Abstract
Recent evidence suggests that mineralocorticoid receptor (MR) antagonism has beneficial effects on tissue oxidative stress and insulin metabolic signaling as well as reducing proteinuria. However, the mechanisms by which MR antagonism corrects both renin-angiotensin-aldosterone system (RAAS) impairments in renal insulin metabolic signaling and filtration barrier/podocyte injury remain unknown. To explore this potential beneficial interactive effect of MR antagonism we used young transgenic (mRen2)27 (Ren2) rats with increased tissue RAAS activity and elevated serum aldosterone levels. Ren2 and age-matched Sprague-Dawley (SD) control rats (age 6–7 wk) were implanted with a low dose of the MR antagonist spironolactone (0.24 mg/day) or vehicle, both delivered over 21 days. Albuminuria, podocyte-specific proteins (synaptopodin, nephrin, and podocin), and ultrastructural analysis of the glomerular filtration barrier were measured in relation to RAAS activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, reactive oxygen species (ROS), and the redox-sensitive Rho kinase (ROK). Insulin metabolic signaling was determined via measurement of insulin receptor substrate-1 (IRS-1) phosphorylation, IRS-1 ubiquitin/proteasomal degradation, and phosphorylation of Akt. Ren2 rats exhibited albuminuria, loss of podocyte-specific proteins, and podocyte foot process effacement contemporaneous with reduced renal IRS-1 and protein kinase B/Akt phosphorylation compared with SD control rats (each P < 0.05). Ren2 kidneys also manifested increased NADPH oxidase/ROS/ROK in conjunction with enhanced renal tissue levels of angiotensin II (ANG II), ANG-(1-12), and angiotensin type 1 receptor. Low-dose spironolactone treatment reduced albuminuria and tissue RAAS activity and improved podocyte structural and protein integrity with improvements in IRS-1/Akt phosphorylation. Thus, in this model of RAAS activation, MR antagonism attenuates glomerular/podocyte remodeling and albuminuria, in part through reductions in redox-mediated impairment of insulin metabolic signaling.
Keywords: renal mineralocorticoid receptor, reduced nicotinamide adenine dinucleotide phosphate oxidase, oxidative stress, podocyte
aldosterone, like angiotensin II (ANG II), exerts nongenomic actions in cardiovascular and renal tissues including the generation of reactive oxygen species (ROS), increases in inflammation, and impairment of insulin metabolic signaling and endothelial function, processes that promote glomerular disease and albuminuria (5, 6, 9, 29, 31). These adverse effects may result, in part, from mineralocorticoid receptor (MR)- and angiotensin type 1 receptor (AT1R)-mediated activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and redox-sensitive serine (Ser) kinases, such as Rho kinase (ROK) (18, 33, 36). Recent evidence suggests that aldosterone potentiates ANG II-mediated NADPH oxidase and redox-sensitive ROK activation in the kidney as well as in cardiovascular tissue (2, 7, 11, 23, 27, 38, 41). Furthermore, there is mounting evidence that insulin metabolic signaling is important for generating energy and impeding apoptosis of glomerular podocytes (32, 38, 41). Aldosterone, like ANG II, has recently been reported to suppress insulin metabolic signaling in skeletal muscle tissue (18) and vascular smooth muscle cells (12), in part via activation of redox-sensitive Ser kinases and inhibition of insulin metabolic signaling. Accordingly, renin-angiotensin-aldosterone system (RAAS)-induced oxidative stress and associated activation of redox-sensitive kinases, such as ROK, may contribute to reduced insulin metabolic signaling, podocyte injury, loss of slit pore diaphragm integrity, and albuminuria (11, 38).
Insulin metabolic signaling involves tyrosine (Tyr) phosphorylation of the insulin receptor substrate-1 (IRS-1) docking protein and phosphorylation/activation of downstream protein kinase B (Akt). This metabolic signaling pathway modulates cellular functions such as glucose utilization, ATP production, and tissue remodeling (6). Activation of redox-sensitive Ser kinases, such as ROK, and associated Ser phosphorylation of IRS-1 can result in IRS-1 proteosomal degradation and reduced IRS-1 binding to, and activation of, phosphatidylinositol 3-kinase (PI3-K) and downstream Akt phosphorylation/activation (6). Impaired insulin-mediated Akt phosphorylation has been associated with increased podocyte apoptosis and foot process effacement, and thus loss of glomerular filtration barrier/podocyte integrity (2, 12, 20, 35, 37). Glomerular filtration barrier integrity is also dependent on the maintenance of podocyte integrity and signaling (i.e., Tyr phosphorylation of nephrin, podocin, and synaptopodin) (20). In this regard, the role of ROK activation in impairment of insulin metabolic signaling and glomerular structure and function has not previously been investigated (10). Accordingly, we hypothesized that activation of the MR potentiates ANG II activation of NADPH oxidase and the redox-sensitive ROK, which has been shown to contribute to glomerular dysfunction and proteinuria (10, 11, 15, 17). We further hypothesized that alternate pathways involving renin-angiotensin system (RAS) peptides such as angiotensin-(1-12) [ANG-(1-12)], working in conjunction with ANG II and aldosterone, may further activate ROK and contribute to altered insulin metabolic signaling and associated glomerular damage and albuminuria.
Therefore, we evaluated the impact of in vivo treatment with the MR antagonist spironolactone (Sp) on renal oxidative stress, insulin metabolic signaling, and podocyte integrity in transgenic (mRen2)27 (Ren2) rats. The Ren2 rat overexpresses the mouse renin gene, resulting in elevated plasma levels of aldosterone (4, 30) as well as enhanced tissue RAS activity as evidenced by increased tissue ANG II and AT1R levels (18, 33, 36, 38). To specifically explore MR antagonistic effects of Sp we employed a very low dose of this drug, previously shown not to lower blood pressure (18, 33, 36).
MATERIALS AND METHODS
Animals and Treatments
All animal procedures were approved by the University of Missouri animal care and use committees, and animals were housed in accordance with National Institutes of Health (NIH) guidelines. Ren2 rats (6–7 wk of age) and age matched Sprague-Dawley (SD) littermates were randomly assigned to untreated (n = 5; Ren2-C and SD-C, respectively) and Sp-treated (n = 5; Ren2-Sp and SD-Sp, respectively) paradigms (18, 33, 36). Ren2-Sp and SD-Sp animals were implanted subcutaneously with a 5-mg timed-release Sp or placebo pellet (Innovative Research of America, Sarasota, FL) for 21 days. We showed previously (18, 33, 36) that this low dose of Sp does not affect blood pressure in this rodent model. Pellets were placed, under anesthesia, via a superscapular incision closed with permanent suture.
Systolic Blood Pressure and Albuminuria
Restraint conditioning was initiated before blood pressure measurements were performed as previously described (33, 38). Systolic blood pressure (SBP) was measured in triplicate on separate occasions throughout the day by the tail-cuff method (Harvard Systems, Student Oscillometric Recorder) before initiation of treatment, on day 19 or 20, and before death at 21 days (32). Urine albumin and creatinine were determined as previously described (38).
Immunohistochemical Quantification
Four-micrometer paraffin-embedded sections of kidney of different treatments were deparaffinized in CitriSolv and rehydrated in ethanol series and HEPES wash buffer (Fisher Bioreagents, Fairlawn, NJ; pH = 7.4). Epitopes were retrieved (antigen retrieval) in citrate buffer pH 6 for 25 min at 95°C with a steamer. Slides were then immediately transferred into a humidity chamber. Nonspecific binding sites were blocked (5% BSA, 5% serum of the animals in which secondary antibodies were generated, 0.01% sodium azide) at room temperature for 4 h. After a gentle wash with HEPES the sections were incubated with primary antibodies, 1:100 goat polyclonal ANG II (Santa Cruz Biotechnology, Santa Cruz, CA), 1:100 rabbit polyclonal AT1R (Santa Cruz), 1:50 rabbit polyclonal ANG-(1-12) (C. M. Ferrario), 1:100 goat polyclonal Nox2 (Santa Cruz), 1:100 goat polyclonal p47phox (Santa Cruz), 1:100 rabbit polyclonal IRS-1 (Santa Cruz), 1:50 rabbit polyclonal pIRS-1 Tyr941 (Upstate), Ser473 phosphorylated Akt (1:500) (Santa Cruz), 1:75 mouse monoclonal desmin (Santa Cruz), 1:75 mouse monoclonal α-smooth muscle actin (Sigma), 1:100 goat synaptopodin (Santa Cruz), 1:100 rabbit polyclonal podocin (Santa Cruz), and 1:100 goat polyclonal nephrin (Santa Cruz), overnight at room temperature. All primary antibodies were diluted in 10-fold-diluted blocker. The sections were washed (3 × 15 min) and incubated with 1:300 Alexa Fluor 647 donkey anti-mouse, -rabbit, or -goat corresponding with the primary antibodies for 4 h. The slides were then washed (3 × 15 min), and sections were mounted with Mowiol and stored in lighttight slide boxes at 4°C until assessment with an abi-photon confocal microscope (Zeiss LSM, 510 MLO, Thornwood, NY); 1,024 × 1,024-pixel images were captured with the LSM imaging system under the same microscope and computer settings for all animals of four groups in each experiment. The signal intensities were measured and analyzed in equal regions with MetaView as previously described (18, 36, 38).
Oxidative Stress
NADPH oxidase activity.
NADPH oxidase activity was determined in kidney cortical tissue as previously described (18, 36, 38). Briefly, NADPH oxidase activity was determined by measuring the conversion of Radical Detector (Cayman Chemical, Ann Arbor, MI) in the absence and presence of NADPH inhibitor diphenyleneiodonium sulfate (500 μM) with spectrophotometric (450 nm) techniques.
ROS formation.
ROS formation was measured by chemiluminescence as previously described (33). Briefly, kidney tissue sections were homogenized in sucrose buffer with a glass/glass homogenizer and centrifuged, and supernatants (whole homogenate) were then removed and placed on ice. Whole homogenate (100 μl) was added to 1.4 ml of 50 mM phosphate (KH2PO4) buffer (150 mM sucrose, 1 mM EGTA, 5 μM lucigenin, 100 μM NADPH, pH 7.0) in dark-adapt counting vials. After dark adaptation, samples were counted on a scintillation counter and the last 5 min were averaged. Samples were then normalized to total protein in the whole homogenate, and ROS values were expressed as counts per minute per milligram of protein.
RhoA Activity
Active RhoA, as a surrogate of ROK, was determined with a Rho G-LISA assay (Cytoskeleton, Denver, CO). Briefly, 50 mg of collected kidney tissue was immediately snap-frozen to prevent degradation of active Rho and then lysed in 1 ml of ice-cold lysis buffer. The lysates were centrifuged at 1,500 g for 10 min at 4°C and then adjusted to 1.0 mg/ml protein by dilution with ice-cold lysis buffer. An equal volume of the sample was added to a Rho G-LISA plate coated with Rho-GTP-binding protein and placed on a cold microplate shaker set at 400 rpm and 4°C for 30 min. After three rinses with wash buffer at room temperature, 50 μl of anti-RhoA primary antibody (diluted 1:250) was added and left on a shaker at room temperature for 45 min. Fifty microliters of diluted horseradish peroxidase (HRP)-labeled secondary antibody (1:250) was then added to the wells and placed on the shaker again at room temperature for 45 min. HRP detection reagent was then added, and the luminescence signal was detected with a microplate.
Immunoprecipitation and Western Blot Analysis
Standard Western blot analysis was used as previously described in detail (38). Briefly, proteins were separated via electrophoresis at room temperature and 200 V for 1 h and then transferred for 1 h at 100 V on ice onto a methanol-activated polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) in transfer buffer (25 mM Tris base, 192 mM glycine, 20% methanol). For immunodetection, blots were blocked overnight at 4°C in blocking buffer [5% nonfat dry milk in TBS-T (20 mM Tris base, 150 mM NaCl, 0.1% Tween 20; pH 7.6)], serially washed in TBS-T at room temperature, and then probed with the primary antibody for the podocyte-specific proteins synaptopodin, nephrin, and podocin (1:200) (Santa Cruz). Additionally, anti-IRS-1 (1:500) (Cell Signaling, Danvers, MA) immunoprecipitation was performed for ubiquitination (23a). After an overnight incubation, the membranes were washed, incubated with 1:5,000 goat anti-rabbit HRP for 1 h at room temperature, washed again, treated with an enhanced chemiluminescent reagent, and digitally imaged with a Bio-Rad Chemi-Doc XRS. Protein bands were quantified with Bio-Rad's Quantity One software. All proteins were corrected for total protein loaded by staining with 1% amino black (Sigma, St. Louis, MO).
Histological Analysis with Light Microscopy
Kidney cortical tissues were fixed in 10% neutral-buffered formalin for 24 h and subjected to fixation in 70% ethanol as previously described (38). Cortical sections were then processed, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin (H & E) and trichrome. Briefly, images from all cross sections of cortical tissue (minimum 10 glomeruli per animal cut at the vascular pole) were captured, and the glomerular tuft area was quantified by MetaVue. Images obtained were then blinded and evaluated by a board-certified veterinary renal pathologist (C. E. Wiedmeyer) using a scoring scheme based on 1-4 for hypercellularity and mesangial expansion (38).
Ultrastructural Analysis with Transmission Electron Microscopy Methods
Kidney cortical tissue was thinly sliced, placed immediately in primary transmission electron microscopy (TEM) fixative, and prepared as previously described (38). A JOEL 1200-EX TEM microscope was utilized to view all samples. Three glomeruli per rat were evaluated, with five 10K and 60K images per glomeruli. TEM images were analyzed with ImageJ (public domain, NIH), and analysis was adapted from previous work.
Statistical Analysis
This investigation was powered based on prior sensitivity and variability measurements of albuminuria (23a, 38, 39) to achieve a significance of P < 0.05 with a power of 0.8. All values are expressed as means ± SE. Statistical analyses were performed in SPSS 13.0 (SPSS, Chicago, IL), using ANOVA with Fisher's least significant difference as appropriate and Student's t-test for paired analysis.
RESULTS
Low-Dose Sp Reduces Albuminuria Without Altering SBP in Ren2 Rats
At initiation of treatment (6–7 wk of age), SBP was higher in Ren2 compared with SD control rats, as previously reported (18, 33, 36). At the end of the treatment period (9–10 wk of age), there was a significant increase in SBP in Ren2-C compared with SD-C rats. However, no reduction in SBP was observed with low-dose Sp treatment in the Ren2-Sp group, as previously reported (18, 36). There were attendant increases in albuminuria in the Ren2 rats (0.19 ± 0.03 mg/mg; P < 0.05) compared with SD control rats (0.07 ± 0.2 mg/mg), with improvements in the Sp-treated Ren2 rats (0.15 ± 0.04 mg/mg; P < 0.05).
Low-Dose Sp Attenuates Kidney RAAS Activation in Ren2 Rats
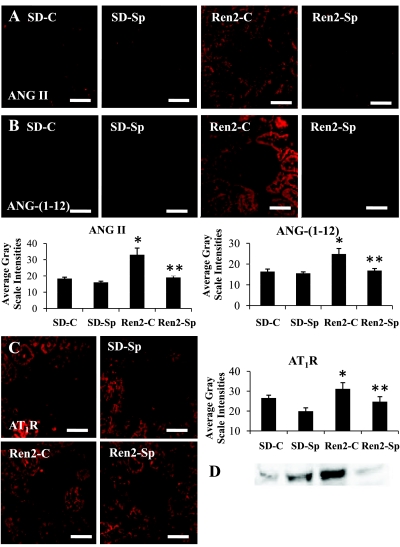
Recent work suggests that alternative sources within the kidney contribute to RAAS activation. For example, newly described pro-peptide ANG-(1-12) cleaved from angiotensinogen may be an alternate substrate for the formation of biologically active angiotensins (13). This fragment isolated from rat tissue may, in turn, generate ANG II by nonrenin pathways. To determine relative kidney tissue RAAS activity, we utilized semiquantitative immunohistochemical analysis for ANG II and ANG-(1-12) in conjunction with Western blot for the AT1R (Fig. 1). Semiquantitative immunohistochemical analyses demonstrated increases in ANG II, ANG-(1-12), and AT1R in untreated Ren2 rats (P < 0.05) compared with SD control rats. These differences were abrogated with 3 wk of in vivo low-dose Sp treatment (P < 0.05).
Fig. 1.
Mineralocorticoid receptor (MR) antagonism attenuates renal renin-angiotensin-aldosterone system (RAAS) activation in transgenic (mRen2)27 (Ren2) rats. A: representative images of semiquantitative immunohistochemistry analysis for angiotensin II (ANG II) with corresponding measures of intensities at bottom left. B: representative images of semiquantitative immunohistochemistry analysis for angiotensin-(1-12) [ANG-(1-12)] with corresponding measures of intensities at bottom right. C and D: representative images of semiquantitative immunohistochemistry analysis for angiotensin type 1 receptor (AT1R) with corresponding measures of intensities on right (C) and Western blot (D). Values are presented as means ± SE. *P < 0.05 compared with Sprague-Dawley control rats (SD-C); **P < 0.05 spironolactone-treated Ren2 (Ren2-Sp) compared with Ren2 control (Ren2-C) rats. Scale bars = 50 μm.
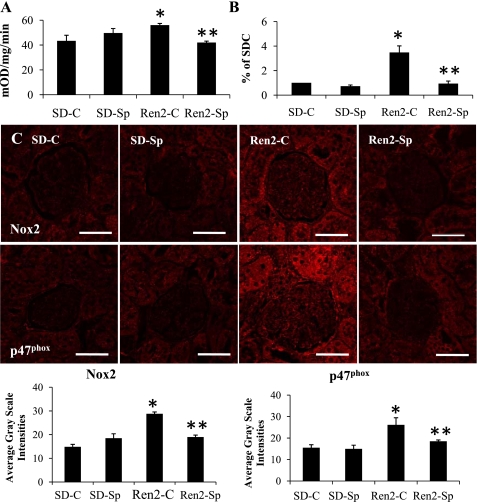
Low-Dose Sp Attenuates the Increased NADPH Oxidase Activation and ROS in Ren2 Kidneys
NADPH oxidase activity was elevated in Ren2 renal tissue compared with SD control tissue. Sp treatment reduced this activity in Ren2 tissue (Fig. 2A) to a value comparable to SD-C tissue. There were parallel increases in NADPH oxidase subunits NOX2 and p47phox in the Ren2 tissue compared with SD-C tissue (P < 0.05), and these differences were attenuated in the Sp treated Ren2 tissue (P < 0.05). Renal ROS levels were higher in Ren2 control rats compared with the placebo-treated SD group. In concert with the effects of Sp treatment on NADPH oxidase activity, there were lower ROS levels in the treated renal tissue (Fig. 2B).
Fig. 2.
MR antagonism improves renal oxidative stress in Ren2 rats. A: reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. mOD, milli-optical density. B: reactive oxygen species (ROS) formation by chemiluminescence expressed as % of that in SD-C. C and D: representative images of semiquantitative immunohistochemistry analysis for NADPH oxidase subunits Nox2 and p47phox and corresponding measures of intensities at bottom. Values are presented as means ± SE. *P < 0.05 Ren2-C compared with SD-C rats; **P < 0.05, Ren2-Sp compared with Ren2-C rats. Scale bars = 50 μm.
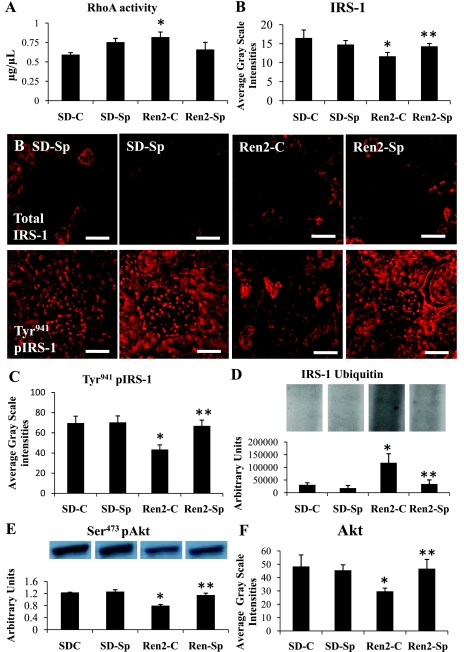
Low-Dose Sp Attenuates the Decreased IRS-1 and Akt Phosphorylation in Ren2 Kidneys
Contemporaneous with increased NADPH oxidase and ROS, there were increases in RhoA activation in the Ren2 renal tissue compared with SD control tissue (Fig. 3A), with a trend to normalization in Sp-treated Ren2 tissue (P > 0.05). There were reductions of total IRS-1 in Ren2-C compared with SD-C tissue by semiquantitative analysis (P < 0.05; Fig. 3B). Consistent with ROK-induced IRS-1 phosphorylation (Fig. 3C), Ren2 cortex exhibited ubiquitin proteosomal degradation of IRS-1 (Fig. 3D) compared with SD control cortex (P < 0.05) (Fig. 3D); each improved with Sp treatment in the Ren2 (P < 0.05). In concert with decreased levels of IRS-1, there were reductions in Ser473 phosphorylation of Akt in Ren2 renal cortex compared with SD control cortex (P < 0.05) (Fig. 3E), corroborated by semiquantitative analysis of total Akt (P < 0.05), and this difference was abrogated with Sp treatment (P < 0.05; Fig. 3F).
Fig. 3.
MR antagonism improves redox-sensitive insulin metabolic signaling in Ren2 rats. A: redox-sensitive RhoA activation as a measure of Rho kinase activity. B and C: representative semiquantitative immunohistochemical analysis of total insulin receptor substrate-1 (IRS-1) with corresponding measures of intensities. D: ubiquitin IRS-1 degradation with representative blot. E: Western blot analysis of serine (Ser)473 phosphorylated Akt. F: measures of total Akt immunostaining. *P < 0.05, Ren2-C compared with SD-C rats; **P < 0.05, Ren2-Sp compared with Ren2-C rats. Scale bars = 50 μm.
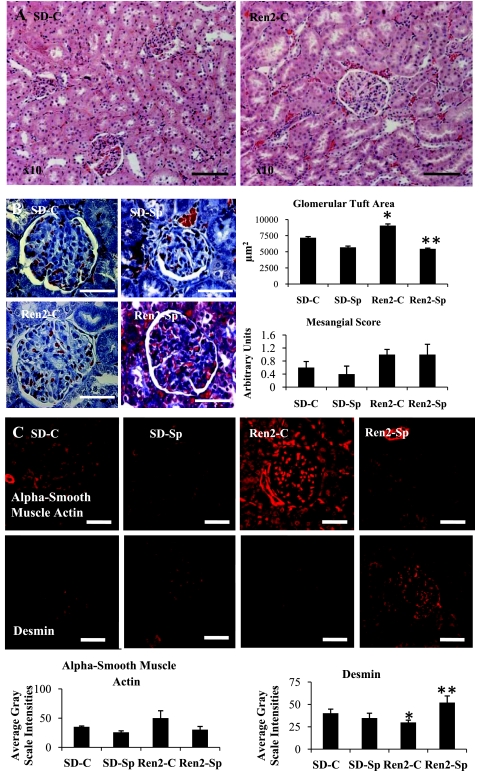
Low-Dose Sp Improves Structural Glomerular Remodeling in Ren2 Rats
On histological analysis utilizing both H & E and trichrome staining, there were no visible differences in renal tubulointerstitial architecture in Ren2 versus SD rats (Fig. 4A). However, there were quantifiable increases in the glomerular area of renal cortex from Ren2 compared with SD control rats, which was normalized in the Sp-treated Ren2 cortex (Fig. 4B). While there was a trend for increases in mesangial hypercellularity in Ren2 compared with SD control rats, these differences were not significant, and there was no treatment effect (each P > 0.05).
Fig. 4.
MR antagonism improves glomerular remodeling in Ren2 rats. A: representative images of kidney cortical tissue utilizing hematoxylin and eosin stain. There were no observed tubulointerstitial morphological changes but noted glomerulomegaly. Scale bars = 100 μm. B: representative images of trichrome stain with glomerulomegaly measures of tuft area and mesangial score on right. C: representative images of semiquantitative immunohistochemical analysis of mesangial α-smooth muscle actin and podocyte desmin with corresponding measures of intensities at bottom. Scale bars = 50 μm. Values are presented as means ± SE. *P < 0.05, Ren2-C compared with SD-C rats; **P < 0.05, Ren2-Sp compared with Ren2-C rats.
To differentiate glomerular cytoskeletal architecture between mesangium and podocytes, we evaluated known markers for mesangium (α-smooth muscle actin) and podocyte (desmin). Commensurate with the trend for mesangial expansion observed on light microscopy, there was a trend to increased α-smooth muscle actin in Ren2 compared with SD, abrogated with Sp treatment (each P > 0.05; Fig. 4C). Additionally, semiquantitative immunohistochemical analyses revealed that there were significant reductions of podocyte-specific desmin in the Ren2 kidney (P < 0.05), and this abnormality improved after treatment with low-dose Sp (P < 0.05).
Low-Dose Sp Improves Podocyte Integrity in Ren2 Rats
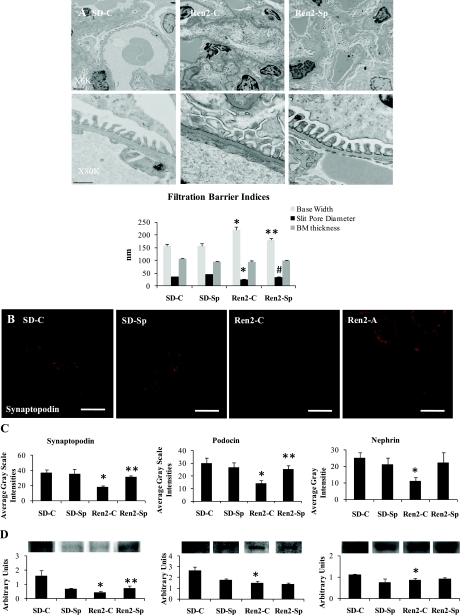
Ultrastructural analysis of the filtration barrier of the Ren2 glomerulus revealed no changes in basement membrane thickening but significant podocyte foot process effacement (Fig. 5A). Consistent with effacement, there were quantifiable increases in podocyte foot process base width, as well as decreases in intact slit pore diaphragm surface in the untreated Ren2 compared with SD control rats (P < 0.05), abnormalities that were largely corrected with Sp treatment (P < 0.05). Concurrent with observations of decreased desmin in the Ren2 and demonstration of ultrastructural podocyte foot process effacement, there were also significant reductions in podocyte-specific proteins synaptopodin, nephrin, and podocin in both semiquantitative immunohistochemical analyses and Western blot compared with SD controls (P < 0.05; Fig. 5, B–D). Interestingly, only synaptopodin was improved with low-dose Sp treatment in Ren2 (P < 0.05).
Fig. 5.
MR antagonism improves synaptopodin in Ren2 rats. A: ultrastructural analysis of the filtration barrier and podocyte foot process effacement utilizing transmission electron microscopy. B and C: representative images of semiquantitative immunohistochemical analysis of synaptopodin (B) with corresponding measures of intensities including nephrin and podocin (C). D: Western analysis of corresponding podocyte-specific proteins. Scale bars = 50 μm. *P < 0.05, Ren2-C compared with SD-C rats; **P < 0.05, Ren2-Sp compared with Ren2-C rats; #P = 0.06, Ren2-Sp compared with Ren2-C rats.
DISCUSSION
Results from this investigation support a potentially novel mechanism by which MR antagonism protects against glomerular injury and albuminuria in a transgenic rat model of RAAS activation. Indeed, renal cortical tissue from the Ren2 rat manifested increased ANG II, ANG-(1-12), and AT1R compared with age-matched SD control rat tissue. Renal cortical tissue from the transgenic model also manifested increased renal NADPH oxidase/ROS and associated increases in redox-sensitive ROK activity. These alterations were accompanied by decreased IRS-1 levels and diminished Akt473 phosphorylation, suggesting decreased insulin metabolic signaling. Alterations in redox status and insulin signaling in Ren2 renal tissue were also associated with diminished podocyte-specific protein levels and loss of integrity of podocyte foot processes and slit pore membrane structure on ultrastructural analysis. In vivo treatment of young Ren2 rats with very low-dose Sp for 3 wk attenuated the development of albuminuria and improved redox-associated metabolic signaling parameters, as well as podocyte and slit pore membrane integrity. That these beneficial effects were independent of any measurable lowering of blood pressure suggests that they were directly mediated via tissue MR antagonism.
Our data support recent reports that aldosterone exerts deleterious glomerular structural and functional effects with resultant albuminuria (2, 8). Previous work indicated that increased Ren2 renal tissue NADPH oxidase activity, ROS production, perivascular fibrosis, podocyte foot process effacement, and albuminuria are mediated, in part, via AT1R activation (39). In this context, our observations that low-dose Sp treatment reduced AT1R, ANG II, and ANG-(1-12) levels in renal cortical tissues from Ren2 rats are noteworthy. Emerging evidence supports the pathophysiological importance of novel peptides such as ANG-(1-12) in the composite effects of RAAS in tissue remodeling (13, 14, 25). In this regard, increased ANG-(1-12) levels may serve as an alternate substrate for the production of bioactive ANG II in the Ren2 kidney. This alternative component of the RAS is expressed in the glomerulus but appears to be preferentially expressed along with angiotensinogen at the luminal surface of the proximal tubular cells (13). Accordingly, increased ANG-(1-12), and its processed product ANG II (13), may contribute to altered tubular function as well as the glomerular abnormalities seen in untreated Ren2 rats. Finally, data from this investigation support the notion that antagonism of the MR can mitigate the detrimental autocrine/paracrine effects of increased expression of RAS within the kidney (6, 13, 14, 26).
Similar to prior reports (37, 38), NADPH oxidase activity was increased in renal cortical tissue from Ren2 animals. This enzyme is a highly regulated membrane-bound enzyme complex that catalyzes the production of superoxide anion (O2−) and other ROS. The enzyme complex includes membrane-bound subunits p22phox and Nox2 (or 4 in the kidney), as well as cytosolic regulatory subunits p47phox, p67phox, and p40phox and the small GTP-binding protein Rac1/Rac2, all of which are expressed in the kidney (18, 39, 41). There is an emerging body of evidence demonstrating that mineralocorticoids, like ANG II, may activate NADPH oxidase in various tissues (3, 22, 41). Results from the present investigation indicate that MR activation increases kidney tissue NADPH oxidase activity, in part via activation of membrane-bound Nox2 as well as the cytosolic p47phox subunits. The resulting generation of ROS appears to be associated with increased redox-sensitive ROK-induced IRS-1 Ser phosphorylation, and increases in its proteosomal degradation (34). Reductions in IRS-1 would, in turn, result in less engagement of IRS-1 docking protein with PI3-K and decreased downstream Akt phosphorylation/activation, as observed in Ren2 kidneys in this investigation.
There are several potential mechanisms by which MR antagonism may improve Akt phosphorylation and filtration barrier/podocyte injury observed on TEM ultrastructural analysis. A recent report suggests that aldosterone increases proteosomal degradation of the IRS-1 docking protein in vascular smooth muscle cells (12). Commensurate with our results in the kidney, in vitro treatment with a MR antagonist abolished the increased ubiquitination/reduction in IRS-1 levels and Akt phosphorylation in vascular smooth muscle cells. It was also observed that treatment with antioxidants such as N-acetylcysteine or an inhibitor of the ubiquitin proteosomal pathway prevented aldosterone-induced reductions of IRS-1 and associated decreases in Akt activation in response to insulin (12). Collectively, these observations suggest that aldosterone likely acts through MR-mediated stimulation of redox-sensitive Ser kinase signaling pathways (1, 12) to promote proteosomal degradation of IRS-1, with consequent reductions in PI3-K/Akt. The resultant impairment in insulin metabolic signaling likely promotes tissue remodeling in the renal glomerulus as well as in conventional insulin-sensitive tissues (6, 16).
RhoA/ROK appears to be a particularly attractive target as RAAS downstream effectors of IRS-1 degradation/Akt phosphorylation because ROK activation contributes to podocyte injury/remodeling (16, 28, 41). Rho GTPases are essential in a diverse role of cellular function including cell proliferation metabolism and ultimately remodeling (42). In this context, RhoA, when activated, is known to regulate actin stress fibers and focal adhesion complexes that are mediated by ROK signaling (16, 28). Recent evidence suggests that ROK regulates α-smooth muscle actin activity in glomerular mesangial cells (34). Our observations support a similar process and further suggest that aldosterone mediates ROK suppression of desmin, a podocyte-specific cytoskeletal filament protein. ROK has been shown to be activated by NADPH oxidase-mediated oxidative stress, an important mediator of tissue remodeling in the Ren2 rat (18). Thus MR signaling promotes redox-sensitive ROK activation and reduces insulin metabolic signaling leading to filtration barrier/podocyte injury.
Our data support the notion that MR antagonism can reduce redox-mediated inhibition of Akt activation in kidney cortical tissue and associated alterations in filtration barrier/podocyte function in a rodent model of activated RAAS activity and albuminuria. These beneficial effects were obtained with a very low dose of Sp, independent of reductions in SBP. It should be noted that without measurements of glomerular filtration rate and renal blood flow, one cannot be certain that the improvements in insulin metabolic signaling, oxidative stress, slit pore membrane integrity, and albuminuria are not due, in part, to hemodynamic changes within the kidney. Data from this and previous research suggest there may be dual effects between MR and AT1R blockade not fully differentiated in this report (14, 43). However, these new data add to previous observations that increased kidney tissue oxidative stress contributes to albuminuria and that RAAS interruption with an AT1R and/or MR blockade attenuates these changes (39). It is also uncertain whether MR antagonism with Sp in the kidney is totally due to nonselective blockade of the MR. Indeed, even with the very low dose of Sp used in this investigation antiandrogenic properties may have contributed to the beneficial renal effects of this drug in the male Ren2 rats. The specific mechanisms of the beneficial effects of MR blockade on glomerular structure and function remain to be fully elucidated.
In conclusion, interruption of MR signaling is an attractive strategy to reduce redox-mediated glomerular damage, because increased activation of these receptors is linked to multiple pathological mechanisms including oxidative stress, inflammation, altered metabolic signaling, apoptosis, and fibrosis in cardiovascular and renal tissue. Moreover, MR antagonism has provided proven benefits in terms of cardiovascular morbidity and mortality (29). A better understanding of the mechanisms underlying the participation of MR activation in RAAS-mediated oxidative stress could thereby identify new therapeutic targets and potentially reduce the development and progression of kidney disease as well as reducing cardiovascular disease.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01 HL-73101-01A1 to J. R. Sowers and P01 HL-51952 to C. M. Ferrario, the Department of Veterans Affairs Merit System (0018 for J. R. Sowers) and Veterans Integrated Service Network (VISN) 15 (A. Whaley-Connell), and the Missouri Kidney Program (A. Whaley-Connell).
Acknowledgments
The authors acknowledge Brenda Hunter and Elaine Rehmer for editing this manuscript.
REFERENCES
- 1.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem 275: 9047–9054, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Brown NJ, Nakamura S, Ma L, Nakamura I, Donnert E, Freeman M, Vaughan DE, Fogo AB. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 58: 1219–1227, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Calle C, Campión J, García-Arencibia M, Maestro B, Dávila N. Transcriptional inhibition of the human insulin receptor gene by aldosterone. J Steroid Biochem Mol Biol 84: 543–553, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension 25: 1014–1020, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol 186: 1–20, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Diah S, Zhang GX, Nagai Y, Zhang W, Gang L, Kimura S, Hamid MR, Tamiya T, Nishiyama A, Hitomi H. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp Cell Res 314: 3654–3662, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int 66: 1493–1502, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Funder JW The nongenomic actions of aldosterone. Endocr Rev 26: 313–321, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharmacol 568: 242–247, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K, Wakino S, Kanda T, Homma K, Sugano N, Saruta T. Molecular mechanisms and therapeutic strategies of chronic renal injury: role of rho-kinase in the development of renal injury. J Pharmacol Sci 100: 29–33, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension 50: 750–755, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 294: H2614–H2618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi Y, Yamada M, Imakiire T, Kushiyama T, Higashi K, Hyodo N, Yamamoto K, Oda T, Suzuki S, Miura S. A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol 192: 595–603, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes 57: 714–723, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Koshikawa S, Nishikimi T, Inaba C, Akimoto K, Matsuoka H. Fasudil, a Rho-kinase inhibitor, reverses l-NAME exacerbated severe nephrosclerosis in spontaneously hypertensive rats. J Hypertens 26: 1837–1848, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, Hayden MR, Wei Y, Ferrario C, Sowers JR. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab 295: E110–E116, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JK, Tsai SY. Multiple hormone response elements can confer glucocorticoid regulation on the human insulin receptor gene. Mol Endocrinol 8: 625–634, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Lehtonen S Connecting the interpodocyte slit diaphragm and actin dynamics: emerging role for the nephrin signaling complex. Kidney Int 73: 903–905, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation 109: 2792–2800, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16: 2906–2912, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol 28: 1511–1518, 2008. [DOI] [PubMed] [Google Scholar]
- 23a.Morris EM, Whaley-Connell A, Thyfault JP, Britton SL, Lock LG, Wei Y, Ibdah JI, Sowers JR. Low aerobic capacity and high fat diet contributes to oxidative stress and IRS-1 degradation in the kidney. Am J Nephrol 30: 112–119, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol 17: 3438–3446, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of pro-angiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res 28: 925–936, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant 22: 1314–1322, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes 57: 1683–1692, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Sander M, Bader M, Djavidani B, Maser-Gluth C, Vecsei P, Mullins J, Ganten D, Peters J. The role of the adrenal gland in hypertensive transgenic rat TGR(mREN2)27. Endocrinology 131: 807–814, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Schiffrin EL Effects of aldosterone on the vasculature. Hypertension 47: 312–218, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148: 3773–3780, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Sun GP, Kohno M, Guo P, Nagai Y, Miyata K, Fan YY, Kimura S, Kiyomoto H, Ohmori K, Li DT, Abe Y, Nishiyama A. Involvements of Rho-kinase and TGF-beta pathways in aldosterone-induced renal injury. J Am Soc Nephrol 17: 2193–2201, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int 73: 1385–1393, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Whaley-Connell AT, Rehmer J, Habibi J, Young J, Rehmer N, Patel K, Hayden MR, DeMarco VG, Ferrario CM, Ibdah JI, Sowers JR. Mineralocorticoid receptor blockade attenuates vascular apoptosis and injury. Hypertension 53: 158–165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2)27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of angiotensin II-mediated NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario C, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol 291: F1308–F1314, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 24: 1842–1847, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue C, Siragy HM. Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension 46: 584–590, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Zhao W, Ahokas RA, Weber KT, Sun Y. ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol 291: H336–H343, 2006. [DOI] [PubMed] [Google Scholar]