Abstract
Animal models of acute renal injury suggest that the epidermal growth factor receptor (EGFR) axis may have a beneficial role in the recovery from acute renal injury, but recent reports describe detrimental effects of EGFR activation in chronic renal injury. Expression of the EGFR ligand heparin-binding EGF-like growth factor (HB-EGF) increases following renal injury, but the effects of this sustained upregulation have not been well studied. Here, stable overexpression of soluble HB-EGF (sHB-EGF) in mouse inner medullary collecting duct (IMCD) cells led to marked phenotypic changes: sHB-EGF-expressing cells demonstrated a fibroblast-like morphology, did not form epithelial sheets, exhibited cytoplasmic projections, decreased expression of epithelial markers, and increased expression of fibroblast-specific protein-1. They also demonstrated anchorage-independent growth and formed tumors when injected subcutaneously into nude mice. Quantitative RT-PCR and a luciferase reporter assay suggested that sHB-EGF repressed transcription of E-cadherin, and a concomitant TGF-β-independent upregulation of the E-cadherin repressor Snail-2 was observed. Stable downregulation of Snail-2 in sHB-EGF-overexpressing cells restored epithelial characteristics (E-cadherin and cytokeratin expression) but did not alter their anchorage-independent growth. In summary, sustained exposure to sHB-EGF induces epithelial-to-mesenchymal transition of IMCD cells, in part by upregulating the E-cadherin transcriptional repressor Snail-2.
Keywords: epidermal growth factor receptor, chronic kidney disease, fibrosis
several lines of evidence suggest that the epidermal growth factor receptor (EGFR) axis modulates the response to renal injury. Ischemic injury increases EGFR expression and activation in rat kidney (33, 43), and exogenous administration of epidermal growth factor (EGF) accelerates renal tubular regeneration (12). Endogenous EGFR ligands increase in response to several types of acute tubular injury, including ischemia-reperfusion, mercuric chloride, folic acid administration, and aminoglycosides (10, 11, 35). Mice containing a point mutation in the EGFR that reduces receptor tyrosine kinase activity by >90% demonstrate slower recovery of renal function, more severe histological tubular injury, and reduced renal DNA synthesis than wild-type controls after acute mercuric chloride-induced injury (42).
Although these reports suggest that activation of the EGFR axis may have a beneficial role in the recovery from acute renal injury, sustained receptor activation may have detrimental effects. In vitro, EGF and transforming growth factor-β1 (TGF-β1) synergistically induce epithelial-to-mesenchymal transition (EMT) (27), the process through which tubular epithelial cells may transform into interstitial fibroblasts and promote renal fibrosis. Blockade of EGFR activation with the EGFR-tyrosine kinase inhibitor gefitinib prevented the decline of renal function and the development of fibrosis in a rat model of hypertensive nephrosclerosis (8). Furthermore, in a subtotal nephrectomy model, expression of a dominant negative EGFR in the proximal tubule protected against tubular atrophy and interstitial fibrosis (39).
EGFR ligands include EGF, heparin-binding EGF-like growth factor (HB-EGF), TGF-α, amphiregulin, betacellulin, epiregulin, and epigen (37). These ligands are synthesized as type 1 transmembrane proteins, and their soluble counterparts result from extracellular cleavage by specific metalloproteases. Interestingly, angiotensin II-mediated activation of the AT1 receptor can induce proteolytic cleavage of TGF-α and HB-EGF, suggesting a potential link between activation of the renin-angiotensin system and the EGFR axis. Lautrette et al. (19) have shown that renal lesions induced by chronic angiotensin II infusion are ameliorated in mice overexpressing a dominant negative EGFR, in mice lacking TGF-α, or in mice given a pharmacological inhibitor of the TGF-α sheddase, TACE.
The potential for HB-EGF to contribute to renal injury has not been well studied. Unlike EGF expression, which decreases following ischemia-reperfusion- or cisplatin-induced injury (33, 34), we have previously reported a transient increase in HB-EGF expression, primarily in the distal nephron, following ischemic injury and aminoglycoside nephrotoxicity in rats (11, 35). To test the hypothesis that sustained exposure to HB-EGF may be injurious, rather than reparative, we overexpressed the soluble ligand in a mouse inner medullary collecting duct (IMCD) cell line and found these cells dedifferentiated from an epithelial to a mesenchymal phenotype, a process that was mediated in part by activation of the E-cadherin transcriptional repressor Snail-2 (Slug).
MATERIALS AND METHODS
Cell culture.
IMCD cells (mIMCD3) and MDCK type II cells were purchased from ATCC and were grown in Dulbecco's modified Eagle's medium:nutrient mix F12 (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated in 5% CO2-95% air at 37°C. The growth media for transfected cells also contained 1 mg/ml G418 (Sigma-Aldrich, St. Louis, MO) with or without 200 μg/ml hygromycin (Research Products International, Mt. Prospect, IL).
Plasmids, antibodies, and reagents.
The rabbit anti-rat HB-EGF polyclonal antibody was a gift from Li Feng (Dept. of Immunology, The Scripps Research Institute, La Jolla, CA). The rabbit anti-mouse Fsp1 polyclonal antibody was generously supplied by Dr. Eric Neilson (Vanderbilt University, Nashville, TN). The luciferase reporter construct containing the E-cadherin 5′-flanking sequence [Ecad3 (1,484 bp)] was provided by Dr. Eric Fearon (University of Michigan, Ann Arbor, MI). Commercially obtained antibodies included anti-E-cadherin and anti-Smad2/3 (BD Biosciences, San Jose, CA); anti-pan-cytokeratin (Sigma-Aldrich); anti-pSmad2/3 (Cell Signaling Technology, Danvers, MA); and from Santa Cruz Biotechnology (Santa Cruz, CA), anti-pEGFR (Tyr 1173), anti-EGFR, and anti-ERK1/2. Horseradish peroxidase-conjugated secondary antibodies for Western blots were purchased from Calbiochem (San Diego, CA), and fluorescein secondary antibodies for immunofluorescence were obtained from Vector Laboratories (Burlingame, CA). Recombinant human TGF-β was purchased from R&D Systems (Minneapolis, MN), and SB431542, an inhibitor of activin receptor-like kinases, was obtained from Sigma-Aldrich. The pcDNA3-neo plasmid was purchased from Invitrogen, and the pRNAT-U6.3/Hygro vector was obtained from GenScript (Piscataway, NJ).
Creation of an IMCD cell line that overexpresses sHB-EGF.
To develop IMCD cells that stably express soluble HB-EGF, we followed the same procedure that we previously described for the creation of sHB-EGF-overexpressing MDCK II cells (38). Briefly, IMCD cells were transfected with 1 μg of pcDNA3-neo containing rat sHB-EGF cDNA (38) using Lipofectamine reagent (Invitrogen). After five passages in DMEM/F12 containing 1 mg/ml G418, stable transfectants were selected with cloning cylinders and subcloned by serial dilution. Detection of sHB-EGF in conditioned medium using an anti-HB-EGF antibody identified clones of interest, and two (clones 11 and 12) with similar protein expression were used for all experiments described herein. Control cells were transfected under identical conditions with the empty pcDNA3-neo vector (IMCDvec).
Immunoblotting.
Equal amounts of total protein were subjected to SDS-PAGE and immunoblotted with antigen-specific antibodies per our standard protocol (15). The signal was visualized with horseradish peroxidase-conjugated secondary antibodies using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Cell proliferation assay.
Cells were plated in triplicate in 96-well plates, 4 × 104 cells/well, in DMEM/F12 media with 10% FCS. The next day, the media was removed and replaced with the same media supplemented with [3H]thymidine (0.1 μCi/well). After 24 h, the media was removed, and cells were lysed with 10% SDS and counted with a beta-counter as previously described (31). In separate experiments, 500 nM PD153035 was added 6 h before and during the incubation with [3H]thymidine. Three independent experiments were performed in triplicate.
Cell migration assay.
Cell migration was assessed using Transwell chambers (12 mm, 0.8-μm pore size; Corning, Acton, MA) that had been coated with rat-tail collagen by submerging the Transwell membrane into a lower chamber containing 10 μg/ml rat-tail collagen in PBS overnight at 4°C. The next day, the coated Transwells were placed into a new plate with lower chambers filled with serum-free medium. Each well was plated with 1 × 105 cells in 100 μl serum-free media. The Transwell chamber was placed under standard incubation conditions for 4 h, and then the cells on the upper surface of the membrane were gently removed using a cotton swab. The membrane was fixed with 3.7% formaldehyde for 30 min at room temperature and stained with 0.1% crystal violet for 1 h. The membrane was washed and the cells on the lower surface were counted under a light microscope at ×400 magnification. At least 10 high-power fields were counted for each well. Three independent experiments were performed in triplicate.
Anchorage-independent growth assay.
Cells (5 × 105) were mixed in 1 ml of warm (37°C) DMEM/F12 containing 0.3% low-melting agarose and 10% FCS. This mixture was overlaid onto a 1-ml layer of cooled DMEM/F12 containing 0.5% agarose and 10% FCS in 35-mm cell culture dishes. After 3 wk, plates were photographed and colonies were counted. Two independent experiments were performed in triplicate.
E-cadherin luciferase assay.
Cells were cotransfected in duplicate at ∼50% confluence with 0.8 μg E-cad3 (luciferase reporter construct containing 1,484 bp of the 5′-flanking sequence) (13) and 0.02 μg phRL-TK, a Rinella luciferase plasmid (Promega, Madison, WI) using Effectene transfection reagent per the manufacturer's instructions (Qiagen, Valencia, CA). Approximately 48 h later, cells were lysed and processed using the Dual-Luciferase Reporter Assay System (Promega), and the resulting fluorescences of firefly luciferase and Rinella luciferase were read by a luminometer. Experiments were performed in triplicate.
RNA isolation and semiquantitative RT-PCR.
RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH) following the manufacturer's suggested protocol. Semiquantitative RT-PCR was performed by reverse transcribing an estimated 1 μg RNA of each sample (calculated using the A260) with a GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA). The subsequent PCR reaction included 4 μl of the resultant cDNA, as well as 10× PCR buffer, 2 mM MgCl2 (final), 0.5 U Taq DNA polymerase, 1 μM of each primer (see below), and DNase-free water in a 20-μl reaction. The PCR program was: 95°C × 5 min followed by 25–32 cycles of 95°C × 30 s, 60°C × 30 s, 72°C × 30 s, then 72°C × 10 min.
Quantitative RT-PCR.
RNA was isolated as described above. An estimated 5 μg RNA of each sample was reverse-transcribed using a SuperScript II Reverse Transcriptase kit (Invitrogen) per the manufacturer's protocol. The resulting cDNA was then used for quantitative PCR with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Thermal cycling and collection of real-time PCR data were performed on a MyiQ detection system (Bio-Rad); the program was: 95°C × 3 min followed by 40 cycles of 95°C × 30 s, 56°C × 30 s, and melting curves were obtained after completion of the amplification cycles. Experimental and control samples were both normalized to GAPDH, and the standard curve method was used for quantitation. Within each experiment, each sample was performed in triplicate.
PCR primers.
The following primer sequences were obtained from PrimerBank (41): mouse E-cadherin (PrimerBank ID 6753374a3; forward, 5′-GTCTACCAAAGTGACGCTGAA-3′; reverse, 5′-GGGTACACGCTGGGAAACAT-3′); mouse Snail-1 (PrimerBank ID 6755586a1; forward, 5′-CACACGCTGCCTTGTGTCT-3′; reverse, 5′-GGTCAGCAAAAGCACGGTT-3′); mouse Snail-2 (PrimerBank ID 6755576a1; forward, 5′-TGGTCAAGAAACATTTCAACGCC-3′; reverse, 5′-GGTGAGGATCTCTGGTTTTGGTA-3′); mouse TGF-α (PrimerBank ID 13654266a1; forward, 5′-CACTCTGGGTACGTGGGTG-3′; reverse, 5′-CACAGGTGATAATGAGGACAGC-3′); and mouse HB-EGF (PrimerBank ID 6754178a1; forward, 5′-CGGGGAGTGCAGATACCTG-3′; reverse, 5′-TTCTCCACTGGTAGAGTCAGC-3′). The sequences for forward and reverse mouse GAPDH primers were 5′-CCAGAACATCATCCCTGCAT-3′ and 5′-GTTCAGCTCTGGGATGACCTT-3′, respectively (22).
Snail-2 knockdown.
Snail-2 was knocked down in IMCDsHB cells by transfecting them with a vector containing Snail-2-targeted shRNA. Two shRNA sequences were obtained from the Cold Spring Harbor Laboratory RNAi Codex (http://codex.cshl.edu) and were designated “601” and “871,” which indicated the position of the first targeted nucleotide in the mRNA transcript. The shRNA constructs consisted of a BamHI site, the sense sequence targeted to Snail-2, a hairpin sequence, the antisense sequence targeted to Snail-2, a termination signal, and a HindIII site: 601 = 5′-TAGCGGATCCCGCAAGTACTGTGACAAGGAATTGATAT CCGTTCCTTGTCACAGTACTTGTTTTTTCCAAAAGCTT-3′ and 871 = 5′-TAGCG GATCCCGCAGAATGTCGCTTCTGCATTTGATATCCGATGCAGAAGCGACATTCTGTTTTTTCCAAAAGCTT-3′. These oligonucleotides and their complementary strands were synthesized; annealing of complementary strands was performed in 50 mM HEPES, pH 7.4, containing 100 mM NaCl by heating to 95°C followed by slow cooling to room temperature. The annealed oligonucleotides were purified by electrophoresis on a 3% agarose gel using a QIAquick Gel Extraction Kit (Qiagen), subjected to sequential digestion with HindIII and BamHI, and gel-purified again. The pRNAT-U6.3/Hygro vector was linearized with HindIII and BamHI sequential digestion, followed by gel purification to remove the resulting fragment from the reaction mixture. Each annealed oligonucleotide, now with sticky ends, was mixed with linearized vector and ligated using a DNA Ligation Kit v2.1 (Takara Bio USA, Madison, WI), incubating at 16°C overnight. Competent DH5a were transformed with the ligated product; ampicillin-resistant colonies were selected, and plasmid minipreps (Qiagen) were performed. BamHI and HindIII digestion was performed to screen for successful incorporation of the shRNA oligonucleotide into the vector. One plasmid for each shRNA construct (601 and 871) was verified by sequencing, and IMCDsHB cells were transfected using Effectene reagent according to the manufacturer's protocol (Qiagen). A scrambled sequence composed of similar base pair composition to the 871 construct was transfected as a control. IMCDsHB-shRNA double-transfectants were selected in media containing 1 mg/ml G418 and 200 μg/ml hygromycin. GFP+ cells were subcloned using cloning cylinders. After passages (≥5), semiquantitative and quantitative RT-PCR were used to assess Snail-2 knockdown. Two clones from each construct were used for most experiments; data from a representative clone are presented.
Immunofluorescence.
Cells were plated on eight-chamber glass slides and allowed to grow to desired confluence. Media was removed, and cells were washed twice with ice-cold 1× PBS containing 0.1 mM CaCl2 and 0.05 mM MgCl2 before fixation with 3.7% paraformaldehyde for 30 min. Cells were washed twice with PBS as above, ice-cold methanol was added for 10 min, and cells were washed again. After permeabilization for 10 min with 50 mM NH4Cl + 0.2% Triton X-100, the cells were washed a final time, and then blocked with 5% goat serum in PBS for 1 h. The blocking buffer was removed, and primary antibodies were added for 2 h at room temperature (anti-E-cadherin 1:300, anti-cytokeratin 1:500, anti-Fsp1 1:500). After washing five times (30 min) with PBS, FITC- or PE-conjugated secondary antibodies (1:200) were added for 45 min at room temperature. Cells were washed for 30 min, embedded in a glycerol/PBS-based mounting medium, and examined with a fluorescent microscope.
In vivo studies.
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of Vanderbilt University. Athymic nude mice were injected subcutaneously with either IMCDvec or IMCDsHB cells. Each mouse was injected in four separate sites to test four different doses of cells per mouse (ranging from 2.5 × 105 to 1 × 106 cells/site). After 3 wk, tumor sizes were measured with calipers. Volume was determined according to the following equation: volume = (length × width2) × 0.5. Mice were anesthetized and euthanized following approved procedures. Four mice were injected with each cell line.
Statistics.
Data are presented as means ± SE. Differences between two groups were analyzed using Student's unpaired t-test for normally distributed data and the Mann-Whitney U-test when a normal distribution was not assumed. Comparisons between more than two groups were made using one-way ANOVA, with posttest comparisons made between experimental conditions and control conditions with Dunnett's multiple comparison test. P < 0.05 was considered statistically significant.
RESULTS
Overexpression of sHB-EGF in IMCD cells.
Overexpression of sHB-EGF in IMCD cells (IMCDsHB) led to the expected release of immunoreactive HB-EGF into the media (Fig. 1A). The secreted HB-EGF was shown to be biologically active by using conditioned media to induce EGFR phosphorylation in quiescent, wild-type IMCD cells (data not shown). In addition, IMCDsHB cells demonstrated increased tyrosine phosphorylation of EGFR and decreased expression of total EGFR, similar to IMCD cells treated with 100 ng/ml HB-EGF (Fig. 1B). These data are consistent with chronic activation of the EGFR; ligand-induced EGFR activation induces internalization/degradation of the receptor (6, 38). IMCDsHB cells demonstrated a marked change in morphology compared with vector-transfected IMCD cells (IMCDvec): they did not grow in epithelial sheets, and they exhibited significant cytoplasmic projections (Fig. 1C). The apparent transition from an epithelial to a mesenchymal phenotype was supported by the decreased protein expression of E-cadherin and cytokeratin and by the increased protein expression of fibroblast-specific protein-1 (Fsp-1) (Fig. 1D).
Fig. 1.
A: immunoblot of conditioned media from wild-type inner medullary collecting duct (IMCD) cells, vector-transfected IMCD cells (vec), and soluble heparin-binding EGF-like growth factor (sHB-EGF)-overexpressing IMCD cells (stable clones 11 and 12). B: treatment of IMCD cells with HB-EGF (100 ng/ml) increases EGF receptor (EGFR) phosphorylation and decreases total EGFR expression. A similar pattern is observed in sHB-EGF-overexpressing IMCD cells. Total ERK, which is unaffected by EGFR activation, served as a loading control. C: morphology of vector-transfected and sHB-EGF-overexpressing IMCD cells. D: representative immunoblots of cell lysates; β-actin served as a loading control.
Functional characteristics of IMCDsHB cells also differed from IMCDvec cells. Overexpression of sHB-EGF led to a doubling of the rate of cell proliferation (P = 0.008) as measured by [3H]thymidine incorporation (Fig. 2A, left). When serum-starved cells were treated with 500 nM PD153035, a specific and potent tyrosine kinase inhibitor of EGFR, for 6 h before and through the 24-h incubation with [3H]thymidine, cell proliferation was significantly inhibited, suggesting that EGFR activation contributes to the increased rate of proliferation observed in IMCDsHB cells (Fig. 2A, right, P = 0.0003). Overexpression of sHB-EGF also doubled the rate of cell migration (P = 0.005), measured by the number of cells that successfully migrated through a collagen-coated Transwell filter with 0.8-μm pores in 4 h, as described in materials and methods (Fig. 2, A and B). To determine the extent to which sHB-EGF dedifferentiated these epithelial cells, we assessed their potential for anchorage-independent growth in a soft agar assay. Large, viable colonies of IMCDsHB cells grew in soft agar by 3 wk, whereas there were essentially no detectable viable colonies of IMCDvec cells (Fig. 3A). To assess the extent of dedifferentiation in vivo, we injected IMCDvec and IMCDsHB subcutaneously into nude mice and analyzed tumor formation over time. Three weeks after injection, IMCDsHB cells formed tumors, and mean tumor volume directly correlated with the number of cells injected (means ± SD for 1.25 × 105 cells: 17.8 ± 10.6 mm3, 2.5 × 105: 38.5 ± 5.5 mm3, 5.0 × 105: 62.5 ± 6.0 mm3, 1 × 106: 197.5 ± 16.3 mm3; P < 0.0001 by ANOVA) (Fig. 3, B and C). In contrast, none of the mice injected with IMCDvec cells developed tumors (n = 4 mice, 16 sites of injection).
Fig. 2.
Cell proliferation (A) and cell migration (B) were increased in sHB-EGF-overexpressing IMCD cells compared with vector-transfected control cells. Treatment with the EGFR tyrosine kinase inhibitor PD153035 (500 nM) for 6 h before and during [3H]thymidine incorporation significantly inhibited cell proliferation. *P ≤ 0.008.
Fig. 3.
A: photomicrographs of colonies of sHB-EGF-overexpressing IMCD cells and vector-transfected control cells in soft agar 3 wk after plating (magnification ×100 for both). B: gross appearance of subcutaneous tumors 3 wk after injection of the designated number of sHB-EGF-overexpressing IMCD cells (1.25 × 105 - 1 × 106 cells). C: subcutaneous tumors formed at every injection site of sHB-EGF-overexpressing IMCD cells (16 sites in 4 mice). No tumors formed from injections of vector-transfected IMCD cells. The average tumor volume increased with dose of cells injected (P < 0.0001 by ANOVA).
sHB-EGF overexpression leads to transcriptional repression of E-cadherin.
The loss of E-cadherin is a critical event in EMT. We considered that sHB-EGF overexpression might downregulate E-cadherin by increasing its degradation, altering mRNA stability, or decreasing its transcription. Semiquantitative and quantitative RT-PCR revealed an 87% decrease in E-cadherin mRNA in IMCDsHB cells compared with IMCDvec (P < 0.0001) (Fig. 4A). Decay of mRNA after actinomycin D treatment, detected by semiquantitative RT-PCR, did not suggest a difference in mRNA stability between IMCDvec and IMCDsHB cells. In addition, treatment of IMCDsHB cells with proteasome inhibitors (MG132 and lactacystin) or lysosome inhibitors (chloroquine or ammonium chloride) did not increase levels of E-cadherin protein (data not shown).
Fig. 4.
E-cadherin mRNA was downregulated by sHB-EGF overexpression in IMCD cells compared with vector-transfected cells, as measured by quantitative RT-PCR (A; *P < 0.0001) and a luciferase reporter assay (B; *P = 0.006).
IMCDsHB and IMCDvec cells were transiently cotransfected with a luciferase reporter plasmid driven by the E-cadherin promoter and a control plasmid expressing Rinella luciferase. Luciferase expression was reduced by 84% in IMCDsHB compared with IMCDvec cells (P = 0.006) (Fig. 4B), corroborating the quantitative RT-PCR data; together, these results suggest that sHB-EGF leads to transcriptional repression of E-cadherin in IMCD cells.
Snail-2, a transcriptional repressor of E-cadherin, is upregulated in IMCDsHB cells.
Of the several known transcriptional repressors of E-cadherin, the Snail family has received the greatest attention. In our hands, commercial antibodies against Snail family members were not sufficiently specific, so semiquantitative RT-PCR was used to screen for sHB-EGF-induced changes in expression of several transcriptional repressors of E-cadherin (Snail-1, Snail-2, SIP1, ZEB1, and E47). In multiple experiments, we found that Snail-2 expression was markedly increased in IMCDsHB cells compared with IMCDvec, whereas the expression of Snail-1 was reduced or unchanged (Fig. 5, A and B). We occasionally noted small increases in the expression of SIP1 and ZEB1 in IMCDsHB cells compared with control, but these findings were not consistent and were not pursued.
Fig. 5.
A: Snail-2, but not Snail-1, mRNA was significantly upregulated in sHB-EGF-overexpressing IMCD cells compared with vector-transfected control cells as determined by quantitative RT-PCR. B: representative ethidium bromide-stained agarose gels of semiquantitative RT-PCR products derived from vector-transfected and sHB-EGF-overexpressing IMCD cells. C: semiquantitative RT-PCR suggested that, similar to IMCD cells, Snail-2 is upregulated in sHB-EGF-overexpressing MDCK II cells compared with vector-transfected cells.
To determine whether increased expression of Snail-2 in response to sHB-EGF was a cell line-specific phenomenon, we assessed its expression in MDCK II cells that were stably transfected to overexpress sHB-EGF (38). Similar to our results with IMCD cells, MDCKsHB cells had increased expression of Snail-2 compared with MDCKvec (Fig. 5C). As previously reported, MDCKsHB cells exhibit increased cell migration and decreased cell-matrix and cell-cell interactions; in addition, these cells develop tubular structures in response to hepatocyte growth factor (38). Interestingly, overexpression of sHB-EGF does not lead to a detectable decrease in E-cadherin protein expression in MDCK II cells, however. This suggests that in contrast to our observation in IMCD cells, other mediators of E-cadherin expression dominate the effects of sHB-EGF-induced upregulation of Snail-2 in MDCK II cells.
Snail-2 expression induced by sHB-EGF is independent of TGF-β.
TGF-β is one of the classic modulators of EMT (17). Activation of TGF-β receptors leads to the phosphorylation of Smad2/3, and these proteins then translocate to the nucleus along with Smad4 to modulate gene activity as cofactors of the transcriptional machinery (36). To examine the possibility that sHB-EGF modulates EMT indirectly by stimulating TGF-β-dependent pathways, we examined protein expression of phosphorylated Smad2/3 (P-Smad2/3) in IMCDsHB cells. As expected, P-Smad2/3 was not detected in serum-starved IMCDvec cells unless they were stimulated with exogenous TGF-β, and this increase in P-Smad2/3 could be blocked by the ALK5 inhibitor SB431542, which inhibits TGF-β signaling. P-Smad2/3 was barely detectable in IMCDsHB cells, however, even after larger quantities of total protein were assayed (Fig. 6A). Therefore, the effects of sHB-EGF are unlikely to be mediated by TGF-β signaling.
Fig. 6.
A: immunoblots of cell lysates for P-Smad2/3. Lysates were prepared after incubating near-confluent cells in serum-free conditions overnight. For the indicated treatments, exogenous HB-EGF (100 ng/ml) or TGF-β (10 ng/ml) was added 1 h before lysis on ice. When the ALK5 inhibitor SB431542 (SB; 10 μM) was used, it was added 15 min before either the addition of TGF-β (in the case of IMCD+SB+TGF-β cells) or lysis (in the case of IMCDsHB+SB cells). Constitutive expression or exogenous addition of HB-EGF did not lead to phosphorylation of Smad2/3. Exogenous treatment with TGF-β served as a positive control. B: cells were grown in the presence or absence of TGF-β (10 ng/ml) and/or SB431542 (10 μM) for 5 days. RNA was collected, and quantitative RT-PCR was used to determine the relative expressions of Snail-1 and Snail-2 compared with wild-type IMCD cells. P < 0.0001 (Snail-1) and P = 0.0011 (Snail-2) by ANOVA; *P < 0.01 compared with IMCD by Dunnett's multiple comparison test.
Treating serum-starved IMCD cells with exogenous TGF-β (10 ng/ml) for 5 days significantly upregulated Snail-1 (P < 0.01), but the slight upregulation of Snail-2 was not statistically significant (Fig. 6B). As expected, SB431542 blocked the effects of exogenous TGF-β on Snail-1 upregulation. SB431542 did not, however, inhibit the dramatic increase in Snail-2 expression induced by sHB-EGF overexpression (IMCDsHB vs. IMCDsHB + SB, P = 0.61 by t-test; each of these groups vs. IMCD, P < 0.01 by Dunnett's multiple comparison test). These results suggest that TGF-β and sHB-EGF differentially affect the transcription of Snail family members in IMCD cells (Fig. 6B).
Snail-2 knockdown restores some, but not all, epithelial characteristics.
To determine whether Snail-2 upregulation was a causative factor in the development of the EMT phenotype in IMCDsHB cells, we stably knocked down Snail-2 in these cells with two different shRNA constructs. One of the constructs, designated IMCDsHB-601, appeared to have off-target effects, as it partially inhibited Snail-1 mRNA expression in addition to Snail-2 (Snail-1 expression in IMCDsHB-601 vs. IMCDsHB-scram, P = 0.005) (Fig. 7). Snail-2 knockdown restored some epithelial characteristics: cellular morphology changed, with cells forming epithelial sheets and losing their spindle-shaped appearance and cytoplasmic projections, E-cadherin and cytokeratin expression were restored, and Fsp-1 expression was reduced (Figs. 8 and 9). Despite these changes, however, some behaviors persisted that are characteristic of dedifferentiated cells: both shRNA clones maintained their ability to grow in soft agar and their rates of [3H]thymidine incorporation and migration were not significantly different from IMCDsHB controls (data not shown). These behaviors persisted in both clones, suggesting that the additional off-target inhibition of Snail-1 in the IMCDsHB-601 clone likely contributed less to the resulting phenotype than the targeted knockdown of Snail-2. These observations support that the EMT promoted by chronic EGFR activation partially, but not completely, depends on Snail-2 in IMCD cells.
Fig. 7.
Quantitative RT-PCR demonstrated near-complete knockdown of Snail-2 mRNA in sHB-EGF-overexpressing IMCD cells stably transfected with shRNA constructs 601 and 871. *P = 0.005 (vs. IMCDsHB-scram, Snail-1). **P = 0.001 (vs. IMCDsHB-scram, Snail-2).
Fig. 8.
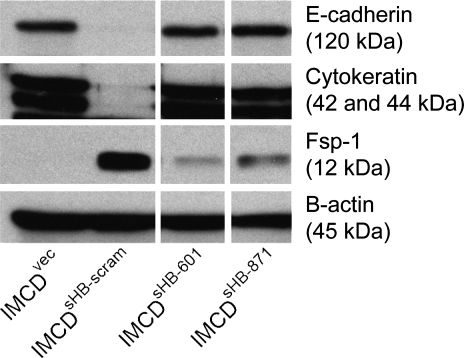
Immunofluorescence images demonstrating significant restoration of E-cadherin and cytokeratin with knockdown of Snail-2 in sHB-EGF-overexpressing IMCD cells stably transfected with Snail-2 shRNA (IMCDsHB-601 and -871). Fsp-1 expression, although reduced compared with IMCDsHB cells, was still detectable in stable shRNA transfectants. Fsp-1 was not detected in vector-transfected IMCD cells that do not overexpress sHB-EGF (IMCDvec).
Fig. 9.
Immunoblots of cell lysates from vector-transfected IMCD cells that do not overexpress sHB-EGF (IMCDvec), sHB-EGF-overexpressing IMCD cells (IMCDsHB), and sHB-EGF-overexpressing IMCD cells stably transfected with Snail-2 shRNA (IMCDsHB-601 and -871). β-Actin served as a loading control.
DISCUSSION
In this study, we found that chronic stimulation of the EGFR axis by soluble HB-EGF leads to a marked dedifferentiation of IMCD cells and that the phenotype is dependent, in part, on HB-EGF-mediated upregulation of the E-cadherin repressor Snail-2 (Slug). The observed EMT is not dependent on the actions of TGF-β; in fact, our data suggest that TGF-β upregulates Snail-1, but not Snail-2, in IMCD cells. We also demonstrated that although shRNA-mediated knockdown of Snail-2 restores wild-type epithelial cell morphology in IMCDsHB cells, they continue to display cell behaviors (e.g., proliferation, migration, anchorage-independent growth) that are characteristic of dedifferentiated cells.
EMT is a crucial process during embryonic development, but it has recently been found to play a role in neoplasia and fibrosis. For epithelial cells to migrate into the tubulointerstitium, E-cadherin must be repressed. Snail-1 was the first transcriptional repressor of E-cadherin described (2, 5), but subsequently two other Snail family members, including Snail-2 (Slug), have been identified as well as other non-Snail transcriptional repressors of E-cadherin (e.g., E47, ΔEF1/Zeb1, and Sip1/Zeb2). These repressors are tightly regulated at the transcriptional level and/or by subcellular localization (7, 47). Insight into how EMT is regulated may provide new chemotherapeutic and antifibrotic therapies.
EGFR activation promotes renal fibrosis in animal models (8, 19, 39). Its involvement in this process has been emphasized by the recent demonstration that transactivation of EGFR by TGF-α may play a central role in angiotensin II-mediated renal injury (19). Similar to TGF-α, HB-EGF is synthesized as a transmembrane precursor protein (proHB-EGF), which undergoes ectodomain shedding to release soluble HB-EGF (sHB-EGF) in response to multiple stimuli, including angiotensin II (32). In addition, EGFR activation promotes further proteolytic cleavage of proHB-EGF and induces the transcription of both HB-EGF and other EGFR ligands, leading to a positive feedback loop that could prolong or amplify signals through the EGFR axis (9, 40). Because EGFR ligands have differential binding specificities to various combinations of ErbB receptors (homo- and heterodimers of EGFR/ErbB1, ErbB2, ErbB3, and ErbB4) (23), evaluating the contribution of other ligands to the progression of renal fibrosis is warranted. Our results are consistent with the hypothesis that chronic activation of EGFR by ligands such as HB-EGF promotes EMT in cells of the distal tubule, and therefore may be involved in renal fibrosis. In preliminary experiments, we have observed an upregulation of the EGFR ligands HB-EGF and TGF-α following ureteral obstruction, which induces fibrogenesis within days.
The malignant transformation that occurs in response to sustained activation of ErbB receptors has been well described. Ligands induce EGFR dimerization, which leads to autophosphorylation of cytoplasmic tyrosine residues that serve as binding sites for signaling molecules. This leads to activation of multiple cellular pathways (e.g., MAPK, PI3K-AKT, and PLCγ-PKC) that promote cell proliferation and survival (45, 46). Our results suggest that Snail-2 plays a significant role in EGFR activation-induced EMT, but not transformation, in these cells. The effector signaling pathways that drive Snail-2 expression warrant further investigation, but we hypothesize that they do not completely overlap with the pathways that lead to increased cell proliferation and the capacity for anchorage-independent growth. Furthermore, the activation of these different pathways likely depends on the dose and identity of the EGFR ligand.
Snail-1 and Snail-2 are members of an evolutionarily conserved family of zinc-finger transcription factors (25, 26). Both are expressed in the intermediate mesoderm and the metanephric mesenchyme during renal development and are downregulated before epithelial differentiation (3). Kidneys develop normally in mice with a loss-of-function mutation in Snai2 (14, 28), suggesting a functional redundancy of Snail-1 and Snail-2. However, a recent report has shown that the E-cadherin repressors Snail-1, Snail-2, and E47 produce different genetic profiles when overexpressed in MDCK cells, providing reason for differential regulation of these transcription factors (24). Our results suggest that TGF-β induces Snail-1 and chronic EGFR activation induces Snail-2 in IMCD cells.
It is well established that the Snail gene family promotes EMT (1, 4). Snail increases cell motility, disrupts E-cadherin-mediated cell adhesion, and stimulates the production of matrix metalloproteases (2, 5, 16). TGF-β1 induces Snail expression and EMT in MDCK cells in a MAPK- and PI3K-dependent manner (29). Snail-2-overexpressing transgenic mice are morphologically normal but develop tumors of mesenchymal origins (30). In the kidney, upregulation of Snail-1 protein, assessed by immunostaining with a noncommercial antibody, has been reported in the nuclei of tubular epithelial cells and, to a lesser degree, in interstitial cells following ureteral obstruction in rats (44). Another group demonstrated increased protein expression of Snail-1 and increased mRNA expression of both Snail-1 and Snail-2 in obstructed kidneys of neonatal mice; in this case, however, the upregulation of the Snail genes was attenuated by the CCR-1 antagonist BX471, which specifically blocks interstitial leukocyte recruitment (18). Our data, however, raise the hypothesis that EGFR activation may be partially responsible for the upregulation of Snail-2, and subsequent EMT, in renal injury.
The effects of EGFR activation on the expression of Snail family members are likely dependent on cell type. In the human epithelial carcinoma cell line A431, chronic treatment with EGF leads to increased Snail-1 expression, which is dependent on downregulation of caveolin-1 (21). In human breast and pancreatic cancer cell lines with high levels of EGFR expression, chronic activation of the EGFR axis has recently been shown to induce transcription of the basic helix-loop-helix transcription factor Twist via a STAT3-mediated mechanism, leading to EMT (20). Preliminary data in our laboratory indicate that Twist mRNA may also be upregulated by sHB-EGF in IMCD cells, but its relative contribution to EMT requires further investigation.
As with any study that primarily uses immortalized cell lines, the present study has certain limitations. The effects we observed could be cell line specific, although it is notable that sHB-EGF overexpression in MDCK II cells also upregulated Snail-2 mRNA. The effects of sHB-EGF on other nephron segments, such as the proximal tubule, have not yet been studied. Furthermore, constitutive overexpression of a protein, although a common experimental approach, may produce different biological effects than observed in vivo.
In summary, chronic stimulation of the EGFR axis by sHB-EGF leads to dedifferentiation of IMCD cells. The resulting phenotype is partially dependent on the TGF-β-independent upregulation of the E-cadherin transcriptional repressor Snail-2. Therefore, although the upregulation of growth factors (e.g., HB-EGF) in response to renal injury may have restorative potential, prolonged activation of the EGFR axis may favor fibrogenesis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-51265 and DK-38226 and funds from the Department of Veterans Affairs (R. C. Harris) and by a Research Fellowship from the National Kidney Foundation to J. P. Smith.
REFERENCES
- 1.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25: 5603–5613, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutet A, Esteban MA, Maxwell PH, Nieto MA. Reactivation of Snail genes in renal fibrosis and carcinomas: a process of reversed embryogenesis? Cell Cycle 6: 638–642, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter G The EGF receptor: a nexus for trafficking and signaling. Bioessays 22: 697–707, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez D, Montserrat-Sentis B, Virgos-Soler A, Guaita S, Grueso J, Porta M, Puig I, Baulida J, Franci C, Garcia de Herreros A. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol 23: 5078–5089, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francois H, Placier S, Flamant M, Tharaux PL, Chansel D, Dussaule JC, Chatziantoniou C. Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. FASEB J 18: 926–928, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem 269: 20060–20066, 1994. [PubMed] [Google Scholar]
- 10.Hise MK, Salmanullah M, Liu L, Drachenberg CI, Papadimitriou JC, Rohan RM. Control of the epidermal growth factor receptor and its ligands during renal injury. Nephron 88: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Homma T, Sakai M, Cheng HF, Yasuda T, Coffey RJ Jr, Harris RC. Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J Clin Invest 96: 1018–1025, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest 84: 1757–1761, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X, Woodard AS, Rimm DL, Fearon ER. Transcriptional defects underlie loss of E-cadherin expression in breast cancer. Cell Growth Differ 8: 773–778, 1997. [PubMed] [Google Scholar]
- 14.Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev Biol 198: 277–285, 1998. [PubMed] [Google Scholar]
- 15.Jo YI, Cheng H, Wang S, Moeckel GW, Harris RC. Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nephron Exp Nephrol 107: e87–e94, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Jorda M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, Cano A, Fabra A. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci 118: 3371–3385, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange-Sperandio B, Trautmann A, Eickelberg O, Jayachandran A, Oberle S, Schmidutz F, Rodenbeck B, Homme M, Horuk R, Schaefer F. Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am J Pathol 171: 861–871, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67: 9066–9076, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499–515, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Mikula M, Dzwonek A, Jagusztyn-Krynicka K, Ostrowski J. Quantitative detection for low levels of Helicobacter pylori infection in experimentally infected mice by real-time PCR. J Microbiol Methods 55: 351–359, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Yagi H, Yotsumoto F, Kawarabayashi T, Mekada E. Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci 97: 341–347, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, Palacios J, Cano A. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res 66: 9543–9556, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Nieto MA The snail superfamily of zinc-finger transcription factors. Nat Rev 3: 155–166, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264: 835–839, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol Renal Physiol 273: F563–F574, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Oram KF, Carver EA, Gridley T. Slug expression during organogenesis in mice. Anat Rec 271: 189–191, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem 278: 21113–21123, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Mancera PA, Gonzalez-Herrero I, Perez-Caro M, Gutierrez-Cianca N, Flores T, Gutierrez-Adan A, Pintado B, Sanchez-Martin M, Sanchez-Garcia I. SLUG in cancer development. Oncogene 24: 3073–3082, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA 97: 2202–2207, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Safirstein R, Price PM, Saggi SJ, Harris RC. Changes in gene expression after temporary renal ischemia. Kidney Int 37: 1515–1521, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Safirstein R, Zelent AZ, Price PM. Reduced renal prepro-epidermal growth factor mRNA and decreased EGF excretion in ARF. Kidney Int 36: 810–815, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Sakai M, Zhang M, Homma T, Garrick B, Abraham JA, McKanna JA, Harris RC. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J Clin Invest 99: 2128–2138, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal 17: 1183–1193, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Singh AB, Tsukada T, Zent R, Harris RC. Membrane-associated HB-EGF modulates HGF-induced cellular responses in MDCK cells. J Cell Sci 117: 1365–1379, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, Tsuneoka M, Miura Y, Masuda M, Horiguchi Y, Mekada E. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J Biol Chem 276: 30475–30482, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 31: e154, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol 14: 3147–3154, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Yano T, Yazima S, Hagiwara K, Ozasa H, Ishizuka S, Horikawa S. Activation of epidermal growth factor receptor in the early phase after renal ischemia-reperfusion in rat. Nephron 81: 230–233, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Yoshino J, Monkawa T, Tsuji M, Inukai M, Itoh H, Hayashi M. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem Biophys Res Commun 362: 63–68, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal 19: 2013–2023, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest 117: 2051–2058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6: 931–940, 2004. [DOI] [PubMed] [Google Scholar]