Abstract
Tubulointerstitial lesions are important in the progression of proteinuric renal disease. Tubular kidney injury molecule-1 (Kim-1) is induced in acute renal injury and reversible as a natural course. Kim-1 is also present in chronic renal damage; however, the dynamics of Kim-1 in chronic renal damage and effects of antiproteinuric treatment on Kim-1 are unknown. We studied Kim-1 in adriamycin nephrosis (AN) before and after renin-angiotensin system blockade. A renal biopsy was taken 6 wk after adriamycin injection to study renal damage and Kim-1 expression. Subsequently, ACE inhibition (ACEi; n = 23), angiotensin II antagonist (AT1A; n = 23), or vehicle (n = 10) was given for 6 wk; healthy rats served as controls (CON; n = 8). In AN, renal Kim-1 mRNA was induced 26-fold vs. CON at week 6, with further increase in vehicle to week 12 (40-fold) but was reduced by ACEi and AT1A to 10- and 12-fold vs. CON (P < 0.05 vs. week 6). Kim-1 protein was undetectable in CON; in AN, it was present in brush border of dilated tubules in areas with adjacent interstitial lesions. Renal Kim-1 protein levels increased from weeks 6–12 in vehicle and decreased in ACEi- and AT1A-treated groups (P < 0.05). In vehicle, urinary Kim-1 was increased (P < 0.05 vs. CON), with a reduction by ACEi and AT1A (P < 0.05 vs. vehicle). Renal and urinary Kim-1 correlated with proteinuria and interstitial damage cross-sectionally. Reductions in proteinuria and renal Kim-1 correlated, which was not associated by corresponding changes in tubulointerstitial fibrosis. In conclusion, on longitudinal follow-up during antiproteinuric treatment increased renal Kim-1 expression is reversible in proportion to proteinuria reduction, likely reflecting reversibility of early tubular injury, supporting its potential as a biomarker for tubulointerstitial processes of damage and repair.
Keywords: ACE inhibitors, chronic kidney disease, fibrosis, proximal tubule, proteinuria
chronic proteinuria elicits tubulointerstitial damage, which plays an important role in proteinuria-induced progressive renal function loss. The severity of tubulointerstitial lesions can predict the clinical course (12, 29, 38, 39) as well as the efficacy of antiproteinuric treatment (20, 23) in experimental and clinical renal conditions. Intervention in the renin-angiotensin system (RAS) provides renoprotection through effects on blood pressure and proteinuria. (17, 27). In particular, the antiproteinuric effects are involved in protection against progressive tubulointerstitial injury (26, 28).
Kidney injury molecule-1 (Kim-1), a type 1 membrane protein that is expressed at negligible levels in normal rat kidneys, is massively induced in tubules after ischemic or toxic injury in rats (14, 15), with resolution during the restoration of structural and functional integrity (14). After the initial observations in acute renal injury, it has become increasingly clear that Kim-1 is also expressed in chronic renal conditions. This has been established in experimental models such as protein-overload nephropathy (33), adriamycin-induced nephropathy (21), angiotensin II-induced renal damage in homozygous Ren2 rats (9), and murine polycystic kidney disease (22). In renal patients, KIM-1 is upregulated in a variety of conditions, including chronic proteinuric kidney disease and acute and chronic renal transplant dysfunction (35, 40). Kim-1 is localized to proximal tubular cells, and, in acute renal injury, particularly expressed in cells with characteristics of injury and regeneration, such as loss of brush border, a flat cell structure, and luminal debris (14, 15, 22). In chronic renal disease, Kim-1 is also mainly expressed in dedifferentiated proximal tubuli, in areas of fibrosis and inflammation (35). The Kim-1 protein has a short cytoplasmic domain and extracellular Ig and mucin domains (14). The functional significance of renal Kim-1 expression during conditions of renal injury has not been established as yet, but recent findings by Ichimura et al. (13) in acute renal injury demonstrate a main role for Kim-1 role in phagocytosis of apoptotic cells. These findings corroborate earlier studies showing that Kim-1 is a functional phosphatidylserine receptor that can induce phagocytosis of apoptotic cells (18). Moreover, Kim-1 was also shown to have properties of a type B macrophage scavenger receptor and to bind oxidized LDL (13). These recent data suggest a functional role of Kim-1 in the repair capacity of the kidney.
Interestingly, the ectodomain of Kim-1 can be shed into urine after cleavage by a matrix metalloproteinase (1), regulated by MAP kinase signaling pathways in response to cell stress (41). Urinary Kim-1 levels are associated with Kim-1 protein expression in injured nephrons in experimental and clinical renal disease (15, 33, 35). Moreover, urinary KIM-1 levels were associated with a worse renal prognosis at long term follow-up after renal transplantation (34). Together, these findings fueled interest in the potential of urinary Kim-1 as a noninvasive biomarker for processes of tubular injury associated with tubulointerstitial damage (5, 24). This would be of interest for several renal conditions, including proteinuric renal disease where tubulointerstitial injury is a main determinant of outcome (24). Recent data from our group in patients with nephrotic range proteinuria showed that nonpharmacological and pharmacological reduction in proteinuria were accompanied by a reduction in urinary KIM-1 (36). However, whether proteinuria reduction is associated with a reduction in renal Kim-1 expression, and corresponding tubulointerstitial damage, has not been established.
Therefore, we studied renal and urinary Kim-1 in adriamycin-induced nephropathy, a rat model of chronic proteinuria-induced renal damage, before and after antiproteinuric intervention with RAS blockade. By taking a renal biopsy during the phase of established proteinuria before renoprotective intervention, we could obtain longitudinal within-individual data on renal Kim-1. Parameters of interstitial inflammation and fibrosis were measured to establish a possible association with Kim-1 expression and its changes over time.
METHODS
Animals.
Sixty-four male Wistar rats (HsdCpb:Wu; Harlan, Zeist, The Netherlands), weighing 336 ± 17 g at disease induction, were studied. Rats were housed in a temperature-controlled room with a 12:12-h light-dark cycle and free access to food and water. Urine was collected over a 24-h period each week in metabolic cages and stored at −20°C.
All surgical procedures took place under isoflurane anesthesia in N2O/oxygen (1:2). Adriamycin nephrosis (AN) was induced by injection of 2 mg/kg adriamycin (doxorubicin) into the tail vein. Six weeks thereafter, a renal biopsy was performed via dorsolateral incision to study pretreatment renal damage. Immediately after surgical removal of a small part of the lower pole from the left kidney, gelfoam was applied to achieve hemostasis. Renal tissue samples were snap-frozen in liquid nitrogen and stored at −80°C; another part was fixed in 4% paraformaldehyde and embedded in paraffin.
After recovery from the biopsy, groups were treated with vehicle (VEH; n = 10), ACE inhibitor (ACEi; lisinopril, 75 mg/l in the drinking water, equal to 5 mg·kg−1·day−1, n = 23) or angiotensin type 1 receptor antagonist (AT1A; L158,809, 150 mg/l drinking water, equal to 10 mg·kg−1·day−1, n = 23) (6). Healthy control animals (CON; n = 8) were used as time controls. In prior experiments, we showed that the biopsy procedure does not affect the course of renal damage (37). Treatment was continued for 6 wk until death at week 12. Immediately after surgery for the biopsy, four animals died in the VEH group, one in the ACEi group, and three in the AT1A group; these animals were not included in the analyses. At the end of the study, a 2-ml blood sample was taken by cannulation of the abdominal aorta; kidneys were perfused with saline, removed, and further processed as with the biopsy. The protocol was approved by the Committee for Animal Experiments of the University of Groningen, Groningen, The Netherlands.
Clinical parameters.
Proteinuria was measured by the Biuret method (Bioquant, Merck, Darmstadt, Germany). Plasma and urine creatinine levels were determined colorimetrically (Sigma, St. Louis, MO). Systolic blood pressure was measured weekly by the tail-cuff method in trained, conscious rats (8).
Microbead-based assay for quantitation of urinary Kim-1.
Kim-1 protein in urine was measured using Microsphere-based Luminex xMAP technology with monoclonal antibodies raised against rat Kim-1 in the Vaidya/Bonventre laboratory (32). This technique is an adaptation of the recently developed and validated sandwich ELISA assay described previously (31). For measurement, 30 μl of urine samples were analyzed in duplicate.
Quantitative PCR for Kim-1.
Total RNA was isolated using an adaptation of the standard guanidine thiocyanate lysis (10). First-strand cDNA was synthesized from 1 μg total RNA (RT-PCR Core kit, PerkinElmer). TaqMan real-time PCR was performed in 386-well plates on an ABI Prism 7900 Sequence Detector System (Applied Biosystems, Foster City, CA). Kim-1 gene-specific Taqman probe and primer sets were obtained from Applied Biosystems as Assays-on-Demand (AOD) gene expression products. The AOD identification number for Kim-1 was Rn00597703 m1. Sequences of the primers and probe for housekeeping gene GAPDH mRNA are as follows: 5′-GAA CAT CAT CCC TGC ATC CA-3′ (forward), 5′-CCA GTG AGC TTC CCG TTC A-3′ (reverse), and 5′-CTT GCC CAC AGC CTT GGC AGC-3′ (probe).
For GAPDH, the quantitative (q) PCR mixture contained 5 μl of cDNA, 10 μl of 2X TaqMan Universal PCR Master Mix (Eurogentec, Seraing, Belgium), 900 nmol/l of each primer, and 200 nmol/l probe in a total reaction volume of 20 μl. The qPCR mixture for the AOD product Kim-1 contained 10 μl of 2× Taqman Universal PCR Master Mix (Eurogentec), 1 μl of 20* AOD Gene Expression Assay Mix, and 5 μl of cDNA in a total reaction volume of 20 μl. All assays were performed in triplicate. Reaction tubes without template cDNA served as negative controls. The PCR plate was incubated for 2 min at 50°C to optimize the uracil-N-glycosylase enzyme activity and at 95°C for 10 min to activate the Taq polymerase. The reaction was then subjected to 40 cycles of denaturation at 95°C for 15 s, annealing, and extension at 60°C for 1 min. The threshold cycle (CT) was defined as the fractional cycle number at which the fluorescence generated by cleavage of the probe passed a preset threshold. The samples with CT >37 were considered not to express the given mRNA. The CT is inversely proportional to the logarithmic scale of the starting quantity of template cDNA. Consequently, the gene dosage was deduced by calculation of the difference in CT from the CT of the reference gene GAPDH. The average CT values for Kim-1 were subtracted from the average housekeeping gene CT values to yield CT. Results were finally expressed as 2ΔCT, which is an index of the relative amount of renal Kim-1 mRNA expression.
In situ hybridization.
The primer used for Kim-1 in situ hybridization was forward: 5′-AAC GCA GCG ATT GTG CAT CC-3′ and reverse: 5′-GTC CAC TCA CCA TGG TAA CC-3′. The 696-bp Kim-1 PCR product was subcloned into the pCRII-TOPO vector (Invitrogen, Carlsbad, CA). RNA probes were labeled with a DIG RNA labeling kit (Sp6/T7, Roche, Mannheim, Germany). In situ hybridization was performed on routinely fixed paraffin-embedded tissue sections using standard laboratory protocols. Briefly, deparaffinized sections were air-dried, treated with Triton X-100, followed by proteinase K. Thereafter, slides were incubated with DIG-labeled probe in a hybridization solution consisting of 1 ml 20× SSC, 50 μl 100× Denhardt's solution, 1 ml 50% dextran sulfate, 2.5 ml formamide, 200 μl t-RNA, 50 μl 1 M DTT, and 125 μl salmon sperm DNA overnight at 55°C. After a washing, slides were treated with 2 U/ml RNase T1 in 1 mM EDTA and 2× SSC at 37°C for 30 min. Positive cells were visualized with anti-DIG-labeled alkaline phosphatase for 1 h at 37°C in 0.1 M maleic acid buffer containing 0.15 M NaCl, 1% blocking buffer and 2% normal sheep serum. The staining reaction was performed for 48 h at 4°C with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate in TBS with MgCl2 and levamisole.
Immunohistochemistry.
Paraffin sections (4 μm) were stained with periodic acid-Schiff to evaluate focal glomerulosclerosis (FGS) and interstitial fibrosis. Immunostaining was performed on paraffin sections for Kim-1 (antibody against the intracellular domain of Kim-1: peptide 9; dilution 1:1,000, Biogen, Cambridge, MA), α-smooth muscle actin (α-SMA; clone 1A4, dilution 1:15,000, Sigma,), collagen type III (dilution 1:100, Biogenesis, Poole, UK), and macrophages (ED1; dilution 1:1,000, Serotec, Oxford, UK). After dewaxing with xylol and alcohol, antigen retrieval was performed by overnight incubation (80°C) in 0.1 M Tris·HCl buffer. After blocking of endogenous peroxidase activity, sections were incubated for 1 h with diluted primary antibodies in PBS with 1% bovine serum albumin. Binding for antibodies was detected using two sequential incubations (30 min) with peroxidase-labeled secondary antibodies. Peroxidase activity was developed using 3,3′-diamino benzidine (DAB; Sigma) solution for 10 min, to which hydrogen peroxide was added. An automated staining system (DAKO Autostainer, Edition 4.0, DAKO, Carpinteria, CA) was used to obtain comparable staining results for all slides.
To study the colocalization of Kim-1 with renal interstitial damage, double staining with ED1 (macrophages), α-SMA, or collagen III was performed. Slides were incubated with a mixture of primary antibodies for 1 h at room temperature: anti-Kim-1 and anti-ED1 at dilution 1:400, α-SMA at 1:500. After washing with PBS, secondary antibodies were added. Kim-1 was detected with peroxidase-labeled goat anti-rabbit antibodies and ED1 and α-SMA with alkaline phosphatase-labeled goat anti-mouse antibodies. First, peroxidase activity was developed with DAB for 10 min. Subsequently, alkaline phosphatase activity was developed with Naphtol AS-MX, and color was developed with Fast Blue BB combined with levamisol and MgSO4 for 30 min. To combine collagen III and Kim-1 (both polyclonal), staining for Kim-1 with peroxidase-labeled secondary antibodies and DAB was first performed, followed by incubation with glycine/HCl, pH 2.0, for 30 min. After blocking of endogenous biotin and streptavidin, sections were incubated with anti-collagen III (dilution 1:50) followed by a biotin-labeled goat anti-rabbit antibody and alkaline phosphatase-labeled streptavidin. Appropriate isotype and PBS controls were consistently negative for all antibodies.
Quantification of renal damage.
FGS was scored semiquantitatively on a scale of 0–4 in 50 glomeruli/kidney, moving from outer to inner cortex. FGS lesions were defined as glomerular areas with mesangial expansion and adhesion formation simultaneously present in one segment (25). Interstitial fibrosis was scored semiquantitatively on a scale of 0–3 (30). Interstitial Kim-1, α-SMA, and collagen type III staining was measured by a blinded observer using computer image analysis (Advanced QUIPS, Leica Imaging Systems, Cambridge, UK). The proportional area of immunostaining was measured in 50 randomly selected cortical interstitial images/kidney, with exclusion of large vessels and glomeruli. The area of immunostaining for Kim-1, α-SMA, and collagen III was divided by the total surface of the image. The number of glomerular macrophages was determined in 50 glomeruli/slide. Interstitial macrophages were counted in 50 consecutive fields.
Statistical analysis.
Data are expressed as means ± SD when a normal distribution was present, or as median and 95% confidence interval when the data distribution was not normal. When normal distribution was present, ANOVA with a post hoc test (differences between groups) or paired samples t-test (differences between weeks 6 and 12) were used. When abnormal distribution was present, the nonparametric Kruskal-Wallis (differences between groups) and Wilcoxon signed rank test (differences between weeks 6 and 12) were used. To perform linear regression, data were transformed with natural logarithms. Spearman's Rho correlation coefficients are given. To calculate the predictive value of the different parameters at week 6 for outcomes at week 12 in the ACEi- and AT1A-treated animals, linear regression analysis was performed, using the backward method. Dependent variables were, respectively, FGS, interstitial fibrosis, and proteinuria at week 12. As input variables, we entered blood pressure, proteinuria, FGS, interstitial fibrosis, macrophages, α-SMA, Kim-1 protein (immunohistochemistry), and Kim-1 mRNA (all variables at week 6). Statistical analyses were performed using SPSS statistical software, version 12.0, and GraphPad Prism, version 3.02. Statistical significance was assumed at the 5% level.
RESULTS
Clinical parameters.
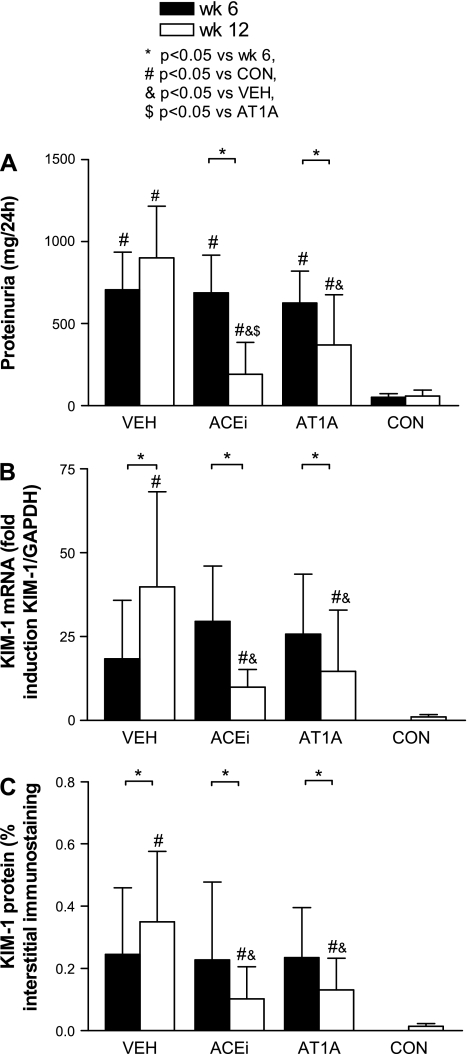
At week 6, overt proteinuria was present in the adriamycin rats (Fig. 1A). During ACEi treatment (75 mg/l drinking water), proteinuria decreased from week 6 to week 12 by 78 ± 15% and with AT1A by 59 ± 24% (both P < 0.05 vs. week 6). In the VEH animals, proteinuria stabilized between the 6- and 12-wk time points. Blood pressure was significantly reduced by ACEi and by AT1A but was stable during VEH (Table 1). Compared with healthy controls, creatinine clearance was significantly reduced in all adriamycin groups.
Fig. 1.
Bar graphs of proteinuria (A), kidney injury molecule (Kim)-1 mRNA (measured with quantitative real-time PCR; B), and Kim-1 protein (measured with computer-assisted morphometry; C) in the different treatment groups. VEH, vehicle treatment; ACEi, angiotensin-converting enzyme inhibition; AT1A, angiotensin type 1 antagonist; CON, healthy control rats.
Table 1.
Clinical parameters and renal damage
| Week | VEH (n = 6) | ACEi (n = 22) | AT1A (n = 21) | CON (n = 8) | |
|---|---|---|---|---|---|
| Blood pressure, mmHg | 6 | 151±11 | 146±9 | 144±11 | 123±17 |
| 12 | 139±24 | 103±20*† | 118±23* | 118±14 | |
| Creatinine clearance, ml/min | 12 | 1.37±0.88‡ | 1.29±0.39‡ | 1.43±0.37‡ | 2.42±0.82 |
| Macrophages/interstitial field | 6 | 73 (49–231) | 88 (36-138) | 79 (60-118) | |
| 12 | 9 (2-44)* | 13 (7–18)* | 18 (15–60)* | 9 (2–16) | |
| α-SMA, % interstitial staining | 6 | 7.7 (4–11) | 7.5 (5.6–8.4) | 6.9 (5.1–8) | |
| 12 | 9.6 (3.7–16.1)‡ | 5.5 (4.4–8.5)‡ | 7.1 (5.3–10.6)‡ | 1 (0.4–2.2) | |
| Collagen type III, % interstitial staining | 6 | 0.41 (0.19–0.64) | 0.56 (0.42–0.63) | 0.52 (0.45–0.75) | |
| 12 | 0.55 (0.25–1.1)‡ | 0.31 (0.28–0.71)‡ | 0.5 (0.37–0.85)‡ | 0.12 (0–0.3) | |
| Interstitial fibrosis, score 0–3 | 6 | 0 (0–2) | 0.5 (0–2) | 0 (0–1) | |
| 12 | 1 (0–3)*‡ | 1 (1–2)*‡ | 1 (0–2)*‡ | 0 (0–0) | |
| FGS, score 0–400 | 6 | 20 (5–28) | 30 (22–59) | 12 (8–32) | |
| 12 | 107 (45–160)*‡ | 28 (26–68) | 36 (23–88)*†‡ | 4 (0–16) |
Values are means ± SD for blood pressure and creatinine clearance and median and 95% confidence interval (in parentheses) for macrophages, α-small muscle actin (SMA), collagen type III, interstitial fibrosis, and focal glomerulonephritis (FGS); n = no. of animals. VEH, vehicle; ACEi, angiotensin-converting enzyme inhibitor; AT1A, angiotensin II antagonist; CON, control.
P < 0.05 vs. week 6.
P < 0.05 vs. VEH.
P < 0.05 versus CON.
Quantification of renal damage.
Macrophage influx was high in adriamycin rats at week 6 and decreased in all groups from week 6 to week 12 (Table 1). In adriamycin-treated rats, α-SMA expression was present at week 6. It was increased compared with control rats and was not different between weeks 6 and 12 in all groups. Collagen type III staining and interstitial fibrosis were increased in adriamycin rats compared with healthy controls, without an apparent effect of ACEi and AT1A. Mild FGS was present at week 6, with a sharp increase in the VEH-treated animals at week 12. Treatment with ACEi as well as AT1A attenuated FGS at week 12 compared with vehicle.
Kim-1 qPCR.
The pretreatment biopsies from week 6 of all adriamycin animals showed a massive induction of Kim-1 mRNA, ranging from 18- to 30-fold induction (adriamycin vs. CON: P < 0.05) (Fig. 1B). At the end of the study, Kim-1 mRNA expression was increased by 40-fold in VEH rats. Antiproteinuric treatment attenuated Kim-1 mRNA expression at week 12 to 10-fold in the ACEi-treated group and 12-fold in AT1A-treated rats (both P < 0.05 vs. VEH).
Kim-1 in situ hybridization.
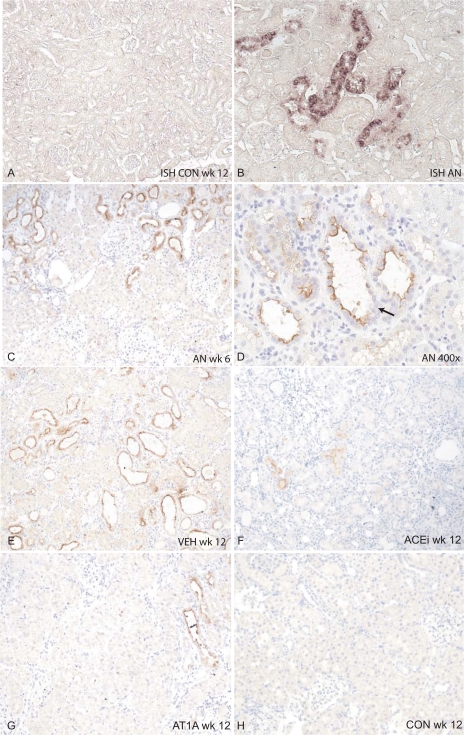
In healthy rats, no renal Kim-1 mRNA was detected (Fig. 2A). In adriamycin-treated rats, Kim-1 mRNA was clearly present, mainly localized in the cytoplasm of tubular cells from focally grouped dilated tubules (Fig. 2B). However, in the most severely damaged tubules, characterized by advanced dilation and severe flattening of tubular cells, Kim-1 mRNA could not easily be detected. It appeared that single nephrons could be traced from cortex to medulla by their positive Kim-1 mRNA staining.
Fig. 2.
Localization of Kim-1. In situ hybridization (ISH; A and B) and immunohistochemistry (C–H) for rat Kim-1. A: Kim-1 mRNA in a healthy control rat. No Kim-1 mRNA is visible (magnification ×100). B: Kim-1 mRNA is induced in rats with adriamycin nephrosis (AN) in dilated tubules. (magnification ×100). C: Kim-1 protein (immunohistochemistry) expression in a rat with AN at week 6 (magnification ×100 in the biopsy). Several tubules, which seem to adhere to 1 nephron, are positive for Kim-1. Kim-1 is expressed in the dilated tubules at the apical membrane, which is shown in more detail in D at ×400 magnification. Here, it is also visible that Kim-1 shows a mosaic pattern; not all cells are positive for Kim-1 (arrow, Kim-1-negative tubules). In severely damaged tubules, as apparent from advanced flattening of the tubular cells, Kim-1 is not easily detected (see C). E: Kim-1 protein expression in a VEH-treated rat at week 12. Expression of Kim-1 was somewhat higher than at week 6. F: after 6 wk of ACEi treatment, Kim-1 protein was reduced, but in dilated tubules still some Kim-1 expression was visible. G: treatment with AT1A also reduced Kim-1 staining. H: in healthy control rats at week 12, no Kim-1 protein was detected.
Kim-1 immunohistochemistry.
In concert with the expression pattern observed with in situ hybridization, Kim-1 protein-positive tubules in adriamycin rats were often organized in segments, suggestive of the contours of a single nephron (Fig. 2, C and E). Kim-1 protein was prominent at the apical border of dilated tubular cells with weak cytoplasm expression. Kim-1 was often present in slightly dilated tubules, with morphologically well-differentiated cells. In more dilated tubules, Kim-1 expression within one tubule showed a mosaic staining pattern with Kim-1-positive and -negative cells adjacent to each other (Fig. 2D). In severely damaged tubules, as apparent from advanced flattening of the tubular cells, Kim-1 was not easily detected (Fig. 2E). Kim-1 expression was absent in the glomerulus, peritubular interstitial cells, and inner medullary cells. Computerized quantification revealed that Kim-1 protein expression was similar at week 6 (before the start of treatment) for all groups (Figs. 1C and 2C). At week 12, Kim-1 protein expression was significantly attenuated in the ACEi and AT1A groups (from 0.23 ± 0.25 to 0.10 ± 0.10 and 0.23 ± 0.16 to 0.13 ± 0.10% of tissue volume, respectively, for week 6 compared with week 12, both P < 0.05, Fig. 2, F and G). In the VEH-treated group, Kim-1 protein increased (from 0.23 ± 0.20 to 0.35 ± 0.23% of tissue volume for week 6 compared with week 12, P < 0.05, Fig. 2E). In healthy controls, virtually no Kim-1 protein was present (0.01 ± 0.01% tissue volume, Fig. 2H).
Colocalization studies.
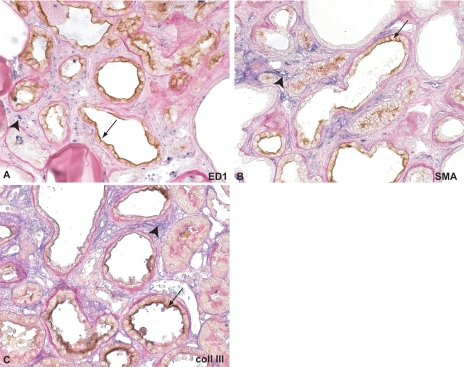
To investigate whether renal Kim-1 expression was associated with classic markers of tubulointerstitial damage, we performed double staining for Kim-1 with a macrophage marker (ED1, inflammation), α-SMA expression (myofibroblast transformation, indicating the presence of prefibrotic changes), and collagen type III (fibrotic lesions). The double staining of Kim-1 with ED1 showed that Kim-1-positive tubules were often surrounded by interstitial macrophages (Fig. 3A), whereas Kim-1-negative (and morphologically normal) tubules were not associated with local macrophage infiltration. However, in more advanced fibrotic lesions, macrophages were also present in areas with severely dilated Kim-1-negative tubules. The Kim-1-positive tubules were surrounded by α-SMA-positive fibroblasts, indicating the presence of prefibrotic changes (Fig. 3B). In this double staining, also severely dilated, but Kim-1-negative, tubules were surrounded by α-SMA-positive fibroblasts. Kim-1-positive tubules were often surrounded by collagen III deposits (Fig. 3C). However, this colocalization was not always present, especially not in advanced lesions.
Fig. 3.
Double staining of Kim-1 in combination with macrophages, α-smooth muscle actin (SMA), and collagen type III. A: double staining for Kim-1 (stained in brown, arrows) and ED1 (macrophages, blue, arrowheads). Surrounding the Kim-1-positive tubules, an influx of macrophages is present, especially in early lesions. In later stages, macrophages are widespread, present in the whole cortex (not shown). B: double staining for Kim-1 (brown, arrows) and α-SMA (blue, arrowheads). Kim-1-positive dilated tubules are often surrounded by myofibroblasts. C: double staining for Kim-1 (brown, arrows) and collagen type III (blue, arrowheads). Flattened tubules that are positive for Kim-1 are sometimes surrounded by increased deposition of collagen type III (arrow). However, in advanced stages, also many dilated tubules surrounded by collagen III deposition are negative for Kim-1.
Urinary Kim-1.
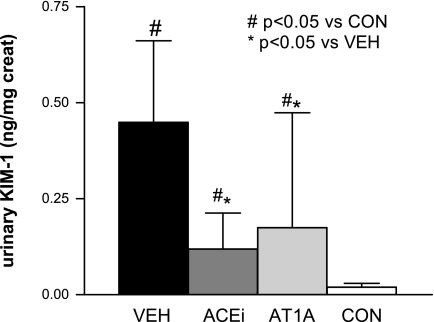
The shedded ectodomain of Kim-1 in urine was measured by a microbead (Luminex)-based assay at the end of the study (32). The concentration of urinary Kim-1 was significantly increased in all adriamycin-treated animals at week 12. In the VEH-treated group, urinary Kim-1 was 257 ± 164 vs. 21 ± 10 pg/ml (below the limits of detection of the assay) in controls (P < 0.05). Treatment with ACEi or AT1A for 6 wk significantly reduced urinary Kim-1 to 53 ± 47 and 91 ± 111 pg/ml, respectively (both P < 0.01 vs. VEH). In Fig. 4, urinary Kim-1 levels are normalized to urinary creatinine concentration.
Fig. 4.
Urinary Kim-1 measured by microbead assay normalized by urine creatinine. For more details, see methods. Data are presented as means ± SD.
Correlations among urinary and renal Kim-1, proteinuria, and renal damage.
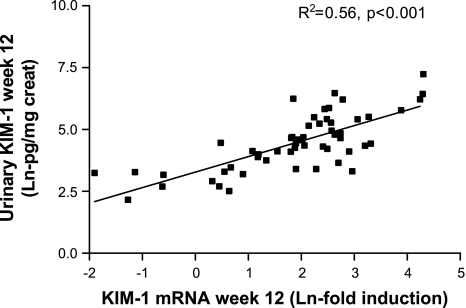
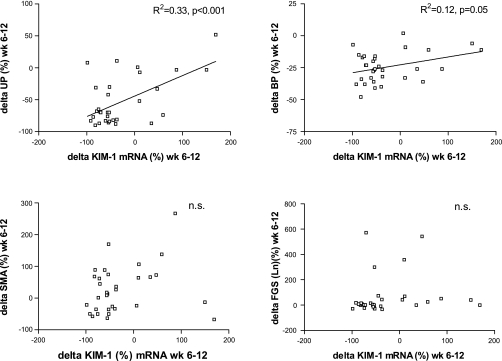
On individual analysis, urinary Kim-1 and renal Kim-1 were strongly associated (R2 = 0.56, P < 0.001, Fig. 5). Taking the parameters separately, renal Kim-1 (mRNA) was strongly associated with proteinuria (R2 = 0.42, P < 0.001) and with markers of renal damage. The association with α-SMA was strong as well (R2 = 0.52, P < 0.001). Kim-1 mRNA was also associated with other parameters of renal damage, namely, tissue macrophages (R2 = 0.15, P < 0.05), interstitial fibrosis (R2 = 0.44, P < 0.01), and FGS (R2 = 0.49, P < 0.01). Also, urinary Kim-1 was associated with proteinuria (R2 = 0.55, P < 0.001) and with renal damage: α-SMA (R2 = 0.32, P < 0.001), macrophages (R2 = 0.23, P < 0.01), interstitial fibrosis (R2 = 0.30, P < 0.01), and FGS (R2 = 0.55, P < 0.01). There was no association with blood pressure (R2 = 0.04). Thus, on cross-sectional analysis, animals with more proteinuria had higher levels of renal Kim-1 and urinary Kim-1. Higher levels of Kim-1 mRNA and urinary Kim-1 were also associated with more renal damage. The association between the change in proteinuria with the change in Kim-1 during antiproteinuric treatment is shown in Fig. 6, giving an R2 of 0.33, P < 0.001, whereas the association between reduction in blood pressure and reversibility of Kim-1 was of borderline significance with a R2 of 0.12, P = 0.05. On multivariate analysis, the reduction of Kim-1 was explained by the reduction in proteinuria but not blood pressure. The changes in Kim-1 expression were not correlated with changes in interstitial fibrosis (prefibrotic changes as apparent from α-SMA) or changes in FGS (Fig. 6, bottom).
Fig. 5.
Scatter plot showing the strong correlation between urinary Kim-1 (normalized by urinary creatinine) and renal Kim-1 expression. Data were transformed with the natural logarithm to obtain a normal distribution.
Fig. 6.
Scatter plots showing the relation between percent change in renal Kim-1 mRNA between week 6 (pretreatment) and week 12 (after 6-wk treatment) vs. 4 different markers in all adriamycin nephritic rats (treated and untreated). Top left: percent change in proteinuria (UP) between weeks 6 and 12. Top right: percent change in blood pressure (BP) between weeks 6 and 12. Bottom left: α-SMA immunohistochemistry between weeks 6 and 12. Bottom right: focal glomerulosclerosis (FGS) between weeks 6 and 12.
To analyze whether pretreatment renal Kim-1 expression predicts renal outcome after treatment, we performed univariate analysis, showing for ACEi- and AT1A-treated rats, renal Kim-1 mRNA at week 6 measured by real-time PCR predicts proteinuria at week 12 (R2 = 0.62, P < 0.05), focal glomerulosclerosis (R2 = 0.53), and interstitial fibrosis (R2 = 0.41).
When linear regression analysis is used, the best predicting model for FGS at week 12 contains FGS, macrophages, Kim-1 protein (immunohistochemistry), and proteinuria. In an analysis for proteinuria at week 12, Kim-1 mRNA at week 6 is the best predictor. For interstitial fibrosis at week 12, only α-SMA at week 6 is the best predictor.
DISCUSSION
The major findings of this paper are the reversibility of renal tubular Kim-1 expression during antiproteinuric treatment by RAS blockade, in proportion to the reduction in proteinuria. This is the first study with longitudinal data on renal Kim-1 expression before and after antiproteinuric treatment. Interestingly, renal Kim-1 expression before treatment predicts the outcome of renal damage after treatment. In line with earlier studies, Kim-1 expression was present in injured and dilated tubules in areas with interstitial inflammation and fibrosis, but less so in tubuli with advanced lesions. Moreover, the levels of urinary Kim-1 were strongly associated with renal tissue Kim-1.
This study describes the expression of Kim-1 in adriamycin-induced nephropathy and its reversibility during treatment with RAS blockade. Adriamycin nephropathy is a well-established model of for interstitial fibrosis, characterized by the gradual development of proteinuria, due to direct toxicity on glomerular structure responsible for changes in glomerular permeability (3, 4, 16). The direct toxicity is due to oxygen free radicals, as demonstrated by increased ATPase activity (2). Proteinuria usually stabilizes after 4–5 wk (4). During the subsequent phase, persistent proteinuria causes tubular cell activation and injury with attraction of interstitial inflammatory cells (3, 43) and the development of interstitial fibrosis. Both macrophages and tubular cells contribute to fibrosis through the production of growth factors (3). Although the pathophysiology of adriamycin nephropathy has not been elucidated in full detail, by the sequence of events with proteinuria preceding the development of renal fibrotic lesions, adriamycin is well suited to study the effects of antiproteinuric intervention on the subsequent development of renal structural damage and on the reversibility of tubular damage markers.
In chronic kidney disease, RAS blockade improves the long-term outcome, but in many patients renoprotection is incomplete, prompting better identification of patients that need intensified therapy. In adriamycin nephropathy, we previously showed that more severe pretreatment tubulointerstitial damage predicts a worse long-term outcome of renoprotective intervention (20), which can be ameliorated by intensified treatment (19). For clinical purposes, therefore, it would be important to have a biomarker that reflects the severity of pretreatment tubulointerstitial renal damage better than proteinuria alone to identify patients that need intensified treatment (24). Here, pretreatment renal Kim-1 levels predicted antiproteinuric response and outcome at an end point with a worse outcome in individuals with higher Kim-1 levels. Also, in line with other studies (33, 35), urinary Kim-1 was strongly associated with renal Kim-1 expression, although unfortunately urinary Kim-1 was available only at the end point. Future studies therefore should further explore this issue and investigate whether pretreatment urinary Kim-1 can identify individuals that need intensified therapy to obtain effective long-term renoprotection.
The mechanism of Kim-1 induction by adriamycin nephropathy, and its reversibility during antiproteinuric treatment, was not specifically studied here, but several inferences can be made. Kim-1 expression occurs in a wide array of renal conditions, in association with early tubular damage. It is plausible that in our study proteinuria was the trigger for tubular damage, as supported by the between-group differences in proteinuria and renal Kim-1 expression, the cross-sectional correlation between renal Kim-1 and proteinuria, and the corresponding changes in proteinuria and Kim-1 during treatment. Reabsorption of leaked proteins is followed by tubular activation characterized by the production of growth factors and cytokines (43). This may lead to tubular injury characterized by proliferation, apoptosis, inflammation, and increased extracellular matrix production (42). A role for proteinuria would be in line with the abundant expression of Kim-1 in protein overload-induced proteinuria (33). The functional consequences of Kim-1 expression have not been established so far, but recently, Ichimura et al. (13) demonstrated that epithelial cells expressing Kim-1 are capable of phagocytosis of apoptotic and necrotic cells of injured tubule lumen in cultured primary rat tubule epithelial cells. Kim-1 recognizes apoptotic cells by surface-specific epitopes (phosphatidylserine and oxidized lipoproteins) (13). Thus Kim-1 may play a role in the remodeling after injury and renal repair capacity. However, it cannot be established from our study whether Kim-1 is actively involved in the process of damage and repair, or, alternatively, is just a consequence of renal injury. Unraveling cause and consequence would require specific intervention in Kim-1, for instance, by Kim-1 knockout or overexpression models.
After antiproteinuric treatment with RAS blockade, the increase in renal and urinary Kim-1 was significantly attenuated. This reduction in Kim-1 levels is probably due to reduction of proteinuria and its intrarenal sequelae. Based on the current data, we cannot exclude the possibility that it is due to a direct pharmacological effect of the intervention by amelioration of angiotensin II-dependent activation of tubular cells and the subsequent cytokine and growth factor release (7). Recent human data from our group may allow the partial dissection between effects of pharmacological intervention and the effects of proteinuria reduction per se. In nondiabetic proteinuric patients, nonpharmacological reduction of proteinuria was associated with a decrease in urinary KIM-1 excretion (36). For the current study, we assume that the RAS blockade contributed particularly by reducing proteinuria, whereas a role for blood pressure cannot be fully excluded. Also, RAS blockade can act by direct reduction of angiotensin II effects and improve medullary blood flow and tubular oxygenation, which may support restoration of tubular integrity (11) by restoring ischemia. This is also in line with data on Kim-1 expression in homozygous Ren-2 rats, a model of angiotensin-mediated renal damage, where treatment with RAS blockers reduced Kim-1 expression compared with untreated Ren-2 rats. The latter effect is not blood pressure dependent, demonstrated by the reduction of Kim-1 expression in the same study with homozygous Ren-2 rats after treatment with p38 MAP kinase inhibitors (9). In the present study, a weak association was present between the reduction in blood pressure and reduction in Kim-1, which, nevertheless, was no longer significant after correction for the reduction in proteinuria by multivariate analysis.
We also studied the cellular localization of Kim-1 in relation to the presence of tubulointerstitial damage. Kim-1 was localized in the apical membrane of dilated tubules, which corresponds to the localization of Kim-1 in ischemic and toxic injury (14, 15). In ischemic injury, Kim-1 expression is most prominent in the S3 segment (i.e., the segment most susceptible to ischemic injury), whereas in our model of proteinuria-induced renal damage Kim-1 expression was also prominent in the midcortical and superficial tubules, which is in congruence with the Kim-1 expression in folic acid-induced renal injury and polycystic kidney disease, models where damage is not predominantly in the S3 segment (15, 22). Therefore, localization of Kim-1 expression appears to be related to the susceptibility of the specific tubular segments to different types of injury.
When related to localization of tubulointerstitial damage, we found that Kim-1 was expressed in areas that display interstitial inflammation, tubular dilation, and (pre)-fibrotic changes. Interestingly, Kim-1 expression was mainly apparent at the apical membrane of dilated tubular cells, whereas it was absent in advanced stages of tubular damage. We observed a mosaic staining pattern of Kim-1 within one tubule: cells with preserved morphology within the tubule showed Kim-1 expression, and the more flattened cells were Kim-1 negative, using our technique. In ischemia-reperfusion, Kim-1 is colocalized with vimentin (dedifferentiation) and BrdU (proliferation) in regenerating tubular cells (14). In polycystic kidney disease (22), Kim-1-positive cells demonstrate partial loss of polarity but preserved staining for actin, villin, and E-cadherin. Together, these data suggest that Kim-1 is expressed early in the sequence of events of dedifferentiation of an injured tubular cell. It should be noted that the reversibility of renal Kim-1 during antiproteinuric treatment was not accompanied by reversibility of interstitial fibrosis, reinforcing the notion that the dynamics of Kim-1 expression predominantly reflect early tubular injury rather than late fibrosis. This is in line with recent data in renal transplant recipients (40) and with the study by Ichimura et al. (13), demonstrating phagocytosis of necrotic and apoptotic cells by epithelial cells expressing Kim-1. By analogy, in our proteinuric model, Kim-1 might protect the renal interstitium from toxic mediators and apoptotic cells induced by the proteinuric ultrafiltrate.
Kim-1 is shed into urine, and, in accord with other studies, we found that its urinary excretion corresponded to intrarenal Kim-1 expression. Urinary Kim-1 was increased in untreated proteinuric animals, whereas animals after antiproteinuric treatment showed lower levels of urinary Kim-1. As urinary Kim-1 correlates with proteinuria and renal damage (and renal Kim-1 levels), it is a potential noninvasive marker for interstitial injury. Recently, we found that urinary KIM-1 reflects not only tubular KIM-1 expression in human renal disease but also other markers of renal damage (35). Also, in renal transplant recipients, urinary KIM-1 is an independent predictor of long-term graft loss (34). Thus KIM-1 is a promising new biomarker of renal damage and graft loss. Therefore, it would be of great interest to see whether the effects of treatment on urinary Kim-1 could prospectively predict renal outcome of treatment. In this study, however, we did not measure urinary Kim-1 levels before treatment and cannot draw conclusions on this issue. In nondiabetic proteinuric patients, we prospectively studied urinary KIM-1 levels before and after antiproteinuric treatment. Urinary KIM-1 levels are markedly elevated in these patients and decrease with antiproteinuric treatment, by angiotensin II blockade, as well as by a low-sodium diet and the combination with diuretic therapy (36). Our current data suggest that urinary KIM-1 levels may be used to monitor the effect of antiproteinuric intervention on the tubulointerstitial sequelae of proteinuria, which could be of prognostic relevance. However, prospective long-term studies would be needed to test this assumption.
In conclusion, the increase in renal Kim-1 expression in adriamycin nephropathy is reversible by antiproteinuric treatment by RAS blockade in proportion to the reduction in proteinuria. This reversibility however, is not paralleled by reversibility of established fibrotic lesions, in line with the association of renal Kim-1 with early injury in particular. Pretreatment renal Kim-1 levels predict antiproteinuric response and the subsequent renal outcome of treatment. Moreover, renal Kim-1 is closely related to urinary Kim-1 levels. These data provide further support for Kim-1 as a marker for early tubular injury, which could be used to guide and individualize renoprotective intervention.
GRANTS
J. V. Bonventre was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-39773, DK-72381, and DK-74099. V. S. Vaidya was supported by Scientist Development Grant 0535492T from the American Heart Association. These data were presented at the 38th Annual Meeting of the American Society of Nephrology, 8-13 November 2005 (Philadelphia, PA).
Acknowledgments
We thank Anke Mink, Marian Bulthuis, Sippie Huitema, Jeffrey Damman, and Petra Ottens for assistance. The compounds lisinopril and L158, 809 were kind gifts from Merck, Sharp, and Dohme (Rahway, NJ). The antibody for Kim-1 (used for immunohistochemistry and Western blotting) was a kind gift from Dr. V. Bailly (Biogen, Cambridge, MA).
REFERENCES
- 1.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem 277: 39739–39748, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Barbey MM, Fels LM, Soose M, Poelstra K, Gwinner W, Bakker W, Stolte H. Adriamycin affects glomerular renal function: evidence for the involvement of oxygen radicals. Free Radic Res Commun 7: 195–203, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Bertani T, Cutillo F, Remuzzi G. Tubulo-interstitial lesions mediate renal damage in adriamycin glomerulopathy. Kidney Int 30: 488–496, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Bertani T, Poggi A, Pozzoni R, Delaini F, Sacchi G, Thoua Y, Mecca G, Remuzzi G, Donati MB. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest 46: 16–23, 1982. [PubMed] [Google Scholar]
- 5.Bonventre JV Kidney injury molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl 241: 78–83, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Bos H, Henning RH, De Boer E, Tiebosch ATM, De Jong PE, De Zeeuw D, Navis GJ. Addition of AT1 blocker fails to overcome resistance to ACE-i in adriamycin nephrosis. Kidney Int 61: 473–480, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Cooper ME. Role of angiotensin II in tubulointerstitial injury. Semin Nephrol 21: 554–562, 2001. [DOI] [PubMed] [Google Scholar]
- 8.De Boer E, Navis GJ, Tiebosch ATM, De Jong PE, De Zeeuw D. Systemic factors are involved in the pathogenesis of proteinuria-induced glomerulosclerosis in adriamycin nephrotic rats. J Am Soc Nephrol 10: 2359–2366, 1999. [DOI] [PubMed] [Google Scholar]
- 9.de Borst MH, van Timmeren MM, Vaidya VS, de Boer RA, Van Dalen MB, Kramer AB, Schuurs TA, Bonventre JV, Navis G, van Goor H. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the renin-angiotensin system or p38 MAP kinase. Am J Physiol Renal Physiol 292: F313–F320, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Deelman LE, Navis G, de Boer E, Wietses M, de Zeeuw D, Henning RH. Role of proteinuria in the regulation of renal renin-angiotensin system components in unilateral proteinuric rats. J Renin Angiotensin Aldosterone Syst 4: 38–42, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Dukacz SA, Adams MA, Kline RL. Short- and long-term enalapril affect renal medullary hemodynamics in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol 276: R10–R16, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Goumenos DS, Brown CB, Shortland J, El Nahas AM. Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol Dial Transplant 9: 1418–1425, 1994. [PubMed] [Google Scholar]
- 13.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 286: F552–F563, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Jeansson M, Bjorck K, Tenstad O, Haraldsson B. Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20: 114–122, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klahr S, Morrissey JJ. Comparative study of ACE inhibitors and angiotensin II receptor antagonists in interstitial scarring. Kidney Int 52: S111–S114, 1997. [PubMed] [Google Scholar]
- 18.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27: 927–940, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer AB, Bos H, van Goor H, Navis GJ. Sodium intake modifies the negative prognostic value of renal damage prior to treatment with ACE inhibitors on proteinuria induced by adriamycin. Nephron Physiol 103: 43–52, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kramer AB, Laverman GD, van Goor H, Navis G. Inter-individual differences in anti-proteinuric response to ACEi in established adriamycin nephrotic rats are predicted by pretreatment renal damage. J Pathol 201: 160–167, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kramer AB, van der Meulen EF, Hamming I, van Goor H, Navis G. Effect of combining ACE inhibition with aldosterone blockade on proteinuria and renal damage in experimental nephrosis. Kidney Int 71: 417–424, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kuehn EW, Park KM, Somlo S, Bonventre JV. Kidney injury molecule-1 expression in murine polycystic kidney disease. Am J Physiol Renal Physiol 283: F1326–F1336, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Lufft V, Kliem V, Hamkes A, Bleck JS, Eisenberger U, Brunkhorst R. Antiproteinuric efficacy of fosinopril after renal transplantation. Clin Transpl 12: 409–415, 1998. [PubMed] [Google Scholar]
- 24.Perico N, Cattaneo D, Remuzzi G. Kidney injury molecule 1: in search of biomarkers of chronic tubulointerstitial damage and disease progression. Am J Kidney Dis 53: 1–4, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl: S57–S65, 2005. [DOI] [PubMed]
- 27.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P, Perna A, Remuzzi G. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt H, Bohle A, Reineke T, Mayer-Eichberger D, Vogl W. Long-term prognosis of membranoproliferative glomerulonephritis type I. Significance of clinical and morphological parameters: an investigation of 220 cases. Nephron 55: 242–250, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Smit-van Oosten A, Navis G, Stegeman CA, Joles JA, Klok PA, Kuipers F, Tiebosch AT, van Goor H. Chronic blockade of angiotensin II action prevents glomerulosclerosis, but induces graft vasculopathy in experimental kidney transplantation. J Pathol 194: 122–129, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290: F517–F529, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya V, Ramirez V, Bobadilla N, Bonventre J. A microfluidics based assay to measure kidney injury molecule-1 (Kim-1) in the urine as a biomarker for early diagnosis of acute kidney injury (Abstract). J Am Soc Nephrol 16: 192A, 2005. [Google Scholar]
- 33.van Timmeren MM, Bakker SJ, Vaidya VS, Bailly V, Schuurs TA, Damman J, Stegeman CA, Bonventre JV, van Goor H. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol 291: F456–F464, 2006. [DOI] [PubMed] [Google Scholar]
- 34.van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries APJ, Gans ROB, van Goor H, Stegeman CA, Bonventre JV, Bakker SJL. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 84: 2007. [DOI] [PMC free article] [PubMed]
- 35.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Waanders F, Vaidya VS, van Goor H, Leuvenink H, Damman K, Hamming I, Bonventre JV, Vogt L, Navis G. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis 53: 16–25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wapstra FH, van Goor H, de Jong PE, Navis G, de Zeeuw D. Dose of doxorubicin determines severity of renal damage and responsiveness to ACE-inhibition in experimental nephrosis. J Pharmacol Toxicol Methods 41: 69–73, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Wehrmann M, Bohle A, Bogenschutz O, Eissele R, Freislederer A, Ohlschlegel C, Schumm G, Batz C, Gartner HV. Long-term prognosis of chronic idiopathic membranous glomerulonephritis. An analysis of 334 cases with particular regard to tubulo-interstitial changes. Clin Nephrol 31: 67–76, 1989. [PubMed] [Google Scholar]
- 39.Wehrmann M, Bohle A, Held H, Schumm G, Kendziorra H, Pressler H. Long-term prognosis of focal sclerosing glomerulonephritis. An analysis of 250 cases with particular regard to tubulointerstitial changes. Clin Nephrol 33: 115–122, 1990. [PubMed] [Google Scholar]
- 40.Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int 73: 608–614, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Humphreys BD, Bonventre JV. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol 18: 2704–2714, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Zoja C, Benigni A, Remuzzi G. Cellular responses to protein overload: key event in renal disease progression. Curr Opin Nephrol Hypertens 13: 31–37, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, Remuzzi G. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int 53: 1608–1615, 1998. [DOI] [PubMed] [Google Scholar]