging from 10−9 to 10−3 M. Thereafter, the tissues were rinsed with fresh buffer, and relaxation dose-response curves to cumulatively administered isoproterenol (10−9 to 10−4 M) were generated after the tissues were half-maximally contracted with their respective ED50 doses of ACh. The initial constrictor dose-response curves to ACh were analyzed with respect to the maximal isometric contractile force (Tmax) of each tissue to the agonist, and the subsequent relaxation responses to isoproterenol were analyzed with respect to percent maximal relaxation (Rmax) from the initial level of active cholinergic contraction.
Statistical analysis.
The results are expressed as means ± SE. Comparisons between groups were made using the Student's t-test (2-tailed) or ANOVA with Tukey posttest analysis, where appropriate, and P values of <0.05 were considered statistically significant. The statistical analyses were conducted using the Prism computer program by GraphPad Software.
RESULTS
IL-13 regulates 11β-HSD expression in human ASM cells.
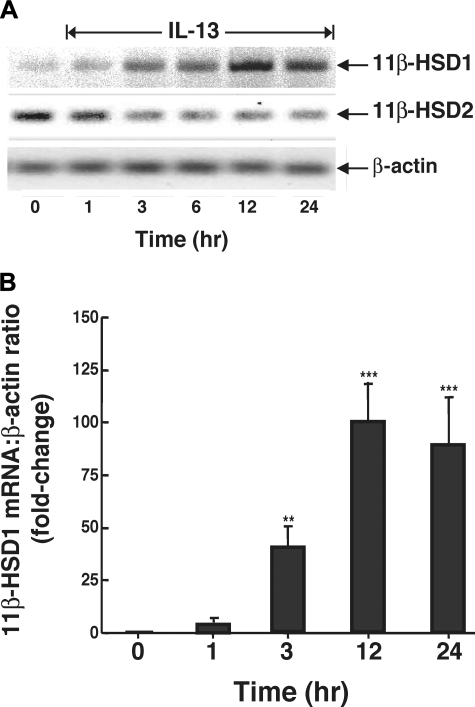
The Th2 cytokines, IL-4 and IL-13, play critical roles in the pathogenesis of allergic airway inflammation, with IL-13 being identified as the central mediator of airway constrictor hyperresponsiveness in asthma (21, 55) due, at least in part, to its direct proasthmatic action in ASM (22, 33). Since both of these cytokines were previously found to upregulate 11β-HSD1 expression and activity in monocytes (48), we initially examined whether IL-13 also modulates 11β-HSD1 and 11β-HSD2 expression in ASM. As exemplified in Fig. 1A, confluent cultures of human ASM cells exposed to a maximally effective concentration of IL-13 (50 ng/ml) exhibited temporal increases in 11β-HSD1 mRNA expression, with distinctly enhanced expression detected at 3 h, peak induction exhibited at 12 h, and upregulated expression sustained at 24 h. The results from 3 experiments using real-time PCR demonstrated that the 11β-HSD1 transcript levels detected at 24 h following IL-13 administration averaged 89.9-fold above those exhibited in unstimulated ASM cells (Fig. 1B). In contrast to its upregulation of 11β-HSD1, IL-13 administration inhibited mRNA expression of the NAD+-dependent dehydrogenase, 11β-HSD2, with maximal inhibition obtained at 3 h that was sustained at 24 h (Fig. 1A). These observations are in general agreement with those in previous studies wherein the pleiotropic proinflammatory cytokines, IL-1β and TNFα, were also found to induce 11β-HSD1 mRNA expression in bronchial smooth muscle cells (7, 24) but failed to increase 11β-HSD2 transcript levels (7).
Fig. 1.
Human airway smooth muscle (ASM) cells exposed to IL-13 exhibit altered expression of 11β-hydroxysteroid dehydrogenase (11β-HSD)-1 and 11β-HSD2 mRNA transcripts. A: as detected by RT-PCR, IL-13 (50 ng/ml) elicits temporal increases in 11β-HSD1 mRNA expression, with peak expression of transcripts detected at 12 h and sustained upregulated expression at 24 h. Conversely, constitutive expression of 11β-HSD2 transcripts is suppressed by IL-13, with maximal inhibition detected at 3 h that is sustained at 24 h. B: temporal changes in intensity of 11β-HSD1 mRNA expression expressed as a ratio of the corresponding level of β-actin mRNA. Data represent means ± SE based on 3 separate experiments. **P < 0.01; **P < 0.001.
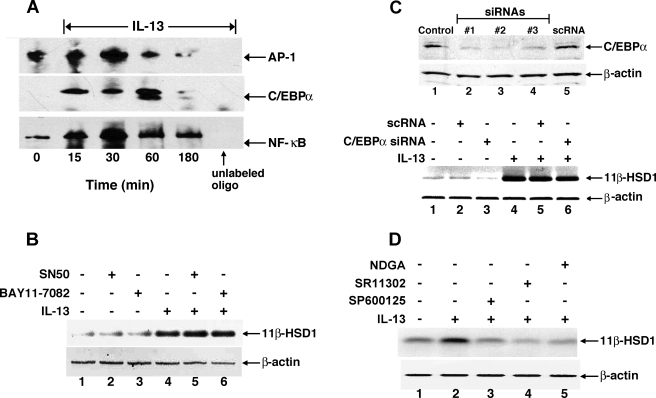
The proximal 5′ promoter region of the 11β-HSD1 gene has several putative transcription binding sites, most notably for the transcription factors AP-1 and C/EBP-α (5, 54). To determine the relative contributions of these transcription factors in mediating IL-13-induced upregulation of 11β-HSD1 expression in ASM, we first examined whether IL-13 evokes changes in their DNA binding activities. For comparison, we also evaluated the DNA binding activity of NF-κB, as this transcription factor has been shown to mediate the proinflammatory effects of IL-13 in the lung (10). EMSA analysis of the binding of each transcription factor to its respective consensus sequence contained in a biotin-labeled DNA probe demonstrated that, relative to unstimulated control cells, the DNA binding activities of AP-1, C/EBP-α, and NF-κB were transiently enhanced following IL-13 administration, with peak increases detected at 30 min for AP-1 and NF-κB and at 60 min for C/EBP-α (Fig. 2A). Small molecule inhibitors and gene silencing were next employed to identify which of these transcription factors is/are responsible for mediating the IL-13-induced upregulated expression of 11β-HSD1. Accordingly, 11β-HSD1 transcript levels were determined by RT-PCR following treatment of ASM cells for 24 h with either vehicle alone (control) or IL-13 (50 ng/ml), both in the absence and presence of pretreatment with specific inhibitors directed against NF-κB or AP-1 signaling or with siRNAs directed at inhibiting C/EBP-α expression. As depicted in Fig. 2B, relative to unstimulated controls (lane 1), ASM cells treated with IL-13 exhibited significantly increased 11β-HSD1 mRNA expression (lane 4); this response was unaffected in IL-13-exposed cells wherein NF-κB signaling was impaired by pretreatment with previously reported (42) maximal effective concentrations of either SN50 (20 μM), an inhibitor of NF-κB translocation to the nucleus, or BAY 11-7082 (10 μM), an inhibitor of IκBα phosphorylation (lanes 5 and 6, respectively). To evaluate the role of C/EBP-α, we initially established in preliminary experiments (n = 3) that Lipofectamine transfection of ASM cells with each of three different siRNA duplexes directed against C/EBP-α produced maximal knockdown of C/EBP-α protein levels at 72 h posttransfection that ranged between 73 and 95%, whereas transfection with a scRNA serving as negative control had no effect, as exemplified at the top of Fig. 2C. Accordingly, ASM cells were treated for 72 h with vehicle alone or with either scRNA or a pool of the three C/EBP-α siRNA duplexes and subsequently examined for induced changes in 11β-HSD1 mRNA at 24 h following IL-13 administration. As shown at the bottom of Fig. 2C, relative to vehicle-exposed cells (lane 1), 11β-HSD1 mRNA expression was enhanced in the IL-13-treated ASM cells (lane 4), and this effect was unaltered by pretreating the cells with either the scrambled or C/EBP-α siRNA duplexes (lanes 5 and 6, respectively), although the C/EBP-α siRNA preparations appeared to inhibit basal 11β-HSD1 mRNA expression (lane 3). Finally, to ascertain the role of AP-1 activation, IL-13-induced expression of 11β-HSD1 transcripts was examined in the absence and presence of pretreatment with each of three small molecule inhibitors of AP-1 signaling including the potent natural antioxidant, NDGA (1 μM), the selective AP-1 inhibitor retinoid, SR 11302 (1 μM), and the JNK inhibitor, SP-600125 (10 μM). IL-13-induced stimulation of 11β-HSD1 transcripts was completely abrogated by pretreating the cells with either of these AP-1 inhibitors (Fig. 2D). Thus these data demonstrate that AP-1 activation is intimately involved in mediating IL-13-induced 11β-HSD1 expression in ASM cells.
Fig. 2.
Inhibition of activator protein-1 (AP-1) activation prevents IL-13-induced upregulation of 11β-HSD1 mRNA. A: representative EMSAs from 3 separate experiments showing that IL-13 elicits increased DNA binding of the transcription factors, AP-1, CCAAT/enhancer binding protein-α (C/EBP-α), and NF-κB, in human ASM cells. Note the lack of detection of specific binding activity for each biotin-labeled probe in the presence of competitive binding using 100-fold excess of the respective unlabeled oligonucleotide. B: upregulation of 11β-HSD1 transcript levels by IL-13 is unaffected in ASM cells pretreated with inhibitors of NF-κB signaling, including SN50 (20 μM) and BAY 11-7082 (10 μM). C, top: representative Western blot depicting knockdown of C/EBP-α protein levels in ASM cells at 72 h following transfection with each of 3 small interfering RNA (siRNA) duplexes (1–3) directed against C/EBP-α, whereas transfection with a scrambled (negative control) siRNA duplex (scRNA) has no inhibitory effect on C/EBP-α protein expression. Bottom: upregulation of 11β-HSD1 transcript levels by IL-13 is unaltered in ASM cells transfected with either a pool of all 3 C/EBP-α siRNAs or the scRNA duplex. D: upregulated expression of 11β-HSD1 mRNA by IL-13 is prevented in ASM cells pretreated with inhibitors of AP-1 signaling, including either nordihydroguaiaretic acid (NDGA; 1 μM), SR 11302 (1 μM), or SP-600125 (10 μM). The immunoblot shown is representative of 3 separate experiments.
Role of MAPK activation in mediating IL-13-induced upregulation of 11β-HSD1 expression.
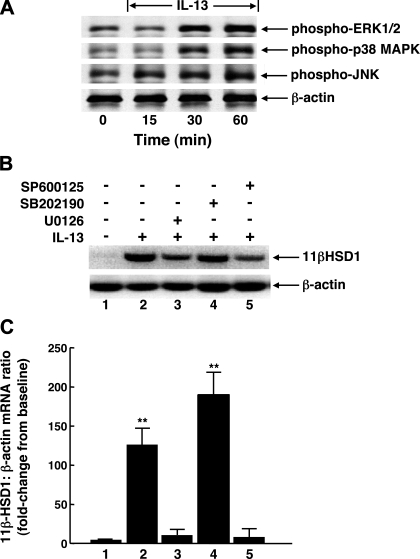
Since MAPKs are known to play critical roles in mediating activation of proinflammatory transcription factors (notably AP-1 and NF-κB) in response to cytokines and other inflammatory stimuli, we next examined whether the AP-1-mediated induction of 11β-HSD1 transcripts by IL-13 is regulated by MAPK activation. As exemplified by the Western blots in Fig. 3A, initial experiments (n = 3) demonstrated that treatment of ASM cells with IL-13 (50 ng/ml) acutely evoked (within 15–30 min) enhanced phosphorylation of the MAPK proteins, ERK1/2, p38 MAPK, and JNK, which was sustained at 60 min. Accordingly, specific small molecule inhibitors directed against these MAPKs were used in subsequent studies aimed at identifying which (if any) of these MAPKs may be responsible for mediating IL-13-induced upregulated expression of 11β-HSD1 transcripts. As depicted by a representative experiment at the top of Fig. 3B, with the corresponding results based on densitometric analysis of the data obtained in four such experiments at the bottom, relative to controls (lane 1), cells treated for 24 h with IL-13 exhibited significantly increased 11β-HSD1 mRNA expression (lane 2), and this response was markedly attenuated in IL-13-exposed cells that were pretreated with either the selective MEK-ERK1/2 inhibitor, U0126 (5 μM; lane 3), or the JNK inhibitor, SP-600125 (10 μM; lane 5). Interestingly, contrasting the suppressive effects of these inhibitors, relative to cells treated with IL-13 alone, the induction of 11β-HSD1 transcripts was further significantly enhanced (P < 0.05) in IL-13-exposed cells that were pretreated with the p38 MAPK inhibitor, SB-202190 (10 μM; lane 4). Taken together, these observations demonstrate that the AP-1-mediated induction of 11β-HSD1 mRNA by IL-13 in ASM cells is coupled to activation of both the ERK1/2 and JNK signaling pathways.
Fig. 3.
Upregulation of 11β-HSD1 transcripts in IL-13-exposed ASM cells is prevented by inhibition of ERK1/2 or JNK signaling. A: representative immunoblots from 1 of 3 experiments depicting IL-13-induced acute phosphorylation (phospho-) of ERK1/2, p38 MAPK, and JNK proteins in ASM cells. Note, expression of β-actin probe is unaltered confirming equal amounts of protein loading in the immunoblot preparations. B: pretreatment of ASM cells with either the MEK-ERK1/2 inhibitor, U0126 (5 μM), or the JNK inhibitor, SP-600125 (10 μM), suppresses IL-13-induced upregulation of 11β-HSD1 transcripts, whereas 11β-HSD1 mRNA expression is enhanced in cells pretreated with the p38 MAPK inhibitor, SB-202190 (10 μM). C: corresponding quantitative analysis of the effects of the latter MAPK inhibitors on IL-13-induced expression of 11β-HSD1 transcripts demonstrating that, relative to untreated control cells (lane 1), the densitometric ratios of 11β-HSD1-to-β-actin mRNA expression are significantly increased in IL-13-exposed ASM cells (lane 2). The latter stimulatory effect of IL-13 on 11β-HSD1 expression is inhibited in cells pretreated with either U0126 (lane 3) or SP-600125 (lane 5), whereas 11β-HSD1 transcript levels are further significantly increased in IL-13-exposed ASM cells that are pretreated with SB-202190 (lane 4). Data are means ± SE from 4 experiments (*P < 0.01).
Effects of IL-13 and IL-4 on 11β-HSD1 oxoreductase activity in ASM cells.
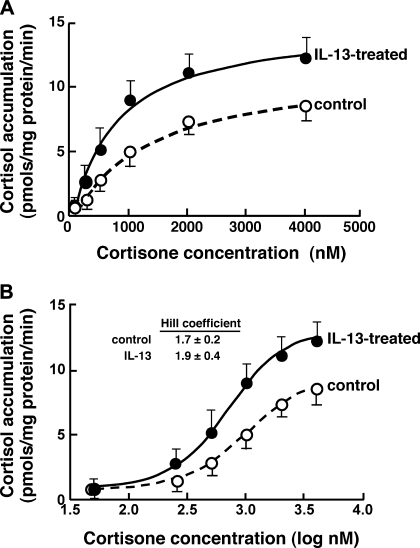
To assess whether the upregulated expression of 11β-HSD1 transcripts by IL-13 is translated into corresponding changes in 11β-HSD1 oxoreductase activity, the latter was determined by measuring cortisol production in response to cortisone (substrate) administration (concentration range: 50–5,000 nM) in extracts of ASM cells that were initially exposed for 24 h to either vehicle alone (control) or IL-13 (50 ng/ml). The results obtained in the control and IL-13-treated cell preparations were compared by fitting the enzyme kinetic data using both the Michaelis-Menten equation (Fig. 4A) and a sigmoidal log dose-response function (Fig. 4B). The latter analysis revealed a better statistical fit of the data and yielded a mean ± SE value for Vmax that was significantly greater in the IL-13-treated vs. control ASM cells, amounting to 13.2 ± 2.1 vs. 9.4 ± 1.4 pmol·mg protein−1·min−1, respectively (P < 0.01). The corresponding computed mean value of Km was lower in the IL-13-treated vs. control cells at 674 ± 88 vs. 997 ± 107 nM, respectively; however, this difference was not statistically significant. Thus these data demonstrate that, in concert with upregulated mRNA expression, 11β-HSD1 oxoreductase activity is increased in IL-13-exposed ASM cells. It is relevant to note that the sigmoidal analysis revealed Hill coefficient values for the control and IL-13-exposed ASM cells that were similar at 1.7 ± 0.2 and 1.9 ± 0.4, respectively (Fig. 4B), the latter values implicating the presence of cooperative kinetics of 11β-HSD1 with cortisone. This finding concurs with earlier evidence demonstrating that human 11β-HSD1 acts predominantly in a dimeric form and thereby exhibits cooperative kinetics with cortisone, as reflected by a Hill coefficient of ∼2 (5).
Fig. 4.
Effect of IL-13 on 11β-HSD1 oxoreductase activity in ASM cells. Kinetics of 11β-HSD1 oxoreductase activity were determined based on measurements of cortisol production from varying concentrations of cortisone (substrate) administered to lysates of ASM cells initially exposed for 24 h to either vehicle alone (control) or IL-13 (50 ng/ml). The kinetics data fitted to the Michaelis-Menten equation (A) and to a sigmoidal logarithmic dose-response curve (B) demonstrate that, relative to control ASM cells, conversion of cortisone to cortisol is enhanced in IL-13-exposed cells. Based on the sigmoidal analysis, the mean maximal velocity (Vmax) is significantly increased in the IL-13-treated vs. control ASM cells, amounting to 13.2 ± 2.1 vs. 9.4 ± 1.4 pmol·mg protein−1·min−1, respectively (P < 0.01), whereas the corresponding mean Michaelis-Menten constant (Km) values of 674 ± 88 vs. 997 ± 107 nM, respectively, are not significantly different. The sigmoidal analysis further reveals similar mean values for the Hill coefficients at 1.9 ± 0.4 and 1.7 ± 0.2 for the IL-13-treated and control ASM cell preparations, respectively. Data represent means ± SE based on 5 experiments.
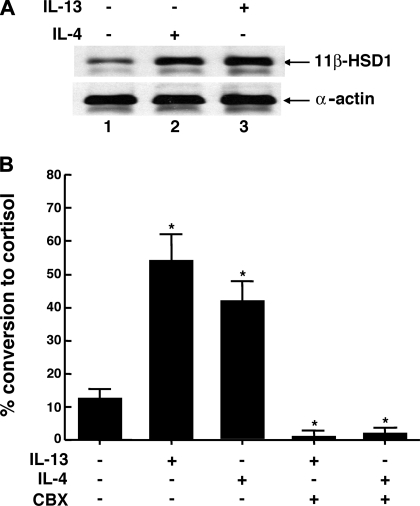
In parallel studies, we next evaluated whether 11β-HSD1 protein expression and oxoreductase activity are comparably altered in ASM cells exposed to IL-4. In these experiments, ASM cells were initially exposed for 24 h to vehicle alone and to 50 ng/ml either IL-4 or IL-13, and, thereafter, in separate preparations, 11β-HSD1 protein expression was determined by Western blotting and oxoreductase activity was determined by measuring cortisol production in the ASM cell culture medium following the administration of a near Km concentration of cortisone (i.e., 1 μM). Figure 5A demonstrates that, as with IL-13, cells treated with IL-4 also exhibited distinctly increased 11β-HSD1 protein expression. Moreover, relative to untreated cells, the means ± SE for percent conversion of cortisone to cortisol were significantly increased in the ASM cell preparations that were exposed to either IL-13 or IL-4 (P < 0.01), and, although the effect of IL-4 was somewhat less pronounced, there was no significant difference between the stimulatory effects of these cytokines (Fig. 5B). As further displayed, coincubation of the ASM cell preparations with the 11β-HSD inhibitor, CBX (1 μM), completely ablated both the IL-13- and IL-4-induced changes in oxoreductase activity and yielded levels of activity that were markedly below those detected in control (unstimulated) cells. In relation to these observations, it should be noted that, in separate experiments, we found that the stimulatory effects of IL-13 and IL-4 on 11β-HSD1 oxoreductase activity were also completely abrogated by coincubating the cytokine-exposed cells with an anti-IL-4Rα receptor blocking MAb (0.1 μg/ml), which inhibits signaling by both IL-4 and IL-13 due to the shared IL-4Rα component in their receptors, whereas coincubation with the same concentration of an isotype control IgG2α antibody had no significant effect (data not shown).
Fig. 5.
Comparison of effects of IL-4 and IL-13 on 11β-HSD1 protein expression and oxoreductase activity in ASM cells. A: representative Western blot from 3 experiments demonstrating upregulation of 11β-HSD1 protein levels following exposure of ASM cells for 24 h to 50 ng/ml either IL-4 or IL-13. B: corresponding oxoreductase activity, expressed as percent conversion of 1 μM cortisone (∼Km) to cortisol, determined following 24 h exposure of ASM cells to vehicle alone and to either IL-4 or IL-13, both in the absence and presence of carbenoxolone (CBX; 1 μM). Relative to untreated cells, oxoreductase activity is significantly increased in cells exposed to either IL-13 or IL-4 (P < 0.01), with no significant difference between the stimulatory effects of these cytokines. Note, coincubation of cells with the 11β-HSD inhibitor, CBX, prevents the induction of oxoreductase activity by IL-13 or IL-4 and yields levels of activity that are significantly below those detected in control cells. Data represent means ± SE based on 4 experiments (*P < 0.01).
IL-13-induced GRE transactivation in ASM.
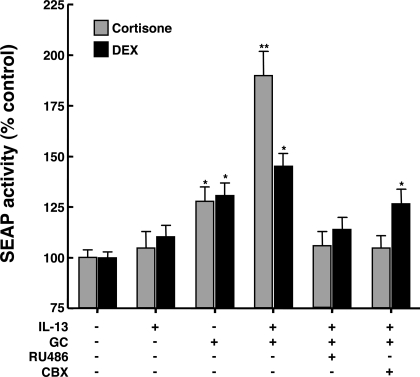
To ascertain whether the stimulatory effect of IL-13 on 11β-HSD1 activity is manifested by altered endogenous GC-induced GR transactivation, the latter was assessed in ASM cells transfected with a promoter-reporter construct, pGRE-SEAP, that is comprised of three GRE sequences upstream from a TATA box and a reporter gene that encodes SEAP. The transfected cells were treated for 24 h with vehicle alone and either IL-13 (50 ng/ml), cortisone (1 μM), or the combination of IL-13 and cortisone, each condition in the absence and presence of pretreatment with either the GR antagonist, RU-486 (10 μM), or CBX (1 μM). In addition, to compare the effects of cortisone with those of an intrinsically active GC, similar experiments were conducted using the synthetic bioactive GC, DEX (1 μM), which was also administered to ASM cells in the absence and presence of the same pretreatment conditions. As demonstrated in Fig. 6 (light bars), relative to controls, cells treated with IL-13 alone did not display a significant change in SEAP activity. Conversely, significantly increased levels of SEAP activity were detected in the culture medium of cells treated with cortisone, averaging 28.1 ± 7.0% above control (P < 0.05); SEAP activity was further markedly enhanced to 90.1 ± 12.1% above control in cells exposed to IL-13 and cortisone (P < 0.01). The induced release of SEAP was completely abrogated in IL-13 and cortisone-exposed cells that were pretreated with either RU-486 or CBX, yielding levels that were similar to those detected in control cells (Fig. 6), whereas neither RU-486 nor CBX alone significantly affected basal SEAP activity (data not shown). By comparison, the stimulatory effect of DEX alone on SEAP activity was not significantly different from that of cortisone, however, in cells treated with DEX and IL-13, the increase in SEAP activity was significantly less than that exhibited by cells treated with cortisone and IL-13 (Fig. 6; dark bars). RU-486 also completely ablated the induced release of SEAP by IL-13 and DEX-exposed cells; however, unlike in cortisone-exposed cells, pretreatment with CBX did not significantly suppress IL-13 and DEX-induced stimulation of SEAP activity, yielding levels that were similar to those detected in cells exposed to DEX alone. Taken together, these observations demonstrate that the heightened 11β-HSD1 activity evoked by IL-13 is associated with enhanced cortisone-induced GR transactivation, whereas that elicited by DEX, an intrinsically bioactive synthetic GC that is relatively resistant to 11β-HSD1 oxoreductase activity (4), is enhanced to a notably lesser extent by IL-13.
Fig. 6.
Comparison of effects of IL-13 on glucocorticoid receptor (GR) transactivation in ASM cells treated with cortisone and dexamethasone (DEX). Relative to untreated (control) ASM cells, GR transactivation detected using the secretable form of alkaline phosphatase (SEAP) reporter assay is unaltered in cells treated for 24 h with IL-13 alone. SEAP activity is significantly increased in glucocorticoid (GC)-treated cells, with similar levels of enhanced SEAP activity detected in cells exposed to 1 μM cortisone or DEX. Relative to cells exposed to DEX, cortisone-treated cells exhibit markedly enhanced SEAP activity when coincubated with IL-13. The induction of SEAP activity by both cortisone and DEX is prevented in cells coincubated with the GR antagonist, RU-486, whereas coincubation with the 11β-HSD inhibitor, CBX, prevents the induction of SEAP activity in cortisone-treated cells while having a lesser inhibitory effect in cells exposed to DEX. Data represent means ± SE from 4 to 5 experiments (*P < 0.05; **P < 0.01).
Role of 11β-HSD in regulating IL-13-induced changes in ASM responsiveness.
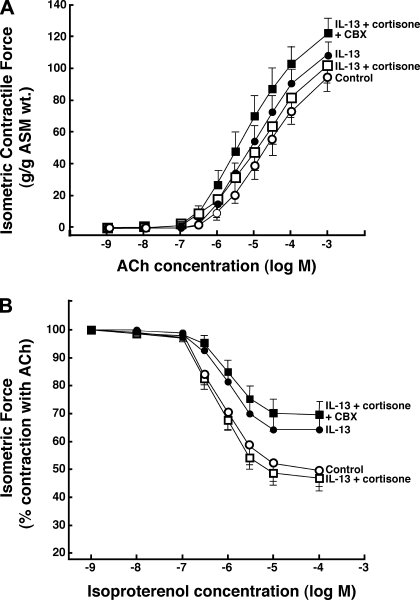
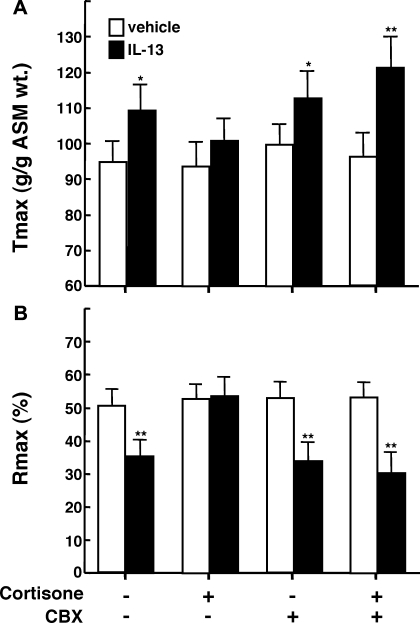
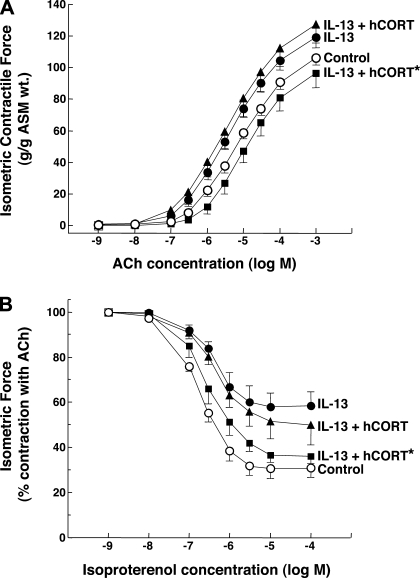
Previous studies have reported that IL-13 directly elicits proasthmatic changes in ASM function, including heightened ASM constrictor responsiveness and impaired ASM relaxation to bronchoactive agonists (22, 33). This evidence, together with the above observations demonstrating the presence of intrinsic 11β-HSD1 oxoreductase activity in resting (unstimulated) ASM and its upregulation by IL-13, raise the hypothesis that 11β-HSD1 activity in ASM plays a bronchoprotective role in the proasthmatic state, a condition known to be associated with the release of IL-13 (21, 22, 55), as well as the release of endogenous GCs (13, 16, 36, 39, 40, 45). This issue was addressed by comparing the agonist-induced constrictor and relaxation responses in isolated rabbit ASM tissues exposed for 24 h to either vehicle alone or IL-13 (50 ng/ml), both in the absence and presence of pretreatment with 0.1 μM cortisone, reflecting the upper range of its normal circulating levels (5). As previously reported (22), relative to their respective controls, ASM tissues exposed to IL-13 exhibited significantly increased constrictor responsiveness to exogenously administered ACh (Fig. 7A), yielding a mean ± SE Tmax of 108.3 ± 9.2 g/g ASM wt vs. the average Tmax of 94.9 ± 9.3 g/g ASM wt generated in the control tissues (P < 0.05, using 2-tailed t-test). Additionally, relative to controls, the relaxation responses to cumulative administration of the β2-adrenoceptor agonist, isoproterenol, were significantly impaired in the IL-13-exposed ASM segments (Fig. 7B), wherein the mean ± SE Rmax amounted to 35.3 ± 5.2% vs. the Rmax of 50.7 ± 5.3% obtained in the control ASM tissues (P < 0.01). Both of these characteristic proasthmatic-like changes in ASM constrictor and relaxation responsiveness were inhibited in IL-13-exposed tissues that were pretreated with cortisone (Fig. 7; open squares). The latter effect of cortisone, however, was abrogated in IL-13-exposed tissues that were coincubated with the combination of cortisone and CBX (Fig. 7; closed squares), demonstrating that the protective effect of cortisone required the presence of 11β-HSD1 oxoreductase activity to enable its conversion to bioactive cortisol. The data from these studies are summarized in Fig. 8, which also displays the results from extended experiments wherein we found that neither the Tmax responses to ACh nor the Rmax responses to isoproterenol were significantly altered in vehicle-exposed ASM tissues that were pretreated with either cortisone or CBX alone (open bars) or in IL-13-exposed tissues that were pretreated with CBX alone (closed bars). Collectively, these observations support the hypothesis that 11β-HSD1-dependent intracrine generation of cortisol from cortisone in ASM serves to curtail the proasthmatic effects of IL-13 on ASM function. Finally, in relation to these observations, it should be noted that, in separate studies, we found that, as with cortisone, the changes in ASM constrictor and relaxation responsiveness were also prevented in IL-13-exposed tissues that were pretreated with 1 μM DEX (data not shown), a finding that concurs with those in a previous study wherein we (24) demonstrated that this synthetic GC abrogates the proasthmatic changes in rabbit ASM tissue responsiveness elicited by IL-1β and TNFα. Unlike with cortisone, however, the protective effect of DEX was largely preserved in IL-13-exposed ASM tissues that were coincubated with CBX, an observation that is consistent with the known relative insensitivity of DEX to 11β-HSD1 oxoreductase activity (4).
Fig. 7.
Cortisone prevents IL-13-induced proasthmatic changes in ASM tissue constrictor and relaxant responsiveness. Relative to vehicle-treated controls, isolated rabbit ASM tissues exposed for 24 h to IL-13 (50 ng/ml) exhibit significantly increased constrictor responses to ACh (A) and impaired relaxation responses to isoproterenol (B). The IL-13-induced changes in ASM constrictor and relaxation responsiveness are prevented in tissues that are incubated with cortisone (0.1 μM). The latter protective effect of cortisone on IL-13-induced changes in ASM responsiveness is abrogated in tissues coincubated with CBX (1 μM). Data are means ± SE from 5 paired experiments. Based on ANOVA with Tukey posttest analysis, for both the dose-response curves to ACh and isoproterenol, differences between control and IL-13-exposed tissues and between control and IL-13-exposed tissues that are pretreated with both cortisone and CBX are statistically significant, yielding P values <0.05 and <0.01, respectively. Comparably, based on Student's 2-tailed t-test analysis, for both the mean maximal isometric contractile force (Tmax) and maximal relaxation (Rmax) values generated at the maximal administered concentrations of ACh and isoproterenol, respectively, differences between control and IL-13-exposed tissues and between control and IL-13-exposed tissues that are pretreated with both cortisone and CBX are also statistically significant, yielding P values <0.05 and <0.01, respectively.
Fig. 8.
Modulatory effects of cortisone and inhibition of 11β-HSD activity on IL-13-induced changes in ASM tissue responsiveness. A: relative to vehicle-exposed isolated rabbit ASM tissues (open bars), Tmax responses to ACh are significantly increased in tissues exposed for 24 h to 50 ng/ml IL-13 (closed bars). B: relative to vehicle-exposed ASM tissues, corresponding Rmax responses to isoproterenol are significantly reduced in tissues treated with IL-13. The heightened Tmax responses and attenuated Rmax responses are ablated in IL-13-exposed ASM tissues that are pretreated with cortisone, and the latter effects of cortisone are abrogated in IL-13-exposed tissues that are coincubated with CBX. Note, incubation of ASM tissues with either cortisone or CBX alone had no effect on the tissue Tmax or Rmax responses. Data are means ± SE from 5 paired experiments (*P < 0.05; **P < 0.01).
Amplification of 11β-HSD1 oxoreductase activity by IL-13 heightens the protective effect of cortisone on IL-13-induced changes in ASM responsiveness.
Given that the above results implicate 11β-HSD-dependent intracrine generation of cortisol in mediating the protective effect of cortisone in IL-13-exposed ASM tissues, we next examined whether the amplification of 11β-HSD1 oxoreductase activity by IL-13 (Figs. 4 and 5) enables a heightened protective effect of cortisone on IL-13-induced proasthmatic changes in ASM responsiveness. To address this issue, we compared the constrictor and relaxation responses in IL-13-exposed rabbit ASM tissues that were cotreated with medium isolated from cultured human ASM cells that were exposed for 24 h to either vehicle alone or to 500 nM cortisone [i.e., a concentration within the range of its stimulated circulating levels (5)] in the absence and presence of IL-13 (50 ng/ml). The levels of cortisol detected in the culture media of the ASM cell preparations that were incubated with cortisone in the absence and presence of IL-13 averaged 19.9 ± 3.4 and 82.4 ± 8.3 ng/ml, respectively, which represent values of percent conversion of cortisone to cortisol that amount to ∼11 and ∼46%, respectively. Before exposing the rabbit ASM tissues for 24 h to IL-13 with and without the latter cortisol-containing conditioned media, the tissues were treated with CBX (1 μM) to prevent their 11β-HSD-dependent metabolism of the GC present in the media preparations. As demonstrated in Fig. 9, relative to the responses obtained in rabbit ASM tissues that were exposed to medium from control (untreated) human ASM cells (open circles), IL-13-exposed tissues (closed circles) exhibited increased constrictor responses to ACh (Fig. 9A) and impaired relaxation responses to isoproterenol (Fig. 9B). These induced changes in ASM responsiveness were not significantly affected in IL-13-exposed tissues that were cotreated with the cortisol-containing medium from ASM cells that were incubated with cortisone in the absence of IL-13 (hCORT), although their relaxation responses to isoproterenol were somewhat less impaired (closed triangles). By comparison, the effects of IL-13 on ASM responsiveness were prevented in ASM tissues that were cotreated with the elevated cortisol-containing medium from ASM cells that were incubated with cortisone and IL-13 (hCORT*; closed squares), with no significant differences detected in either the constrictor or relaxation responses between these tissues and controls. Of note, in related parallel experiments, we found that: 1) neither of the cortisol-containing media preparations appreciably affected the constrictor or relaxation responses in naïve control ASM tissues; and 2) the above protective effect of the cortisol-containing medium was abrogated in IL-13-exposed ASM tissues that were pretreated with the GR antagonist, RU-486 (10 μM) (data not shown). Thus these data provide evidence supporting the concept that IL-13-induced amplification of 11β-HSD1 oxoreductase activity, resulting in an enhanced conversion of cortisone to cortisol, enables a physiologically relevant concentration of cortisone to exert heightened protection of ASM from the proasthmatic effects of IL-13 on ASM function.
Fig. 9.
Inhibition of IL-13-induced changes in constrictor and relaxation responsiveness in rabbit ASM tissues exposed to conditioned medium from cultured human ASM cells treated with cortisone in the presence of IL-13. Relative to controls (open circles), rabbit ASM tissues exposed for 24 h to IL-13 (closed circles) exhibit significantly increased constrictor responses to ACh (A) and impaired relaxation responses to isoproterenol (B). IL-13-induced changes in constrictor and relaxation responsiveness are not significantly altered in ASM tissues treated with cortisol-containing medium isolated from human ASM cell cultures exposed for 24 h to 500 nM cortisone (hCORT; closed triangles). By comparison, IL-13-induced changes in constrictor and relaxation responsiveness are prevented in tissues treated with cortisol-containing medium from human ASM cell cultures exposed to cortisone in the presence of IL-13 (hCORT*; closed squares). Data are means ± SE from 4 paired experiments. Based on ANOVA with Tukey posttest analysis for both the dose-response curves to ACh and isoproterenol, differences between control and IL-13-exposed tissues and between control and IL-13-exposed tissues that are pretreated with hCORT are statistically significant, yielding P values <0.05 and <0.01, respectively. Comparably, based on Student's 2-tailed t-test analysis, for both the mean Tmax and Rmax values generated at the maximal administered concentrations of ACh and isoproterenol, respectively, differences between control and IL-13-exposed tissues and between control and IL-13-exposed tissues that are pretreated with hCORT are also statistically significant, yielding P values <0.05 and <0.01, respectively.
DISCUSSION
GCs are the most commonly used agents to treat asthma due to their ability to attenuate both the pulmonary inflammatory response and airway hyperreactivity associated with this disorder. The bronchoprotective action of GCs is not only attributed to their anti-inflammatory and immunosuppressive properties, but also reflects the ability of GCs to directly alter ASM function by effects that include inhibition of ASM contractility, augmentation of ASM relaxation, suppression of ASM proliferation, prevention of the release of various cytokines and chemokines from stimulated ASM, and other actions (24, 27, 38). In light of this body of evidence and that demonstrating that the localized cellular response to endogenous GCs is critically dependent on the GC-activating and -inactivating activities of 11β-HSD1 and 11β-HSD2, respectively (5, 49), the present study sought to identify the role of ASM in autologously regulating its prereceptor bioavailability of endogenous GCs and, consequently, their ability to modulate ASM function under proasthmatic conditions. Our findings are the first to demonstrate that: 1) ASM is adept in amplifying the prereceptor bioavailability of endogenous GCs by upregulating 11β-HSD1 oxoreductase activity in response to its exposure to IL-13, the Th2-type cytokine identified as that most responsible for the induction of airway hyperresponsiveness in allergic asthma (21, 55); and 2) this IL-13-induced facilitation of cortisone conversion to cortisol enables the ASM to respond to physiologically relevant concentrations of endogenous GC that can effectively suppress the proasthmatic impact of this key proinflammatory cytokine on ASM function. Thus these novel findings highlight an intrinsic Th2 cytokine-driven homeostatic feedback mechanism in ASM that serves to enhance its responsiveness to endogenous GCs under proasthmatic conditions and thereby curtail the severity of expression of the airway asthmatic phenotype.
Cellular expression of 11β-HSD1 is widespread, including in different immune cell types (11, 14, 48, 49, 57), wherein it acts in vivo primarily in its oxoreductase capacity to convert cortisone to cortisol. A number of studies have reported that this prereceptor control of endogenous GC bioavailability at the GR is locally amplified under proinflammatory conditions by the induction of 11β-HSD1 expression in response to specific cytokines that accumulate in the extracellular microenvironment (5, 9, 49). As in immune cells (9, 11, 14, 48, 49, 57), cytokine-induced upregulation of 11β-HSD1 expression has been demonstrated in vascular smooth muscle cells exposed to IL-1β and TNFα, suggesting that the induction of enhanced 11β-HSD1 oxoreductase activity may play a role in regulating the inflammatory responses in arterial vessels, potentially influencing the development of atherosclerosis (7). Moreover, 11β-HSD1 induction by IL-1β and TNFα was also demonstrated in human ASM cells (7); we (24) previously reported that 11β-HSD1 mRNA expression is augmented in IL-1β/TNFα-exposed ASM cells that are pretreated with DEX, implying a cooperative interaction between GC and cytokine signaling in upregulating 11β-HSD1 expression in ASM. Our present results extend these previous findings in demonstrating that ASM expression of 11β-HSD1 is upregulated in the presence of IL-13, whereas constitutive mRNA expression of 11β-HSD2, the dehydrogenase that suppresses GC bioavailability by converting cortisol to cortisone, is inhibited in IL-13-exposed ASM (Fig. 1A). Taken together, these observed opposing effects of IL-13 on 11β-HSD isozyme expression are complementary in nature, given that both upregulation of 11β-HSD1 and inhibition of 11β-HSD2 would cooperatively serve to enhance prereceptor GC bioavailability in the IL-13-exposed ASM. Moreover, in this context, it is noteworthy that IL-13-induced downregulation of 11β-HSD2 was found to precede the upregulation of 11β-HSD1 (Fig. 1A), a finding that is consistent with the notion that 11β-HSD1-dependent accumulation of cortisol in the IL-13-exposed state may be facilitated by initially impairing the ability of 11β-HSD2 (i.e., via its downregulation) to attenuate the accumulation of cortisol due to its conversion to cortisone.
In evaluating the mechanism underlying the induction of 11β-HSD1 expression, our results demonstrated that IL-13 evokes enhanced DNA binding of the transcription factors, AP-1 and C/EBP-α (Fig. 1B), a finding that concurs with those in previous studies that identified putative binding sites for these transcription factors in the proximal 5′ promoter region of the 11β-HSD1 gene (5, 54). Moreover, our extended observations demonstrated that, of these transcription factors and their known upstream MAPKs: 1) activation of AP-1 is responsible for mediating the induction of 11β-HSD1 expression in IL-13-exposed ASM (Fig. 2); and 2) the latter phenomenon is attributed to activation of both the ERK1/2 and JNK signaling pathways (Fig. 3). Taken together, these findings are consistent with earlier evidence demonstrating that these MAPKs play complementary roles as cross-regulators of transcription factor activation (8) and that activation of the heterodimer components of AP-1, c-Jun and c-Fos, are phosphorylated within their transcription activating domains by JNK and ERK1/2, respectively (32). Notably, in contrast to these MAPKs, our data further suggest that coactivation of p38 MAPK by IL-13 exerts an opposing (i.e., downregulating) effect on 11β-HSD1 expression, as inhibition of p38 MAPK enhanced the induction of 11β-HSD1 by IL-13 (Fig. 3). Although the mechanism underlying this suspected inhibitory action of p38 MAPK remains to be identified, it is relevant to note that a one-way cross talk between p38 MAPK and ERK1/2 was previously reported, wherein phosphorylated p38α was found to couple with ERK1/2 and thereby sterically block ERK1/2 phosphorylation by MEK1/2 (56) and possibly also act via a protein kinase that lies upstream of MEK1/2 (43). We (42) recently verified the presence of this cross talk mechanism in ASM in a study demonstrating that p38 MAPK activation inhibits ERK1/2 phosphorylation and its mediation of altered ASM responsiveness in LPS-exposed ASM. In light of this evidence, it is reasonable to propose that, by preventing p38 MAPK coactivation and, hence, its negative regulatory effect on ERK1/2 signaling, the heightened IL-13-induced 11β-HSD1 expression detected in the presence of p38 MAPK inhibition may be due, at least in part, to augmented ERK1/2-dependent activation of AP-1. This suspected indirect mechanism of action of p38 MAPK in regulating 11β-HSD1 expression remains to be systematically evaluated.
In demonstrating that the upregulated expression of 11β-HSD1 transcripts is translated into an enhanced ability of IL-13- and IL-4-exposed ASM cells to convert cortisone to cortisol (Figs. 4 and 5), our observations are consistent with the known in vivo oxoreductase function of 11β-HSD1 (5, 49). Analysis of the kinetics of 11β-HSD1 oxoreductase activity demonstrated that, relative to control cells, the Vmax for cortisol production was significantly increased by an average of ∼40% in IL-13-exposed ASM cells, whereas the apparent Km was not significantly altered (Fig. 4). Furthermore, sigmoidal analysis of the kinetics data revealed that both the control and IL-13-treated ASM cells exhibited similar Hill coefficient values of ∼2 (Fig. 4B). The latter observation concurs with earlier evidence demonstrating that human 11β-HSD1 functions as a dimeric enzyme and, as such, exhibits cooperative kinetics with cortisone, as reflected by a Hill coefficient of ∼2 (5, 34). The relevance of this finding relates to the concept that by displaying cooperative kinetics with cortisone, despite its relatively low affinity to the GC with a Km of ∼1 μM (Fig. 4), the 11β-HSD1 enzyme is capable of converting low (nanomolar) as well as high (micromolar) concentrations of cortisone to cortisol (5, 34). Accordingly, the oxoreductase activity of 11β-HSD1 can accommodate the broad range of circulating “free” cortisone concentrations that are encountered under varying physiological conditions, including in relation to the circadian rhythm, and in response to stress or inflammatory stimuli (5).
To determine whether the stimulatory effect of IL-13 on 11β-HSD1 activity confers an increase in GRE-driven transcriptional activation in response to endogenous GC exposure, our studies on ASM cells transfected with the promoter-reporter construct, pGRE-SEAP, demonstrated that cells treated with 1 μM cortisone (i.e., ∼Km for 11β-HSD1 reductase activity) exhibited significantly increased expression of reporter gene activity that was roughly similar in magnitude to that evoked in cells exposed to the same concentration of DEX (Fig. 6). These observations agree with those in a previous study wherein GR transactivation induced by administering 1 μM cortisol to COS-7 cells transfected with human GR was also comparable in magnitude to that induced by 1 μM DEX (35). Thus it appears that the 11β-HSD1 oxoreductase activity that is constitutively present in ASM is capable of converting a Km concentration of cortisone into cortisol-induced GR transactivation with equal efficacy to that elicited by the same concentration of DEX, a synthetic GC that is intrinsically bioactive and relatively insensitive to 11β-HSD1 reductase activity (4). Our data further demonstrated that, relative to DEX-treated cells, cortisone-induced GR transactivation was markedly potentiated in IL-13-exposed ASM cells and that this phenomenon was dependent on the integrity of 11β-HSD1 activity (Fig. 6). These findings, together with the above observations, support the notion that the amplified GRE-driven transactivation detected in ASM cells exposed to IL-13 was attributed to its induced upregulation of 11β-HSD1 activity, the latter resulting in an enhanced prereceptor bioavailability of cortisol. In this context, it should be emphasized that our approach used to evaluate GR transactivation is sensitive to molecular interactions occurring at all steps from binding of GC to GR through translation of GR transactivation into SEAP activity. Thus, although fundamentally dependent on the integrity of 11β-HSD1 activity, the question is raised as to whether our observed stimulatory effect of IL-13 on cortisone-induced GR transactivation also reflected potential actions of the cytokine on signaling events downstream of initial enhanced GR activation due to increased prereceptor generation of bioactive GC. Characteristically, the interaction between cytokine and GR signaling is mutually antagonistic in nature, with studies demonstrating reduced GR function in isolated peripheral blood mononuclear cells (PBMCs) exposed to proinflammatory cytokines and in PBMCs from steroid-resistant asthmatic individuals (29, 30, 43, 47). Specifically, with respect to the action of IL-13, Spahn and coworkers (47) previously demonstrated that the suppressive effect of hydrocortisone on LPS-induced IL-6 production is significantly diminished in human monocytes pretreated with IL-13, an effect that was attributed to IL-13-induced reduction in GR binding affinity. Conversely, other studies have reported an increase in GR expression under different proinflammatory conditions and following treatment of different cell types, including airway epithelial cells, with various proinflammatory cytokines (12, 17, 41, 52). Although these seemingly discordant reported findings are not readily reconciled, and may be reflective (at least in part) of cell type-specific differences in the effects of cytokines on GR signaling, it is relevant to note that our results demonstrated that the enhancement in GR transactivation by IL-13 in DEX-exposed ASM cells was significantly less than that detected in cortisone-exposed cells (Fig. 6). Therefore, it is likely that our observed stimulatory effect of IL-13 on cortisone-induced GR transactivation was predominantly attributed to 11β-HSD1-dependent amplification of the prereceptor bioavailability of cortisol.
The physiological relevance of 11β-HSD1-dependent regulation of endogenous GC activation in ASM was substantiated in our studies that examined the effects of cortisone on the proasthmatic changes in ASM function elicited by IL-13. In accordance with previous reports, IL-13-exposed ASM tissues exhibited heightened constrictor responses to ACh and attenuated β-adrenoceptor-mediated relaxation (22, 33); both of these proasthmatic-like changes in ASM function were prevented by coincubating the IL-13-treated tissues with a physiologically applicable concentration of cortisone (Fig. 7). Moreover, the results demonstrated that the latter protective action of cortisone was abrogated in IL-13-exposed ASM tissues wherein 11β-HSD activity, both intrinsic and IL-13-induced, were inhibited by CBX. Whereas these observations demonstrated the requirement of 11β-HSD1-dependent intracrine generation of cortisol in mediating the protective action of cortisone, the results in our extended experiments highlighted the physiological significance of IL-13-induced upregulation of 11β-HSD1 oxoreductase activity in regulating the proasthmatic effects of IL-13 on ASM responsiveness. Specifically, these studies demonstrated that IL-13-induced amplification of 11β-HSD1 oxoreductase activity, resulting in an enhanced conversion of cortisone to cortisol, enables a physiologically relevant concentration of cortisone to exert heightened protection of ASM from the proasthmatic effects of IL-13 on ASM function (Fig. 9). Taken together, the above observations underscore the critical role played by ASM in regulating its responsiveness to endogenous GCs and thereby the proasthmatic impact of IL-13 on ASM function. In this regard, although the present study did not address the specific mechanism(s) by which endogenous GCs attenuate IL-13-induced changes in ASM contractility, recent reports have identified several potential mechanisms of action of GCs in modulating ASM function, including induced changes in gene expression via transactivation and transrepression mechanisms, inhibition of contractile protein expression and function, altered agonist-stimulated intracellular Ca2+ signaling, and other mechanisms (19, 20, 29, 34, 41). Moreover, it should be noted that, apart from enabling endogenous GCs to modulate ASM contractility, the presence of an intrinsic 11β-HSD1-dependent homeostatic mechanism in ASM likely serves to also facilitate other known GC-sensitive actions in ASM, including inhibition of ASM proliferation and prevention of its stimulated release of various cytokines and chemokines (27, 38), potentially including cytokines released by IL-13-stimulated ASM cells (25). Finally, given its ability to convert cortisone to cortisol, it is conceivable that ASM may release the bioactive GC into its microenvironment in vivo and thereby exert an anti-inflammatory paracrine action on adjacent cells, possibly including airway infiltrating inflammatory cells. This possibility is a provocative consideration in light of our recent evidence demonstrating that superantigen-pulsed ASM cells exhibit immunological synapse formation with coincubated CD4+ T lymphocytes and that this intercellular communication elicits IL-13 release into the cell culture medium (51). Although it remains to be established whether the latter phenomenon can lead to a paracrine interaction in vivo that involves the localized release of bioactive GC generated by the IL-13-exposed ASM in its conversion of cortisone to cortisol, it is of interest to note that a comparable paracrine mechanism was previously identified in the thymus wherein the local production of GCs by thymic epithelial cells was found to exert an inhibitory effect on T cell receptor signaling in adjacent thymic T cells (50).
In considering the implications of the present findings, it must be emphasized that our observations pertain to studies conducted using cultured human ASM cells and isolated rabbit ASM tissue preparations. Accordingly, certain fundamental issues are raised, including the extent to which our findings are applicable to the in vivo state and to the human condition. In this regard, it should be noted that our observations directly relate to the body of evidence in the literature that implicates the important role played by endogenous GC release in attenuating the airway response to allergen challenge in asthmatic individuals and in in vivo animal models of allergic asthma (13, 16, 39, 40). Our findings also concur with the identified role of 11β-HSD1 oxoreductase activity in regulating endogenous GC-mediated changes in phenotypic expression of other proinflammatory conditions (9, 49). Additionally, our data generated in the rabbit ASM tissues are compatible with those obtained in the cultured human ASM cells, as both of these experimental preparations exhibited complementary changes in ASM function accompanying IL-13 administration and in the role of 11β-HSD1 oxoreductase activity in mediating the effects of endogenous GCs on ASM function. In this regard, it is noteworthy that the circulating GC profile in the rabbit is similar to that in humans (i.e., cortisol rather than corticosterone in rodents) and that there exists greater homology in the amino acid composition of the human and rabbit 11β-HSD proteins than that between human and rodents (37). Finally, it is should be noted that the changes in constrictor and relaxation responsiveness observed in the IL-13-exposed ASM tissues mimicked the perturbations in airway function that characterize the asthmatic ASM phenotype, including enhanced constrictor responsiveness to cholinergic stimulation and impaired β-adrenoceptor-mediated airway relaxation (1, 2, 18). Thus, in light of these considerations, we believe that the findings described herein are likely applicable to the human condition.
In conclusion, the present study examined the mechanism regulating 11β-HSD1 expression in ASM and its role in mediating the bioavailability and action of endogenous GCs in ASM under proasthmatic conditions. The results provide new evidence demonstrating that: 1) ASM exposed to IL-13, the cytokine considered most responsible for inducing airway hyperresponsiveness in allergic asthma, exhibits upregulated expression of the endogenous GC-activating enzyme, 11β-HSD1, whereas expression of the GC-inactivating isozyme, 11β-HSD2, is downregulated; 2) the induction of 11β-HSD1 by IL-13 is attributed to activation of the transcription factor, AP-1, which is coupled to signaling via the activated MAPKs, ERK1/2 and JNK; 3) the upregulated expression of 11β-HSD1 is reflected by increased IL-13 (also IL-4)-induced oxoreductase activity that results in an amplified conversion of cortisone to its bioactive derivative, cortisol; 4) this facilitated prereceptor bioavailability of cortisol confers heightened GR-induced transcriptional activation in the IL-13-exposed ASM; and 5) the latter phenomenon enables the ASM to respond to physiologically relevant concentrations of endogenous GCs that act to suppress the opposing proasthmatic effect of IL-13 on ASM constrictor and relaxation responsiveness. Collectively, these novel findings identify an intrinsic Th2 cytokine-driven homeostatic feedback mechanism in ASM that serves to enhance its responsiveness to endogenous GCs under proasthmatic conditions and thereby curtail the severity of expression of the airway asthmatic phenotype. Thus, given the possibility that an inherited or acquired disruption of the integrity of this homeostatic feedback mechanism may adversely impact the asthmatic phenotype, the development of interventions targeted at upregulating 11β-HSD1 expression and function in ASM may offer new therapeutic approaches to mitigate the airway asthmatic response.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-31467 and HL-61038.
REFERENCES
- 1.Bai TR Abnormalities in airway smooth muscle in fatal asthma. Am Rev Respir Dis 141: 552–557, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Bai TR, Mak JC, Barnes PJ. A comparison of beta-adrenergic receptors and in vitro relaxant responses to isoproterenol in asthmatic airway smooth muscle. Am J Respir Cell Mol Biol 6: 647–651, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci 94: 557–572, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Best R, Nelson SM, Walker BR. Dexamethasone and 11-dehydrodexamethasone as tools to investigate the isozymes of 11 beta-hydroxysteroid dehydrogenase in vitro and in vivo. J Endocrinol 153: 41–48, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Blum A, Maser E. Enzymology and molecular biology of glucocorticoid metabolism in humans. Prog Nucleic Acid Res Mol Biol 75: 173–216, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal MN, Mclean JA, Mathews KP, Sheldon JM, Mich AA. Adrenal-pituitary function in bronchial asthma. Arch Intern Med 117: 34–38, 1996. [PubMed] [Google Scholar]
- 7.Cai TQ, Wong B, Mundt SS, Thieringer R, Wright SD, Hermanowski-Vosatka A. Induction of 11beta-hydroxysteroid dehydrogenase type 1 but not -2 in human aortic smooth muscle cells by inflammatory stimuli. J Steroid Biochem Mol Biol 77: 117–122, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 20: 117–122, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Chapman KE, Coutinho A, Gray M, Gilmour JS, Savill JS, Seckl JR. Local amplification of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1 and its role in the inflammatory response. Ann NY Acad Sci 1088: 265–273, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Chapoval SP, Al-Garawi A, Lora JM, Strickland I, Ma B, Lee PJ, Homer RJ, Ghosh S, Coyle AJ, Elias JA. Inhibition of NF-kappaB activation reduces the tissue effects of transgenic IL-13. J Immunol 179: 7030–7041, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC, Hewison M, Stewart PM. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 16: 1037–1044, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Damo M, Rabier M, Loubatiere J, Blotman F, Crastes de Paulet A. Glucocorticoid receptors in fibroblasts from synovial tissue. Changes during the inflammatory process. Preliminary results. Agents Actions 17: 478–483, 1986. [DOI] [PubMed] [Google Scholar]
- 13.De Bie JJ, Hessel EM, Van Ark I, Van Esch B, Hofman G, Nijkamp FP, Van Oosterhout AJ. Effect of dexamethasone and endogenous corticosterone on airway hyperresponsiveness and eosinophilia in the mouse. Br J Pharmacol 119: 1484–1490, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman L, Hewison M, Hughes SV, Evans KN, Hardie D, Means TK, Chakraverty R. Expression of 11beta-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 106: 2042–2049, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Fujitaka M, Nomura S, Sakura N, Ueda K, Matuura R, Yumiba C. Morning and afternoon serum levels of cortisone and cortisol in asthmatic patients. Clin Chim Acta 299: 101–108, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Georen S, Ahnblad P, Stjarne P, Wikstrom AC, Stierna P. Significance of endogenous glucocorticoid sensitivity for airway eosinophilia in a murine model of allergy. Acta Otolaryngol (Stockh) 125: 378–385, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gladman DD, Urowitz MB, Doris F, Lewandowski K, Anhorn K. Glucocorticoid receptors in systemic lupus erythematosus. J Rheumatol 18: 681–684, 1991. [PubMed] [Google Scholar]
- 18.Goldie RG, Spina D, Henry PJ, Lulich KM, Paterson JW. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, β-adrenoceptor agonists and theophylline. Br J Clin Pharmacol 22: 669–676, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsmith AM, Hershenson MB, Wolbert MP, Bentley JK. Regulation of airway smooth muscle alpha-actin expression by glucocorticoids. Am J Physiol Lung Cell Mol Physiol 292: L99–L106, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Goto K, Chiba Y, Sakai H, Misawa M. Glucocorticoids inhibited airway hyperresponsiveness through downregulation of CPI-17 in bronchial smooth muscle. Eur J Pharmacol 591: 231–236, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennic DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282: 2261–2263, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunstein MM, Hakonarson H, Leiter J, Chen M, Whelan R, Grunstein JS, Chuang S. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 282: L520–L528, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hakonarson H, Grunstein MM. Autocrine regulation of airway smooth muscle responsiveness. Respir Physiol Neurobiol 137: 263–276, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Hakonarson H, Halapi E, Whelan R, Gulcher J, Stefansson K, Grunstein MM. Association between IL-1β/TNF-α-induced glucocorticoid-sensitive changes in multiple gene expression and altered responsiveness in airway smooth muscle. Am J Respir Cell Mol Biol 25: 761–771, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hakonarson H, Herrick DJ, Grunstein MM. Mechanism of impaired β-adrenoceptor responsiveness in atopic sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 269: L645–L652, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Hardy R, Rabbitt EH, Filer A, Emery P, Hewison M, Stewart PM, Gittoes NJ, Buckley CD, Raza K, Cooper MS. Local and systemic glucocorticoid metabolism in inflammatory arthritis. Ann Rheum Dis 67: 1204–1210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirst SJ, Lee TH. Airway smooth muscle as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med 158: S201–S206, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Hu A, Nino G, Grunstein JS, Fatma S, Grunstein MM. Prolonged heterologous β2-adrenoceptor desensitization promotes proasthmatic airway smooth muscle function via PKA/ERK1/2-mediated phosphodiesterase-4 induction. Am J Physiol Lung Cell Mol Physiol 294: L1055–L1067, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol 109: 649–657, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol 151: 3460–3466, 1993. [PubMed] [Google Scholar]
- 31.Kang BN, Jude JA, Panettieri RA Jr, Walseth TF, Kannan MS. Glucocorticoid regulation of CD38 expression in human airway smooth muscle cells: role of dual specificity phosphatase 1. Am J Physiol Lung Cell Mol Physiol 295: L186–L193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Swartzman IN, Panettieri RA Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 164: 141–148, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Maser E, Volker B, Friebertshauser J. 11 Beta-hydroxysteroid dehydrogenase type 1 from human liver: dimerization and enzyme cooperativity support its postulated role as glucocorticoid reductase. Biochemistry 41: 2459–2465, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Muller C, Cluzeaud F, Pinon GM, Rafestin-Oblin ME, Morfin R. Dehydroepiandrosterone and its 7-hydroxylated metabolites do not interfere with the transactivation and cellular trafficking of the glucocorticoid receptor. J Steroid Biochem Mol Biol 92: 469–476, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Nagai H, Tsuji F, Goto S, Koda A. Pharmacological study of bacterial lipopolysaccharide-induced airway hyperresponsiveness in guinea-pigs. Arch Int Pharmacodyn Ther 313: 161–175, 1991. [PubMed] [Google Scholar]
- 37.Ozols J Lumenal orientation and post-translational modifications of the liver microsomal 11 beta-hydroxysteroid dehydrogenase. J Biol Chem 270: 2305–2312, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Panettieri RA Jr. Effects of corticosteroids on structural cells in asthma, and chronic obstructive pulmonary disease. Proc Am Thorac Soc 1: 231–234, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Peebles RS Jr, Togias A, Bickel CA, Diemer FB, Hubbard WC, Schleimer RP. Endogenous glucocorticoids and antigen-induced acute and late phase pulmonary responses. Clin Exp Allergy 30: 1257–1265, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki H, Yanai M, Shimura S, Okayama H, Aikawa T, Sasaki T, Takishima T. Late asthmatic response to Ascaris antigen challenge in dogs treated with metyrapone. Am Rev Respir Dis 136: 1459–1465, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Schottelius A, Wedel S, Weltrich R, Rohde W, Buttgereit F, Schreiber S, Lochs H. Higher expression of glucocorticoid receptor in peripheral mononuclear cells in inflammatory bowel disease. Am J Gastroenterol 95: 1994–1999, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Shan X, Hu A, Veler H, Fatma S, Grunstein JS, Chuang S, Grunstein MM. Regulation of Toll-like receptor 4-induced proasthmatic changes in airway smooth muscle function by opposing actions of ERK1/2 and p38 MAPK signaling. Am J Physiol Lung Cell Mol Physiol 291: L324–L333, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Sher ER, Leung DY, Surs W, Kam JC, Zieg G, Kamada AK, Szefler SJ. Steroid-resistant asthma. Cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest 93: 33–39, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shore SA, Moore PE. Regulation of β-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol 137: 179–195, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Silverman ES, Breault DT, Vallone J, Subramanian S, Yilmaz AD, Mathew S, Subramaniam V, Tantisira K, Pacák K, Weiss ST, Majzoub JA. Corticotropin-releasing hormone deficiency increases allergen-induced airway inflammation in a mouse model of asthma. J Allergy Clin Immunol 114: 747–754, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Singh RP, Dhawan P, Golden C, Kapoor GS, Mehta KD. One-way cross-talk between p38(MAPK) and p42/44(MAPK). Inhibition of p38(MAPK) induces low density lipoprotein receptor expression through activation of the p42/44(MAPK) cascade. J Biol Chem 274: 19593–19600, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Spahn JD, Szefler SJ, Surs W, Doherty DE, Nimmagadda SR, Leung DY. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol 157: 2654–2659, 1996. [PubMed] [Google Scholar]
- 48.Thieringer R, Le Grand CB, Carbin L, Cai TQ, Wong B, Wright SD S, Hermanowski-Vosatka A. 11 Beta-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J Immunol 167: 30–35, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 25: 831–866, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med 179: 1835–1846, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veler H, Hu A, Fatma S, Grunstein JS, DeStephan CM, Campbell D, Orange JS, Grunstein MM. Superantigen presentation by airway smooth muscle to CD4+ T lymphocytes elicits reciprocal proasthmatic changes in airway function. J Immunol 178: 3627–3636, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Verheggen MM, van Hal PT, Adriaansen-Soeting PW, Goense BJ, Hoogsteden HC, Brinkmann AO, Versnel MA. Modulation of glucocorticoid receptor expression in human bronchial epithelial cell lines by IL-1 beta, TNF-alpha and LPS. Eur Respir J 9: 2036–2043, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Webster JC, Cidlowski JA. Mechanisms of glucocorticoid-receptor-mediated repression of gene expression. Trends Endocrinol Metab 10: 396–402, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Williams LJ, Lyons V, MacLeod I, Rajan V, Darlington GJ, Poli V, Seckl JR, Chapman KE. C/EBP regulates hepatic transcription of 11beta-hydroxysteroid dehydrogenase type 1. A novel mechanism for cross-talk between the C/EBP and glucocorticoid signaling pathways. J Biol Chem 275: 30232–30239, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 282: 2258–2260, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Shi X, Hampong M, Blanis L, Pelech S. Stress-induced inhibition of ERK1 and ERK2 by direct interaction with p38 MAP kinase. J Biol Chem 276: 6905–6908, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Zhang TY, Ding X, Daynes RA. The expression of 11 beta-hydroxysteroid dehydrogenase type I by lymphocytes provides a novel means for intracrine regulation of glucocorticoid activities. J Immunol 174: 879–889, 2005. [DOI] [PubMed] [Google Scholar]