Abstract
Nephrotoxicity is the major limiting factor for the use of cisplatin in cancer therapy. Recent studies have demonstrated an important role for p53 in cisplatin-induced renal injury. Nevertheless, pharmacological and genetic blockade of p53 only provides partial renoprotective effects, suggesting the presence of p53-independent injury mechanisms. To understand the p53-independent mechanisms, we have now examined cisplatin-induced apoptosis in p53-deficient kidney cells. We show that cisplatin could induce Bax activation, cytochrome c release, and apoptosis in primary cultures of p53-deficient renal tubular cells, albeit at a level that was lower than in the wild-type cells. Cisplatin could also induce typical apoptosis in p53-deficient baby mouse kidney (BMK) cells. The apoptosis was caspase dependent and could be completely blocked by general caspase inhibitors. Bax and Bak, two key molecules in the mitochondrial pathway of apoptosis, were interdependently activated by cisplatin, with Bax translocation to and Bax/Bak oligomerization in mitochondria, leading to cytochrome c release. Importantly, cytochrome c release and apoptosis were diminished in Bax/Bak single or double-knockout BMK cells. Furthermore, overexpression of Bcl-2 could ameliorate cisplatin-induced cytochrome c release and apoptosis. Together, the results have demonstrated a p53-independent mechanism of cisplatin nephrotoxicity that involves the mitochondrial pathway of apoptosis.
Keywords: Bax, Bak, cytochrome c
cisplatin is a widely used chemotherapeutic agent, which however, induces major side effects in normal tissues and organs including the kidneys, leading to acute kidney injury and renal failure (2, 13, 24, 28, 34). A well-recognized pathological feature of cisplatin nephrotoxicity is renal cell injury and death, especially in renal tubules. Research during the recent years has discovered multiple mechanisms or signaling pathways that contribute to cisplatin-induced tubular cell death, although it remains unclear how these mechanisms are orchestrated to induce an impressive pathology (see Ref. 28 for a recent review).
Using both in vitro and in vivo experimental models, we and others have demonstrated an important role for p53 in cisplatin-induced tubular cell apoptosis and nephrotoxicity (6, 18, 38). The signaling pathways up- and downstream of p53 are also being delineated. Our latest work has further revealed an early DNA damage response involving ATR-Chk2 in p53 activation during cisplatin nephrotoxicity (29). Despite these findings, it is recognized that p53 is only partially responsible for cisplatin-induced renal injury. For example, compared with wild-type littermates, p53-deficient mice showed less severe renal injury following cisplatin injection, which was nevertheless significantly higher than in vehicle solution-injected control group (38). These observations suggest that cisplatin nephrotoxicity involves both p53-dependent and -independent mechanisms (14). It has been shown that p53 contributes to cisplatin nephrotoxicity, at least in part, by inducing tubular cell apoptosis through the transactivation of cell-killing genes such as p53-upregulated modulator of apoptosis (PUMA) and PIDD (17, 32). However, very little is known about the p53-independent mechanism of cisplatin nephrotoxicity.
The current study sought to analyze the p53-independent mechanism. We show that cisplatin can induce Bax activation, cytochrome c release, and apoptosis in primary proximal tubular cells isolated from p53-deficient mice. In a further study, we took advantage of the p53-deficient baby mouse kidney (BMK) cell lines that have Bax/Bak single- or double-knockout genotypes (8). We show that cisplatin can induce apoptosis in BMK cells in the absence of p53. The apoptosis is caspase dependent and is accompanied by Bax/Bak activation and cytochrome c release from mitochondria. It is attenuated in Bax/Bak-knockout cells and can be diminished by Bcl-2 expression. Together, the results suggest that p53-independent tubular cell apoptosis during cisplatin nephrotoxicity takes the mitochondrial pathway.
METHODS
BMK cell lines.
Stable E1A alone transformed BMK epithelial cells from p53-deficient mice and E1A plus dominant-negative p53 transformed BMK cells from wild-type (WT), Bax-deficient (Bax−/−), Bak-deficient (Bak−/−), and Bax/Bak double-knockout mice were kindly provided by Dr. Eileen White (8). All cell lines were maintained in DMEM supplemented with 5% fetal bovine serum for experiments.
Antibodies and reagents.
Antibodies used in this study were from the following sources: polyclonal anti-p53 was from Cell Signaling Technology (Beverly, MA); polyclonal anti-PUMA was generated and described previously (41); polyclonal anti-Bax (N-20) was from Santa Cruz Biotechnology; polyclonal anti-Bak (NT) and polyclonal anti-Bax (NT) were from Upstate Biotechnology; monoclonal anti-cytochrome c was from BD BioSciences Pharmingen (San Diego, CA); monoclonal anti-cyclooxygenase IV was from Molecular Probes (Eugene, OR); and all secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Chemical cross-linker bismaleimidohexane (BMH) was from Pierce. Carbobenzoxy-Val-Ala-Asp-fluoromethyl ketone (VAD), carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (DEVD.AFC), and 7-amino-4-trifluoromethyl coumarin (AFC) were purchased from Enzyme Systems Products (Dublin, CA). An Annexin V-FITC apoptosis detection kit was from BD BioSciences Pharmingen. An in situ cell death detection kit (fluorescein) was from Roche Applied Science. pβactBcl-2 was provided by Dr. Junying Yuan at Harvard Medical School. Plasmid vector enhanced green fluorescent protein (pEGFP) was purchased from Clontech Laboratories, and transfection reagent METAFECTENE was from Biontex. Unless indicated, all other reagents including cisplatin were from Sigma (St. Louis, MO).
Cisplatin treatment of BMK cells.
Cells were plated at a density of 1.4 × 106 cells/dish in 35-mm dishes and reached ∼90% confluence by the next day for the experiment. Freshly prepared cisplatin was added to cells at a final concentration of 100 μM in culture medium. At the end of incubation, cells were monitored morphologically or harvested with indicated buffers to collect cell lysates for biochemical analyses. For cell lysis, both floating and adherent cells were collected.
Primary proximal tubular cell culture and cisplatin treatment.
Animals were handled for experiments according to a protocol approved by the Institutional Animal Care and Use Committee of the Charlie Norwood Veterans Affairs Medical Center. Isolation and primary culture of proximal tubular cells from mice were described in our recent work (37, 38). Briefly, the renal cortex was minced thoroughly and digested with 0.75 mg/ml collagenase 4 (Worthington, Lakewood, NJ) in Hanks' solution at 37°C for 15 min. The digestion was stopped by 10% horse serum, and the renal tubules were collected by centrifugation at 50 g for 2 min. After washes, the proximal tubules were then purified by centrifugation at 2,000 g for 10 min in DMEM/F12 medium containing 32% Percoll (Amersham, Piscataway, NJ). The resultant cell pellets were washed and plated into collagen-coated flasks for primary culture using DMEM/F12 medium supplemented with 10% fetal bovine serum, 5 μg/ml transferrin, 5 μg/ml insulin, 0.05 μM hydrocortisone, and 50 μM vitamin C. After 7–10 days of growth, the primary cells were seeded on 35-mm dishes at a density of ∼0.3 × 106 cells/dish to reach ∼90% confluence by the next day for treatment with 30 and 100 μM cisplatin, respectively.
Transient transfection.
Transfection was conducted as before (4, 40). In brief, BMK cells were plated to reach 50–60% confluence in 35-mm dishes after overnight growth and transfected with 0.2 μg pEGFP or cotransfected with 0.2 μg pEGFP and 1.0 μg pβactBcl-2 using METAFECTENE. pEGFP, which expresses green fluorescent protein in transfected cells, was used to identify the transfectants. After transfection, the cells were maintained in serum-free medium for 4–5 h and then incubated in full culture medium overnight. Transfection efficiency was usually ∼20%. Cisplatin treatment was performed the next day as described above.
Morphological examination of apoptosis.
Apoptotic cells were identified by their morphology described previously (17, 18), including cellular shrinkage, nuclear condensation and fragmentation, and formation of apoptotic bodies. Cellular morphology was examined by phase contrast microscopy. Nuclear morphology was monitored by fluorescence microscopy after 10 μg/ml Hoechst 33342 staining. Four fields with ∼200 cells/field were examined in each condition to estimate the apoptosis percentage. Cells were also stained with 2 μg/ml propidium iodide (PI) to indicate necrotic cell death.
TdT-mediated dUTP nick-end labeling assay.
Apoptosis was also monitored by TdT-mediated dUTP nick-end labeling (TUNEL) assay using an in situ cell death detection kit. Briefly, cells grown on coverslips were fixed with 4% paraformaldehyde for 1 h at room temperature. After permeabilization with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice, cells were exposed to a TUNEL reaction mixture that contained terminal deoxynucleotidyl transferase and nucleotides including FITC or TMR red-labeled dUTP. Following 1-h incubation at 37°C in a humidified atmosphere in the dark, the coverslips were mounted on slides and positive staining with nuclear DNA fragmentation was detected by fluorescence microscopy.
Flow cytometric analysis of apoptosis.
Apoptosis was further quantified by fluorescence-activated cell sorting after annexin V-FITC/PI staining as described before (15, 16). Briefly, cells were collected by trypsinization and centrifugation at 1,000 g for 5 min. Following resuspension in binding buffer (10 mM HEPES-NaOH, 140 mM NaCl, 2.5 mM CaCl2) at a final cell density of 1–2 × 106 cells/ml, 100 μl of a single-cell suspension (1–2 × 105 cells) was incubated with 5 μl annexin V-FITC and 5 μl PI for 15 min at room temperature in the dark. After addition of 400 μl of binding buffer, the samples were analyzed by using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) within 1 h. For each sample, 10,000 events were counted.
Caspase activity measurement.
The enzymatic activity of caspases was measured using DEVD.AFC, a fluorogenic peptide substrate. Briefly, cells were extracted with 1% Triton X-100. The lysates of 25 μg protein were added to enzymatic reactions containing 50 μM DEVD.AFC. After 1-h incubation at 37°C, fluorescence at 360 nm (excitation)/530 nm (emission) was then monitored. For each measurement, a standard curve was constructed using free AFC. Based on the standard curve, the fluorescence reading from each enzymatic reaction was converted into the nanomolar amount of liberated AFC per milligram protein to indicate caspase activity.
Immunoblot analysis.
Protein concentration of cellular extracts was determined using a bicinchoninic acid (BCA) reagent (Pierce, Rockford, IL). Equal amounts of protein were loaded in each lane for reducing SDS-gel electrophoresis and then electroblotted onto polyvinylidene difluoride membranes. The blots were subsequently incubated with blocking buffer, indicated primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Antigens on the blots were revealed using an enhanced chemiluminescence kit from Pierce.
Dual-immunofluorescence of cytochrome c and activated Bax.
Cells were grown on collagen-coated coverslips for immunofluorescence analysis as described in our earlier work (15). Briefly, the cells were fixed with a modified Zamboni's fixative containing 4% paraformaldehyde and picric acid and permeabilized with 0.1% SDS. Following 1-h incubation in blocking buffer (2% BSA, 0.2% milk, and 2% normal goat serum in PBS), the cells were then exposed to a mixed primary antibody of rabbit anti-Bax (NT) and mouse anti-cytochrome c and subsequently a mixture of FITC-labeled goat anti-rabbit and Cy3-labeled goat anti-mouse secondary antibodies. The coverslips were finally mounted on slides with Antifade, and signals were examined by fluorescence microscopy under the Cy3 and FITC channel.
Cellular fractionation.
Subcellular localization of Bax and cytochrome c was determined by cellular fractionation as shown previously (17, 36). In brief, cells were permeabilized with 0.05% digitonin in an isotonic buffer (in mM: 250 sucrose, 10 HEPES, 10 KCl, 1.5 MgCl2, 1 EDTA, and 1 EGTA, pH 7.1) for 2 min at room temperature. The digitonin-soluble extract was collected as the cytosolic fraction. The digitonin-insoluble part was further dissolved in 2% SDS buffer as the membrane-bound organellar fraction enriched with mitochondria. These two fractions were analyzed for Bax and cytochrome c by immunoblotting.
Analysis of Bax/Bak oligomerization.
BMH, a homobifunctional, maleimide cross-linker, was used to analyze Bax/Bak oligomerization, as described in our recent work (15, 40). Briefly, cells were first fractionated with 0.05% digitonin to collect the membrane fraction for cross-linking. The resultant membrane fraction was then incubated with 5 mM BMH in phosphate-buffered saline for 30 min at room temperature under constant mixing. Finally, the cross-linked samples were dissolved in 2% SDS buffer and subjected to immunoblot analysis for Bax and Bak.
Statistics.
Qualitative data including immunoblots and cell images are representatives of at least three experiments. Quantitative data were expressed as means ± SD. Statistical analysis was conducted using GraphPad Prism software. Statistical differences in multiple groups were determined by multiple comparisons with Tukey's posttests following analysis of variance. Statistical differences between two groups were determined by a two-tailed unpaired Student t-test. P < 0.05 was considered significantly different.
RESULTS
Cisplatin-induced Bax translocation and cytochrome c release in primary proximal tubular cells isolated from p53-deficient mice.
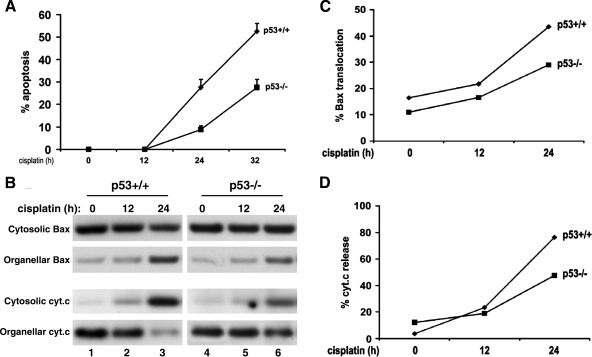
Cisplatin nephrotoxicity is significantly but partially prevented in p53-deficient mice (38), suggesting the involvement of both p53-dependent and -independent mechanisms of renal injury. To understand the p53-independent mechanism, we initially examined primary proximal tubular cells that were isolated from p53-deficient mice. Consistent with our published results (38), we showed lower apoptosis in p53-deficient cells. For example, after 32 h of 30 μM cisplatin treatment, wild-type and p53-deficient cells showed 50 and 30% apoptosis, respectively (Fig. 1A). By cellular fractionation and immunoblotting, we further showed that Bax translocation to mitochondria and cytochrome c release from the organelles were also suppressed in p53-deficient primary tubular cells (Fig. 1B, lane 3 vs. lane 6). Nevertheless, residual changes in the molecules persisted (Fig. 1B, lane 6). The redistribution of Bax and cytochrome c was then quantified by densitometric analysis of the immunoblots (Fig. 1, C and D). Clearly, cisplatin at the concentration of 30 μM induced a time-dependent Bax translocation and cytochrome c release in both wild-type and p53-deficient cells, but at a lower level in the latter (Fig. 1, B–D). The partial protective effects shown in p53-deficient cells were lost at higher cisplatin concentrations. It was shown that 100 μM cisplatin treatment for 24 h induced 60–70% apoptosis in both wild-type and p53-deficient cells. By PI staining, 100 μM cisplatin also induced ∼10% necrosis in both cell genotypes.
Fig. 1.
Cisplatin-induced Bax activation and cytochrome c release in p53-proficient and -deficient proximal tubular cells. Proximal tubular cells were isolated from p53-proficient and -deficient mouse littermates for primary culture. The primary cells were incubated with 30 μM cisplatin for the indicated times. Cells with typical apoptotic morphology were counted to determine the percentage of apoptosis (A). Cells were also fractionated into cytosolic and organellar fractions for immunoblot analysis of Bax and cytochrome c (B). Densitometric results of the blots are shown in C and D. The results show that cisplatin could activate the mitochondrial pathway of apoptosis in p53-deficient primary tubular cells, albeit at a lower efficacy than that in p53-proficient cells.
Cisplatin induces apoptosis in p53-deficient BMK cells.
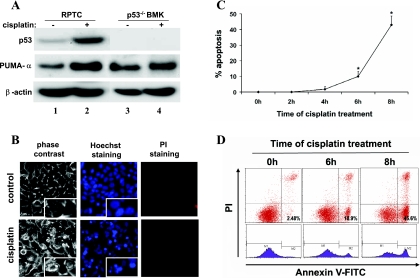
The above results suggest that the intrinsic or mitochondrial pathway may contribute to p53-independent apoptosis during cisplatin nephrotoxicity. To further investigate this possibility, we took advantage of the BMK cell lines that have various Bax/Bak single or double-knockout genotypes. The BMK cells were originated from p53-deficient BMK tissues (8). We first confirmed the absence of p53 in these cells by examining p53 and PUMA-α expression. The results are shown in Fig. 2A. p53 was not detected in BMK cells regardless of whether the cells were exposed to cisplatin (lanes 3 and 4). As a positive control, p53 was detected in rat kidney proximal tubular cells (RPTC) and induced by cisplatin (lanes 1 and 2). Consistently, PUMA-α, a p53-regulated proapoptotic gene, was induced in RPTC cells, but not in the BMK cells (Fig. 2A). These analyses confirmed the p53 deficiency in BMK cells, which were utilized in subsequent experiments to examine the p53-independent mechanism of renal cell apoptosis during cisplatin nephrotoxicity.
Fig. 2.
Cisplatin-induced apoptosis in p53-deficient baby mouse kidney (BMK) cells. A: confirmation of p53 deficiency in BMK cells. Renal proximal tubule cells (RPTC) and BMK cells were incubated for 8 h without or with 20 and 100 μM cisplatin, respectively. Whole-cell lysates were collected for immunoblot analysis of p53 and p53-upregulated modulator of apoptosis (PUMA)-α. The same blots were reprobed for β-actin to control protein loading and transferring. Cisplatin induced p53 and PUMA-α in RPTC but not BMK cells. B: representative images of cell morphology. BMK cells were untreated (control) or treated with 100 μM cisplatin for 8 h. The cells were then stained with Hoechst 33342 and propidium iodide (PI). Cellular and nuclear morphology was recorded by phase contrast and fluorescence microscopy, respectively. Insets: cell and nuclear morphology at high magnifications. C: percentage of apoptosis assessed by morphological criteria. BMK cells were treated with 100 μM cisplatin for 0–8 h. The cells with typical apoptotic morphology were counted to assess apoptosis percentage. Data are expressed as means ± SD; n = >5. *Statistically significantly different from the control (0h). D: flow cytometric analysis of apoptosis after annexin V and PI staining. BMK cells were treated with 100 μM cisplatin for 0, 6, or 8 h. The cells were than subjected to annexin V and PI staining, followed by flow cytometry. The results show that cisplatin induced apoptosis in p53-deficient BMK cells time dependently.
We monitored cell morphology to determine whether cisplatin can induce apoptosis in BMK cells. As shown in Fig. 2B, after 100 μM cisplatin treatment for 8 h, many BMK cells displayed the typical morphological changes of apoptosis. These cells were shrunken, with apoptotic bodies containing condensed and fragmented nuclei (insets). Staining with PI was barely seen, indicating minimal or the absence of necrosis. We further determined the time dependence of cisplatin-induced apoptosis in BMK cells. As shown in Fig. 2C, no significant apoptosis was detected during the first 4 h of cisplatin incubation. Noticeable apoptosis was shown from 6 h and increased to ∼45% at 8 h. To confirm the morphological observations, we analyzed the cells by flow cytometry after annexin V and PI staining (Fig. 2D). Cisplatin treatment increased annexin V-positive but PI-negative cells in a time-dependent manner, indicating the induction of typical apoptosis.
Cisplatin-induced apoptosis in BMK cells can be completely blocked by general caspase inhibitors.
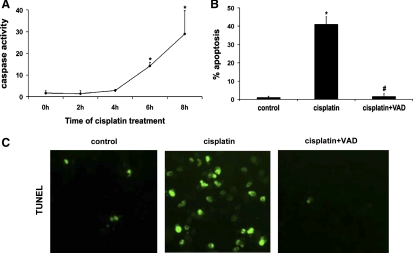
By an enzymatic assay, we showed that cisplatin induced a time-dependent caspase activation in BMK cells (Fig. 3A). Caspase activation was obvious after 6 h of cisplatin treatment and increased further by 8 h (Fig. 3A). To determine the involvement of caspases in BMK cell apoptosis, we tested the effects of VAD, a peptide inhibitor of caspases with fluoromethyl ketone moiety. As shown in Fig. 3B, by morphology cisplatin incubation for 8 h induced ∼42% apoptosis in BMK cells, which was reduced to the basal level of ∼2% by 100 μM VAD, while a control fluoromethyl ketone compound (FA) did not have significant effects (not shown). We further confirmed the results by TUNEL assay of apoptosis (Fig. 3C). Clearly, cisplatin treatment led to positive TUNEL staining in a significant number of cells (Fig. 3C, cisplatin), which was completely inhibited by VAD (Fig. 3C, cisplatin+VAD). In the same experiments, VAD abolished cisplatin-induced caspase activation (data not shown). Together, the results indicate that cisplatin-induced apoptosis in p53-deficient renal cells is predominantly caspase dependent. The inhibitory effects of VAD were also shown in p53-knockout primary proximal tubular cells. For example, 30 μM cisplatin-induced apoptosis was reduced by VAD from ∼20 to ∼1%. Nevertheless, VAD did not promote long-term cell viability as shown by an MTT assay (date not shown).
Fig. 3.
Inhibition of BMK cell apoptosis by carbobenzoxy-Val-Ala-Asp-fluoromethyl ketone (VAD) during cisplatin treatment. A: caspase activation during cisplatin incubation of BMK cells. BMK cells were treated with 100 μM cisplatin for 0–8 h. The cells were then extracted with 1% Triton X-100 to collect lysate for enzymatic analysis of caspase activity. B and C: effects of VAD on apoptosis. BMK cells were treated with 100 μM cisplatin for 8 h in the absence or presence of 100 μM VAD. At the end of incubation, the cells were fixed with 4% paraformaldehyde to evaluate apoptosis by cell morphology (B) and TdT-mediated dUTP nick-end labeling (TUNEL) staining (C). Data are expressed as means ± SD (n = 5). The results show that cisplatin-induced apoptosis in BMK cells was caspase-dependent. *Statistically significantly different from the control (0h). #Statistically significantly different from the cisplatin-only group.
Bax translocation and cytochrome c release in BMK cells during cisplatin treatment.
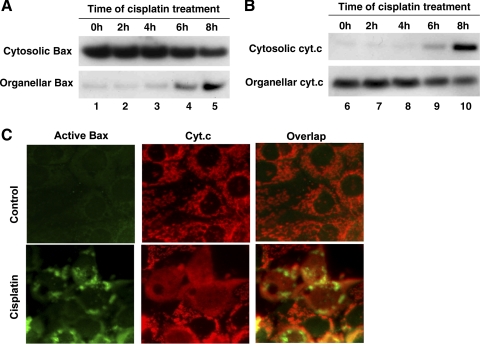
The mitochondrial pathway of apoptosis, characterized by Bax/Bak activation and cytochrome c release from mitochondria, has been implicated in renal cell apoptosis during cisplatin nephrotoxicity (20, 30). This inference was further supported by our recent study demonstrating the resistance of Bax-null mice to cisplatin-induced renal injury (37). To investigate whether the mitochondrial pathway is responsible for p53-independent apoptosis, we examined Bax translocation and cytochrome c release during cisplatin incubation of BMK cells. The subcellular distributions of Bax and cytochrome c were first analyzed by cellular fractionation and immunoblotting. As shown in Fig. 4A, in control cells, the majority of Bax was shown in the cytosolic fraction (lane 1). Cisplatin treatment for 2–4 h did not induce Bax translocation (lanes 2 and 3). However, a significant amount of Bax accumulated in the organellar fraction (enriched with mitochondria) after 6–8 h of cisplatin incubation, which was accompanied by the loss of Bax from the cytosolic fraction (lanes 4 and 5). The translocation of Bax to mitochondria was paralleled by the release of cytochrome c from the organelles. As shown in Fig. 4B, 6 h of cisplatin treatment led to the appearance of cytochrome c in the cytosolic fraction (lane 9), which was increased markedly at the end of 8 h (lane 10). We further analyzed the molecular redistributions by dual immunofluorescence staining of active Bax and cytochrome c (Fig. 4C). As expected, in control cells there was no active Bax staining, and cytochrome c showed a perinuclear organellar or mitochondrial staining. Cisplatin treatment for 8 h induced Bax activation, resulting in punctate Bax staining in mitochondria. Notably, the same cells released cytochrome c into the cytosol. Together, these results demonstrate the activation of the mitochondrial pathway of apoptosis by cisplatin in p53-deficient renal cells.
Fig. 4.
Bax activation and cytochrome c release in BMK cells during cisplatin treatment. A and B: BMK cells were treated with 100 μM cisplatin for 0–8 h. The cells were fractionated into cytosolic and membrane-bound organellar/mitochondrial fractions for immunoblot analysis of Bax and cytochrome c. C: dual immunofluorescence of active Bax and cytochrome c. BMK cells were untreated (control) or treated with 100 μM cisplatin for 8 h. The cells were then fixed for dual immunofluorescence using specific antibodies against active Bax and cytochrome c. The results show cisplatin-induced Bax activation and translocation to mitochondria and cytochrome c release in BMK cells.
Bax and Bak oligomerization in p53-deficient cells during cisplatin treatment.
Upon activation, Bax and Bak form oligomers in mitochondria, leading to mitochondrial outer membrane permeabilization and subsequent cytochrome c release (1, 7, 11, 21). To analyze the molecular oligomerization in p53-deficient BMK cells, we collected mitochondrial fractions for chemical cross-linking to stabilize the oligomers for immunoblotting. As shown in Fig. 5A, Bax oligomers were not observed at the first 4 h of cisplatin treatment (lanes 1 and 2). Cisplatin incubation for 6–8 h induced significant Bax oligomerization with appearance of a dimer, trimer, and tetramer (lanes 3 and 4). In the same samples, the amount of Bax monomer also increased, confirming our previous results of Bax translocation to mitochondria (Fig. 4). Similarly, Bak oligomerized after 6–8 h of cisplatin treatment (Fig. 5B, lanes 3 and 4).
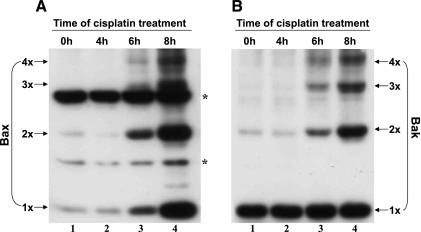
Fig. 5.
Cisplatin-induced Bax and Bak oligomerization in BMK cells. BMK cells were incubated with 100 μM cisplatin for 0–8 h. At the end of incubation, the mitochondrial fraction was collected and subjected to chemical cross-linking with 5 mM bismaleimidohexane (BMH). The cross-linked samples were then analyzed for Bax (A) and Bak (B) by immunoblotting. 1x, 2x, 3x, and 4x, respectively, represent monomer, dimer, trimer, and tetramer of the protein; * refers to nonspecific bands. The results show that cisplatin induced the oligomerization of both Bax and Bak in BMK cells in a time-dependent manner.
Cisplatin-induced apoptosis in BMK cells depends on Bax and Bak.
To further determine the role of Bax and Bak, we compared cisplatin-induced apoptosis in BMK cell lines originated from wild-type, Bax and Bak single, or double-knockout mice. The presence or absence of Bax and Bak in these cells lines was confirmed by immunoblot analysis (Fig. 6A). When these cells were subjected to cisplatin treatment, significant apoptosis was induced in wild-type cells, but not in Bax and Bak single- or double-knockout cell lines (Fig. 6B). Cell counting results are summarized in Fig. 6C. Cisplatin treatment induced ∼50% apoptosis in the wild-type cells, which was reduced to ∼10% by Bax deficiency and to ∼5% by Bak deficiency, respectively. In Bax and Bak double-knockout cells, apoptosis was completely abrogated. The morphological results were confirmed by caspase activity analysis. Consistently, cisplatin-induced caspase activation was ameliorated in Bax or Bak single-knockout cells and completely diminished in double-knockout cells. Taken together, the results suggest that Bax and Bak are indeed involved in and required for cisplatin-induced apoptosis in BMK cells.
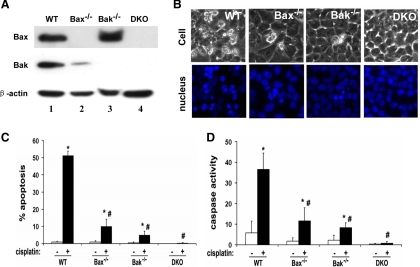
Fig. 6.
Cisplatin-induced apoptosis is attenuated by Bax/Bak-deficient BMK cells. A: immunoblot analysis of Bax and Bak. BMK cells originated from wild-type (WT), Bax-deficient (Bax−/−), Bak-deficient (Bak−/−), and Bax/Bak-double knockout (DKO) mice were extracted to collect whole-cell lysate for immunoblot analysis of Bax and Bak. The same blots were reprobed for β-actin to control protein loading and transferring. B–D: 4 BMK cell lines were incubated with 100 μM cisplatin for 8 h to examine cell morphology (B), apoptosis (C), and caspase activity (D). A and B show representative results. In C and D, data are expressed as means ± SD (n = 5). The results indicate that cisplatin-induced apoptosis was suppressed in Bax/Bak single- or double-knockout BMK cells. *Statistically significantly different from the control (cisplatin −). #Statistically significantly different from the cisplatin-treated WT cells.
Bax/Bak deficiency inhibits cisplatin-induced cytochrome c release in BMK cells.
We further examined the effects of Bax/Bak knockout on cytochrome c release. As shown in Fig. 7A, cytochrome c was mainly located in the organellar fractions of control cells irrespective of their Bax/Bak status (lanes 1, 3, 5, and 7). After cisplatin exposure, a significant amount of cytochrome c was released into the cytosolic fraction in wild-type cells (lane 2), and the release was markedly suppressed in Bax/Bak single- and double-knockout cells (lanes 4, 6, and 8). We also noticed differences in cytochrome c expression levels in these cell lines. Analysis of the whole-cell lysate showed that Bak-knockout cells had lower cytochrome c than other cell lines (Fig. 7B), whereas cyclooxygenase IV, an integral protein of mitochondria, did not show any obvious difference. To semiquantify cytochrome c release, we analyzed the immunoblots by densitometry to calculate the percentage. As shown in Fig. 7C, Bax/Bak deficiency reduced cisplatin-induced cytochrome c release from 58% in wild-type cells to ∼25%. At a late time point of 24 h, wild-type cells were completely dead, whereas there were some (10–30%) surviving cells in Bax/Bak-knockout cells (data not shown), further supporting a role for Bax/Bak in cisplatin-induced cytochrome c release and apoptosis in BMK cells.
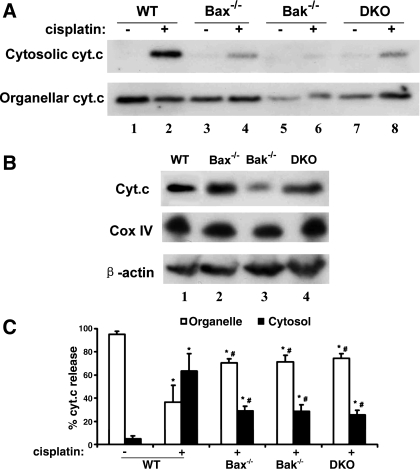
Fig. 7.
Reduced cytochrome c release during cisplatin treatment in Bax/Bak-deficient BMK cells. The indicated 4 BMK cell lines were untreated or treated with 100 μM cisplatin for 8 h. The cells were then fractionated into cytosolic and organellar/mitochondrial fractions for immunoblot analysis of cytochrome c (A). Whole-cell lysates were also collected to compare cytochrome c expression level in these cell lines (B). The percentage of cytochrome c release was determined by densitometric analysis of immunoblots (C). In C, data are expressed as means ± SD (n = 3). The results show that cytochrome c release during cisplatin treatment was suppressed in Bax/Bak-deficient BMK cells. *Statistically significantly different from the control (cisplatin −). #Statistically significantly different from cisplatin treated WT cells.
Interdependence of Bax and Bak for their activation during cisplatin treatment.
As two key molecules for the mitochondrial pathway of apoptosis, Bax and Bak have been shown to interact and cooperatively oligomerize to facilitate cell death in some apoptotic models (25, 33, 35). Our results shown above suggested a role for both Bax and Bak in mediating cisplatin-induced apoptosis in p53-deficient renal cells. To study the functional interaction between Bax and Bak, we examined their oligomerization in wild-type and Bax or Bak knockout cells. The results are shown in Fig. 8. Cisplatin induced Bax oligomerization in wild-type cells, but not in Bak-knockout cells (Fig. 8A, lanes 2 and 4). Conversely, cisplatin induced Bak oligomerization in wild-type cells, but not in Bax-knockout cells (Fig. 8B, lanes 6 and 8). The results suggest that Bax and Bak are interdependent in terms of oligomerization and activation in mitochondria.
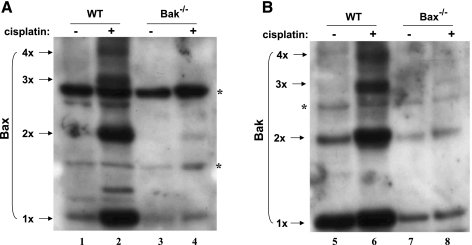
Fig. 8.
Interdependence of Bax and Bak for their activation during cisplatin treatment. WT, Bax-, or Bak-knockout BMK cells were untreated or treated with 100 μM cisplatin for 8 h. After treatment, the mitochondrial fractions were collected and subjected to cross-linking with BMH, followed by immunoblot analysis of Bax (A) and Bak (B). 1x, 2x, 3x, and 4x, respectively, represent monomer, dimer, trimer, and tetramer; * refers to nonspecific signals. The results show that Bax and Bak were interdependent for their activation in response to cisplatin in BMK cells.
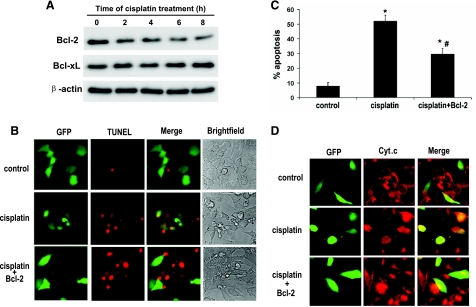
Bcl-2 inhibits cisplatin-induced cytochrome c release and apoptosis in BMK cells.
We further determined the effects of Bcl-2 overexpression on cisplatin-induced apoptosis in BMK cells. The transfection efficiency in BMK cells was ∼20%. To mark the transfected cells for examination, we cotransfected BMK cells with GFP and Bcl-2. The cells were then subjected to cisplatin treatment, and apoptosis was revealed by TUNEL assay. The evaluation was focused on the GFP-labeled/transfected cells. As shown in Fig. 9B, compared with the nontreated control group, cisplatin induced significant apoptosis in GFP-only-transfected cells. In contrast, Bcl-2- and GFP-cotransfected cells showed lower apoptosis. The results of cell counting from several separate experiments are summarized in Fig. 9C. Cisplatin induced >50% apoptosis in the GFP-only-transfected cells, which was reduced to ∼30% by GFP and Bcl-2 cotransfection. To confirm that Bcl-2 protected the cells by maintaining their mitochondrial integrity, we further examined cytochrome c release by immunofluorescence analysis. Representative images are shown in Fig. 9D. After cisplatin treatment, many GFP-only-transfected cells exhibited cytochrome c release from mitochondria, resulting in a diffuse cytosolic staining. On the contrary, most of the GFP- and Bcl-2-cotransfected cells maintained cytochrome c in their mitochondria, showing a perinuclear, punctate staining (Fig. 9). These results further support a role for the mitochondrial pathway of apoptosis in cisplatin-induced BMK cell apoptosis.
Fig. 9.
Inhibition of cytochrome c release and apoptosis by Bcl-2 in BMK cells during cisplatin treatment. A: immunoblot analysis of Bcl-2 and Bcl-xL. BMK cells were treated with 100 μM cisplatin for 0–8 h to collect lysate for immunoblot analysis. B–D: BMK cells were transiently transfected with green fluorescent protein (GFP) or cotransfected with GFP and Bcl-2. After transfection, the cells were untreated or treated with 100 μM cisplatin for 8 h. The cells were then subjected to TUNEL staining. B: representative cell images. C: percentage of apoptosis (TUNEL positive) in transfected cells. The cells that showed GFP green fluorescence were evaluated to determine the percentage of TUNEL-positive cells. Data are expressed as means ± SD (n = 4). *Statistically significantly different from the control. #Statistically significantly different from the cisplatin-only-treated cells. D: immunofluorescence of cytochrome c. The results show that Bcl-2 overexpression attenuated cisplatin-induced apoptosis and cytochrome c release in BMK cells.
DISCUSSION
Previous studies have suggested that cisplatin-induced renal cell injury and nephrotoxicity involves both p53-dependent and -independent mechanisms. Using p53-deficient BMK and primary proximal tubular cells, we have specifically examined the p53-independent mechanism in this study. Our results show that, in the absence of p53, cisplatin induces renal cell apoptosis via the intrinsic or mitochondrial pathway. Under this condition, both Bax and Bak are activated, leading to cytochrome c release from mitochondria and subsequent caspase activation. Interestingly, the activation of Bax and Bak is shown to be interdependent. Moreover, we show that cisplatin-induced apoptosis is attenuated in Bax/Bak-deficient cells and also by Bcl-2 expression. These findings have provided insights into the p53-independent mechanism of cisplatin nephrotoxicity.
Recently, we and others have demonstrated a critical role for p53 in cisplatin nephrotoxicity (6, 18, 38). p53 is activated by cisplatin both in cultured renal tubular cells and in mouse kidneys, inducing apoptosis, tissue damage, and renal dysfunction. Inhibition of p53 by pharmacological or genetic approaches can suppress cisplatin-induced renal cell apoptosis in vitro and nephrotoxicity in vivo (6, 18, 38). Further studies have investigated the upstream and downstream signals that contribute to p53 activation and regulation. An early DNA damage response has been revealed, in which ATR and Chk2 signaling has been shown to contribute to p53 activation (29). In addition, reactive oxygen species, especially hydroxyl radicals, may also directly or indirectly participate in p53 activation (16). Of the numerous downstream targets of p53, several have been suggested to be transcriptionally regulated by p53 during cisplatin nephrotoxicity. These include PUMA-α, PIDD, caspases, p21, and others (5, 12, 17, 22, 32, 39). Among them, PUMA-α and PIDD have been shown to relay p53 signaling to mitochondria, leading to the activation of the intrinsic pathway of apoptosis (17, 32). While PIDD may activate caspase-2 to induce apoptosis-inducing factor release from mitochondria, PUMA-α may interact with Bcl-XL at mitochondria to activate Bax/Bak and induce cytochrome c release (17, 32). Of interest, the current study indicates that Bax and Bak can also be activated by cisplatin independently of p53, forming oligomers at mitochondria, leading to cytochrome c release (Figs. 4–6). Apparently, both p53-dependent and -independent mechanisms of apoptosis converge at mitochondria during cisplatin nephrotoxicity. This scenario is important for understanding the complexity and integration of multiple signaling pathways under this pathological condition. During cisplatin treatment, proximal tubular cells isolated from p53-deficient mice show a lower extent of Bax translocation and cytochrome c release than wild-type cells (Fig. 1). Consistently, following cisplatin treatment, p53-deficient cells survived better in the long term (38). Thus the effect of p53 deficiency on apoptosis is not just a shift in time course. Together, these results suggest that p53-dependent and -independent mechanisms might work in parallel or synergistically to induce renal cell apoptosis and nephrotoxicity (Fig. 10).
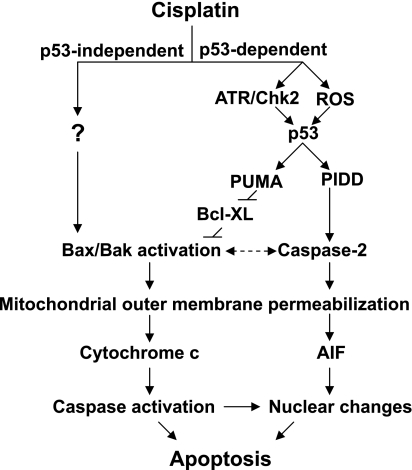
Fig. 10.
Activation of the mitochondrial pathway of apoptosis during cisplatin nephrotoxicity by p53-dependent and -independent mechanisms. Cisplatin-induced renal tubular cell apoptosis involves both p53-dependent and -independent mechanisms. In the p53-dependent mechanism, cisplatin induces an early DNA damage response (involving ATR and Chk2) and the production of reactive oxygen species (ROS), leading to p53 activation. p53 then induces proapoptotic genes such as PUMA and PIDD to activate Bax/Bak or caspase-2 to permeabilize mitochondria, resulting in the release of apoptogenic factors. In the p53-independent mechanism, Bax and Bak are activated by yet-to-be-identified factors to permeabilize mitochondria. AIF, apoptosis-inducing factor.
The intrinsic pathway of apoptosis is tightly controlled at mitochondria by the Bcl-2 family of proteins. Specifically, these proteins regulate the permeability of the mitochondrial outer membrane and thereby control the release of multiple apoptogenic molecules from the intermembrane space (1, 7, 11, 21). Previous studies have shown Bax activation during cisplatin-induced renal cell apoptosis (20, 30). Our results have now further demonstrated Bax/Bak oligomerization in mitochondria, followed by cytochrome c release. Notably, we have also demonstrated evidence for a crucial role of Bax/Bak in cisplatin-induced mitochondrial leakage and apoptosis by using Bax- and Bak-knockout cells (Figs. 6 and 7). These observations are supported by our earlier in vivo study showing that cisplatin-induced cytochrome c release, apoptosis, and renal failure are suppressed in Bax-deficient mice (37). Of note, our results do not exclude the possible involvement of other apoptotic pathways during cisplatin nephrotoxicity, such as the death receptor-mediated extrinsic pathway. In addition, it has been shown that cells at different cell cycle status respond to cisplatin differently and dividing cells are generally more sensitive to anticancer agents including cisplatin (9, 26). The cells used in our study may contain both actively dividing cells and contact-inhibited cells and therefore may undergo cell death via multiple mechanisms. In normal healthy kidneys, tubular cells are contact inhibited and fully differentiated. However, during acute kidney injury initial cell death (likely necrosis) creates wounds or space, and neighboring cells flatten, crawl in, and may proliferate. Under this condition, the tubules contain a mixed population of cells: those that are distant from the wound are still contact inhibited, whereas the cells that are close to or in the wound are in a migratory and proliferative phase.
It is unclear how the death signal during cisplatin nephrotoxicity is relayed to Bax/Bak to provoke mitochondrial injury and apoptosis in the absence of p53. In this regard, studies of nonrenal cells have suggested several possibilities. In p53-null primary murine colonocytes, cisplatin was shown to induce nuclear translocation and activation of p73, a member of the p53 family of proteins, leading to apoptosis (27). Interestingly, p73-induced apoptosis was shown to be mediated by PUMA and NOXA, which can activate Bax to release cytochrome c (10, 23). In addition, several protein kinases, such as MEK/ERK, JNK, and PKC-δ, were also shown to be activated by cisplatin in p53-mutated or -deficient tumor cells, inducing cell apoptosis through the mitochondrial pathway (3, 19, 31). Further research should test these possibilities.
An interesting observation in our study is the interdependence between Bax and Bak for their activation during cisplatin-induced apoptosis. While Bax and Bak are simultaneously activated in wild-type cells, Bax translocation and oligomerization are significantly suppressed by Bak deficiency, and vice versa (Fig. 8). This finding raises the questions of how the interdependence takes place and whether it is relevant to their function on mitochondria permeabilization and cell apoptosis. Although Bax and Bak were initially thought to be functionally redundant, several further studies have suggested the physical association and possible functional cooperation between Bax and Bak in response to cell death stimuli (25, 33, 35). TNF-α-induced cytochrome c release and apoptosis in Hela cells were shown to be related not only to Bax and Bak individual oligomerization but also to Bax-Bak interaction shown by their coimmunoprecipitation and cross-linking to form a heterodimer (33). Similar interactions were also observed in renal tubular cells during ATP depletion and KB-3 human carcinoma cells treated with antimitotic chemotherapy agents (25, 35). Furthermore, antiapoptotic proteins, such as Bcl-2 and Bcl-XL, were shown to prevent cells from apoptosis via blocking the Bax-Bak interaction and cytochrome c release (25, 35). Together with these previous findings, our results suggest that Bax and Bak may function cooperatively and interdependently to facilitate mitochondrial membrane disruption, resulting in the release of apoptogenic factors during cisplatin nephrotoxicity.
In conclusion, this study has demonstrated that cisplatin can induce apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. In concert with the p53-dependent mechanism, the p53-independent signaling may activate Bax/Bak to permeabilize the outer membrane of mitochondria, unleashing the apoptotic cascade during cisplatin nephrotoxicity.
GRANTS
The study was supported by grants from the National Institutes of Health and Department of Veterans Affairs.
Acknowledgments
We thank Drs. Kurt Degenhardt and Eileen White (Center for Advanced Biotechnology and Medicine, Rutgers University, Piscataway, NJ) for kindly providing the BMK cell lines.
REFERENCES
- 1.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bae IH, Kang SW, Yoon SH, Um HD. Cellular components involved in the cell death induced by cisplatin in the absence of p53 activation. Oncol Rep 15: 1175–1180, 2006. [PubMed] [Google Scholar]
- 4.Bhatt K, Feng L, Pabla N, Liu K, Smith S, Dong Z. Effects of targeted Bcl-2 expression in mitochondria or endoplasmic reticulum on renal tubular cell apoptosis. Am J Physiol Renal Physiol 294: F499–F507, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J Pharmacol Exp Ther 308: 921–928, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302: 8–17, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 116: 205–219, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. J Biol Chem 277: 14127–14134, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Evans DL, Tilby M, Dive C. Differential sensitivity to the induction of apoptosis by cisplatin in proliferating and quiescent immature rat thymocytes is independent of the levels of drug accumulation and DNA adduct formation. Cancer Res 54: 1596–1603, 1994. [PubMed] [Google Scholar]
- 10.Flinterman M, Guelen L, Ezzati-Nik S, Killick R, Melino G, Tominaga K, Mymryk JS, Gaken J, Tavassoli M. E1A activates transcription of p73 and Noxa to induce apoptosis. J Biol Chem 280: 5945–5959, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Chesney RW. Regulation of TauT by cisplatin in LLC-PK1 renal cells. Pediatr Nephrol 20: 1067–1072, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 1: 47–61, 2003. [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther 327: 300–307, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Pabla N, Murphy RF, Yang T, Yin XM, Degenhardt K, White E, Dong Z. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem 282: 2636–2645, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang M, Wei Q, Pabla N, Dong G, Wang CY, Yang T, Smith SB, Dong Z. Effects of hydroxyl radical scavenging on cisplatin-induced p53 activation, tubular cell apoptosis and nephrotoxicity. Biochem Pharmacol 73: 1499–1510, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287: F1140–F1147, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Lasfer M, Davenne L, Vadrot N, Alexia C, Sadji-Ouatas Z, Bringuier AF, Feldmann G, Pessayre D, Reyl-Desmars F. Protein kinase PKC delta and c-Abl are required for mitochondrial apoptosis induction by genotoxic stress in the absence of p53, p73 and Fas receptor. FEBS Lett 580: 2547–2552, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Lee RH, Song JM, Park MY, Kang SK, Kim YK, Jung JS. Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct cells. Biochem Pharmacol 62: 1013–1023, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev 2: 63–67, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Megyesi J, Udvarhelyi N, Safirstein RL, Price PM. The p53-independent activation of transcription of p21 WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1211–F1216, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, Knight RA, Green DR, Thompson C, Vousden KH. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem 279: 8076–8083, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KB, Madias NE. Cisplatin nephrotoxicity. Miner Electrolyte Metab 20: 201–213, 1994. [PubMed] [Google Scholar]
- 25.Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E, Saikumar P. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J Biol Chem 278: 5367–5376, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mueller S, Schittenhelm M, Honecker F, Malenke E, Lauber K, Wesselborg S, Hartmann JT, Bokemeyer C, Mayer F. Cell-cycle progression and response of germ cell tumors to cisplatin in vitro. Int J Oncol 29: 471–479, 2006. [PubMed] [Google Scholar]
- 27.Oniscu A, Sphyris N, Morris RG, Bader S, Harrison DJ. p73alpha is a candidate effector in the p53 independent apoptosis pathway of cisplatin damaged primary murine colonocytes. J Clin Pathol 57: 492–498, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283: 6572–7583, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol 13: 858–865, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Schweyer S, Soruri A, Meschter O, Heintze A, Zschunke F, Miosge N, Thelen P, Schlott T, Radzun HJ, Fayyazi A. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br J Cancer 91: 589–598, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem 280: 31230–31239, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Sundararajan R, Cuconati A, Nelson D, White E. Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem 276: 45120–45127, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol 148: 107–121, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Upreti M, Chu R, Galitovskaya E, Smart SK, Chambers TC. Key role for Bak activation and Bak-Bax interaction in the apoptotic response to vinblastine. Mol Cancer Ther 7: 2224–2232, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53–62, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–F1291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C, Kaushal V, Haun RS, Seth R, Shah SV, Kaushal GP. Transcriptional activation of caspase-6 and -7 genes by cisplatin-induced p53 and its functional significance in cisplatin nephrotoxicity. Cell Death Differ 15: 530–544, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Yi X, Yin XM, Dong Z. Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 278: 16992–16999, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7: 673–682, 2001. [DOI] [PubMed] [Google Scholar]