Abstract
N-methyl-d-aspartate receptors (NMDA) are expressed in the kidney, where little is known of their functional role. Several series of micropuncture experiments were performed in hydropenic rats using the NMDA channel blocker, MK801, and the NMDA coagonist, l-glycine, to probe NMDA for effects on single-nephron glomerular filtration rate (SNGFR) and proximal reabsorption (Jprox). During intravenous infusion of MK801 or l-glycine, Henle's loop was perfused to manipulate SNGFR via tubuloglomerular feedback (TGF), thereby facilitating analysis of glomerulotubular balance. To confirm local actions on the kidney, MK801 was delivered to the glomerulus by microperfusion past the macula densa and to the proximal tubule by microperfusion into the early S1 segment. By all measures, MK801 acted on the glomerulus to reduce SNGFR, and acted on the proximal tubule to suppress Jprox, while having no effect on the responsiveness of TGF. l-Glycine raised SNGFR, dampened the TGF response, and could not be proved to independently stimulate proximal reabsorption. NMDA exerts a tonic vasodilatory influence on the glomerulus and a proreabsorptive effect on the proximal tubule. These combined effects allow NMDA to modulate SNGFR with minimal impact on late proximal flow. The full effects of l-glycine infusion on proximal tubule and TGF response do not extrapolate from the response to NMDA blockade.
Keywords: N-methyl-d-aspartate receptors, l-glycine, tubuloglomerular feedback, glomerulotubular balance, micropuncture
the n-methyl-d-aspartate receptor (NMDA-R) is a heterotetrameric amino acid receptor that functions as a membrane calcium channel. The NMDA-R has been studied extensively in neural tissue where binding of two l-glycine and two l-glutamate molecules leads to channel opening and calcium influx (9). Numerous functions have been ascribed to nitric oxide formed at various locations in the brain as a result of NMDA-R activation, but little is known about NMDA-R outside of the nervous system. We previously identified immunoreactive NMDA-R in the rat kidney cortex. We also observed renal vasoconstriction in response to NMDA-R blockers, implying that NMDA-R subserves a vasodilatory role in the kidney (2). NMDA-Rs were subsequently identified in the subapical proximal tubule (11). The present studies were undertaken to determine whether the renal hemodynamic response to NMDA-R blockade originates in the proximal tubule, which affects glomerular filtration via tubuloglomerular feedback. The alternative would be that NMDA-R blockade causes renal vasoconstriction as a primary vascular event. Several studies were performed, all involving renal micropuncture in the rat. Results indicate that NMDA-R blockade independently reduces glomerular filtration rate (GFR) and proximal reabsorption (Jprox).
METHODS
Overview.
All animal experiments were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with an Institutional Animal Care and Use Committee-approved protocol. Male Wistar rats (Harlan Labs) or Wistar-Froemter rats (locally bred) were given free access to standard rat chow and water up until the time of the experiments. Wistar rats were employed in most experiments. Wistar-Froemter rats, which manifest glomeruli on the kidney surface, were used when it was necessary to access the earliest proximal tubule for microperfusion.
Surgical preparation for micropuncture.
Inactin anesthesia (100 mg/kg ip; Research Biochemicals International), surgical preparation, equilibration, and monitoring during micropuncture were performed according to protocols as previously described (1). Hydropenia was maintained by infusing Ringer solution at 2 ml/h. 3H-inulin (80 μCi/ml) was added to the Ringer solution for those experiments requiring a marker of GFR. Tubular microperfusion was performed with Hampel nanoliter pumps (Dept. of Pharmacology, University of Tuebingen) and artificial tubular fluid (ATF). ATF for orthograde perfusion of Henle's loop was of the following composition (in mM): 130 NaCl, 10 NaHCO3, 4 KCl, 2 CaCl2, 8 urea, 0.1% FD&C green, pH 7.4. ATF for the perfusion from the early proximal tubule was of the following composition (in mM): 115 NaCl, 25 NaHCO3, 4 KCl, 2 CaCl2, 4 urea, 5.5 glucose, 0.1% FD&C green, pH 7.4. Studies designed to test for effects of systemically administered drugs included two experimental periods. Control data were obtained during the first period. Then, the drug was begun by bolus or continuous intravenous infusion, 30 min were allowed for reequilibration, and then data gathering was begun for the second period.
Assessing Jprox by manipulating single-nephron GFR.
To test for primary effects on tubular reabsorption, one must control for differences in load delivered to the tubule by glomerular filtration. To accomplish this, we used tubuloglomerular feedback (TGF) as a tool for manipulating single-nephron GFR (SNGFR) so that Jprox could be determined as a function of SNGFR in each nephron as previously described (12, 26, 28). Late proximal nephrons were localized on the kidney surface and an obstructing wax block was inserted immediately upstream from the most downstream accessible segment. A microperfusion pipette with ATF was inserted downstream from the wax block to perfuse Henle's loop with the aid of a Hampel nanoliter pump. Controlled perfusion of Henle's loop was performed to activate TGF, thereby causing SNGFR to change. While perfusing Henle's loop to manipulate SNGFR, timed collections of tubular fluid were made upstream from the wax block to measure SNGFR and late proximal flow (VLP) rate. Collections were made from each nephron during maximal TGF activation (loop of Henle microperfusion at 40 nl/min) and minimal TGF activation (loop of Henle microperfusion at 0–8 nl/min). Nephrons were vented upstream from the wax block before each collection to prevent pressure from building up in the proximal tubule. Two minutes were allowed for equilibration before each collection and each collection was for 3 min. Tubular fluid samples were assayed for volume by transfer to a constant-bore glass capillary and then counted for radioactivity to determine SNGFR. Data from these paired collections were exploited to characterize Jprox as a function of SNGFR by linear interpolation (12, 26, 28).
Two-period micropuncture experiments with systemic drug administrations.
For experiments designed to determine the impact of removing endogenous NMDA-R activity on SNGFR and Jprox, the NMDA-R blocker MK801 (Sigma cat. no. M-107) was infused intravenously in saline at 10 mg·kg−1·h−1. After it was determined that systemic infusion of MK801 also lowered blood pressure, additional two-period experiments were performed substituting the vasodilator, hydralazine (50–100 μg), for MK801 as a control for the potential confounding by blood pressure. In another set of experiments, systemic infusion of NMDA-R coagonist, l-glycine, was done with 2.66 M l-glycine at 1.4 ml/h as per a standard protocol from multiple prior studies (3–8, 20, 21). In all two-period studies, 30 min were allowed for equilibration to study drug before starting data gathering for the second period.
Loop of Henle microperfusion with NMDA-R blocker.
To circumvent systemic effects whereby the impact of MK801 infusion on kidney function might be circuitous, we determined the effect on SNGFR of perfusing Henle's loop from the late proximal tubule at 8 or 40 nl/min with ATF or ATF containing MK801 (133 μM). Since each nephron returns to pass by its glomerulus at the end of Henle's loop, drugs that are reabsorbed across the macula densa can affect SNGFR when given by this route (22, 25, 27). Experiments were performed in hydropenic male Wistar rats prepared for micropuncture. Four collections were made from each late proximal nephron as the loop of Henle was perfused in the following order: 1) ATF at 40 nl/min, 2) ATF at 8 nl/min, 3) MK801 at 40 nl/min, and 4) MK801 at 8 nl/min. Four minutes of microperfusion were allotted for the nephron to equilibrate before collections 1 and 3. Two minutes were allotted before collections 2 and 4. Each collection was for 3 min. When fluid was not being collected for counting, it was collected nonetheless to prevent stop-flow conditions from developing in the nephron.
Early proximal microperfusion with NMDA-R blocker in filtering nephrons.
To study the impact on Jprox of directly applying NMDA-R blocker to the proximal tubule, MK801 (2 μM at 5 nl/min) was delivered into the free-flowing early S1 segment while SNGFR and VLP were determined by collecting from the late proximal tubule. These experiments were performed in male Wistar Froemter rats from our breeding colony at VA San Diego, which are amenable to this procedure by virtue of having glomeruli on the kidney surface. Rats were prepared for micropuncture as above. Early S1 and late proximal segments were identified by injecting a brief pulse of dye into Bowman's space. A wax block was placed in the late proximal tubule and tubular fluid collection begun upstream from the wax block. Fluid collected during the first 3 min was discarded, followed by a timed 3-min collection. Thereafter, a microperfusion pipette was inserted into the earliest accessible S1 segment containing dye-stained ATF or ATF with MK801 (2 μM). Late proximal fluid collected during the next 4 min was discarded before a second 3-min timed collection. Then, the microperfusion pump was turned off, the perfusion pipette left in place, 3–4 min allowed for reequilibration, then a final 3-min timed collection was made from the late proximal tubule.
Early proximal microperfusion with NMDA-R blocker in nonfiltering nephron.
A wax block was inserted into earliest accessible S1 segment at the neck of Bowman's space to block glomerular filtration. Perfusion of the proximal tubule was begun just downstream from the wax block at 20 nl/min using ATF doped with 3H inulin (16 μCi/ml) as a marker of the administered volume. Perfusate was collected for 2–3 min from the last accessible loop of the late proximal tubule, measured for volume, and counted for radioactivity. Next, a second microperfusion pump was inserted into the same early proximal segment to deliver MK801 (1 μM × 10 nl/min) and the first perfusion pump dialed down to maintain the total perfusion at 20 nl/min. After 3 min to equilibrate, a second collection was made. Consecutive collections with placebo or MK801 perfusions served as time controls.
Statistics.
Comparisons were by ANOVA or covariance (ANCOVA) with or without design for repeated measures as appropriate. Repeated measures were used to test for measurements that were repeated within a nephron. ANCOVA was employed with SNGFR as a covariate to test for primary effects on tubular function, where a “primary” change in tubular reabsorption is, by definition, independent of the filtered load. Testing was done with proprietary software (Systat v 6.01, SPSS). Statistical significance was assigned for P < 0.05.
RESULTS
Effects of systemic infusions.
As described under methods, two-period micropuncture experiments were performed with an initial control period followed by a second period during which animals received some agent intravenously. TGF was used as a tool to manipulate SNGFR so that primary effects on tubular reabsorption could be distinguished from the effects of glomerulotubular balance (GTB). Separate sets of experiments were performed to establish the glomerular and proximal tubular effects of the NMDA-R channel blocker, MK801, the NMDA-R coagonist, l-glycine, and the vasodilator, hydralazine. The latter was employed as a hypotensive control after MK801 was found to lower blood pressure.
Response to systemically administered NMDA-R blocker, MK801.
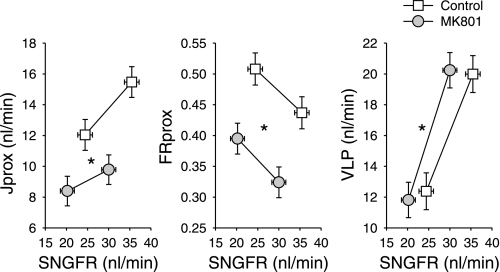
Two-period micropuncture experiments were performed in six rats. Late proximal collections were obtained with and without TGF activation in each of 22 control nephrons and 24 nephrons during systemic MK801 infusion. Results are depicted in Fig. 1. By least-squares ANOVA, MK801 reduced SNGFR by 16% (P = 0.006) irrespective of the applied TGF stimulus. The average TGF response was 10 nl/min and was unaffected by MK801.
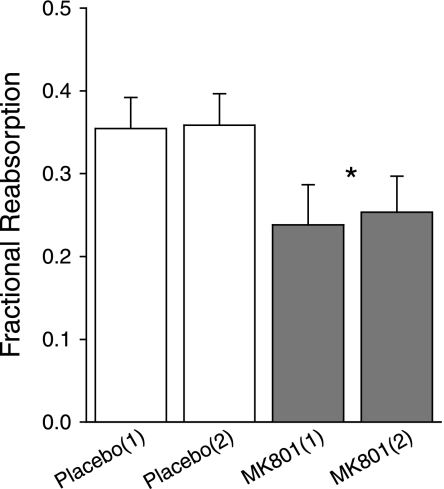
Fig. 1.
Effects of systemically infused MK801 on single-nephron glomerular filtration rate (SNGFR; abcissa) and net proximal reabsorption (Jprox; ordinate). This representation is designed to reveal effects on the tubule that are independent of SNGFR. Such effects are “primary tubular effects” and represented by a vertical displacement in the relationship between a given variable and SNGFR. Primary tubular effects are confirmed using ANCOVA with SNGFR and state of tubuloglomerular feedback (TGF) activation as covariates. To test the tubule over a range of SNGFR in each nephron, SNGFR was manipulated by perfusing Henle's loop at 0 or 40 nl/min to activate TGF. FRprox, fractional proximal reabsorption; VLP, late proximal flow. *P < 0.0005 for a “primary effect” of MK801 on Jprox, FRprox, or VLP.
MK801 also decreased Jprox by 34% (P < 0.0005). Due to GTB, some decline in Jprox is expected to result from the decline in SNGFR. Treating SNGFR as a covariate to nullify the influence of GTB, there remained a 20% decline in Jprox by ANCOVA, which represents a “primary tubular effect” of the NMDA-R blocker (P < 0.0005). A core assumption of ANCOVA is that the slope of the relationship between Jprox and SNGFR is unaffected by MK801. As is apparent in Fig. 1, the data are compatible with this assumption.
Fractional proximal reabsorption (FRprox) increased with TGF activation in both groups (P < 0.005). This reflects the nature of Jprox as a stochastic process whereby the probability that a given fluid element will escape reabsorption by a nephron segment decreases with its residence time in that segment (reviewed in Ref. 23). The fact that FRprox varies with SNGFR in a given nephron makes it dicey to infer primary effects on the proximal tubule from simple differences in FRprox. In the present case, the slope of FRprox vs. SNGFR was unaffected by MK801 (Fig. 1, middle), so the primary effect of MK801 was tested by ANCOVA with SNGFR as covariate to reveal a 23% decline in FRprox during MK801 (P < 0.0005), which confirms that MK801 suppresses Jprox, as shown above using Jprox.
Systemic infusion of MK801 also lowered mean arterial blood pressure (BP) by 13 ± 2 mmHg, motivating additional hypotensive control experiments with hydralazine (vide infra).
Response to NMDA-R coagonist, l-glycine.
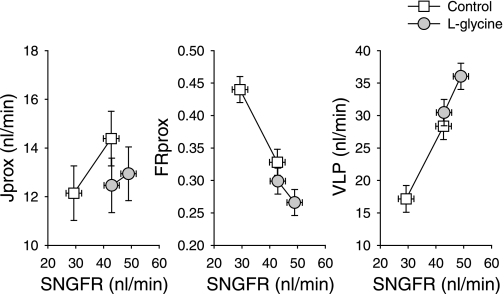
Data were obtained from 60 nephrons in 8 rats. These were divided into two separate sets of experiments that were performed several months apart. In the entire first set, the baseline (preglycine) SNGFR was unusually high for a Wistar rat. In the second set of experiments, baseline SNGFR was typical for this strain of rat. The response to l-glycine was the same in both sets of animals, so the results are pooled for presentation. Results are depicted in Fig. 2. By least-squares ANOVA, l-glycine increased SNGFR by 28% irrespective of the TGF stimulus being applied (P = 0.001). l-Glycine also reduced the maximum range of the TGF response by about half (13 ± 2 vs. 6 ± 2 nl/min, P = 0.025). After controlling for SNGFR by ANCOVA, l-glycine appeared to reduce Jprox by ∼30% (P < 0.001) with the caveats that ANCOVA could be deemed unreliable since l-glycine also reduced the efficiency of GTB and SNGFR was distributed differently between the two groups. By least-squares ANOVA applied to the raw data, l-glycine caused VLP to increase by 11 nl/min, or 46%. The relative contributions of SNGFR and primary tubular effects on the overall 11-nl/min increase in VLP were parsed by comparing the least-squares VLP from ANOVA vs. ANCOVA. By this analysis, 75% of the increase in VLP was attributable to GTB and the remainder (2.7 nl/min) was attributable to the primary decrease in Jprox. The tubular component appears modest in Fig. 2, but was statistically significant (P < 0.001).
Fig. 2.
Effects of l-glycine infusion on the proximal tubule. The purpose of this representation is to reveal primary effects on Jprox, which appear as vertical shifts in the relationship between SNGFR and some index of Jprox. Most of the increase in VLP during glycine infusion appears to result from the increase in SNGFR. But the minor component due to owing to the primary decrease in Jprox was statistically significant (P < 0.001).
Effects of BP lowering with hydralazine.
Systemic infusion of MK801 lowered BP by 13 ± 2 mmHg. As a hypotensive control, additional experiments were performed using intravenous boluses of hydralazine intended to match or exceed the impact on mean arterial pressure of MK801 (n = 5 rats). Rats wound up receiving 50–100 μg hydralazine, which reduced BP by 27 ± 5 mmHg, actually exceeding the hypotensive effect of MK801. Hence, the necessary impact on BP was achieved. Paired collections (with and without TGF activation) were made from 13 control nephrons and from 24 nephrons during hydralazine. Based on ANOVA applied to the raw data, hydralazine did not affect SNGFR (30.7 vs. 30.4 nl/min), Jprox (10.4 vs. 10.3 nl/min), VLP rate (20.2 vs. 20.2 nl/min), or the range of the TGF response (6.6 vs. 6.6 nl/min). Hence, the baroceptor signal elicited by hydralazine was insufficient to induce a measurable increase in Jprox in these experiments. The preserved TGF response after BP lowering is discrepant with a previously observed decline in stop-flow pressure responses (19). This may owe to the decidedly nonlinear mapping of glomerular capillary pressure onto SNGFR during the TGF response (29).
Effects of localized MK801 delivery via Henle's loop.
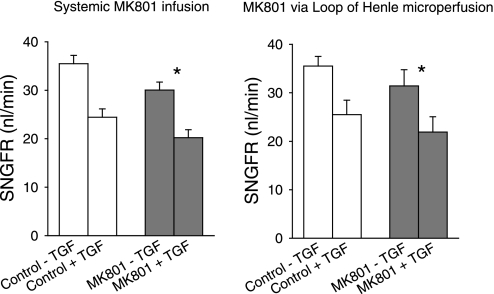
As described under methods, Henle's loop was perfused downstream from a wax block placed in the late proximal tubule using ATF ± MK801. This method has been fruitfully employed by us in the past to deliver drugs to the glomerulus by way of the macula densa (22, 25, 27). Nephrons were perfused at both 8 and 40 nl/min without and with addition of MK801 to the perfusate to examine the effects of MK801 over the range of the TGF response. The concentration of MK801 was chosen based on normalizing the systemic dose to the fraction of body volume occupied by a nephron and on the in vitro IC50 for MK801.
Full sets of four collections per nephron were obtained from 16 nephrons in five rats. SNGFR pre- and post-MK801 was highly correlated (r = 0.8). Likewise, the TGF responses pre- and post-MK801 were correlated (r = 0.6). Hence, the experimental design proved fortunate for making effects of MK801 detectable by repeated-measures analysis. Compared with control perfusions done at the respective flow rates, perfusing Henle's loop with MK801 reduced SNGFR by −13% (P < 0.024 for effect of MK801 by repeated-measures ANOVA, averaging the values for the 8- and 40-nl/min perfusion rates). Adding MK801 to the perfusate had no apparent effect on the range of the TGF response (9.5 ± 3.7 vs. 10.0 ± 2.7 nl/min). Results are shown in Fig. 3 where comparison is also made between the effects of systemic MK801 and local delivery of MK801.
Fig. 3.
Effect of MK801 on SNGFR. Left: MK801 was delivered intravenously and the loop of Henle was perfused with artificial tubular fluid at 0 or 40 nl/min to probe the extremes of TGF. Right: MK801 was delivered by orthograde microperfusion of Henle's loop at 8 or 40 nl/min. MK801 reduced SNGFR by similar amounts regardless of its route of administration and regardless of the state of TGF activation. MK801 had no effect on the maximum TGF response. *P < 0.05 for the effect of MK801 on SNGFR.
Effects of early proximal microperfusion with MK801 in free-flowing nephrons.
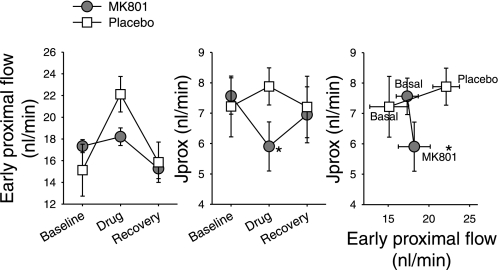
As described under methods, late proximal collections were made before and during addition of 5 nl/min ATF with or without 2 μM MK801 to the free-flowing neck of Bowman's space in rats infused with 3H-inulin as a marker of SNGFR. A third collection was made in each nephron with the perfusion pipette remaining in place, but with the pump turned off and 4 min elapsed for the drug effect to subside. Data were obtained from 16 nephrons perfused with MK801 and from 14 perfused with placebo. Results are depicted in Fig. 4. Adding MK801 to the early proximal tubule caused SNGFR to decline by 4 ± 1 nl/min, an effect that did not occur with placebo (P < 0.003 by repeated-measures ANOVA). As a result, early proximal flow was minimally affected by the 5-nl/min perfusate associated with MK801 perfusion, thereby obviating the need to account for GTB when interpreting changes in Jprox. MK801 reduced Jprox by 22% (P = 0.008). Addition of placebo tended to increase Jprox, as expected for GTB. SNGFR and Jprox returned toward baseline after the microperfusion was stopped in either group.
Fig. 4.
Effect of MK801 on early proximal flow rate and Jprox when added to free flowing early S1 segment at 5 nl/min. Three collections were done in each nephron: baseline, drug perfusion (MK801 or placebo), and recovery. MK801 decreased SNGFR so adding it at 5 nl/min had no effect on early proximal flow. Therefore, we can conclude that MK801 suppresses Jprox independent of the early proximal flow rate. Right: only the first 2 collections. *P < 0.008 for effect of MK801 on Jprox by repeated-measures ANOVA.
Effects of early proximal microperfusion with MK801 in nonfree flowing nephrons.
As described under methods, late proximal collections were made while perfusing downstream from a wax block in the early S1 segment at 20 nl/min with ATF or ATF + MK801. Data were obtained from 18 nephrons in 5 Wistar Froemter rats. Four collections were made from each nephron, two each for placebo and MK801 perfusions. Results are shown in Fig. 5. MK801 reduced reabsorption by 28% (P < 0.001 by repeated measures). Repeated collections with placebo or MK801 served as time controls and confirmed that reabsorption was insensitive to repeated puncturing of the nephron, per se.
Fig. 5.
Controlled perfusion from the early S1 segment at 20 nl/min with late proximal collection. Four collections were made from each nephron. The first 2 collections were made during perfusion with placebo and the last 2 with MK801 (1 μM). MK801 suppressed reabsorption while stability of repeated collections serves as time control (*P < 0.001 for effect of MK801 by repeated-measures ANOVA; n = 18 nephrons in 5 rats).
DISCUSSION
In the present studies, blocking systemic or renal NMDA-Rs reduced SNGFR and lessened Jprox. Furthermore, these effects on glomerular and tubular function occurred independently of each other. In other words, NMDA-R blockade suppressed Jprox independent of the filtered load and reduced SNGFR independent of TGF. Finally, these effects of NMDA-R blockade were observed during systemic administration of the NMDA-R blocker and when the blocker was applied directly to the glomerulus or proximal tubule by microperfusion. Thus, NMDA-Rs in the hydropenic rat kidney cortex tonically affect vasodilation and provide a stimulus for Jprox. Whether these glomerular and tubular actions stem solely from activation of those receptors known to reside in the proximal tubule or whether there is a separate population of vascular receptors remains to be determined. It is also unknown how the tonic influence of NMDA would compete with other homeostatic mechanisms that impinge on kidney functions under conditions other than hydropenia.
These micropuncture data are consistent with our prior observation that systemic administration of MK801 lessens renal blood flow and GFR at the whole kidney level in the hydropenic rat (2). In those experiments, the impact of NMDA-R blockade on kidney function was confirmed with an alternate NMDA-R antagonist, 5,7-dichlorokynurenic acid, and confirmed to be unaltered by renal denervation (2). Given that NMDA-Rs influence a complex array of brain functions (9) that could conceivably influence the kidney independent of the renal nerves, it is also helpful to know that NMDA-R blockade has the same effects when administered directly to single nephrons as when given systemically.
The only other report of NMDA-R and renal hemodynamics was by Yang et al. (31), who cited the role of NMDA-R in excitotoxic death of ischemic neurons to justify a potential role in renal ischemia-reperfusion. In normal kidneys, high-dose agonist NMDA caused a major decline in inulin clearance but had no effect on renal blood flow. Furthermore, major reductions in GFR and salt excretion 24 h after ischemia-reperfusion were completely reversed by infusing D-AP5, which competes for glutamate binding to NDMA-R. The authors concluded that NMDA-Rs exist in the kidney to mediate injury (31). This impression is contrary to our earlier finding that inhibiting NMDA-R with a channel blocker or glycine antagonist causes renal vasoconstriction (2) and to the phenomena presently observed at the single-nephron level. Assuming that everyone's data are valid, the effects of NMDA-R on the kidney must be dichotomous. There is precedent for this in the central nervous system (14, 16). An oversimplified explanation for its dichotomous role in the brain is that low-level NMDA-R activity provides calcium entry that drives nitric oxide synthase to produce nitric oxide needed for neuronal plasticity or vasodilation, whereas high-level NMDA-R activity floods the cell with toxic amounts of calcium leading to injury and death.
Contrary to the notions of Yang et al. (31) that NMDA-Rs exist in the kidney to cause harm, our first intuition about the functional role of NMDA-Rs in the kidney is that they might mediate the selective renal vasodilatory response to amino acid infusion or protein feeding. It has been known since the 1940s that the healthy kidney hyperfilters in response to dietary protein or glycine infusion (18), yet a full explanation of this phenomenon has been elusive. An amino acid receptor in the kidney seemed an obvious candidate and we initially confirmed that the vasodilatory response to l-glycine was mostly prevented by NMDA-R blockers or antagonists (2). We also observed a forward conditioning of this system whereby prior exposure to dietary protein simultaneously augmented the vasodilatory response to acute infusion of l-glycine and increased the renal NMDA-R protein expression (21). These observations fall short of actual proof that NMDA-R is the final answer to all questions about l-glycine and kidney function, but they do suffice to implicate NMDA-R as a significant piece of the puzzle.
We are presently interested in the extent to which the renal hemodynamic effects of NMDA-R are rooted in feedback from the tubule. There is a background to this. First, NMDA-Rs are known to exist in the proximal tubule, but remain to be discovered in the glomerulus. Second, there is a wealth of circumstantial evidence that a normal vasodilatory response to l-glycine requires a cooperative proximal tubule. For example, the vasodilatory response to l-glycine is impaired during nitric oxide synthase blockade (3), two-kidney one-clip Goldblatt hypertension (6), chronic glomerulonephritis (7), and short-term cyclosporin administration (8). In each of these models, a failed GFR response to l-glycine correlates with a decrease in Jprox, which could act as a brake on GFR via TGF (reviewed in Ref. 10). But in all these studies, impressions regarding the effect of l-glycine on the proximal tubule were based on late proximal collections made in the absence of a TGF signal, a method that precludes one from distinguishing GTB from primary effects on Jprox. The present experiments were conducted to mitigate this shortcoming, by using TGF to manipulate GFR, thereby allowing us to quantify the contribution of GTB to a given change in Jprox.
Since we previously found macula densa salt concentration to be stable during l-glycine (20), we anticipated primary decrease in Jprox during NMDA-R blockade and a primary increase during l-glycine. The former expectation was realized but the latter was not. In fact, l-glycine actually exerted a minor negative effect on Jprox while appearing to lessen the efficiency of GTB. It is probable that l-glycine exerts competing influences on the proximal tubule, leading to qualitatively different behavior according to circumstances. To the extent that l-glycine activates NMDA-R, the response to MK801 indicates that l-glycine should be proreabsorptive. Meanwhile, a potential anti-reabsorptive influence of l-glycine is tied to the osmotic burden that it places on the tubule. This standard model of l-glycine infusion previously yielded l-glycine concentrations of 14 and 20 mM in the late proximal tubule and plasma, respectively (4, 5). Combining this with late proximal TF/Pinulin of 2 yields 13 mmol of l-glycine reabsorbed per liter of glomerular filtrate. While this transepithelial gradient would not support a net osmotic diuresis due to l-glycine, this puts l-glycine on comparable footing with bicarbonate, which is the dominant solute for driving for Jprox (13). If the fractional reabsorption of l-glycine is less than bicarbonate, then adding l-glycine would reduce net reabsorption, notwithstanding that l-glycine contributes to reabsorption. Adding l-glycine would also lessen the efficiency of GTB if l-glycine were to operate near its transport maximum. As it turns out, 20 mM plasma l-glycine was required to saturate low-affinity l-glycine reabsorption in the dog [recalculated from the original work of Pitts (17)]. The foregoing effects can all be deduced from the thermodynamics of solute-solvent coupling. It is also likely that l-glycine affects tubular reabsorption by other biological mechanisms that we are unaware of, since the proximal tubule also processes glycine into bioactive peptides, such as glutathione, which it holds internally (15).
TGF became less responsive during l-glycine infusion. We had originally wondered whether NMDA-R would exert a tonic influence on TGF via macula densa nitric oxide synthase, but the MK801 results rule that out. Since it is unlikely that l-glycine could elicit an increase in Loop of Henle reabsorption adequate to overcome 40 nl/min microperfusion of Henle's loop, l-glycine must attenuate feedback either by interfering with signal transmission across the juxtaglomerular apparatus or by a direct effect on glomerular microvessels.
Returning to the response to MK801, we note direct or “primary” effects on both the glomerulus and proximal tubule. In other words, MK801 reduced Jprox independent of GTB and decreased SNGFR independent of TGF. The negative feedback system comprised of GTB and TGF normally operates near the inflection point of the TGF curve (24) and deviates from that point in the course of compensating for an acute disturbance. For example, a primary decline in Jprox will push the operating point toward the elbow of the TGF curve and a primary decline in SNGFR will push it toward the shoulder. A noteworthy feature of the current data is that the combined primary disturbances allow for a change in SNGFR with negligible residual error in VLP, no shift in the operating point with respect to the TGF inflection point, and no resetting of the TGF response. This is illustrated in Fig. 6, which was generated by taking GTB relationships and limits of the TGF response from the present micropuncture data, setting peak open loop gain for the TGF-GTB system at 2 (24), and assuming, for purposes of illustration, that MK801 did not affect alignment of the ambient flow relative to the TGF inflection point. This construct yields a remarkably stable value for ambient VLP and suggests uncanny coordination of the “independent” actions of NMDA-R in the tubule and glomerulus. Overall homeostasis will be more efficient if the mechanisms for compensating disturbances in dietary salt and protein are separable. A system that increases GFR in response to protein while simultaneously preventing the increase in filtered sodium from passing beyond the proximal tubule would accomplish this. Hence, if the body invokes renal NMDA-R signaling in its response to dietary protein (21), the dual actions of NMDA-R on the glomerulus and proximal tubule make sense. If tubular and glomerular NMDA-R signaling are simultaneous, but independent, then how do they manage to appear so well balanced? In fact, we haven't proven them to be balanced since it is possible that the TGF operating point and inflection point no longer coincide after NMDA-R blockade. But unless NMDA-R blockade interferes with the TGF system per se (and we failed to find evidence for this), internal resetting of the TGF system should restore the operating point to the steep portion of the TGF curve (30). Given that fine tuning occurs through TGF resetting, the only requirement is that the glomerular and tubular effects of MK801 were roughly balanced.
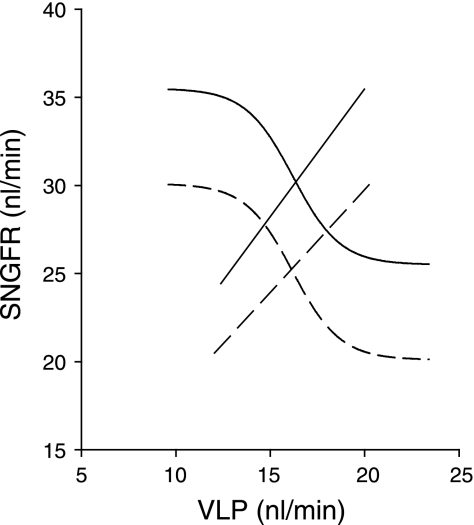
Fig. 6.
Hypothetical effect of N-methyl-d-aspartate receptor (NMDA-R) on operating point of the nephron. Solid, control; dashed, systemic NMDA-R blockade. Rightward shift in glomerulotubular balance (GTB) relationship reflects the primary effect of MK801 on the tubule. Downward shift in the TGF curve reflects primary effect of MK801 on SNGFR. Computed using GTB relationships and the minima and maxima for the TGF curves from the present data. Other features of the TGF curve computed assuming open loop gain of 2 for the TGF system and that operating point aligns with TGF inflection point (24). Accounting for dual primary effects of MK801 on glomerulus and proximal tubule reveals that the combination allows SNGFR to change while ambient VLP remains nearly constant.
In summary, renal NMDA-Rs exert tonic positive influence on SNGFR and Jprox in the hydropenic rat kidney, with the latter effect exceeding what is expected of GTB.
GRANTS
This work was performed with funds from the Department of Veterans Affairs Research Service and from the National Institutes of Health Grant DK-28602.
Acknowledgments
Technical assistance was provided by S. Khang.
REFERENCES
- 1.Blantz RC, Tucker BJ. Measurements of glomerular dynamics. In: Methods in Pharmacology, edited by Martinez-Maldonado M. New York: Plenum, 1978, p. 141–163.
- 2.Deng A, Valdivielso JM, Munger KA, Blantz RC, Thomson SC. Vasodilatory N-methyl-d-aspartate receptors are constitutively expressed in rat kidney. J Am Soc Nephrol 13: 1381–1384, 2002. [DOI] [PubMed] [Google Scholar]
- 3.De Nicola L, Blantz RC, Gabbai FB. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest 89: 1248–1256, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Nicola L, Blantz RC, Gabbai FB. Renal functional reserve in the early stage of experimental diabetes. Diabetes 41: 267–273, 1992. [DOI] [PubMed] [Google Scholar]
- 5.De Nicola L, Blantz RC, Gabbai FB. Renal functional reserve in treated and untreated hypertensive rats. Kidney Int 40: 406–412, 1991. [DOI] [PubMed] [Google Scholar]
- 6.De Nicola L, Keiser JA, Blantz RC, Gabbai FB. Angiotensin II and renal functional reserve in rats with Goldblatt hypertension. Hypertension 19: 790–794, 1992. [DOI] [PubMed] [Google Scholar]
- 7.De Nicola L, Peterson OW, Obagi S, Kaiser JA, Wilson CB, Gabbai FB. Renal functional reserve in experimental chronic glomerulonephritis. Nephrol Dial Transplant 9: 1383–1389, 1994. [PubMed] [Google Scholar]
- 8.De Nicola L, Thomson SC, Wead LM, Brown MR, Gabbai FB. Arginine feeding modifies cyclosporine nephrotoxicity in rats. J Clin Invest 92: 1859–1865, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 8–61, 1999. [PubMed] [Google Scholar]
- 10.Gabbai FB, DeNicola L, Garcia GE, Blantz RC. Role of angiotensin in the regulation of renal response to proteins. Semin Nephrol 15: 396–404, 1995. [PubMed] [Google Scholar]
- 11.Leung JC, Travis BR, Verlander JW, Sandhu SK, Yang SG, Zea AH, Weiner ID, Silverstein DM. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am J Physiol Regul Integr Comp Physiol 283: R964–R971, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Miracle CM, Rieg T, Mansoury H, Vallon V, Thomson SC. Ornithine decarboxylase inhibitor eliminates hyperresponsiveness of the early diabetic proximal tubule to dietary salt. Am J Physiol Renal Physiol 295: F995–F1002, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann KH, Rector FC Jr. Mechanism of NaCl and water reabsorption in the proximal convoluted tubule of rat kidney. J Clin Invest 58: 1110–1118, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist 13: 572–579, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parks LD, Barfuss DW. Transepithelial transport and metabolism of glycine in S1, S2, and S3 cell types of the rabbit proximal tubule. Am J Physiol Renal Physiol 283: F1208–F1215, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system too little activation is bad, too much is even worse. Neuropharmacology 53: 699–723, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Pitts RF A renal reabsorptive mechanism in the dog common to glycin and creatine. Am J Physiol 140: 156–167, 1943. [Google Scholar]
- 18.Pitts RF The effect of infusing glycine and varying the dietary protein intake on renal hemodynamics in the dog. Am J Physiol 142: 355–365, 1944. [Google Scholar]
- 19.Schnermann J, Briggs JP. Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol Renal Fluid Electrolyte Physiol 256: F421–F429, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Slomowitz LA, Deng A, Hammes JS, Gabbai F, Thomson SC. Glomerulotubular balance, dietary protein, and the renal response to glycine in diabetic rats. Am J Physiol Regul Integr Comp Physiol 282: R1096–R1103, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Slomowitz LA, Gabbai FB, Khang SJ, Satriano J, Thareau S, Deng A, Thomson SC, Blantz RC, Munger KA. Protein intake regulates the vasodilatory function of the kidney and NMDA receptor expression. Am J Physiol Regul Integr Comp Physiol 287: R1184–R1189, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 106: 289–298, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol 19: 2272–2275, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Thomson SC, Blantz RC. Homeostatic efficiency of tubuloglomerular feedback in hydropenia, euvolemia, and acute volume expansion. Am J Physiol Renal Fluid Electrolyte Physiol 264: F930–F936, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Thomson SC, Deng A. Cyclic GMP mediates influence of macula densa nitric oxide over tubuloglomerular feedback. Kidney Blood Press Res 26: 10–18, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson SC, Deng A, Komine N, Hammes JS, Blantz RC, Gabbai FB. Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am J Physiol Renal Physiol 287: F732–F738, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J Clin Invest 116: 1110–1116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson S, Vallon V, Blantz RC. Asymmetry of tubuloglomerular feedback effector mechanism with respect to ambient tubular flow. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1123–F1130, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Thomson SC, Vallon V, Blantz RC. Resetting protects efficiency of tubuloglomerular feedback. Kidney Int Suppl 67: S65–S70, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Yang CC, Chien CT, Wu MH, Ma MC, Chen CF. NMDA receptor blocker ameliorates ischemia-reperfusion-induced renal dysfunction in rat kidneys. Am J Physiol Renal Physiol 294: F1433–F1440, 2008. [DOI] [PubMed] [Google Scholar]